Abstract
Heart failure (HF) is a condition that carries a considerable burden of disability many now related to co-existing co-morbidities. The drive to find newer effective therapies targeting novel mechanisms has led to a recent emphasis on treating common co-morbidities that are clustered around contemporary HF patients. Here is renewed contemporary co-morbidities that until recently have received little attention but which are now subject of considerable interest and potential therapeutic advance. These include, diabetes, functional mitral regurgitation and sleep disordered breathing. These three contemporary co-morbidities that have recently been subject to major trial evaluation will be reviewed in this paper.
Keywords: Heart failure, Comorbidity, Diabetes mellitus, Sleep-disordered breathing, Mitral regurgitation
INTRODUCTION
Heart failure (HF) is a condition that is growing in prevalence due to the ageing of societies and due to higher survival rates after myocardial infarction (MI) and other cardiac disorders. It carries a considerable burden of disability and is associated with frequent emergency hospital admissions and a residual high risk of subsequent mortality. Multiple pharmacological and device therapies have been shown to reduce this burden of death and disability, but the residual burden remains disturbingly high. There is thus a constant drive to find newer effective therapies targeting mechanisms of the syndrome of HF that have not yet been adequately managed by our present treatment options. A recent emphasis has been to concentrate on co-morbidities that are clustered around contemporary HF patients. These include diabetes, hypertension, coronary disease, atrial fibrillation, renal impairment, sleep disordered breathing and many others. Newer treatment options are now being targeted at these common co-morbidities with attractive benefits seeming to be possible to achieve for the patient with HF and the particular targeted co-morbidity. Co-morbidities that until recently have received little attention are now subject of considerable interest and potential therapeutic advance. These include, diabetes, functional mitral regurgitation (FMR) and sleep disordered breathing (SDB). These three contemporary co-morbidities that have recently been subject to major trial evaluation will be reviewed in this paper.
DIABETES
Patients with diabetes mellitus are at increased risk of developing new-onset HF and of subsequent episodes of worsening of HF, more than two-fold in men and 5-fold in women.1) Diabetes is also more common in sufferers of HF, especially so for heart failure with preserved ejection fraction (HFpEF).2),3),4) Diabetes has been reported in up to 25%, of stable HF patients and nearly 40% in acute HF presentations. The presence of diabetes in HF patients increases risk, both for emergency HF-related hospital admissions and for death.5) Although there were historically reports of a specific non-atherosclerotic form of HF, coined diabetic cardiomyopathy, this is now not thought to be a major clinical problem. More contemporaneous studies have concentrated on the role of diabetes in promoting and worsening cardiovascular (CV) risk factors such as atherosclerotic coronary artery disease, hypertension, and endothelial dysfunction, combined with the metabolic abnormalities that can affect both skeletal muscle and cardiac muscle function. The relationship between diabetes mellitus and HF is more than the sum of the parts since each condition adversely affects the natural course of the other and worsens its outcome.
Pathophysiology
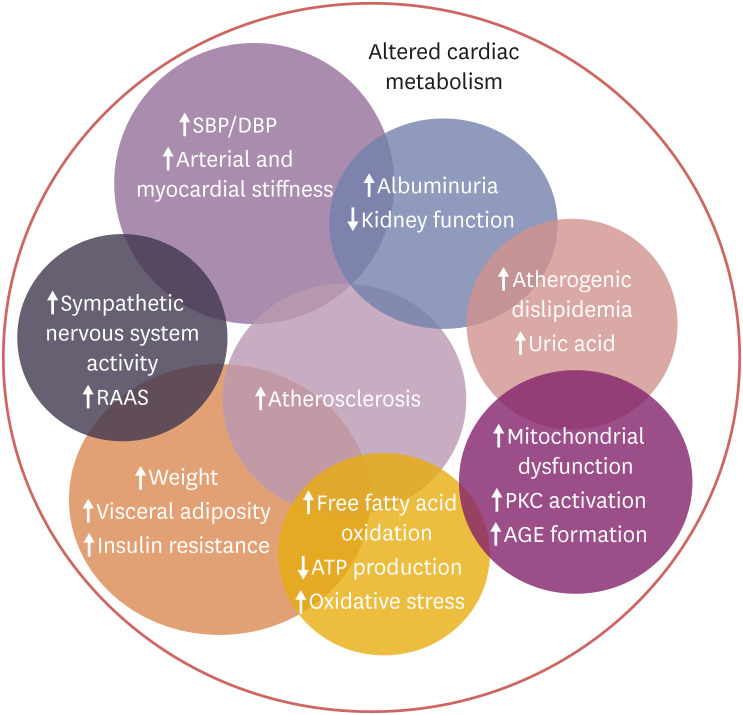
In diabetes altered glucose metabolism and resulting secondary metabolite usage, including a shift in myocardial utilization of glucose toward less efficient fatty acid oxidation by the myocardium, contribute to structural and functional abnormalities of the heart, which can both cause and worsen cardiac dysfunction,6) and negatively impact cardiac contraction and relaxation.7) Hyperglycemia and insulin resistance also negatively affect oxidative stress and microvascular endothelial function, increase myocardial fibrosis and locally activate the renin-angiotensin and sympathetic nervous systems, all of which contribute to the development of subsequent HF. The mechanisms of diabetes-associated myocardial dysfunction are many fold and complex (Figure 1).8)
Figure 1. Diabetes related Mechanism for the development of heart failure.
Reproduced with permission from Rosano and Seferovic.8)
AGE = advanced glycation end-product; DBP = diastolic blood pressure; PKC = protein kinase C; RAAS = renin-angiotensin-aldosterone system; SBP = systolic blood pressure.
The interactions between diabetes and heart failure
A meta-analysis of 77 studies9) looking at the risks of HF in diabetic patients described a relative risk (RR) of 2.06 (95% confidence interval [CI], 1.73–2.46) for developing HF in diabetics compared to non-diabetics, with a RR of 1.23 (95% CI, 1.15–1.32) per 20 mg/dL increase in blood glucose level, and the suggestion of a J-shaped curve relating risk to glucose level with a nadir around 90 mg/dL. Once a patient has established HF, however, the situation may well be different, with little evidence of lower HBA1c levels being beneficial and in fact suggestions that outcomes are worse in diabetic HF patients with lower levels. Higher levels of HbA1c are thus being recommended in diabetic patients with HF.10) Most recent diabetes studies have targeted HbA1c levels <7.5% and most until fairly recently have failed to show convincing CV benefit, calling into question our assumptions about optimal levels of glucose control in established CV disease patients, and most especially in HF patients, where the optimal range may be higher.11) A meta-analysis including more than 34,000 subjects showed that intensive glucose lowering was associated with a 47% increase in new-onset HF (p<0.001) but no significant reduction in CV risk.12) Indeed in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study very tight glucose control in patients with diabetes mellitus increased total mortality by 21% and CV mortality by 29% after long-term follow-up.13) More recently, a study conducted in a large cohort of HF patients with type 2 diabetes showed a U-shaped relationship between HbA1c and mortality,14) with the lowest risk in patients with moderate glycaemic control (HbA1c 7.1–8.0%). Of interest the U-shaped relationship between glucose control and events seems to be present in drug-treated but not in diet-treated diabetic patients, suggesting drug-induced glucose lowering may be associated with some deleterious effects in some cases. Patients with established HF and diabetes compared to those without diabetes have increased symptom severity, reduced functional capacity and more impaired quality of life,4),15),16),17) and a higher mortality rate.18),19) Among patients hospitalized for acute HF, diabetes increased in-hospital and 1-year mortality and the rate of emergency re-hospitalizations for HF.20)
The treatment of patients with both heart failure and diabetes
The efficacy and safety of major drug classes in guideline-directed medical therapy (GDMT) for HF has also been confirmed in diabetic patients, and there are no recommendations to treat HF and differently depending on the presence or absence of diabetes. The converse is however, not true. Indeed, the management of diabetes should certainly take into account co-incident HF and even in fact the risk of a patient developing HF when choosing hypoglycaemic therapy. The efficacy and safety of most of our glucose lowering therapies in patients with established HF remains poorly understood. No randomised clinical trial to date has specifically targeted diabetic patients with HF, but recent large trials of novel hypoglycaemic agents have provided more robust data on the prevention or exaggeration of new-onset HF in at-risk diabetic subjects. Rosiglitazone, a thiazolidinezone, was withdrawn in the EU and the USA because of increased risk of CV events including new-onset HF.21) There are several pathophysiological reasons why therapeutic glucose lowering agent may precipitate new-onset HF, including the known fluid retaining effects of insulin and reduced glucose availability for the heart and muscle metabolism. For these reasons it has become essential to investigate the effects of hypoglycaemic agents specifically in HF patients with diabetes.
Older agents
Most HF guidelines to date have preferred the well-established agent metformin as first-line therapy, being widely accepted as being safe and effective,9) although because of its primarily renal route of excretion, caution should be exercised in patients with impaired renal function and its use is contraindicated in patients with severe renal or hepatic impairment. An increased risk of worsening HF has been reported with sulphonylureas and as a result they should be avoided in diabetic patients with HF.22) Metiglinides have a mechanism of action similar to that of sulphonylureas as they bind to an ATP-dependent K+ channel on the membrane of pancreatic beta cells albeit with a weaker affinity. Because of their effect on insulin secretion, these drugs may induce water retention and should be used with caution in patients with HF. Alpha glucosidase-inhibitors such as acarbose interfere with complex carbohydrate breakdown, and thereby reduce glucose absorption. Because it has no direct effect on insulin Acarbose is widely considered safe to use in patients with HF and diabetes. Thiazolidinediones activate peroxisome proliferator-activated receptors (PPARs), a group of nuclear receptors, with greatest specificity for PPARγ. They increase the storage of fatty acids in adipocytes and an increase the oxidation of carbohydrates. They have also been associated with renal sodium and water retention hence making new-onset HF a potential risk, and this class of agent one to be avoided in HF.9),21)
Newer hypoglycaemic agents
Three newer classes of hypoglycaemic agent have undergone extensive clinical trial evaluation in recent years: dipeptidyl peptidase 4 (DPP-4) inhibitors,23) glucagon like peptide-1 receptor agonists (GLP-1 RA),24) and sodium glucose co-transporter type-2 (SGLT-2) inhibitors.25)
Dipeptidyl peptidase 4
The DPP4i (saxagliptin, alogliptin, sitagliptin, vildagliptin, and linagliptin) class of agents increase incretin levels and thereby improve glycaemic indices. Early studies with these agents have, however, have associated the class with a possible increased risk of HF.26) In general across all the major trials with this class of agent they have shown non-inferiority to alternative therapies in terms of major adverse cardiovascular events (MACE), but their effects on HF-related hospitalisations has been either neutral or harmful with some possible differences between the agents. The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial of saxagliptin showed a statistically significant increase of 27% in hospitalisation for HF.27) The much smaller Vildagliptin in Ventricular Dysfunction Diabetes (VIVIDD) study of vildagliptin showed no significant effect on left ventricular ejection fraction (LVEF), brain natriuretic peptide (BNP) levels, or HF status in patients with heart failure with reduced ejection fraction (HFrEF), with, however, an increase in left ventricular (LV) volumes and a trend to more deaths (11, 8.6% versus 4, 3.2%).28) The clinical significance of these findings remains to be determined. The EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE (EXAMINE) trial of alogliptin showed a non-significant trend towards more HF hospitalisation29) and the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) of sitagliptin showed a neutral effect (3.1% vs. 3.1%).30) The Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA) trial of linagliptin also saw no effect on HF hospitalisations with a trend to benefit (6.0% vs. 6.5%).31) Thus of this drug class, only alogliptin or sitagliptin should be considered for use in patients with, or at high risk of developing HF and then only under specialist supervision.
Glucagon like peptide-1 receptor agonists
GLP-1 RA (lixisenatide, liraglutide, semaglutide, exenatide, albiglutide) act like incretins, amplifying the insulin release stimulated in response to hyperglycaemia and suppressing the production of glucagon and thereby suppressing gluconeogenesis and fasting hyperglycaemia. This class of agent has been the subject of 5 major outcome trials. Superiority in terms of MACE events over placebo has been shown for liraglutide, semaglutide and albiglutide. In terms of the HF effects of these agents, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial of liraglutide showed HF hospitalisations were non-significantly lower by 13%32) and in Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) semaglutide had a neutral effect on HF hospitalisation rates (3.6% vs. 3.3%; hazard ratio [HR], 1.11; 95% CI, 0.77–2.78),33) whereas in Harmony Outcomes albiglutide showed a non-significant trend to a reduction in the combined secondary end-point of CV death or hospital admission for HF compared with placebo (4.0% vs. 5.0%; HR, 0.85; 95% CI, 0.70–1.04)34) with HF hospitalisations alone not being reported. The other 2 cardiovascular outcome trials (CVOTs) the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial of lixisenatide in patients with type 2 diabetes and acute coronary syndrome and the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial of exenatide in type 2 diabetes both showed non-inferiority to placebo but also with no superiority, and no effect on HF hospitalisation rates.35),36) Thus the large GLP-1 RA CVOT trials to date have shown neutral effects on the risk of HF hospitalization. Two recent and smaller trials of these agents in patients with established HF have raised some concerns, however. The Liraglutide on Left Ventricular Function in Chronic Heart Failure Patients With and Without Type 2 Diabetes Mellitus (LIVE) study of liraglutide in 241 HFrEF patients showed no change in LVEF, but that liraglutide increased heart rate by 7 bpm and the rate of serious cardiac events: 12 (10%) with liraglutide versus 3 (3%) with placebo (p=0.04).37) Similarly in the Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) trial of liraglutide in 300 established HFrEF patients, liraglutide (n=154) vs. placebo (n=146), liraglutide had no significant effect on the primary end point, and although not significant there was a trend towards a higher risk of death and rehospitalisation for HF.38)
Sodium glucose co-transporter type-2 inhibitors
The sodium/glucose co-transporter 2 inhibitors (SGLT2i) enhance glucose control by increasing the urinary excretion of glucose. In the first major CVOT to show an overwhelmingly positive effect on both major CV outcomes and HF specific end-points, the SGLT2i empagliflozin was studied in the (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial of conducted in 7,020 patients with type 2 diabetes (HbA1c 7.0% to 10.0%) at high risk for CV events.39) Empagliflozin reduced the risk of 3-point MACE (HR, 0.86; 95% CI, 0.74–0.99; p=0.04). Empagliflozin use was also associated with significantly lower rates of CV death (3.7%, vs. 5.9%, a 38% relative risk reduction), hospitalization for HF (2.7% and 4.1%, a 35% relative risk reduction), and death from any cause (5.7% and 8.3%, a 32% relative risk reduction). Consistent effects of empagliflozin were observed across all pre-defined subgroups including patients with and without HF. Similar, but not identical effects have subsequently been seen with 2 other SGLT2i.
In the CANagliflozin cardioVascular Assessment Study (CANVAS) program canagliflozin improved 3-point MACE (HR, 0.86; 95% CI, 0.75–0.97; p<0.001 for noninferiority and p=0.02 for superiority) and also reduced the risk of a HF-related hospitalization (HR, 0.67; 95% CI, 0.52–0.87), but with no significant reduction in all-cause mortality (HR, 0.87; 95% CI, 0.74–1.01) or CV death (HR, 0.87; 95% CI, 0.72–1.06).40) These beneficial effects of canagliflozin were further supported by the results of the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial which showed a 34% relative risk reduction in cardiorenal outcomes with canagliflozin in patients with diabetes and renal dysfunction (albuminuria and epidermal growth factor receptor 30 to <90 mL/min/1.73 m2) combined with a reduction in the risk of CV death or HF hospitalisation (HR, 0.69; 95% CI, 0.57–0.83).41)
In Dapagliflozin Effect on CardiovascuLAR Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) dapagliflozin (10 mg/day) was compared to placebo for two co-primary efficacy outcomes, MACE and that of CV death or HF hospitalization.42) The primary safety end-point was met, revealing noninferiority of dapagliflozin compared to placebo with respect to MACE and being associated with a lower rate of CV death or hospitalization for HF compared to placebo (4.9% vs. 5.8%; HR, 0.83; 95% CI, 0.73–0.95; p=0.005), mainly driven by a reduction of HF hospitalization (HR, 0.73; 95% CI, 0.61–0.88), with no effect on CV death (HR, 0.98; 95% CI, 0.82–1.17). The reduction of HF hospitalizations was evident both in patients with or without a history of HF. The mechanisms of benefit of these agents are not yet fully established but plausible effects include sodium excretion, reduced plasma volume, modulation of the renin angiotensin aldosterone system, and weight and blood pressure reduction. The clear similarity for the HF-related outcomes for all three SGLT-2 inhibitors in CVOT trials to date is the significant reduction in HF hospitalization events, suggesting the existence of a class effect at least as far as this end-point is concerned. We cannot however extend this to similar effects on all-cause or CV mortality, where as a class effect remains uncertain.
VALVE DISEASE IN HEART FAILURE WITH REDUCED EJECTION FRACTION
It has long been known that one of the principal historical causes of HF has been valve disorders, either as a result of congenital heart abnormalities or acquired through the effects of infections (e.g. rheumatic heart disease) or ageing (such as aortic stenosis). The ability to detect and intervene in many of these syndromes or indeed to prevent them by public heath measures has led to a dramatic reduction in their prevalence. These heart valves disorders are classified primary if they form the initial cardiac abnormality which can subsequently cause HF, usually via pressure (e.g. aortic stenosis) or volume overload (e.g. aortic regurgitation or mitral regurgitation [MR]) or secondary if they develop as a consequence of the cardiac changes of the HF syndrome caused by a different primary myocardial disorder. This review will concentrate on secondary, frequently called FMR as a common complication of advanced HFrEF.
FUNCTIONAL MITRAL REGURGITATION COMPLICATING HEART FAILURE WITH REDUCED EJECTION FRACTION
Pathophysiology of functional mitral regurgitation
The heart in HFrEF is enlarged and has an altered shape, becoming more globular in a process called LV remodelling. These changes have a negative impact on the function of the mitral valve apparatus by multiple mechanisms. First of all, there is a dilatation of the mitral valve ring that can by itself lead to the failure of the leaflets to completely close during diastole. The resulting malapposition of valve leaflets allows them to leak during diastole, allowing MR to develop and increase. This mitral valve leak during diastole allows increasing volumes of blood to flow from the left ventricle into the left atria during ventricular systole, caused in some cases by the increased separation in distance of the leaflets and in others also by incoordinate ventricular contraction and relaxation. Other changes are described including an increase in the forces which drive high pressure blood from the ventricle to the atrium due to the rise in ventricular end-diastolic pressures which frequently complicate HFrEF. When combined with dysfunction of the papillary muscles, common in ischaemic HF and mitral valve leaflet malapposition which is a consequence of the globular enlargement of the ventricle the frequency of some degree of FMR in HFrEF is unsurprisingly quite high.
Whatever the cause, increased FMR is a harmful thing in itself, as it reduces net forward cardiac output and simultaneously increases ineffective backward flow across the mitral valve. The first worsens the effects of reduced cardiac function on organ perfusion and the second leads to increased left atrial and hence pulmonary venous pressures, increased left atrial size and increasing pulmonary stiffness and even congestion. These changes can also lead to inefficient ventilation, pulmonary oedema and atrial fibrillation and each has been associated with worse overall clinical outcomes. It is not surprising therefore that FMR and its severity have been linked to impaired outcomes in patients with HFrEF.
How common is functional mitral regurgitation?
Even in the general population MR is not rare. Overall estimates have been put at 1.7% of the adult population of the USA, but reaching much higher values (8.1–10.9%) over the age of 75 years.43) In a recent study in the UK 22% of a primary care population over the age of 65 years had at least mild MR.44) MR becomes more common in the presence of LV disease and in particular in the presence of LV enlargement, as will be described in the next section. In cases of suspected HF referred for echocardiographic evaluation 12.5% were seen to have significant MR45) and in a hospital practice study from 2003, 59% of HFrEF patients (LVEF ≤40%) had moderate or severe MR, and when present over 4 years of follow-up, moderate to severe MR predicted an increased risk of mortality.46)
The consequences of functional mitral regurgitation
FMR and ventricular enlargement exaggerate the severity of each other, and each worsens overall survival. This can form an adverse cycle of deterioration. Rossi and colleagues47) showed that hospitalisation-free survival was decreased with the increasing severity of MR in the presence of HF. FMR was quantitatively determined by measuring vena contracta (VC), effective regurgitant orifice (ERO), and regurgitant volume (RV) in 1,256 HF patients. Severe FMR (defined as ERO >0.2 cm2 or RV >30 mL, or VC >0.4 cm) represented 24% of the population in non-ischemic HF and was associated with the worst outcomes (HR, 2.0; 95% CI, 1.5–2.6; p<0.0001) after adjustment for LVEF and the presence of a restrictive filling pattern on echocardiography. The independent association of severe FMR with prognosis was seen both in patients with ischaemic HF (HR, 2.0; 95% CI, 1.4–2.7; p<0.0001) and non-ischaemic dilated cardiomyopathy (HR, 1.9; 95% CI, 1.3–2.9; p=0.002). Similar results were seen by Bursi et al.48) who studied 469 CHF patients and showed that FMR is a significant and independent predictor of death and transplantation, but the effect was only significant in this study in less severe HF and lower risk patients. This feature has been reported in other studies and may have implications for the identification of patients who may potentially benefit most from therapeutic reduction in FMR severity, including the optimal degree of MR and the severity of the LV enlargement. In a prospective longitudinal study of 576 HFrEF patients, severe FMR independently predicted total mortality (HR, 1.76; 95% CI, 1.34–2.30; p<0.001), but only in intermediate severity HFrEF, is those in New York Heart Association class II and III with moderately impaired LV function (30–40%) and the second quartile (871–2,360 pg/mL) of N-terminal-pro hormone BNP, and not in more severe HFrEF cases.49)
Trials of functional mitral regurgitation reduction in heart failure
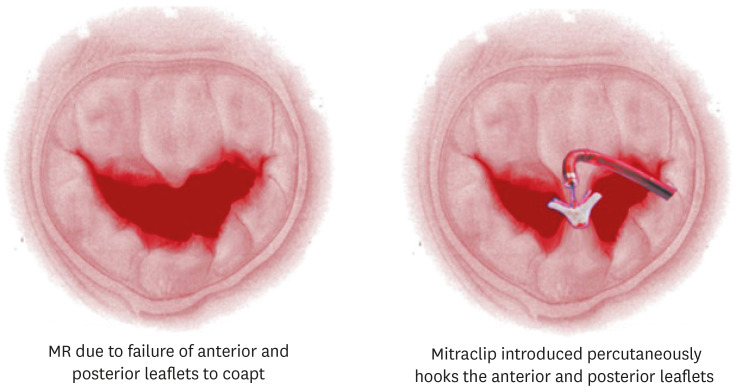
In the last year 2 randomised trials have reported that may have major therapeutic implications for how we manage this common HF co-morbidity, both utilising the most commercially advanced of the percutaneous devices designed to reduce MR in this setting, Mitraclip. Mitraclip is a percutaneously deliverable device that can “clip” mitral valve leaflets together, thereby reducing the maximal ERO area and hence the volume of MR (see Figure 2). The Multicentre Study of Percutaneous Mitral Valve Repair MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR) trial randomised 304 HFrEF patients who had what they described as severe secondary MR (defined as an ERO area of >20 mm2 or a RV of >30 mL per beat) and an LVEF between 15% and 40%, to percutaneous MR reduction procedure using the Mitraclip device or normal medical therapy. They saw no effect at 12 months follow-up on the primary end-point of death or HF hospitalisation (54.6% vs. 51.3% in the control group; odds ratio, 1.16; 95% CI, 0.73–1.84; p=0.53).50) Nor was any difference seen in the major secondary end-points. The Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial that reported a few months later had a very different result.51) In this trial 614 HFrEF patients (LVEF 20% to 50%, moderate-to-severe MR) were randomised to MR reduction via Mitraclip or control. The primary end-point, all HF hospitalizations was significantly reduced (HR, 0.53; 95% CI, 0.40–0.70; p<0.001), as was all-cause mortality (HR, 0.62; 95% CI, 0.46–0.82; p<0.001).
Figure 2. A pictorial representation of functional MR in HFrEF (left panel) and the deposition of a Mitraclip device (right panel).
Reproduced with permission from Brown and Lim. “Catheter Management Of Mitral Regurgitation.” SourceStatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536988/figure/article-179.image.f1/.
MR = mitral regurgitation; HFrEF = heart failure with reduced ejection fraction.
Reconciling the differences between MITRA-FR and COAPT
The headline results of the two trials are very different, and many explanations have been aired to explain the differences.
Here I related the main differences we can see between the 2 trials, but which if any can explain the very different results remains speculative.
• The patients were different. Although MITR-FR described their patients as having severe MR and COAPT defined theirs as moderate to severe, in fact the average degree of MR was more severe in COAPT compared to MITRA-FR (ERO area 41±15 mm2 vs. 31±10 mm2) and the LV function was different in that although the LVEFs were similar COAPT (31±9%) vs. MITRA-FR (33±7%), the LV end-diastolic volumes were different, COAPT (101±34 mL/m2) vs. MITRA-FR (135±35 mL/m2). Thus MITRA-FR recruited patients with more severe LV dysfunction but slightly less severe FMR.
• The procedures were done differently or had different outcomes. Procedural complications were reported as COAPT 8.5% vs. MITRA-FR 14.6%. The 12-month echo data showed ≥3+MR in COAPT 5% vs. MITRA-FR 17%. Whether these represent different approaches or different patient risks remain uncertain.
• The background medical therapies were handled differently. In COAPT a central executive committee confirmed patients were failing maximally-tolerated GDMT at baseline and changes post-randomisation were discouraged in the control group, whereas in MITR-FR baseline therapy was investigator reported as GDMT, and variable adjustment in each group during follow-up was allowed as per “real-world” practice. In addition there was a baseline imbalance in GDMT in COAPT with significantly more patients randomised to intervention being on angiotensin converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor-neprilysin inhibitor 71.5%, compared to control 62.8%, p=0.02 with the difference widening at 12 months 76.5% vs 63.1%, p=0.002 and a new difference in beta-blocker use developing, at 12 months during follow-up a difference in beta-blocker use developed, to become 93.3% vs 86.7%, p=0.02, with changes to beta-blocker therapy during the first 12 months being dose increase by >100% or new drug class started 8.6% vs. 3.8%, p=0.01. Whether this more stringent optimisation of medial therapy at baseline was good because it ensured patients had tried everything else first or bad, because it was associated with instructions not to change subsequent medication as much, remains uncertain.
We must await the results of longer follow-up of MITRA-FR and also those of the still recruiting Clinical Evaluation of the Safety and Effectiveness of the MitraClip System in the Treatment of Clinically Significant Functional Mitral Regurgitation (Reshape-HF2) trial (ClinicalTrials.gov Identifier: NCT02444338), but in the meantime the Heart Failure Association of the European Society of Cardiology (ESC) has concluded “Referral of patients with HF and secondary (i.e. functional) MR to a multidisciplinary HF team that will decide on management is recommended” and “Reduction in MR using a MitraClip device may be considered for patients with HFrEF who fulfil the COAPT selection criteria”.52)
SLEEP DISORDERED BREATHING
HF patients, being older, present frequently with many unrelated co-morbidities. They also are likely to have other conditions that share risk factors with those that led to their HF condition, such as hypertension, smoking, obesity, diabetes and others. These are also risk factors for SDB syndromes that, as a result, have an increased prevalence in HF at any age. SDB is frequently simplified into two distinct patterns; obstructive sleep apnoea (OSA) and central sleep apnea (CSA). In fact, although many subjects have a mixed pattern this is a useful clinical and pathophysiological separation, for, as we now appreciate, the underlying causes, clinical course and indeed therapies are quite distinct, so for the purposes of this article we will review the two types sequentially rather than together. It must be said, however, that clinically a patient may present with an unknown type of sleep apnoea and only formal sleep studies can dissect out the components of each type. Another complication is that during a severe episode of one type the other may briefly develop, as shall be explained later. The co-existence of any type of SDB can worsen patient symptoms and further impair quality of life and may contribute to the progression of HF and increase death rates.
Pathophysiology of obstructive sleep apnoea
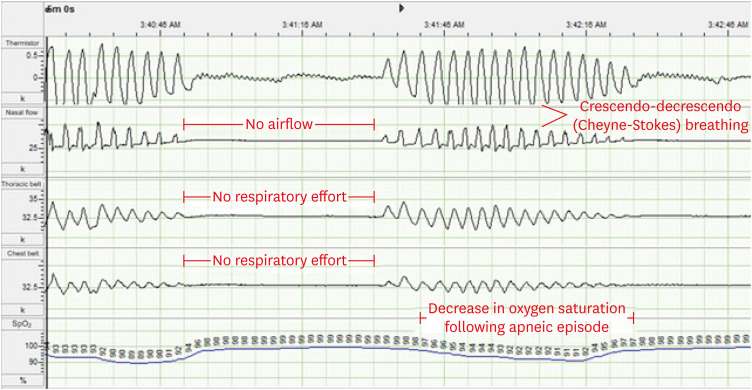
OSA affects 2–4% of the adult population world-wide. OSA is commonly associated with HF as well with hypertension and coronary artery disease and stroke.52),53) It is thus inevitably a frequent finding in HF patients, including HFrEF, HF with midrange ejection fraction, and HFpEF, both because of the background risk and the fact it shares similar risk factors to HF itself. Obstructive sleep apnoea is characterised by intermittent cessation of upper airway airflow occasioned by an obstruction in the upper airway, usually caused by the tongue flopping back to block the airway. It is associated with deep sleep, loss of upper airway tone, and has the risk factors of obesity, alcohol use, narcoleptic agents, male sex, and hypertension. In OSA intermittent upper airway occlusions, with resultant loss of airflow leads to episode of arterial deoxygenation and due to these, arousals and sympatho-excitation due to the stimulatory effects of chemoreflex firing secondary to the disturbed arterial blood gases. Obstructive apnoeic or hypopnoeic episodes occur throughout sleep and are usually irregular and non-rhythmic. Each OSA cycle of apnoea or hyperpnoea is to an extent independent, but in a patient with HF, a mixed apnoea pattern is more likely to develop because the exaggerated chemoreflex sensitivity that is part of the neurohormonal imbalance characteristic of HF54) means that after the apnoeic phase of OSA there is a greater reflex hyperventilation that predisposes to the subsequent oscillatory breathing pattern typical of Cheyne-Stokes ventilation and CSA (figure 3). In OSA in HF patients as in non-HF patients, frequent apnoeic episodes lead to frequent hypoxia, and arousal during sleep which combine to impart significant sympathetic nervous system hyper-activation. In non-HF patients this can worsen hypertension and in HF it is thought to stress the myocardium and increase the risk of ventricular arrhythmias. In addition, the highly negative intra-thoracic pressure swings characteristic of increased ventilatory effort acting against a closed airway lead to worsening LV after-load due to an increase in the transmural LV pressure gradient. A high frequency of obstructive episodes, usually measured as the apnoea/hyopnoea index or apnoea-hypopnoea index (AHI), has been associated with an elevated risk of HF hospitalizations, ventricular arrhythmias and mortality.52)
Figure 3. CSA with cheyne-stokes breathing.
Selected channels of a polysomnogram of a patient with CSA with Cheyne-Stokes breathing. Reproduced from Bradley et al.73)
CSA = central sleep apnea.
The diagnosis and treatment of obstructive sleep apnoea in heart failure
Although there is overlap for practical purposes physicians are advised to treat HF patients with SDB as OSA if the hypoxias are predominantly obstructive in nature and as CSA if the hypoxias are predominantly central in nature. This will require a formal sleep study in any HF patient being considered for SDB therapy, and this has been recommended in recent guidelines.55),56) HF clinicians should be aware of the possibility of OSA affecting their HF patients and explore the possibility in cases of nocturnal breathlessness, reports of poor sleep, or excessive day-time fatigue, sleepiness, along with some more non-specific of symptoms nocturia, morning headaches, and diminished concentration and memory.57) Questioning the sleep partner of a patient is also highly valuable in identifying OSA patients. The first step is an accurate diagnosis. This will usually require polysomnography (PSG) as part of a formal sleep study in a sleep laboratory. Portable monitors may be used to assess HF patients for the likelihood of OSA, but they are insufficiently detailed for diagnosis. Based on current guidelines, the PSG is diagnostic for OSA if respiratory monitoring demonstrates at least 5 obstructive-type hypoxaemic episodes per hour of sleep, i.e. an AHI of 5 or more, (or more than 15 in the absence of sleep related symptoms) and only if the obstructive type episodes predominate.58)
The main therapeutic option for OSA is a positive airway pressure mask. The need for therapy should be discussed with the patient and if the AHI is above 15/hr then treatment is indicated to reduce this to below 15. This is a consensus recommendation and designed to improve symptoms only, as there have been no adequately powered clinical trials that show therapy of OSA in HF patients will improve outcomes.59) Compliance with mask therapy is limited and the need for on-going therapy will depend on whether there is a noticeable improvement in sleep related or daytime sleepiness symptoms. Mask therapies for OSA are of 3 main types: continuous positive airway pressure (CPAP), bi-level positive airway pressure (BiPAP) in which pressure levels decrease during exhalation, and BiPAP-adaptive servoventilation (ASV) in which the two pressures change according to algorithmic control to even out the resulting breathing pattern. The adverse effects of ASV seen in HFrEF patients with predominantly CSA mean that this therapy is contra-indicated in predominant CSA-type sleep apnoea (see later), so care in recommending it for OSA in HFrEF patients is essential. Mask based therapy should be combined with life-style changes such as weight loss in the obese, regular exercise and the avoidance of excess alcohol or any sleeping tablets. Oral devices that push the mandible forward or specially designed mouth guards can assist to a limited extent. Upper airway reconstructive surgery is occasionally used in severe cases unresponsive to or intolerant of mask therapy.
Pathophysiology of central sleep apnea
CSA is a neurological disorder of the control of breathing, quite distinct from the anatomical or functional airway problem as we saw with OSA. CSA is a powerful adverse marker in HFrEF patients.60),61) CSA is associated with oscillating respiratory drive that leads to intermittent hypopnoeas and apnoeas, each followed by a period of hyperpnoea. These can be isolated and irregular in some cases of brain damage or narcotic poisoning, but the most common pattern is for them to be regular and rhythmic in pattern, almost indistinguishable from Cheyne-Stokes respiration (CSR). It is notable in this regard that CSR is also common in advanced HF and carries the same adverse associations as CSA in HF patients.62),63) CSA is common in HF and occurs in nearly half of cases, both HFrEF and HFpEF64),65) Both CSR and CSA are related to respiratory control system instability due to oscillation of the arterial blood carbon dioxide level (PaCO2) above and below the central threshold of ventilation termed the apnoeic threshold66),67) In HF factors such as a prolonged circulation time, exaggerated chemoreflex sensitivity, impaired baroreflex control of heart rate and blood pressure, incipient lung congestion and lung receptors that drive a heightened ventilatory response all contribute to these oscillations in PaCO2 and PaO2.68),69),70),71) When a bout of hyperventilation drives PaCO2 below the apnoeic threshold, breathing stops and CO2 re-accumulates, only to overshoot and drive the next phase of hyperventilation.
The diagnosis and treatment of central sleep apnea in heart failure
As for OSA the proper diagnosis of CSA in a HF patient requires a formal sleep study. Although physicians should have a high index of suspicion in patients whose sleep partner reports nocturnal apnoeas, the symptom of daytime sleepiness appear to be less prominent than they are in OSA. Periodic breathing patterns seen in quiet phases during the day are also common in these patients and may be the clue to detecting CSA in a HF patient.
HF patients with CSA have had very few effective treatment options since the surprise results of the SERVE-HF trial.72) Until this trial positive pressure masks had been used to treat CSA, as they had been for OSA, and the ASV form had proven highly effective at improving sleep breathing physiology in these patients. The Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure (CANPAP) was terminated early due to slow enrolment.73) It reported a neutral effect on HF outcomes. CPAP did improve the number of apnoeic events per hour (40 to 19) and LVEF, but compliance was poor (average use device in the treatment group, 3.6 hours/night). The Treatment of Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial randomised 1,325 HFrEF patients with predominantly CSA index and an AHI of 15 or more events ASV mask therapy or control. At the completion of the trial the composite primary end point (death, cardiac death equivalent, or HF hospitalisation) did not differ significantly between groups, but there a strong trend to harm with the intervention (54.1% vs. 50.8%; HR, 1.13; 95% CI, 0.97–1.31; p=0.10) and a significant excess in total mortality (HR, 1.28; 95% CI, 1.06–1.55; p=0.01) and cardiac related mortality (HR, 1.34; 95% CI, 1.09–1.65; p=0.006). This is despite ASV therapy improving the CSA sleep AHI averages. Based on this trial, ASV is contraindicated in HFrEF patients with predominantly central-type sleep apnoea. Current treatment options for CSA in HFrEF are therefore limited. Oxygen therapy, acetazolamide and theophylline have been tried, but have not been shown to be safe and effective.
One newer treatment option is phrenic nerve stimulation by an implantable device.74) This device, the remedē® System, activates the phrenic nerve to normalize breathing patterns.75) In its pivotal development trial, it met its primary endpoint; a greater proportion of patients in the treatment group having a ≥50% reduction in AHI compared to the control group at 6 months,76) along with the major sleep respiratory metrics (AHI, central apnoeas, oxygenation, arousals, and percentage of time in REM sleep) and patient reported outcomes (Patient Global Assessment and Epworth Sleepiness Scale). A subsequent report pooled effectiveness data from all patients with a diagnosis of HF at baseline (n=96) and included both treatment and former control groups who crossed over onto active therapy. Sleep metrics and quality of life improved from baseline to 6 and 12 months as did health-related quality of life, measured by Minnesota Living with Heart Failure Questionnaire (MLHFQ) with scores changed by −6.8±20.0 (p=0.005). The 6-month rate of HF hospitalization was 4.7% in treatment patients (standard error=3.3) and 17.0% in control patients (standard error=5.5; p=0.065).77) Thus although this has not been shown to improve death or mortality it remains the preferred option for HFrEF patients with CSA in need of therapy for their SDB-related symptoms.
CONCLUSIONS
HF is a condition that is growing in prevalence due to the ageing of societies and due to higher survival rates after MI and other cardiac disorders. It carries a considerable burden of disability and is associated with frequent emergency hospital admissions and a residual high risk of subsequent mortality. Multiple pharmacological and device therapies have been shown to reduce this burden of death and disability, but the residual burden remains disturbingly high. There is thus a constant drive to find newer effective therapies targeting mechanisms of the syndrome of HF that have not yet been adequately managed by our present treatment options. A recent emphasis has been to concentrate on co-morbidities that are clustered around contemporary HF patients. These include diabetes, hypertension, coronary disease, atrial fibrillation, renal impairment, SDB and many others. Newer treatment options are now being targeted at these common co-morbidities with attractive benefits seeming to be possible to achieve for the patient with HF and the particular targeted co-morbidity. Co-morbidities that until recently have received little attention are now subject of considerable interest and potential therapeutic advance. These include, diabetes, FMR and SDB. These three contemporary co-morbidities that have recently been subject to major trial evaluation will be reviewed in this paper.
Footnotes
Conflict of Interest: Professor Coats declares having received honoraria and/or lecture fees from: Astra Zeneca, Menarini, Novartis, Nutricia, Servier, Vifor, Actimed, CVRx, Enopace, Faraday, Gore, Respicardia, Stealth Peptides, and V-Wave.
References
- 1.Authors/Task Force Members. Rydén L, Grant PJ, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metra M, Zacà V, Parati G, et al. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 2011;12:76–84. doi: 10.2459/JCM.0b013e32834058d1. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald MR, Petrie MC, Varyani F, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 5.Fadini GP, Avogaro A, Degli Esposti L, et al. Risk of hospitalization for heart failure in patients with type 2 diabetes newly treated with DPP-4 inhibitors or other oral glucose-lowering medications: a retrospective registry study on 127,555 patients from the Nationwide OsMed Health-DB Database. Eur Heart J. 2015;36:2454–2462. doi: 10.1093/eurheartj/ehv301. [DOI] [PubMed] [Google Scholar]
- 6.Rosano GM, Vitale C, Fragasso G. Metabolic therapy for patients with diabetes mellitus and coronary artery disease. Am J Cardiol. 2006;98:14J–18J. doi: 10.1016/j.amjcard.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Nagoshi T, Yoshimura M, Rosano GM, Lopaschuk GD, Mochizuki S. Optimization of cardiac metabolism in heart failure. Curr Pharm Des. 2011;17:3846–3853. doi: 10.2174/138161211798357773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosano GM, Seferovic P. The management of diabetic patients with heart failure. Int Cardiovasc Forum J. 2017;10:58–62. [Google Scholar]
- 9.Aune D, Schlesinger S, Neuenschwander M, et al. Diabetes mellitus, blood glucose and the risk of heart failure: a systematic review and meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2018;28:1081–1091. doi: 10.1016/j.numecd.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 11.Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54:422–428. doi: 10.1016/j.jacc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ACCORD Study Group , Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elder DH, Singh JS, Levin D, et al. Mean HbA1c and mortality in diabetic individuals with heart failure: a population cohort study. Eur J Heart Fail. 2016;18:94–102. doi: 10.1002/ejhf.455. [DOI] [PubMed] [Google Scholar]
- 15.Suskin N, McKelvie RS, Burns RJ, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21:1368–1375. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
- 16.Banks AZ, Mentz RJ, Stebbins A, et al. Response to exercise training and outcomes in patients with heart failure and diabetes mellitus: insights from the HF-ACTION Trial. J Card Fail. 2016;22:485–491. doi: 10.1016/j.cardfail.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristensen SL, Preiss D, Jhund PS, et al. Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial. Circ Heart Fail. 2016;9:e002560. doi: 10.1161/CIRCHEARTFAILURE.115.002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosterd A, Cost B, Hoes AW, et al. The prognosis of heart failure in the general population: the Rotterdam Study. Eur Heart J. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 19.Johansson I, Edner M, Dahlström U, Näsman P, Rydén L, Norhammar A. Is the prognosis in patients with diabetes and heart failure a matter of unsatisfactory management? An observational study from the Swedish Heart Failure Registry. Eur J Heart Fail. 2014;16:409–418. doi: 10.1002/ejhf.44. [DOI] [PubMed] [Google Scholar]
- 20.Targher G, Dauriz M, Laroche C, et al. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19:54–65. doi: 10.1002/ejhf.679. [DOI] [PubMed] [Google Scholar]
- 21.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 22.Roumie CL, Min JY, D'Agostino McGowan L, et al. Comparative safety of sulfonylurea and metformin monotherapy on the risk of heart failure: a cohort study. J Am Heart Assoc. 2017;6:e005379. doi: 10.1161/JAHA.116.005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen XW, He ZX, Zhou ZW, et al. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the treatment of type 2 diabetes mellitus. Clin Exp Pharmacol Physiol. 2015;42:999–1024. doi: 10.1111/1440-1681.12455. [DOI] [PubMed] [Google Scholar]
- 24.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 25.Cavender MA, Norhammar A, Birkeland KI, et al. SGLT-2 Inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018;71:2497–2506. doi: 10.1016/j.jacc.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 26.Monami M, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and heart failure: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:689–697. doi: 10.1016/j.numecd.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 28.McMurray JJ, Ponikowski P, Bolli GB, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail. 2018;6:8–17. doi: 10.1016/j.jchf.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 29.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 30.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 31.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 36.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 38.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 40.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 41.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 42.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 43.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 44.D'Arcy JL, Coffey S, Loudon MA, et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. doi: 10.1093/eurheartj/ehw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marciniak A, Glover K, Sharma R. Cohort profile: prevalence of valvular heart disease in community patients with suspected heart failure in UK. BMJ Open. 2017;7:e012240. doi: 10.1136/bmjopen-2016-012240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robbins JD, Maniar PB, Cotts W, Parker MA, Bonow RO, Gheorghiade M. Prevalence and severity of mitral regurgitation in chronic systolic heart failure. Am J Cardiol. 2003;91:360–362. doi: 10.1016/s0002-9149(02)03172-7. [DOI] [PubMed] [Google Scholar]
- 47.Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675–1680. doi: 10.1136/hrt.2011.225789. [DOI] [PubMed] [Google Scholar]
- 48.Bursi F, Barbieri A, Grigioni F, et al. Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. Eur J Heart Fail. 2010;12:382–388. doi: 10.1093/eurjhf/hfq014. [DOI] [PubMed] [Google Scholar]
- 49.Goliasch G, Bartko PE, Pavo N, et al. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J. 2018;39:39–46. doi: 10.1093/eurheartj/ehx402. [DOI] [PubMed] [Google Scholar]
- 50.Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. 2018;379:2297–2306. doi: 10.1056/NEJMoa1805374. [DOI] [PubMed] [Google Scholar]
- 51.Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 52.Khayat R, Jarjoura D, Porter K, et al. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36:1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jilek C, Krenn M, Sebah D, et al. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail. 2011;13:68–75. doi: 10.1093/eurjhf/hfq183. [DOI] [PubMed] [Google Scholar]
- 54.Chua TP, Clark AL, Amadi AA, Coats AJ. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1996;27:650–657. doi: 10.1016/0735-1097(95)00523-4. [DOI] [PubMed] [Google Scholar]
- 55.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23:628–651. doi: 10.1016/j.cardfail.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019 doi: 10.1002/ejhf.1531. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester (IL): American Academy of Sleep Medicine; 2005. [Google Scholar]
- 58.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 59.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 60.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 61.Khayat R, Abraham W, Patt B, et al. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18:534–540. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 63.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 64.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9:251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Herrscher TE, Akre H, Øverland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17:420–425. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive heart failure. Relationship to arterial PCO2. Chest. 1993;104:1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- 67.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148:330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 68.Yu J, Zhang JF, Fletcher EC. Stimulation of breathing by activation of pulmonary peripheral afferents in rabbits. J Appl Physiol (1985) 1998;85:1485–1492. doi: 10.1152/jappl.1998.85.4.1485. [DOI] [PubMed] [Google Scholar]
- 69.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 70.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341:949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 71.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 72.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 74.Costanzo MR, Khayat R, Ponikowski P, et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure. J Am Coll Cardiol. 2015;65:72–84. doi: 10.1016/j.jacc.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abraham WT, Jagielski D, Oldenburg O, et al. Phrenic nerve stimulation for the treatment of central sleep apnea. JACC Heart Fail. 2015;3:360–369. doi: 10.1016/j.jchf.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 76.Costanzo MR, Ponikowski P, Javaheri S, et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388:974–982. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- 77.Costanzo MR, Ponikowski P, Coats A, et al. Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur J Heart Fail. 2018;20:1746–1754. doi: 10.1002/ejhf.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]