Abstract
Background and Objectives
The impact of left bundle branch block (LBBB) on left atrial (LA) dyssynchrony in heart failure (HF) patients with non-ischemic dilated cardiomyopathy (DCM) has not been clearly elucidated.
Methods
Eighty consecutive symptomatic HF patients with non-ischemic DCM (left ventricular ejection fraction, LVEF<35%) were included. LBBB was defined on electrocardiography. Left ventricular (LV) systolic and diastolic dyssynchrony index and LA dyssynchrony index were obtained by color-coded tissue Doppler imaging.
Results
There was no significant difference in LV size, LVEF, LV global longitudinal strain, peak atrial longitudinal strain and LA volume between patients with LBBB (n=38) and no LBBB (n=42). There was a significant difference in LV systolic dyssynchrony index (p=0.014) and there was a mild difference in LV diastolic synchronicity index (p=0.045) between patients with LBBB and no LBBB. However, there was no difference in LA dyssynchrony index between the 2 groups (p=0.60). LA dyssynchrony index was not related to LV systolic and diastolic dyssynchrony indexes, but it was related to the deceleration time of mitral early diastolic velocity (E), the ratio of E to mitral annular early diastolic velocity (E/e′) and LA volume. E/e′ was most related to LA dyssynchrony index (r2=0.325, p=0.002).
Conclusions
LBBB influences both LV systolic and diastolic dyssynchrony, but not LA dyssynchrony. LA dyssynchrony was related to LV diastolic function regardless of the presence of LBBB in in patients with non-ischemic DCM.
Keywords: Heart failure, Left atrium, Synchrony, Left bundle branch block, Diastolic
INTRODUCTION
Left bundle branch block (LBBB) with widening of the QRS complex is seen in 30% of the patients with progressive heart failure (HF), and it occurs in nearly half of the patients with non-ischemic dilated cardiomyopathy (DCM).1),2),3)
The deleterious effect of LBBB on left ventricular (LV) systolic and diastolic function caused by regional delays of electrical activity leads to dyssynchronous LV function and has been established in HF patients with DCM.4),5) Cardiac resynchronization therapy is recommended and improvement of symptoms and ventricular function are expected in considerable HF patients with LBBB.6)
Left atrium (LA) is associated with LV remodeling and function. LA is an important determinant of LV filling, especially in patients with end-stage systolic or diastolic ventricular dysfunction7) and more impaired LA mechanics are associated with more severe diastolic dysfunction and predict long-term adverse events in patients with chronic systolic HF.8) Therefore, LA enlargement with functional impairment is a characteristic finding that causes atrial electrical and contractility abnormalities in patients with HF.9),10)
Recently it has been postulated that some HF with preserved ejection fraction (EF) patients who present with LA electromechanical conduction delay could be improved by atrial resynchronization deserves further investigation.11)
However, the impact of LBBB on LA dyssynchrony in non-ischemic DCM has not been clearly elucidated. Therefore, the aim of this study was to assess LA dyssynchrony and its relationship to LV diastolic function in non-ischemic DCM with and without LBBB.
METHODS
Study population
We enrolled 82 consecutive patients with symptomatic HF due to non-ischemic DCM. The inclusion criteria of the study were as follows: the presence of HF with LV systolic dysfunction (LV EF<35%); LV dilation and diffuse hypocontractility on echocardiography; sinus rhythm either with LBBB or with narrow QRS complex (width<120 ms) on electrocardiography; no history of angina pectoris or myocardial infarction; and no significant coronary artery stenosis (≥50% stenosis) on coronary angiography or coronary angio computed tomography. LBBB was defined according to standard electrocardiographic criteria as QRS duration ≥120 ms, a broad, notched, or slurred R wave in the left precordial leads, and an initial R wave followed by a wide, deep S wave in the right precordial leads on a 12-lead electrocardiogram. The exclusion criteria included the presence of congenital or valvular heart disease, hypertrophic or restrictive cardiomyopathy, thyroid disease, and acute myocarditis. All patients gave informed consent, and study approval was obtained from the Institutional Review Board of Korea University Medicine (2011AN0318).
Conventional echocardiography
A commercially available echocardiography system (Vivid 7; GE Vingmed Ultrasound AS, Horten, Norway) with a 3.5-MHz phased array transducer was used for all echocardiographic studies. LV diameter, wall thickness, and LV mass were measured using the criteria of the American Society of Echocardiography.12) The LV volume and EF were calculated using the biplane Simpson's method from apical 4- and 2-chamber views.12) The LA volume was measured using the biplane area-length method from the apical 2- and 4-chamber views.12) The LA emptying fraction was determined as (LA maximal volume at end-systole − LA minimal volume at end-diastole)/LA maximal volume at end-systole.
All volumetric and area parameters were indexed by the body surface area. Mitral inflow was obtained by pulsed-wave Doppler echocardiography with the sample volume placed between the mitral leaflet tips during diastole, and the early diastolic velocity (E velocity) and its deceleration time (DT) were measured. Early diastolic mitral annular velocity at the septal mitral annulus (e′ velocity) was obtained by Doppler tissue imaging and the the ratio of E to mitral annular early diastolic velocity (E/e′) was calculated.
Strain, tissue Doppler imaging and dyssynchrony analysis
For speckle tracking analysis, apical 4- and 2 chamber, and long-axis views images were obtained using conventional 2-dimensional grayscale echocardiography. The frame rate was set between 60 and 80 frames per second. LV global longitudinal strain (GLS, %) was calculated as the mean longitudinal peak negative strain from 18 apical segments during a cardiac cycle. LA global peak atrial longitudinal strain (PALS), measured at the end of the reservoir phase, was calculated as the mean longitudinal peak positive strain from 12 segments in the 4- and 2-chamber views.
Tissue Doppler velocity was used for the assessment of dyssynchrony because of its high temporal resolution, which allows accurate measurement of mechanical dyssynchrony, particularly LV diastolic dyssynchrony and LA dyssynchrony.13),14),15)
Color-coded tissue Doppler imaging (TDI) was applied to record myocardial velocities at the ventricular and atrial walls (using the 2- and 4-chamber images), with adjustment of depth and sector width to achieve frame rates of >100 frames/second. To assess the synchronicity of ventricle, the sample volume was placed in the LV basal and mid portions of the anterior, inferior, septal, and lateral walls, per region, the time interval between the onset of the QRS complex and the peak systolic velocity (Ts) and peak of early diastolic velocity (Te) was derived. LV systolic dyssynchrony index (SD-Ts) was defined as the standard deviation (SD) of Ts among the 12 LV walls (intraobserver mean difference = 1.3±1.0ms; interobserver mean difference = 1.7±1.2 ms).
LV diastolic dyssynchrony index (SD-Te) was defined as the SD of Te among the 12 LV walls (intraobserver mean difference = 1.4±0.9 ms; interobserver mean difference = 2.1±0.8 ms).
To assess the synchronicity of the LA, the sample volume was placed below the mitral annulus of the anterior, inferior, septal, and lateral LA walls, and the time interval between the onset of the P-wave and peak of atrial velocity (Ta) was derived. LA dyssynchrony index (SD-Ta) was defined as the SD of Ta among the 4 LA walls (intraobserver mean difference = 0.9±0.8 ms; interobserver mean difference = 1.6±0.4 ms). At least 3 consecutive beats were stored digitally and analyzed offline.
Statistical analysis
Data are presented as the mean±SD. The differences between the groups were tested using the Student's t-test. To assess the relationship of LA dyssynchrony index with various echocardiographic parameters, we performed univariate and multivariate analyses based on stepwise multiple linear regression. All analyses were performed with commercially available statistical software (SPSS, version 13.0; SPSS Inc., Chicago, IL, USA). A p value<0.05 was considered to indicate statistical significance.
RESULTS
Clinical and echocardiographic characteristics
From among the 82 patients with non-ischemic DCM, 2 patients were excluded because the echocardiographic images were not considered acceptable for analysis. Table 1 shows the clinical characteristics of study patients. Among these 80 patients, 38 had LBBB and 42 had no LBBB. The width of QRS complex was significantly larger in patients with LBBB than in patients with no LBBB (168.3±17.9 ms vs. 93.8±14.5 ms, p<0.001). However, there was no difference in P-wave duration on ECG between the 2 groups (128.5±12.5 ms vs. 122.5±15.4 ms, p=0.24).
Table 1. Clinical characteristics of heart failure patients with non-ischemic dilated cardiomyopathy.
| With LBBB (n=38) | With no LBBB (n=42) | p value | ||
|---|---|---|---|---|
| Age (years) | 61±12 | 58±11 | 0.203 | |
| Men/women | 16/22 | 17/25 | 0.531 | |
| Heart rate (/min) | 72±10 | 76±12 | 0.381 | |
| Systolic BP (mmHg) | 119±15 | 114±16 | 0.227 | |
| Diastolic BP (mmHg) | 76±12 | 72±9 | 0.209 | |
| QRS complex width (ms) | 168.3±17.9 | 93.8±14.5 | <0.001 | |
| P-wave duration (ms) | 128.5±12.5 | 122.5±15.4 | 0.240 | |
| Hypertension | 9 (23.7) | 10 (23.8) | 0.599 | |
| Diabetes mellitus | 8 (21.0) | 9 (21.4) | 0.593 | |
| NYHA class II/III/IV | 8/21/9 | 9/25/8 | 0.735 | |
| Medications | ||||
| Beta blocker | 30 (78.9) | 34 (81.0) | 1.000 | |
| Diuretics | 28 (73.7) | 32 (76.2) | 0.803 | |
| Spironolactone | 19 (50.0) | 16 (38.1) | 0.368 | |
| ACEI or ARB | 31 (81.6) | 35 (83.3) | 0.534 | |
Data are expressed as the mean±standard deviation or number (%).
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; LBBB = left bundle branch block; NYHA = New York Heart Association.
There was no significant difference in the baseline clinical parameters, including the prevalence of hypertension and diabetes mellitus and medical therapy (Table 1). The medical treatment for patients included β-blockers (n=64, 80%), angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) (n=66, 82.5%), spironolactone (n=35, 43.8%), and diuretics (n=60, 75%).
There was no significant difference in echocardiographic parameters including LV size, LV EF, LV GLS, PALS, LA volume, and mitral inflow and mitral annular Doppler parameters between patients with LBBB and with no LBBB. LA emptying fraction and a′ velocity were not different between the 2 groups (Table 2).
Table 2. Echocardiographic characteristics of heart failure patients with non-ischemic dilated cardiomyopathy.
| With LBBB (n=38) | With no LBBB (n=42) | p value | |
|---|---|---|---|
| LV end-diastolic volume (mL/m2) | 115.7±16.8 | 109.0±13.7 | 0.315 |
| LV end-systolic volume (mL/m2) | 84.6±14.6 | 82.4±13.6 | 0.794 |
| LV ejection fraction (%) | 26.1±4.2 | 24.6±4.2 | 0.395 |
| LA volume (mL/m2) | 44.5±15.4 | 44.2±17.8 | 0.448 |
| LA emptying fraction (%) | 41.6±10.1 | 40.8±11.2 | 0.311 |
| Global LV GLS (%) | −6.5±2.3 | −6.2±2.0 | 0.355 |
| Global PALS (%) | 13.0±11.3 | 13.6±10.9 | 0.422 |
| E velocity (cm/s) | 66.0±26.3 | 70.1±26.2 | 0.662 |
| A velocity (cm/s) | 72.8±20.1 | 72.6±23.1 | 0.982 |
| DT (ms) | 200.6±77.4 | 186.8±67.4 | 0.216 |
| e′ velocity (cm/s) | 3.7±1.2 | 3.5±1.0 | 0.453 |
| a′ velocity (cm/s) | 6.5±1.8 | 6.1±1.9 | 0.562 |
| E/e′ | 18.5±8.1 | 20.2±6.5 | 0.327 |
| MR grade (>2) | 8 (21.0) | 11 (26.2) | 0.392 |
| PA systolic pressure (mmHg) | 42±15 | 44±14 | 0.764 |
Data are expressed as the mean±standard deviation or number (%).
DT = deceleration time of mitral early diastolic velocity; E = mitral early diastolic velocity; E/e′ = the ratio of early diastolic mitral inflow velocity to early diastolic mitral annular velocity; GLS = global longitudinal strain; LA = left atrium; LBBB = left bundle branch block; LV = left ventricular; MR = mitral regurgitation; PA = pulmonary artery; PALS = peak atrial longitudinal strain.
Impact of left bundle branch block on left ventricular and left atrial dyssynchrony
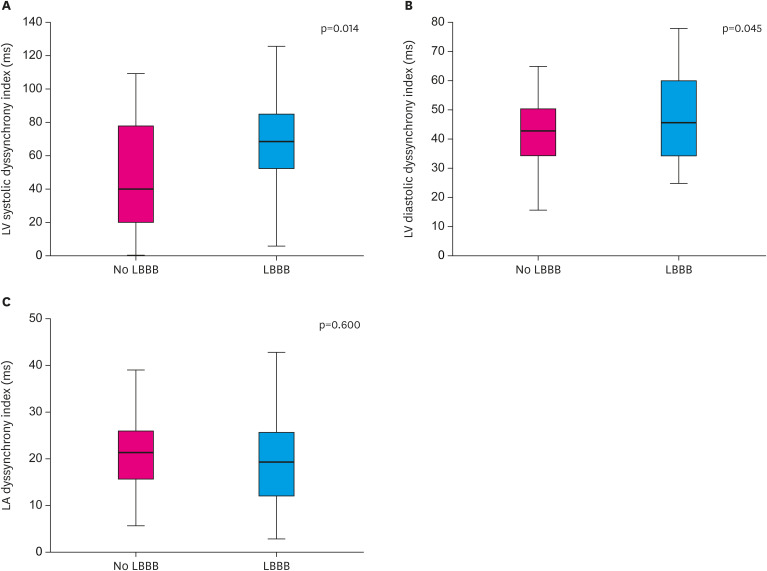
There was a significant difference in SD-Ts (68.2±38.2 ms vs. 48.5±32.0 ms, p=0.014) and there was a mild difference in SD-Te between patients with LBBB and no LBBB (59.2±36.5 ms vs. 45.3±24.1 ms, p=0.045). However, there was no difference in SD-Ta between patients with LBBB and no LBBB (20.9±11.5 ms vs. 22.5±14.5 ms, p=0.60) (Figure 1 and Table 3). There were no significant relations of SD-Ts and SD-Te with QRS duration (r=0.282, p=0.095 and r=0.207, p=0.233, respectively). The SD-Ta was not related to P-wave duation (r=0.256, p=0.189) and QRS duration (r=0.142, p=0.409), neither.
Figure 1. Comparison of dyssynchrony indexes by the presence of LBBB in heart failure patients with non-ischemic dilated cardiomyopathy. (A) LV systolic dyssynchrony index. (B) LV diastolic dyssynchrony index. (C) LA dyssynchrony index.
LA = left atrial; LBBB = left bundle branch block; LV = left ventricular.
Table 3. LV and LA dyssynchrony indexes.
| With LBBB (n=38) | With no LBBB (n=42) | p value | |
|---|---|---|---|
| LV systolic dyssynchrony index (ms) | 68.2±38.2 | 48.5±32.0 | 0.014 |
| LV diastolic dyssynchrony index (ms) | 59.2±36.5 | 45.3±24.1 | 0.045 |
| LA dyssynchrony index (ms) | 20.9±11.5 | 22.5±14.5 | 0.600 |
Data are expressed as the mean±standard deviation.
LA = left atrium; LBBB = left bundle branch block; LV = left ventricular.
Relation between left atrial dyssynchrony and other echocardiographic parameters
There was no significant relation between SD-Ta and LV volume and LVEF. In addition, SD-Ta was not related to SD-Ts (r=0.083, p=0.465) and SD-Te (r=0.077, p=0.499).
LA emptying fraction was mildly related to SD-Ta (r=−0.177, p=0.051), but not to a′ velocity (r=−0.127, p=0.468).
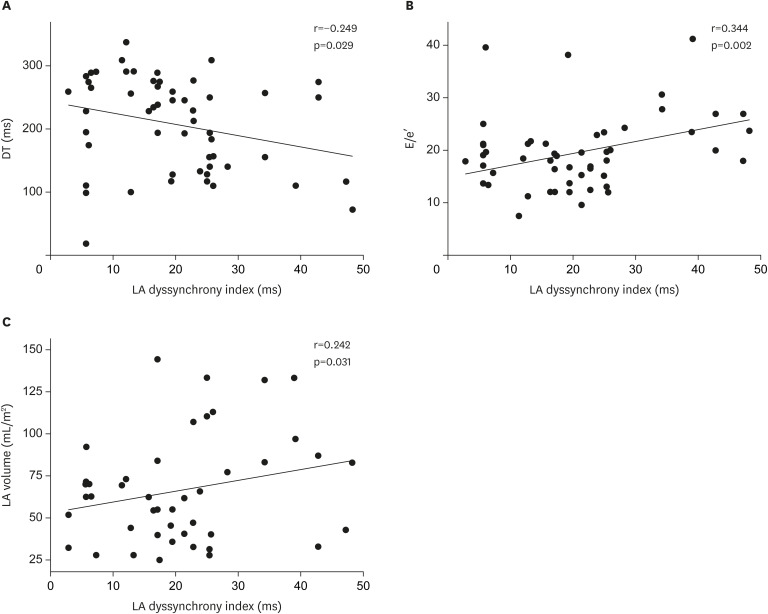
However, SD-Ta was significantly related to the DT (r=−0.249, p=0.029), E/e′ (r=0.344, p=0.002) and LA volume (r=0.242, p=0.031), and E/e′ was most related to SD-Ta (r2=0.329, p=0.002) (Figure 2, Table 4).
Figure 2. The relationship between LA dyssynchrony index and LV diastolic parameters in heart failure patients with non-ischemic dilated cardiomyopathy. (A) DT, (B) E/e′, and (C) LA.
DT = deceleration time of mitral early diastolic velocity; E/e′ = the ratio of early diastolic mitral inflow velocity to early diastolic mitral annular velocity; LA = left atrial; LV = left ventricular.
Table 4. Univariate analysis of LA dyssynchrony index.
| r | p value | |
|---|---|---|
| Age | 0.025 | 0.826 |
| LV end-diastolic volume | −0.127 | 0.482 |
| LV end-systolic volume | −0.081 | 0.654 |
| LV ejection fraction | −0.132 | 0.449 |
| LV GLS | 0.189 | 0.089 |
| LA volume | 0.242 | 0.031 |
| LA emptying fraction | −0.177 | 0.051 |
| PALS | −0.181 | 0.049 |
| E velocity | 0.111 | 0.544 |
| A velocity | −0.192 | 0.294 |
| DT | −0.249 | 0.029 |
| e′ velocity | −0.281 | 0.119 |
| a′ velocity | −0.127 | 0.468 |
| E/e′ | 0.344 | 0.002 |
| LV systolic dyssynchrony index | 0.083 | 0.465 |
| LV diastolic dyssynchrony index | 0.077 | 0.499 |
DT = deceleration time of mitral early diastolic velocity; E = mitral early diastolic velocity; E/e′ = the ratio of early diastolic mitral inflow velocity to early diastolic mitral annular velocity; GLS = global longitudinal strain; LA = left atrium; LV = left ventricular; PALS = peak atrial longitudinal strain.
Patients with higher E/e′ (>15, n=52) showed higher SD-Ta than patients with lower E/e′ (≤15, n=28) (24.3±14.0 ms vs. 17.1±9.9 ms, p=0.018), but there were no significant differences in SD-Ts and SD-Te between the 2 groups (p=0.992 and p=0.069, respectively).
DISCUSSION
The present study showed that 1) there was a significant difference in LV systolic dyssynchrony index and there was a mild difference in LV diastolic dyssynchrony index between patients with LBBB and no LBBB, 2) there was no difference in LA dyssynchrony index between patients with LBBB and no LBBB, and 3) LA dyssynchrony index was related to LV diastolic parameters regardless of the presence of LBBB in HF patients with non-ischemic DCM.
LBBB is common in HF patients with non-ischemic DCM and occurs in up to nearly 50% of the patients.1),2),3) It has been demonstrated that LBBB leads to a disturbed synchrony of the LV wall, and consequently, to a poor coordination of ventricular contraction and relaxation and to an impaired LV diastolic and systolic function in HF patients with DCM.4),5),16)
With similar QRS duration in LBBB, the mechanical effect may depend on the site of block and have different electrical activation process by transseptal time. Therefore, the degree of LV mechanical dyssynchrony assessed by echocardiography may not be well correlated with LV electrical dyssynchrony assessed by QRS duration on ECG. Our study also showed no significant relation between them.
There are a few studies on the relation between LBBB and increase in LA size in ischemic heart disease and LV hypertrophy,17),18) whereas there are hardly any studies about the impact of LBBB on LA electromechanical and volumetric remodeling in HF patients with non-ischemic DCM. LA enlargement with functional impairment is a characteristic finding that causes atrial electrical and contractility abnormalities in patients with HF.9),10) LA remodeling with enlargement and dysfunction is an important determinant of LV filling, especially in patients with end-stage systolic or diastolic ventricular dysfunction7) and more impaired LA mechanics are associated with more severe diastolic dysfunction and predict long-term adverse events in patients with chronic systolic HF.8) Therefore, it has been shown that LA remodeling acts as a barometer of LV diastolic burden.19),20)
Patients with a decreased LVEF had an increased risk proportional to the increase in the size of the LA, which was independent of LVEF, age, or symptomatic status. The main determinant of LA volume is ventricular diastolic function. It has been suggested that enlarged LA volume may be the morphophysiologic expression of chronic diastolic dysfunction. In fact, the LA is exposed directly to LV diastolic pressure through the open mitral valve; and it tends to dilate with increasing pressure because of its thin wall structure.
In addition to structural remodeling, congestive HF also causes electrical remodeling of the atria including depressed excitability, increased refractoriness, and slowed conduction or block.8),9),21),22),23) Congestive HF may also be associated with abnormalities of cell electrophysiology such as cellular uncoupling and anisotropy.24) Atrial remodeling is responsible for the conduction delay and atrial arrhythmias.
More LA enlargement may be related to more LA dyssynchrony,25),26) and in our study, LA dyssynchrony index was also related to LA volume. Therefore, more impaired LA mechanics due to LA dyssynchronous motion are related to more LV diastolic dysfunction. In our study, LA dyssynchrony index was related to LV diastolic parameters such as DT of E and E/e′ and patients with high E/e′ showed higher LA dyssynchrony index than patients with low E/e′. Increased LV filling pressure estimated by E/e′ represents increased afterload for the LA; thus, LV diastolic dysfunction may contribute to the deterioration of LA mechanics by hemodynamic overload and mechanical stretch of the LA.21),27)
However, we did not identify the relationship of the presence of LBBB with LA remodeling and the direct impact of LBBB on LA electromechanical dyssynchrony in HF patients with advanced non-ischemic DCM. P-wave duration is decided by bi-atrial conduction time and may not have correlation with LA mechanical dyssynchrony or mechanical delay. In addition, LV conduction delay by LBBB may not have a direct impact on LA conduction, but LV dysfunction by LBBB is significant impact on LA function and remodeling, either structural and function.28)
LA remodeling may be caused by LV diastolic dysfunction and LA myopathic process, and LA enlargement with dysfunction consequently begets more LV diastolic dysfunction.
Our findings indicate that LA enlargement and electromechanical dysfunction are mainly caused by advanced LV diastolic dysfunction, in addition to LV dilatation and systolic dysfunction regardless of the presence of LBBB in HF patients with non-ischemic DCM. Therefore, our findings highlight the importance of identifying and treating significant LV diastolic dysfunction and its hemodynamic sequelae to minimize their contribution to greater LA remodeling and to worsening of congestive HF.
Although the patients in this study had a normal sinus rhythm, many HF patients with DCM have either paroxysmal or persistent atrial fibrillation (AF). LA dyssynchrony index has been shown as a possible predictor of the new development or recurrence of AF in various conditions,28),29) and we expect to perform follow-up studies in these patients to assess the prognostic value of LA dyssynchrony index for predicting AF.
Recently it has been postulated that some HF with preserved EF patients who present with LA electromechanical conduction delay and delayed LA systole could be improved by atrial resynchronization deserves further investigation.11) However, in our study with HF patients with DCM, LA dyssynchrony was related to LV diastolic parameters such as DT, E/e′ and LA volume regardless of the presence of LBBB and we may recommend that the management of HF with volume control and improvement of LV diastolic function may improve LA dyssynchrony and mechanical dysfunction rather than or before considering atrial resynchronization in these patients.
The present study included a relatively small number of subjects. Our results were limited to HF patients with non-ischemic DCM, and therefore, they may not be readily generalizable to patients with different causes of HF and LV EF. Two patients were excluded from the analyses because they had unacceptable image quality. It was not possible to obtain acceptable tissue velocity images from 2 patients because the LA wall was too thin to place the sample volume within the myocardium during a cardiac cycle. Although the advantage of speckle-tracking strain over TDI velocity is that strain is less affected by passive motion or tethering,30) the analysis have a limitation when apical LV wall are not fully included particularly in patients with severely dilated heart. In addition, there is still limitation to resolve with speckle-tracking strain tracing the LA cavity and is a myocardial wall dropout in the intra-atrial septum, LA appendage and the pulmonary veins. Moreover, TDI velocity was used for the assessment of LV diastolic dyssynchrony and LA dyssynchrony because of its high temporal resolution, which allows accurate measurement of mechanical dyssynchrony.13),14),15) Finally, there are a number of limitations in examining the effects of medical therapy in patients with HF. However, in this study, most HF patients used ACEIs or ARBs and beta blockers and there were no significant differences in dyssynchrony index due to these medications.
In conclusion, LBBB influences both LV systolic and diastolic dyssynchrony, but not LA dyssynchrony. LA dyssynchrony was related to LV diastolic function regardless of the presence of LBBB in patients with non-ischemic DCM and the management of HF with improvement of LV diastolic function may improve LA dyssynchrony and mechanical dysfunction regardless of the presence of LBBB in these patients.
ACKNOWLEDGEMENTS
We sincerely thank Woong-Jin Oh, Jeong-Hyang Kim, and Young-Woon Choi for obtaining images and collecting data.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Park SM, Kim MN, Shim WJ.
- Data curation: Park SM, Kim MN.
- Formal analysis: Park SM.
- Investigation: Park SM, Kim HD, Cho DH, Shim WJ.
- Methodology: Park SM.
- Writing - original draft: Park SM.
- Writing - review & editing: Park SM.
References
- 1.Baldasseroni S, Opasich C, Gorini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 2.Kalra PR, Sharma R, Shamim W, et al. Clinical characteristics and survival of patients with chronic heart failure and prolonged QRS duration. Int J Cardiol. 2002;86:225–231. doi: 10.1016/s0167-5273(02)00270-x. [DOI] [PubMed] [Google Scholar]
- 3.Sze E, Samad Z, Dunning A, et al. Impaired recovery of left ventricular function in patients with cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2018;71:306–317. doi: 10.1016/j.jacc.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trautmann SI, Kloss M, Auricchio A. Cardiac resynchronization therapy. Curr Cardiol Rep. 2002;4:371–378. doi: 10.1007/s11886-002-0036-2. [DOI] [PubMed] [Google Scholar]
- 5.Auffret V, Martins RP, Daubert C, et al. Idiopathic/iatrogenic left bundle branch block-induced reversible left ventricle dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:3177–3188. doi: 10.1016/j.jacc.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 7.Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol. 1998;82:756–761. doi: 10.1016/s0002-9149(98)00452-4. [DOI] [PubMed] [Google Scholar]
- 8.Motoki H, Borowski AG, Shrestha K, et al. Impact of left ventricular diastolic function on left atrial mechanics in systolic heart failure. Am J Cardiol. 2013;112:821–826. doi: 10.1016/j.amjcard.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Rossi A, Cicoira M, Zanolla L, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1425–1430. doi: 10.1016/s0735-1097(02)02305-7. [DOI] [PubMed] [Google Scholar]
- 10.Sanders P, Morton JB, Davidson NC, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 11.Eicher JC, Laurent G, Mathé A, et al. Atrial dyssynchrony syndrome: an overlooked phenomenon and a potential cause of ‘diastolic’ heart failure. Eur J Heart Fail. 2012;14:248–258. doi: 10.1093/eurjhf/hfr169. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Guan Z, Zheng X, et al. Impaired left atrial systolic function and inter-atrial dyssynchrony may contribute to symptoms of heart failure with preserved left ventricular ejection fraction: A comprehensive assessment by echocardiography. Int J Cardiol. 2018;257:177–181. doi: 10.1016/j.ijcard.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Kwon BJ, Lee SH, Park CS, et al. Left ventricular diastolic dyssynchrony in patients with treatment-naive hypertension and the effects of antihypertensive therapy. J Hypertens. 2015;33:354–365. doi: 10.1097/HJH.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 15.Gorcsan J, 3rd, Sogaard P, Bax JJ, et al. Association of persistent or worsened echocardiographic dyssynchrony with unfavourable clinical outcomes in heart failure patients with narrow QRS width: a subgroup analysis of the EchoCRT trial. Eur Heart J. 2016;37:49–59. doi: 10.1093/eurheartj/ehv418. [DOI] [PubMed] [Google Scholar]
- 16.Xiao HB, Lee CH, Gibson DG. Effect of left bundle branch block on diastolic function in dilated cardiomyopathy. Br Heart J. 1991;66:443–447. doi: 10.1136/hrt.66.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obiagwu C, Ariyarajah V, Apiyasawat S, Spodick DH. Correlation of echocardiographic left atrial abnormality with myocardial ischemia during myocardial perfusion assessment in patients with left bundle branch block. Am J Cardiol. 2013;112:660–663. doi: 10.1016/j.amjcard.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Jain AC, Mehta MC, Billie M. Usefulness of left atrial abnormality for predicting left ventricular hypertrophy in the presence of left bundle branch block. Am J Cardiol. 2000;85:354–359. doi: 10.1016/s0002-9149(99)00746-8. [DOI] [PubMed] [Google Scholar]
- 19.Pinamonti B, Di Lenarda A, Sinagra G, Camerini F. Restrictive left ventricular filling pattern in dilated cardiomyopathy assessed by Doppler echocardiography: clinical, echocardiographic and hemodynamic correlations and prognostic implications. Heart Muscle Disease Study Group. J Am Coll Cardiol. 1993;22:808–815. doi: 10.1016/0735-1097(93)90195-7. [DOI] [PubMed] [Google Scholar]
- 20.Park SM, Park SW, Casaclang-Verzosa G, et al. Diastolic dysfunction and left atrial enlargement as contributing factors to functional mitral regurgitation in dilated cardiomyopathy: data from the Acorn trial. Am Heart J. 2009;157:762.e3–762.e10. doi: 10.1016/j.ahj.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Boixel C, Fontaine V, Rücker-Martin C, et al. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol. 2003;42:336–344. doi: 10.1016/s0735-1097(03)00578-3. [DOI] [PubMed] [Google Scholar]
- 23.Li D, Melnyk P, Feng J, et al. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 24.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 25.Ozer N, Yavuz B, Can I, et al. Doppler tissue evaluation of intra-atrial and interatrial electromechanical delay and comparison with P-wave dispersion in patients with mitral stenosis. J Am Soc Echocardiogr. 2005;18:945–948. doi: 10.1016/j.echo.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Esmaeilzadeh M, Nikparvar M, Maleki M, et al. Assessment of inter and intra-atrial asynchrony in patients with systolic heart failure using velocity vector imaging. Res Cardiovasc Med. 2013;2:114–120. doi: 10.5812/cardiovascmed.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 28.Park SM, Kim YH, Choi JI, Pak HN, Kim YH, Shim WJ. Left atrial electromechanical conduction time can predict six-month maintenance of sinus rhythm after electrical cardioversion in persistent atrial fibrillation by Doppler tissue echocardiography. J Am Soc Echocardiogr. 2010;23:309–314. doi: 10.1016/j.echo.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Cho GY, Jo SH, Kim MK, et al. Left atrial dyssynchrony assessed by strain imaging in predicting future development of atrial fibrillation in patients with heart failure. Int J Cardiol. 2009;134:336–341. doi: 10.1016/j.ijcard.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki C, Powell BD, Bruce CJ, et al. Comparison of echocardiographic dyssynchrony assessment by tissue velocity and strain imaging in subjects with or without systolic dysfunction and with or without left bundle-branch block. Circulation. 2008;117:2617–2625. doi: 10.1161/CIRCULATIONAHA.107.733675. [DOI] [PubMed] [Google Scholar]