Abstract
Recognition that neurohormonal activation plays a central role in the pathogenesis of heart failure (HF) led to the development of angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers, mineralocorticoid receptor antagonists and beta blockers. While there has been substantial success with these neurohormonal blocking drugs in patients with HF with reduced ejection fraction (HFrEF), persistently high rates of morbidity and mortality in this population underscore the need for more effective therapies. As part of the systemic neurohormonal activation that takes place in patients with HF, systems that counteract the adverse effect of the renin angiotensin aldosterone system (RAAS) and sympathetic nervous system (SNS) are also activated. Evidence that neprilysin metabolizes many of the effector molecules produced by these counter-regulatory systems raised the possibility that inhibition of this enzyme might be beneficial. However, since angiotensin II is a substrate of neprilysin, inhibition of the enzyme alone would increase levels of this peptide. Thus, treatment strategies that combine RAAS blockade with neprilysin inhibition were sought. Recent large scale randomized clinical trials (RCTs) have provided compelling evidence that sacubitril-valsartan, an angiotensin receptor-neprilysin inhibitor (ARNI), is superior to an ACEI in reducing mortality and HF hospitalization and in improving quality of life in patients with stage C HFrEF. In these trials, sacubitril-valsartan was found to be safe and well tolerated. This review presents the rationale for using ARNIs, describes the RCTs showing their efficacy, summarizes updated recommendations from recent guidelines, and provides practical points about ARNI initiation and up-titration.
Keywords: Heart failure, Neprilysin, Angiotensin receptor inhibitor, Natriuretic peptides
INTRODUCTION
Heart failure (HF) is a major global public health problem.1),2),3) The estimated 45–67 million adult patients around the world who suffer from HF experience increased likelihood of being hospitalized, reduced survival and markedly diminished quality of life. The staggering costs related to HF, which are mainly due to repeated hospitalizations and the loss of productivity in patients with HF, impose a severe economic burden on virtually every country around the world. While therapeutic advances have improved the outlook for patients with HF, more work still needs to be done in order to deal with this global pandemic.
NEUROHORMONAL ACTIVATION IN HF
Approximately half of the worldwide HF population has HF with reduced ejection fraction (HFrEF). In these patients, neurohormonal activation is recognized as playing a critical role in the development and progression of their disease.4),5),6) Systems such as the renin angiotensin aldosterone system (RAAS) and sympathetic nervous system (SNS) are activated early in the course of the disease in response to myocardial injury or increases in load on the heart. As HF progresses, neurohormonal activation intensifies and circulating levels of the main effector molecules of these systems are increased in a step-wise manner.7) Down-stream effects of angiotensin II (Ang II), aldosterone and the catecholamines, the major signaling molecules of the RAAS and SNS, promote vasoconstriction and salt and water retention. They also stimulate maladaptive cardiac remodeling, a process characterized by increases in cardiac chamber volumes, muscle mass and interstitial fibrosis.8) Conformational changes in the remodeled left ventricle which becomes more spherical in shape reduces mechanical efficiency and leads to the development of mitral valvular regurgitation. Positive feedback loops between neurohormonal systems further enhance these adverse effects. The net effect is a vicious cycle that results in progressive deterioration in cardiac structure and function and ultimately to the clinical events, including sudden cardiac death, premature mortality and repeated hospitalizations. Understanding the importance of neurohormonal activation in the progression of HF resulted in the development of agents designed to inhibit this process. There is now abundant evidence that angiotensin converting enzyme inhibitors (ACEIs),9) angiotensin receptor blockers (ARBs),10) mineralocorticoid receptor antagonists (MRAs)11),12) and beta blockers13),14),15),16) all reduce morbidity and mortality and improve quality of life of patients with Stage C HFrEF. As a result, these agents are widely recognized as being the cornerstones of HFrEF therapy and they are given the highest level of recommendation in contemporary guidelines for the pharmacologic treatment of HF.
In addition to activation of the RAAS and SNS, other systems that mediate effects that would be expected to have beneficial effects in patients with HF are also activated. These include molecules such as the natriuretic peptides (NPs), prostaglandins, bradykinin, adrenomedullin and apelin which promote vasodilation and diuresis and have anti-fibrotic and anti-remodeling properties. Until recently strategies to increase levels of these molecules in the circulation have been lacking. Neprilysin, a neutral endopeptidase found on the surface of many cells and in the circulation, is involved in the metabolism of many counter-regulatory vasoactive peptides including the NPs.17) Although inhibition of neprilysin would be expected to increase circulating and tissue levels of these peptides,18),19),20) whether or not this strategy benefit patients with HF required proof in well-designed clinical trials. The focus of this review is to describe the results of recent clinical trials with a new class of drugs, the angiotensin receptor-neprilysin inhibitors (ARNIs) in patients with HF and to provide practical advice about how to use them effectively.
CLINICAL TRIALS WITH COMBINED NEPRILYSIN-ANGIOTENSIN INHIBITION IN PATIENTS WITH HFrEF
In addition to contributing to the breakdown of counter-regulatory peptides, neprilysin also is involved in the metabolism of Ang II. Since the use of a neprilysin inhibitor alone would increase levels of Ang II, a peptide known to mediate long-term adverse effects on the heart and blood vessels, as well as the levels of potentially beneficial peptides neprilysin inhibition alone would not be expected to be useful in patients with HF. A drug which combined both neprilysin and RAAS system inhibition, however, might be quite beneficial in patients with HFrEF.
Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) and other trials with omapatrilat
Initial clinical trials of combined neprilysin-angiotensin inhibition were performed using omapatrilat, a drug that blocked both the angiotensin converting enzyme (ACE) and neprilysin pathways.21) Based on early evidence of success, the OVERTURE study was designed. In this clinical trial, 5,770 patients with New York Heart Association (NYHA) class II–IV HF were randomly assigned to receive either enalapril or the combined ACEI-neprilysin inhibitor omapatrilat. The primary endpoint was a composite of death or HF hospitalization. The results, however, indicated that omapatrilat was not superior to an ACEI alone in reducing the risk of death and hospitalization in patients with chronic HF.22) In the Omapatrilat Cardiovascular Treatment Assessment Versus Enalapril (OCTAVE) study, 25,267 hypertensives were randomized to omapatrilat or enalapril.23) While omapatrilat was associated with a greater reduction in blood pressure, rates of angioedema were higher than with the ACEI, particularly in blacks and smokers. This was likely due to the fact that bradykinin, a peptide known to cause angioedema, is a substrate for ACE as well as neprilysin so that blockade of both enzymes simultaneously by omapatrilat elevated levels of circulating bradykinin. As a consequence of the higher incidence of this potentially lethal side effect, further development of omapatrilat was terminated.
Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF)
ARNIs are agents that combine a neprilysin inhibitor with an ARB, a strategy designed to augment levels of NPs and other counter-regulatory peptides while blocking the effects of Ang II. Since this approach would be less likely than omapatrilat to increase the incidence of bradykinin related angioedema. The possibility that sacubitril-valsartan, an ARNI would be superior to enalapril, an ACEI that had previously been shown to be effective in reducing morbidity and mortality, in improving outcomes in patients with HFrEF was tested in the PARADIGM-HF trial, the largest study carried out to date in a HF population.24) PARADIGM-HF was an international study that enrolled 8,442 patients from 47 countries around the world. Key inclusion and exclusion criteria are listed in Table 1. Patients who fulfilled these criteria were entered into single blind run-in period where they were treated initially with enalapril at a dose of 10 mg twice daily and then with sacubitril-valsartan at a twice daily dose of 49/51 mg which was then increased 97/103 mg twice daily. Due to differences in bioavailability, the 97 mg of valsartan in the combination drug is equivalent to 160 mg when valsartan is administered as a stand-alone drug. Enalapril was selected as the comparator to sacubitril-valsartan based on the fact that it had been shown to be highly effective in improving outcomes including mortality and hospitalizations in the Studies of Left Ventricular Dysfunction (SOLVD) program.25) To minimize the risk of angioedema, the ACEI was held for a day prior to the transition to the ARNI and the ARNI was held for a day before patients were randomized to either enalapril 10 mg twice daily or sacubitril-valsartan 97/103 mg twice daily in the double-blind portion of the study. The primary end-point of PARADIGM-HF was a composite of cardiovascular (CV) mortality and HF hospitalization. Baseline characteristics of the study population are summarized in Table 2. Many of the many patients would be considered to be at relatively ‘low risk' for future events based on the presence of NYHA class II symptoms and the lack of recent HF hospitalization. Regardless of the seemingly low risk profile of the population, over one in 4 of the patients treated with enalapril experienced either CV mortality or HF hospitalization during the relatively brief median follow-up period of 27 months.26) The high rate of events in PARADIGM-HF patients who were randomized demonstrates the substantial risk for future events in HFrEF patients, even when they appear to be stable and are being treated with what was considered to be optimal medical therapy.
Table 1. Patient entry criteria used in the PARADIGM-HF study.
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| • Age ≥18 years; LVEF ≤40% (later changed to ≤35% | • Systolic BP <100 mmHg at screening or <95 mmHg at randomization |
| • Current symptomatic HF (NYHA class II–IV) | • eGFR <30 mL/min/1.73m2 at screening or at randomization or a decrease in eGFR >25% (later amended to 35%) between screening and randomization |
| • Taking an ACEI or ARB with stable dose of ACEI or ARB and beta blocker for at least 4 weeks | • History of angioedema or other unacceptable side effects with ACEIs or ARBs |
| • BNP ≥150 pg/mL or NT-proBNP ≥600 pg/mL or BNP ≥100 pg/mL or NT-proBNP ≤400 pg/mL for patients hospitalized within the past 12 months |
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = B-type natriuretic peptide; BP = blood pressure; eGFR = estimated glomerular filtration rate; HF = heart failure; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; PARADIGM-HF = Prospective Comparison of angiotensin receptor-neprilysin inhibitor (ARNI) with angiotensin converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and Morbidity in Heart Failure.
Table 2. Study dose titration in PIONEER-HF.
| • Starting dose level based on SBP algorithm | |
| - If SBP 100 to <120 mmHg: sacubitril-valsartan 24/26 mg or enalapril 2.5 mg twice daily | |
| - If ≥120 mmHg: sacubitril-valsartan 49/51 mg or enalapril 5 mg twice daily | |
| • At week 1, dose titrated upwards if SBP >110 mmHg | |
| • At week 2,4,6, dose titrated upwards if SBP >100 mmHg | |
| • Target dose | |
| - Sacubitril-valsartan 97/103 mmHg or enalapril 10 mg twice daily | |
| • Clinical assessment and judgment permitted | |
PIONEER-HF = Comparison of Sacubitril-Valsartan versus Enalapril on Effect on N-terminal pro-B-type natriuretic peptide (NT-proBNP) in Patients Stabilized from an Acute Heart Failure Episode; SBP = systolic blood pressure.
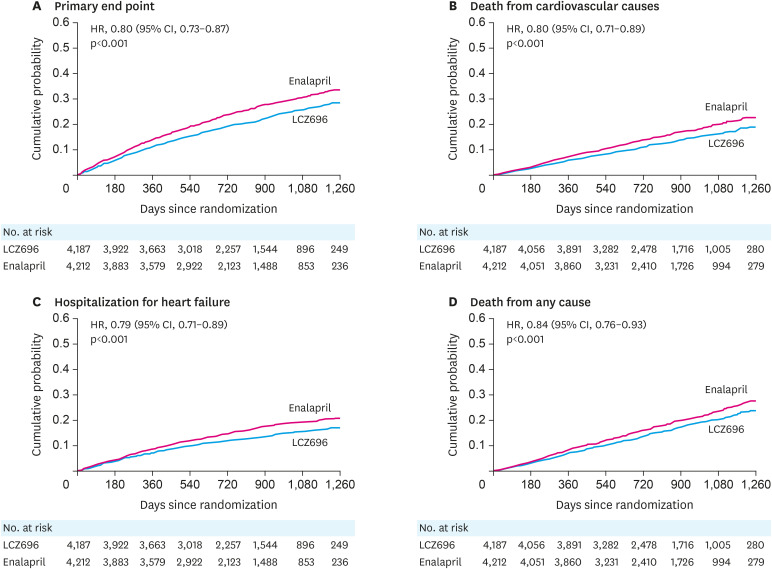
PARADIGM-HF was stopped prematurely due to clear-cut evidence of the superiority of sacubitril-valsartan over enalapril. As shown in Figure 1, there was a 20% risk reduction for the primary morbidity/mortality endpoint in the sacubitril-valsartan compared to the enalapril treated patients (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.73–0.87; p<0.001). Further analysis showed that the beneficial effects were due equally to reductions in CV mortality which was reduced by 20% (HR, 0.80; 95% CI, 0.71–0.89; p<0.001) and HF hospitalization which was reduced by 21% (HR, 0.79; 95% CI, 0.71–0.89; p<0.001). All-cause mortality was also reduced by 16% with sacubitril-valsartan compared to enalapril (HR, 0.84, 95% CI, 0.71–0.93; p<0.001). The results of PARADIGM-HF also showed a significant 20% risk reduction in sudden death and a 21% risk reduction in death due to worsening HF. While it is not possible to extrapolate precisely what the benefit of sacubitril-valsartan over placebo on overall survival might have been, it can be estimated as being approximately 30–35% based on the 15–20% reduction that was seen with ACEIs in earlier trials such as the SOLVD study and the additional 16% reduction seen when sacubitril-valsartan was compared to enalapril. Quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) was also significantly improved in the sacubitril-valsartan compared to the enalapril treated patients. As shown in Figure 2, extensive subgroup analysis looking at groups of patients based on a variety of different baseline characteristics failed to identify subgroup cohorts which were either more or less likely to have done better with sacubitril-valsartan than with enalapril. In other words, the beneficial effects of the ARNI compared to the ACEI extended over the entire population. Moreover, the sacubitril-valsartan combination was generally well tolerated in the PARADIGM-HF population. For the most frequent side effects seen in the study that occurred in over 5% of the patients, only symptomatic hypotension occurred more commonly in the patients who were receiving the ARNI. However, less than 1% of patients in either treatment arm of PARADIGM-HF discontinued study drug during the course of the trial. Neither worsening renal function, hyperkalemia nor cough was significantly different between the treatment arms. Angioedema occurred in more patients who received the sacubitril-valsartan combination than in the patients who received enalapril (19 compared to 10 patients) but overall, this side effect was uncommon with both drugs. No patients in either study group experienced airway compromise or required mechanical airway protection due to angioedema. It should be noted, however, that there were only 413 black patients enrolled in PARADIGM-HF.
Figure 1. Kaplan-Meier curves for key endpoints of the PARADIGM-HF trial. Data from reference 24 with permission.
CI = confidence interval; HR = hazard ratio; PARADIGM-HF = Prospective Comparison of angiotensin receptor-neprilysin inhibitor (ARNI) with angiotensin converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and Morbidity in Heart Failure.
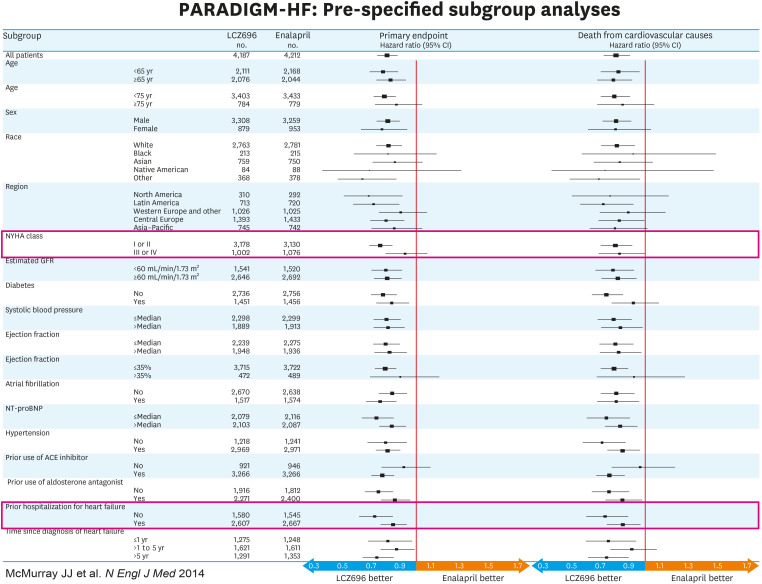
Figure 2. Pre-specified subgroup analyses. Sub-groups categorized according to NYHA class and history of previous hospitalization due to heart failure are outlined in the red bars. The results show superiority of the sacubitril-valsartan combination even in patients considered to be at a lower level of risk based on symptoms or history of heart failure hospitalization. Data from reference 24.
CI = confidence interval; CV = cardiovascular; NYHA = New York Heart Association; PARADIGM-HF = Prospective Comparison of angiotensin receptor-neprilysin inhibitor (ARNI) with angiotensin converting enzyme inhibitor (ACEI) to Determine Impact on Global Mortality and Morbidity in Heart Failure.
As already mentioned, extensive subgroup analysis failed to demonstrate patient characteristics that were associated with reduced superiority of sacubitril-valsartan to enalapril. Even the most stable patients (with risk defined as time since last HF hospitalization or Meta-Analysis Global Group in Chronic Heart Failure [MAGGIC] risk score) had better outcomes with sacubitril-valsartan than with enalapril.27) An issue that is often raised about the PARADIGM-HF results is whether the superiority of sacubitril-valsartan over enalapril persisted as patients got older. Jhund et al.28) showed that when patients were separated into quartiles according to age (with the upper quartile including patients who were greater than 75 years), there was no difference in the superiority of sacubitril-valsartan over enalapril between younger and older patients. When the investigators looked at age as a continuous variable, no evidence of reduced efficacy was detected as patients got older. Another common question is whether the benefits of sacubitril valsartan are seen throughout the spectrum of left ventricular ejection fraction (LVEF) within the HFrEF population. In PARADIGM-HF investigators there were strong inverse relationships between LVEF and risk of the composite endpoint of CV mortality and HF hospital, its components, and all-cause mortality with the likelihood of these events increasing progressively as the baseline LVEF became lower.29) However, the superiority of sacubitril-valsartan over enalapril was maintained at all levels of LVEF.
The influence of baseline systolic blood pressure (SBP) on outcomes in PARADIGM-HF was also examined.30) Patients with the lowest SBP were found to be at the greatest risk for mortality whereas there was a U-shaped relation between SBP and the rate of HF hospitalization. Tolerability of sacubitril-valsartan and enalapril was generally similar across the range of SBPs in the trial. Patients with low baseline SBP who were randomized to sacubitril-valsartan experienced the same relative benefit over enalapril as patients with higher baseline SBPs. However, it is important to recognize that patients who were ultimately randomized in the study had already demonstrated that they were able to tolerate maximum doses of both drugs during the single blind run-in period of the trial.
The likelihood of improvement in quality of life has also been studied in an analysis of the effects of the 2 therapies studied in PARADIGM-HF on the KCCQ.31) Overall summary scores were better in patients treated sacubitril-valsartan compared with those treated with enalapril. There was consistency in this results across most domains and these differences in the effect on quality of life between the 2 drugs was sustained beyond 8 months of treatment. In addition, there was a significantly greater likelihood of a very large improvement in the KCCQ score (i.e., ≥20-point change) in the sacubitril-valsartan compared to the enalapril treated groups (20.5% vs. 12.1% of patients in each group, respectively).
Comparison of Sacubitril-Valsartan versus Enalapril on Effect on N-terminal pro-B-type natriuretic peptide (NT-proBNP) in Patients Stabilized from an Acute Heart Failure Episode (PIONEER-HF)
While PARADIGM-HF demonstrated the efficacy and safety of the sacubitril-valsartan combination in the HFrEF population, additional questions regarding this therapy remained. The patients enrolled into PARADIGM-HF were already being treated with an ACEI or ARB at the time of entry into the study and all were clinically stable. The PIONEER-HF trial was designed to compare the safety and efficacy of the sacubitril-valsartan combination with enalapril in patients who were hospitalized for treatment of worsening HF.32) Entry criteria are shown in Table 2. Patients were included in PIONEER-HF only after intravenous vasoactive drugs had been discontinued and diuretic dose had been stabilized. SBP was required to be ≥100 mmHg and the median value in the study population was 118 mmHg. Patients whose SBP exceeded 120 mmHg were started on 49/51 mg of sacubitril-valsartan or 5 mg enalapril, both twice daily while those with SBP between 100–120 were started on 2.5 mg enalapril or 24/26 sacubitril-valsartan, both twice daily. In contrast to the patients in PARADIGM-HF, all of whom were being treated with an ACEI or ARB at the time of entry into the study, more than half of the patients in PIONEER-HF were not receiving either an ACEI or ARB at the time of randomization.
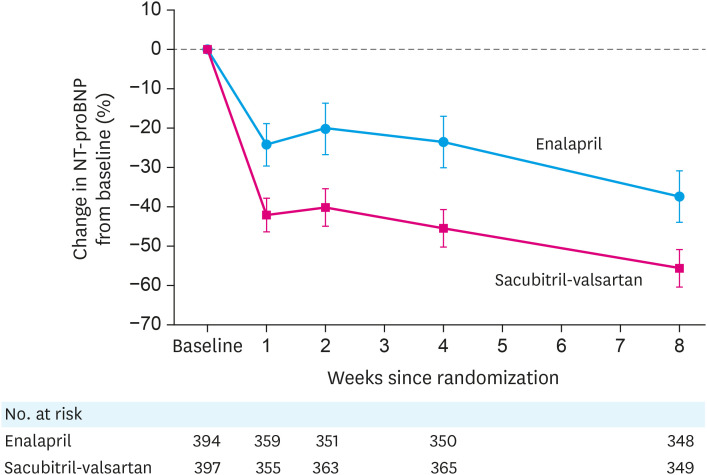
The primary efficacy end-point of PIONEER-HF was time-averaged proportional change in the NT-proBNP concentration from baseline through weeks 4 and 8. As shown in Figure 3, although levels of the NP were reduced in the enalapril arm, they were reduced more rapidly and to a significantly greater extent in patients in the sacubitril-valsartan arm of the study. Differences from baseline NT-proBNP levels between the 2 drugs were seen at all time points after treatment was initiated. A composite of clinical events (including the incidence of a composite of death, rehospitalization for HF, implantation of a left ventricular (LV) assist device, or inclusion on the list of patients eligible for heart transplantation) was designated as a pre-defined exploratory endpoint. Over the 8 weeks follow-up period of the study, the risk of these events was 46% less in the sacubitril-valsartan patients compared to the enalapril treated patients (HR, 0.54; 95% CI, 0.37–0.79; p=0.001). In particular, patients in the sacubitril-valsartan arm experienced significantly less likelihood of HF re-hospitalization. This latter finding was consistent with a results from a post-hoc sub-study of PARADIGM-HF which showed a similar reduction in HF rehospitalization with sacubitril-valsartan compared to enalapril.33) The rates of worsening renal function, hyperkalemia, symptomatic hypotension, and angioedema did not differ significantly between the 2 groups, demonstrating the general tolerability and safety of initiating drug in-hospital.
Figure 3. Levels of NT-proBNP over 8 weeks according to drug treatment in PIONEER-HF. The time-averaged reduction in the NT-proBNP concentration was significantly greater in the sacubitril-valsartan group than in the enalapril group; the ratio of the geometric mean of values obtained at weeks 4 and 8 to the baseline value was 0.53 in the sacubitril-valsartan group as compared with 0.75 in the enalapril group (percent change, −46.7% vs. −25.3%; ratio of change with sacubitril-valsartan vs. enalapril, 0.71; 95% CI, 0.63–0.81; p<0.001). Data from reference 32 with permission.
CI = confidence interval; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PIONEER-HF = Comparison of Sacubitril-Valsartan versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode.
Prospective Comparison of ARNI with ARB Global Outcomes in Heart Failure with Preserved Ejection Fraction (PARAGON-HF)
Treatment options for patients with HF with preserved ejection fraction (HFpEF) are limited and there are no strategies that have been shown to improve survival in this population. The success of the sacubitril-valsartan combination in patients with HFrEF and the results of Prospective Comparison of ARNI with ARB on Management of HFpEF (PARAMOUNT),34) a phase 2 study in which sacubitril-valsartan was more effective than valsartan alone in reducing levels of NT-proBNP over a 12 week period, provided the rationale for studying the effects of ARNI therapy in the HFpEF population. The PARAGON-HF study randomized 4,822 patients with symptomatic HF, an LVEF ≥0.45, elevated NP levels and evidence of structural heart disease to either sacubitril-valsartan at 97/103 mg twice daily or valsartan alone at 160 mg twice daily.35) For the primary efficacy endpoint of the study, which was a composite of CV mortality and HF hospitalization, there was a trend towards a reduction in the primary outcome (HR, 0.87, 95% CI, 0.75–1.01; p=0.06) that was due predominantly by a 15% reduction in HF hospitalizations. An insignificant 5% risk reduction for CV death was also noted. Whereas results of PARADIGM-HF showed that the superiority of sacubitril-valsartan over enalapril was uniform across all patient subgroups in which it was assessed, the results of PARAGON-HF showed heterogeneity in outcomes between groups. In particular, a more favorable effect of sacubitril-valsartan compared to valsartan was found in women compared to men and in patients whose LVEF was below the median value for the entire study population. As with the studies that were performed in the HFrEF population, the results of PARAGON-HF demonstrated the safety and general tolerability of the sacubitril-valsartan combination.
Other studies with ARNIs in patients with HF
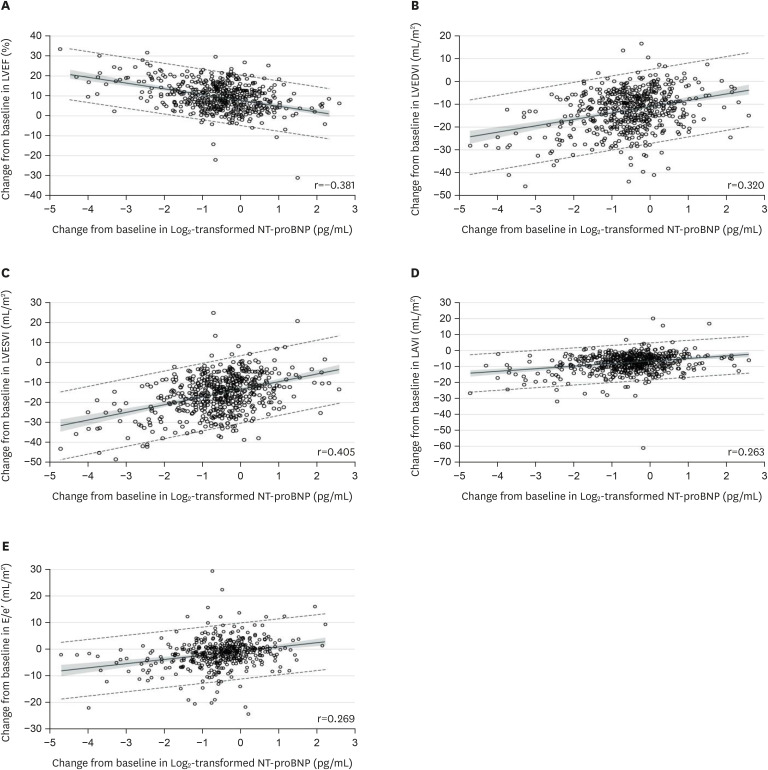
In PARADIGM-HF, treatment with sacubitril-valsartan resulted in a significantly greater reduction in levels of NT-proBNP than was seen with enalapril.24) While NP levels have been used as surrogates for clinical outcomes, they are also related to LV wall stress which is a major factor in promoting maladaptive cardiac remodeling. Increased LV wall stress stimulates the production and release of B-type natriuretic (BNP) in the heart.36) A reduction in BNP or in NT-proBNP, which is a fragment of the BNP molecule, would be indicative of a reduction in LV wall stress. In the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF) study, the association of change in NTpro-BNP following initiation of sacubitril-valsartan with change in cardiac structure and function was assessed in a population of 794 patients with HFrEF.37) In this open label study, sacubitril-valsartan was started in a cohort of patients with HFrEF defined as LVEF ≤0.40. Blood samples for NT-proBNP were obtained at baseline and periodically over a year follow-up period and a transthoracic echocardiogram (TTE) was performed at baseline, 6- and 12-month. The TTE was interpreted by a core laboratory in a blinded fashion. The results showed that initiation of sacubitril-valsartan was associated with a rapid and significant reduction of NT-proBNP, with the majority of the reduction occurring within the first 2 weeks of initiating drug. Treatment with sacubitril-valsartan was also associated with reductions in LV end-diastolic and end-systolic volume indices (LVEDVI and LVESVI) of 12.25 and 16.25 mL/m2, respectively (both p<0.001) after 12 months of therapy, a 9.6% absolute increase in LVEF (p<0.001) and a reduction in left atrial volume index (LAVI). A shown in Figure 4, there was a modest but highly significant association between the changes in LVEF, LVEDVI, LVESVI and LAVI and reductions in NT-proBNP level. Patients who had experienced the largest reduction in NT-proBNP and LVESVi by 6 months had lowest rates of subsequent death or HF hospitalization by 12 months.
Figure 4. Association between changes in LV structure and NT-proBNP levels in PROVE-HF. Scatterplots detailing correlations between baseline and 12-month concentrations of Log2-transformed NT-proBNP and contemporaneous change in LVEF, LVEDVI, LVESVI, LAVI, and E/e′. A mean regression line is detailed with 95% prediction limits demonstrated in dashed lines. The shaded regions indicate 95% confidence limits. Data from reference 37 with permission.
LV = left ventricular; LVEF = left ventricular ejection fraction; LVEDVI = left ventricular end-diastolic volume index; LVESVI = left ventricular end-systolic volume index; LAVI = left atrial volume index; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Biomarkers associated with profibrotic signaling including aldosterone, sST2, TIMP-1, MMP-9, procollagen type I N propeptide, and aminoterminal propeptide of type III collagen are increased in patients with HFrEF and are believed to be associated with the increase in cardiac fibrosis. A substudy of PARADIGM-HF in which levels of these profibrotic biomarkers were measured at baseline and after 6 months of therapy in 2,067 patients reported that the levels of these biomarkers are significantly more decreased with sacubitril-valsartan than with enalapril.38) Since elevation of many of these biomarkers is associated with clinical events in patients with HFrEF, these findings suggest that the reduction in their levels with sacubitril-valsartan is related to the reduction in clinical events seen in PARADIGM-HF.24) It also possible that the combined effects of a reduction in profibrotic signaling along with a the reversal of the remodeling process resulted in the reduction in ventricular arrhythmias that has been reported with the use of sacubitril-valsartan.39) Although the complex molecular mechanisms through which sacubitril-valsartan stimulates reversal of the remodeling process and brings about these favorable changes in cardiac structure and function have not been fully defined, a systems biology approach provided evidence that the 2 drugs in the molecule work through separate pathways.40) Valsartan was found to improve cardiac remodeling by inhibiting members of the guanine nucleotide-binding protein family, while sacubitril attenuated cardiomyocyte cell death, hypertrophy, and impaired myocyte contractility by inhibiting phosphatase and tensin homolog expression.
The Effect of Sacubitril-Valsartan versus Enalapril on Aortic Stiffness in Patients with Heart Failure and Reduced Ejection Fraction (EVALUATE-HF) study assessed the effects of treatment with sacubitril-valsartan compared to enalapril on central aortic stiffness and cardiac remodeling.41) Although there was no difference in the change in aortic stiffness between the treatment groups, there were significantly greater reductions in LVEDVI, LVESVI and LAVI in the sacubitril-valsartan treated patients than in the enalapril treated ones. While these results failed to support the possibility that sacubitril-valsartan favorably affects aortic stiffness, they are consistent with the possibility that the favorable effects on cardiac remodeling demonstrated in both EVALUATE-HF and PROVE-HF are due to direct effects on the heart rather than to effects on the vasculature.
GUIDELINE RECOMMENDATIONS
In 2016, largely in response to the results of the PARADIGM-HF study, the American College of Cardiology/American Heart Association/Heart Failure Society of America published an update to the 2013 guideline for pharmacologic therapy for stage C HFrEF.42),43) The specific recommendation for RAAS inhibition with an ACEI, ARB or ARNI is shown in Figure 5. In this document ACEIs, ARBs and ARNIs all receive a Class I recommendation based on their ability to reduce morbidity and mortality in stage C patients with chronic HFrEF. The guideline further states that these agents should be used in association with a guideline recommended beta blocker. The guideline also recommends that patients with chronic symptomatic HFrEF who have class II or III symptoms while being treated with an ACEI or ARB should have those drugs replaced by an ARNI to further reduce morbidity and mortality.
Figure 5. The 2016 American College of Cardiology/American Heart Association/Heart Failure Society of America heart failure guideline update on pharmacologic treatment for stage C heart failure with reduced ejection fraction. Data from reference 42 with permission.
ACE = angiotensin converting enzyme; ARNI = angiotensin receptor-neprilysin inhibitor; ARB = angiotensin receptor blocker; COR = class of recommendation; LOE = level of evidence; MYHA = New York Heart Association.
An even more recent update on treatment was published in 2019 by the Heart Failure Association of the European Society of Cardiology.44) This document reaffirmed that sacubitril-valsartan is recommended as a replacement for an ACEI or ARB to reduce the risk of HF hospitalization and death in ambulatory patients with HFrEF who remain symptomatic despite optimal medical treatment with an ACEI, beta blocker and MRA. In response to results of PIONEER-HF which enrolled patients during the course of a HF hospitalization, the European Society of Cardiology consensus statement states that Initiation of sacubitril-valsartan rather than an ACEI or an ARB may be considered for patients hospitalized with new-onset HF or decompensated chronic HF to reduce the short-term risk of adverse events and to simplify management (by avoiding the need to titrate ACEI first and then switch to sacubitril-valsartan).
PRACTICAL ISSUES REGARDING THE INITIATION, UPTITRATION AND MAINTENANCE OF ARNIs
Who should be treated with an ARNI?
The clinical trial results reviewed in this manuscript provide compelling evidence that sacubitril-valsartan should be considered as first line therapy in patients with class C HFrEF. An ARNI can be started either in-hospital once the patient has stabilized (according to criteria outlined in the PIONEER-HF study) or in the out-patient setting. There is no need to first initiate an ACEI or ARB and then switch the patient to sacubitril-valsartan. The ARNI can be started de novo as the drug of choice.
Should ARNI's be used as monotherapy or in association with other agents? If not, is there a specific order in which neurohormonal modulating drugs should be initiated?
In patients with class C HFrEF ARNI's should be used in association with an evidence based beta blocker (i.e., carvedilol, either short or long-acting, metoprolol succinate or bisoprolol) and an MRA. The order in which these agents should be used is left to the discretion of the clinician managing the patient's care. The practice of the author is that for hospitalized patients (who are generally volume overloaded when they admitted) to initiate an sacubitril-valsartan and an MRA early in the course. This approach is usually well tolerated provided that there has been a good response to diuretics and that the patient does not require vasoactive agents or mechanical circulatory support (MCS). A good rule of thumb for identifying the time to start sacubitril-valsartan in hospital is that the SBP over the preceding 6 hours should be ≥100 mmHg, diuretic dose should be stable and no intravenous vasodilators should have been administered over that time period. In addition, patients should not have required intravenous inotropic, pressor agents of MCS within the preceding 24 hours. In patients who present with new onset HF in the out-patient setting, the order in which drugs are initiated is flexible. Some of the variables used to decide include blood pressure, presence and severity of renal dysfunction, potassium level and magnitude of congestive signs and symptoms. In the opinion of the author of this review, simultaneous initiation of sacubitril-valsartan and an MRA should only be carried out in settings where follow-up laboratory measures can be readily obtained due to the fact that both of these drugs in elevating serum potassium.
What is the starting dose of sacubitril-valsartan?
The starting dose is based on initiation of sacubitril-valsartan in the PARADIGM-HF and PIONEER-HF trials. For patients being switched from an ACEI or ARB to sacubitril-valsartan in the out-patient setting, the starting dose will depend on the dose of the RAAS blocker that the patient had been taking. For ACEI doses ≤10 enalapril daily (or an equivalent dose of another ACEI), the starting dose of sacubitril-valsartan that is recommended is 24/26 mg twice daily. For patients who have been receiving ACEI doses above this level, the starting dose should be 49/51 mg twice daily. For all patients being switched from an ACEI to sacubitril-valsartan (or vice versa) there needs to be a mandatory 36-hour wash-out period of whichever drug is currently being taken to avoid overlapping effects on neprilysin inhibition, a situation that predisposes to angioedema due to the combined effects of these agents in increasing bradykinin levels. For ARB's, the starting dose is based on valsartan units. Patients taking ≤160 mg daily (or the equivalent dose of another ARB) should be started on 24/26 mg sacubitril-valsartan while patients taking higher doses of the ARB can be started at the 49/51 mg twice daily level. No washout period is required when patients are being switch from an ARB. Patients who are ACEI or ARB naïve should start on the 24/26 mg bid dose of sacubitril-valsartan. The author also starts at this low dose in patients with borderline SBP, evidence of chronic kidney disease or potassium levels that approach the upper limit of normal.
Patients who are started on sacubitril-valsartan during the course of a HF hospitalization should have the initial dose determined by their SBP. Patients whose SBP is >120 mmHg can be started on the intermediate 49/51 mg twice daily regimen while those with SBP between 100 and 120 mmHg should be started on a dose of 24/26 mg twice daily. Patients previously treated with an ACEI should have the drug stopped for at least 36 hours before the first dose of the ARNI.
What is the target dose of sacubitril-valsartan?
The target dose of sacubitril-valsartan, regardless of the start dose or clinical setting (i.e., clinic or hospital) where drug was initiated is 97/103 mg twice daily. In general, up-titration is performed every 2 weeks. Increases in serum creatinine or potassium levels or the onset of symptomatic hypotension may either delay or prohibit up-titration. In general, increases in serum creatinine levels ≤0.3 mg/dL or decreases in estimated glomerular filtration rate (eGFR) ≤25% from the patient's baseline are considered acceptable. Increases in serum potassium to levels ≤5.0 mEq/dL are generally tolerated. Reductions in blood pressure are seen commonly with initiation and during up-titration of sacubitril-valsartan. As will be discussed below, drug can usually be maintained or up-titrated regardless of blood pressure provided that the patient does not experience symptoms and there is no evidence of organ hypoperfusion. Sacubitril-valsartan should not be used in patients with a history of angioedema in response to an ACEI or ARB or with a history of hereditary angioedema.
Should drug be continued if patient is not able to tolerate target dose?
In a post-hoc analysis from PARADIGM-HF, the study population was divided according to whether patients received the maximal dose (97/103 mg sacubitril-valsartan or 10 mg enalapril twice daily) throughout the trial or had the dose of either of the drugs reduced during the course of the trial.45) Overall, 43% of those randomized to enalapril and 42% of those randomized to sacubitril/valsartan had dose of study drug reduced. In a time-updated analysis, any dose reduction was associated with a higher subsequent risk of the primary event (HR, 2.5; 95% CI, 2.2–2.7). Regardless, sacubitril-valsartan remained superior to enalapril and the overall effect on the primary endpoint of CV mortality and HF hospitalization following a dose reduction was similar (HR, 0.80; 95% CI, 0.70–0.93, p<0.001) to that observed in patients who had not experienced any dose reduction (HR, 0.79; 95% CI, 0.71–0.88; p<0.001). These results indicate that although a reduction in the dose of sacubitril-valsartan helps identify patients at increased risk for future events, the persistent benefit of this drug compared to standard ACEI therapy strongly suggests that it should be continued at the highest dose that is tolerated by the patient.
Are there strategies for initiating and up-titrating sacubitril-valsartan?
The most common side effect of treatment with sacubitril-valsartan is postural hypotension. In PARADIGM-HF it was noted to occur during the course of the study in 14.0% of patients treated with sacubitril-valsartan compared to 9.2% of patients treated with enalapril. However, hypotension leading to discontinuation of study drug occurred in less than 1% of patients in either study group, suggesting that it was either not severe or was manageable. In the PIONEER-HF population, hypotension was noted in 18% of patients in each of the 2 treatment groups. While this data is reassuring, the incidence of hypotension is likely greater in ‘real world’ settings as the patients who had been enrolled in the clinical trials had been carefully selected with requirements for ability to tolerate sacubitril-valsartan. These included ability to maintain study drug during the open label run-in period of PARADIGM-HF and a baseline SBP of at least 100 mmHg in PIONEER-HF.
When hypotension occurs, there is no hard and fast level of blood pressure mandating that drug should be discontinued or that the dose needs to be reduced. More important is whether or not the patient has evidence of end-organ perfusion. This can be detected by symptoms such as light headedness (or less commonly fatigue and impaired cognitive function) or elevations in laboratory values indicating organ injury. Elevation in creatinine or fall in eGFR are the most common laboratory indicators of tissue hypoperfusion but increases in liver enzymes can also be seen. It is worth remembering that in patients with HFrEF, these laboratory abnormalities can be due to other causes. In the absence of evidence of organ hypoperfusion, sacubitril-valsartan can be continued despite reduction in SBP to levels that are below the usual baseline value for the patient. When hypotension is accompanied by signs or symptoms of end-organ hypoperfusion, other causes for the reduction in blood pressure should be considered including the effects of other vasodilating agents or the presence of decreased intravascular volume due to GI disturbances, blood loss or excessive diuresis. Over-diuresis, though relatively common, can be difficult to detect. It is not uncommon for previously well tolerated doses of diuretic to become excessive when patients experience improved cardiac function and renal perfusion. It can also occur as a consequence of an increase in the level of peptides (e.g., NPs) that promote diuresis that occurs when neprilysin is inhibited. When over-diuresis is suspected, cautious down titration of the diuretic dose should be tried. Criteria for gain in weight along with contingency plans for increasing diuretic dose should be carefully discussed with the patient at the time the diuretic dose is cut back. In the author's experience, one third to one fourth of patients started on sacubitril-valsartan end up having a reduction in the dose of their loop diuretic.
Other common side effects of sacubitril-valsartan include worsening renal function and hyperkalemia. The occurrence of these side effects, however, was not found to be more common with this drug than with enalapril in either the PARADIGM-HF or PIONEER-HF trials. Post-hoc analysis of data from PARADIGM demonstrated that sacubitril-valsartan led to a slower rate of decrease in the eGFR and improved CV outcomes, even in patients with chronic kidney disease, despite causing a modest increase in the urinary albumin creatinine ration.46)
Management strategies for these side effects are similar to the approach taken when they occur with other RAAS blockers. When worsening renal function occurs as a consequence of hypotension, it can be treated as outlined above. Fluid loss due to gastrointestinal disturbances or excessive diuresis can also result in worsening renal function. When this occurs fluid replacement and/or temporary reduction in the dose of diuretic is the treatment of choice.
Hyperkalemia is a side effect common to all RAAS inhibitors. Treatment approaches include reduction or discontinuation of potassium supplements and reduction of potassium in the diet. The availability of non-absorbable drugs that bind potassium in the gut offer an additional opportunity to manage hyperkalemia.47),48) Several studies have shown that the use of these agents to control potassium levels in patients enables RAAS inhibitor therapy to be continued without reduction or discontinuation.49),50),51)
CONCLUSIONS AND FUTURE DIRECTIONS
The development and testing of an ARNI in randomized clinical trials (RCTs) has resulted in an important new approach for managing patients with stage C HFrEF. Available evidence shows that sacubitril-valsartan is superior to enalapril in improving outcomes in this population. As a result, the use of sacubitril-valsartan is now guideline recommended as a first-line therapy. Initiation of the drug should be considered both in the clinic and during the course of a HF hospitalization without the requirement for prior treatment with an ACEI or ARB.
Results from studies performed in an experimental animal model of post-myocardial infarction (MI) ventricular remodeling in which treatment with sacubitril-valsartan was associated with a reduction in cardiac fibrosis and hypertrophy52),53) raise the possibility that sacubitril-valsartan can improve outcomes in patients following a MI by attenuating cardiac remodeling and dysfunction. This possibility is currently being tested in the Prospective ARNI vs. ACE inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI (PARADISE-MI), a large multinational RCT in which the effects of sacubitril-valsartan will be compare to those of the ACEI, ramipril in reducing the occurrence of a composite endpoint of CV death and HF hospitalization in post-MI patients with evidence of LV dysfunction and/or pulmonary congestion but no known history of chronic HF.
Finally, while the PARAGON-HF study failed to meet its primary endpoint, reductions in the composite of CV mortality and HF hospitalization were seen with sacubitril-valsartan. Patients whose ejection fraction (EF) was below the median value and women appeared to be most likely to experience a reduction in risk with this drug. Further analysis of the PARAGON-HF results will likely provide information that should be helpful in guiding the future direction of sacubitril therapy in patients with HF with mid-range EF as well as those with HFpEF.
Footnotes
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Reyes EB, Ha JW, Firdaus I, et al. Heart failure across Asia: same healthcare burden but differences in organization of care. Int J Cardiol. 2016;223:163–167. doi: 10.1016/j.ijcard.2016.07.256. [DOI] [PubMed] [Google Scholar]
- 3.Sato N. Epidemiology of heart failure in Asia. Heart Fail Clin. 2015;11:573–579. doi: 10.1016/j.hfc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Francis GS, Goldsmith SR, Levine TB, Olivari MT, Cohn JN. The neurohumoral axis in congestive heart failure. Ann Intern Med. 1984;101:370–377. doi: 10.7326/0003-4819-101-3-370. [DOI] [PubMed] [Google Scholar]
- 5.Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. doi: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–1995. doi: 10.1016/S0140-6736(17)31071-1. [DOI] [PubMed] [Google Scholar]
- 7.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 9.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 10.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, Gattis Stough W, Rossignol P, et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J. 2012;33:2782–2795. doi: 10.1093/eurheartj/ehs257. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Williams G, Remme W, et al. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther. 2001;15:79–87. doi: 10.1023/a:1011119003788. [DOI] [PubMed] [Google Scholar]
- 13.Colucci WS, Packer M, Bristow MR, et al. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. Circulation. 1996;94:2800–2806. doi: 10.1161/01.cir.94.11.2800. [DOI] [PubMed] [Google Scholar]
- 14.Krum H, Roecker EB, Mohacsi P, et al. Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA. 2003;289:712–718. doi: 10.1001/jama.289.6.712. [DOI] [PubMed] [Google Scholar]
- 15.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF) JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 16.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 17.D'Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017;19:710–717. doi: 10.1002/ejhf.799. [DOI] [PubMed] [Google Scholar]
- 18.Rademaker MT, Charles CJ, Espiner EA, Nicholls MG, Richards AM, Kosoglou T. Neutral endopeptidase inhibition: augmented atrial and brain natriuretic peptide, haemodynamic and natriuretic responses in ovine heart failure. Clin Sci (Lond) 1996;91:283–291. doi: 10.1042/cs0910283. [DOI] [PubMed] [Google Scholar]
- 19.Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin-converting enzyme inhibition. Hypertension. 2004;44:913–918. doi: 10.1161/01.HYP.0000146483.78994.56. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson IB, McEniery CM, Bongaerts KH, MacCallum H, Webb DJ, Cockcroft JR. Adrenomedullin (ADM) in the human forearm vascular bed: effect of neutral endopeptidase inhibition and comparison with proadrenomedullin NH2-terminal 20 peptide (PAMP) Br J Clin Pharmacol. 2001;52:159–164. doi: 10.1046/j.0306-5251.2001.1420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/s0140-6736(00)02602-7. [DOI] [PubMed] [Google Scholar]
- 22.Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 23.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 25.SOLVD Investigators. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 26.Okumura N, Jhund PS, Gong J, et al. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial (PARADIGM-HF) Circulation. 2016;133:2254–2262. doi: 10.1161/CIRCULATIONAHA.115.020729. [DOI] [PubMed] [Google Scholar]
- 27.Simpson J, Jhund PS, Silva Cardoso J, et al. Comparing LCZ696 with enalapril according to baseline risk using the MAGGIC and EMPHASIS-HF risk scores: an analysis of mortality and morbidity in PARADIGM-HF. J Am Coll Cardiol. 2015;66:2059–2071. doi: 10.1016/j.jacc.2015.08.878. [DOI] [PubMed] [Google Scholar]
- 28.Jhund PS, Fu M, Bayram E, et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36:2576–2584. doi: 10.1093/eurheartj/ehv330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solomon SD, Claggett B, Desai AS, et al. Influence of ejection fraction on outcomes and efficacy of sacubitril/valsartan (LCZ696) in heart failure with reduced ejection fraction: the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial. Circ Heart Fail. 2016;9:e002744. doi: 10.1161/CIRCHEARTFAILURE.115.002744. [DOI] [PubMed] [Google Scholar]
- 30.Böhm M, Young R, Jhund PS, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM-HF. Eur Heart J. 2017;38:1132–1143. doi: 10.1093/eurheartj/ehw570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khariton Y, Fonarow GC, Arnold SV, et al. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:933–941. doi: 10.1016/j.jchf.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 33.Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68:241–248. doi: 10.1016/j.jacc.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 34.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 35.Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 36.Maeder MT, Mariani JA, Kaye DM. Hemodynamic determinants of myocardial B-type natriuretic peptide release: relative contributions of systolic and diastolic wall stress. Hypertension. 2010;56:682–689. doi: 10.1161/HYPERTENSIONAHA.110.156547. [DOI] [PubMed] [Google Scholar]
- 37.Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019:1–11. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zile MR, O'Meara E, Claggett B, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73:795–806. doi: 10.1016/j.jacc.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 39.Martens P, Nuyens D, Rivero-Ayerza M, et al. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol. 2019;108:1074–1082. doi: 10.1007/s00392-019-01440-y. [DOI] [PubMed] [Google Scholar]
- 40.Iborra-Egea O, Gálvez-Montón C, Roura S, et al. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: a systems biology approach. NPJ Syst Biol Appl. 2017;3:12. doi: 10.1038/s41540-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1077–1084. doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Writing Committee Members. Yancy CW, Jessup M, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–93. doi: 10.1161/CIR.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 43.Writing Committee Members. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 44.Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 45.Vardeny O, Claggett B, Packer M, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18:1228–1234. doi: 10.1002/ejhf.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Das S, Dey JK, Sen S, Mukherjee R. Efficacy and safety of patiromer in hyperkalemia: a systematic review and meta-analysis. J Pharm Pract. 2018;31:6–17. doi: 10.1177/0897190017692921. [DOI] [PubMed] [Google Scholar]
- 48.Hoy SM. Sodium zirconium cyclosilicate: a review in hyperkalaemia. Drugs. 2018;78:1605–1613. doi: 10.1007/s40265-018-0991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 51.Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 52.von Lueder TG, Wang BH, Kompa AR, et al. Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail. 2015;8:71–78. doi: 10.1161/CIRCHEARTFAILURE.114.001785. [DOI] [PubMed] [Google Scholar]
- 53.Torrado J, Cain C, Mauro AG, et al. Sacubitril/valsartan averts adverse post-infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol. 2018;72:2342–2356. doi: 10.1016/j.jacc.2018.07.102. [DOI] [PubMed] [Google Scholar]