Abstract
Background and Objectives
Sacubitril/valsartan (SV, LCZ696), the first in class drug, called as angiotensin receptor-neprilysin inhibitor (ARNI) can reduce heart failure (HF) hospitalization and cardiovascular mortality. However, SV prescription rate remains still low despite current HF guideline recommendations. Considering the complex inclusion criteria of Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, the real-world eligibility for SV remains uncertain in Asian heart failure with reduced ejection fraction (HFrEF) patients. Therefore, we aimed to assess real-world HF population eligibility for SV in a large Korean acute HF registry.
Methods
From March 2011 to February 2014, a total of 5,625 patients who were admitted for HF were enrolled in Korea. After excluding HF patients with left ventricular ejection fraction > 40% and in-hospital death, 2,941 patients were analyzed. Criteria for SV based on Korean Food and Drug Administration (KFDA) label and PARADIGM-HF were applied.
Results
Of 2,941 patients, KFDA label criteria excludes the absence of symptoms (New York Heart Association class I, 20%); PARADIGM-HF criteria excludes chronic kidney disease stage IV (9%), hyperkalemia (1%), hypotension (6%), and sub-optimal pharmacotherapy (52%, e.g. lower dose use of angiotensin converting enzyme inhibitor/angiotensin receptor blocker [ACEI/ARB], beta blocker use). When a daily requirement of ACEI/ARB ≥5 mg enalapril (instead of ≥10 mg) was used, the percent of eligibility for SV rose from 12% to 30% based on the PARADIGM-HF criteria.
Conclusions
Among the Korean hospitalized HFrEF patients, 80% met KFDA label criteria, while only 12% met the inclusion criteria of PARADIGM-HF trial for SV if requiring ≥10 mg enalapril. Sub-optimal pharmacotherapy could be the main reason for ineligible SV use based on the PARADIGM-HF criteria.
Keywords: Sacubitril-valsartan, Heart failure, Drung therapy, Patient care
INTRODUCTION
Sacubitril/valsartan (SV, LCZ696) is the first in class drug, called as angiotensin receptor-neprilysin inhibitor (ARNI).1),2) In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, SV reduced heart failure (HF) hospitalization and cardiovascular mortality compared to angiotensin converting enzyme inhibitor (ACEI), enalapril.3) Based on these results, the current United States, European and Korean guidelines recommend SV use for the patients with heart failure with reduced ejection fraction (HFrEF),4),5),6) and both the Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved SV for symptomatic HFrEF patients. In Korea, SV was approved by Korean Food and Drug Administration (KFDA) in April 2016 then we can use SV under insurance coverage from October 2017.2) However, the inclusion criteria of PARADIGM-HF trial were complex, requiring symptomatic HF (New York Heart Association [NYHA] class II–IV), left ventricular ejection fraction (LVEF) ≤40% (later amended to ≤35%), elevated natriuretic peptides (NPs) levels, a dose of ACEI or angiotensin receptor blocker (ARB) equivalent to ≥10 mg of enalapril daily dose for the run-in and ≥20 mg of enalapril daily dose for randomization, and beta-blocker (BB) therapy as tolerated according to current HF guidelines.3) So, HFrEF patients not meeting the enrollment criteria of PARADIGM-HF could be also eligible for SV use on the basis of current FDA/EMA and KFDA approval.7) It could be the reason why SV prescription rate remains still low despite current HF guideline recommendations.8) So many researchers have paid more attention to the difference in HF patients from between real-world or registry vs. clinical trial.
There has been known differences in clinical characteristics between Western and Asian HF patients.9),10),11),12),13),14) Although the PARADIGM-HF trial also enrolled Asian HFrEF patients, the real-world eligibility for SV remains uncertain in Asian HFrEF patients especially for large sample-sized population. Therefore, we aimed to assess in a large, Korean real-world HF population eligibility for SV according to the inclusion criteria of PARADIGM-HF and the current HF guideline/label criteria. For this analysis, we enrolled HF patients after discharge from acute decompensated heart failure (ADHF) from the Korean Acute Heart Failure (KorAHF) registry considering inborn limitation of study population.
METHODS
Study design and population
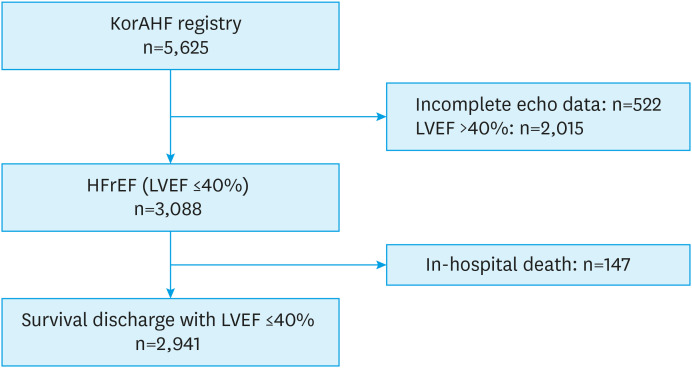
The KorAHF registry is a multicenter prospective cohort study in Korea.11) Briefly, patients were consecutively enrolled on initial admission for ADHF and followed-up at outpatient clinic. Patients who have signs or symptoms of HF and one of the following criteria are acceptable for the study: 1) pulmonary congestion or 2) objective findings of left ventricular systolic dysfunction or structural heart disease. The study protocol was approved by the ethics committee at each hospital (4-2011-0075). More detailed information on the study design and results of the KorAHF registry were described previously.11)12) From March 2011 to February 2014, a total of 5,625 patients who were admitted for ADHF were enrolled consecutively in 10 tertiary university hospitals in Korea. Among them, we selected patients with HFrEF, which was defined as LVEF ≤40% using echocardiography. After excluding 522 patients without quantitative LVEF data and 2,015 patients with LVEF >40% and 147 patients with in-hospital death or heart transplantation, 2,941 patients were analyzed (Figure 1). Estimated glomerular filtration rate (eGFR) was calculated by Modification of Diet in Renal Disease (MDRD) equation.15)
Figure 1. Study population.
HFrEF = heart failure with reduced ejection fraction; KorAHF = Korean Acute Heart Failure; LVEF = left ventricular ejection fraction.
On-treatment angiotensin converting enzyme inhibitor/angiotensin receptor blocker/enalapril equivalent dose
In the KorAHF registry, patients administered with various types of ACEI/ARB so we have to adjust these ACEI/ARB dose to enalapril equivalent dose according to the pre-specified criteria used in Safety and Tolerability of Initiating LCZ696 in Heart Failure Patients (TITRATION) trial as follows (Table 1).16) ‘High-dose’ received a total daily dose >160 mg of valsartan or >10 mg of enalapril, or equivalent doses of other ARBs or ACEIs, respectively; ‘low-dose’ received a total daily dose ≤160 mg of valsartan or ≤10 mg of enalapril, or equivalent. Drug administration doses were collected at discharge, and during follow-up (30 days, 3 months, 6 months, and 1 years) and average on-treatment ACEI/ARB dose was calculated from enalapril equivalent dose at discharge and at each follow-up before the occurrence of an event. With the same method, we can calculate on-treatment blood pressure (BP) using BP at discharge and at each follow-up before the occurrence of an event.17)
Table 1. Definition of low-dose and high-dose ACEI/ARB inhibition strata based on pre-study ACEI/ARB total daily dose at screening.
| Medication | Low-dose RAAS inhibitor stratum | High-dose RAAS inhibitor stratum | |
|---|---|---|---|
| ACEIs | |||
| Enalapril | ≤10 | >10 | |
| Captopril | ≤100 | >100 | |
| Cilazapril | ≤2.5 | >2.5 | |
| Imidapril | ≤10 | >10 | |
| Lisinopril | ≤10 | >10 | |
| Perindopril | ≤4 | >4 | |
| Ramipril | ≤5 | >5 | |
| ARBs | |||
| Candesartan | ≤16 | >16 | |
| Eprosartan | ≤400 | >400 | |
| Fimasartan | ≤60 | >60 | |
| Irbesartan | ≤150 | >150 | |
| Losartan | ≤50 | >50 | |
| Olmesartan | ≤10 | >10 | |
| Telmisartan | ≤40 | >40 | |
| Valsartan | ≤160 | >160 | |
All dose units are based on mg.
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; RAAS = Renin-Angiotensin-Aldosterone System.
Statistical analysis
All data were analyzed using the SPSS 25.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as number (percentages) and continuous variables as means±standard deviation (SD). Normally distributed continuous variables were compared using one-way analysis of variance, and categorical variables were compared using χ2 tests. The p value of <0.05 was considered statistically significant.
RESULTS
Baseline clinical characteristics
The baseline characteristics of 2,941 HFrEF outpatients of the KorAHF registry are shown in Table 2. Mean age was 66±15 years old and more than half had a history of hypertension and approximately one third had diabetes. Patients in our registry were similar with those in European Society of Cardiology-EURObservational Research Programme-Heart Failure Association Heart Failure Long-Term (ESC-EORP-HFA HF-LT) registry regarding age, prevalence of ischemic origin HF & hypertension and to those in PARADIGM-HF regarding age, LVEF and eGFR.18) The patients enrolled in the KorAHF registry were more female, diabetes and had lower body mass index (BMI), systolic blood pressure (SBP) and higher natriuretic peptide levels compared those with ESC-EORP-HFA HF-LT registry and PARADIGM-HF trial. Regarding HF guideline-directed medical therapy (GDMT) in the KorAHF HFrEF patients vs. ESC-EORP-HFA HF-LT or PARADIGM-HF, patients were less likely to use BB and mineralocorticoid receptor antagonist (MRA) and had implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT).
Table 2. Baseline characteristics in Korean AHF Registry and ESC-EORP-HFA HF-LT Registry and in PARADIGM-HF patients.
| Characteristics | KorAHF (n=2,941) | ESC-EORP-HFA HF-LT (n=5,443) | PARADIGM-HF (n=8,339) | |
|---|---|---|---|---|
| Male | 60% | 78% | 78% | |
| Age (years) | 66±15 | 64±13 | 64±11 | |
| BMI (kg/m2) | 23.2±3.8 | 27.8±4.9 | 28.2±5.5 | |
| Ischemic origin | 44.8% | 47% | 60% | |
| Diabetes | 41.2% | 15% | 13% | |
| Hypertension | 58.5% | 56% | 71% | |
| COPD | 10.4% | 15% | 13% | |
| SBP (mmHg) | 113±17 | 121±20 | 121±15 | |
| NYHA functional classification | ||||
| I | 17% | 16% | 5% | |
| II | 72% | 55% | 70% | |
| III | 7% | 26% | 24% | |
| IV | 4% | 2% | 1% | |
| LVEF (%) | 27.1±7.6 | - | 29.5±6.2 | |
| ≤15% | 7.3% | 5% | - | |
| 15–20% | 14.2% | 13% | - | |
| 21–25% | 20.5% | 18% | - | |
| 26–30% | 20.8% | 28% | - | |
| 31–35% | 20.9% | 24% | - | |
| 36–40% | 16.3% | 12% | - | |
| eGFR (mL/min/1.73m2) | 72.2±36.8 | - | 70.0±20.0 | |
| ≥60 | 63.1% | 56% | - | |
| 45–59 | 15.5% | 23% | - | |
| 30–44 | 10.4% | 15% | - | |
| <30 | 10.9% | 7% | - | |
| Potassium (mmol/L) | 4.2±0.5 | 4.5±0.5 | 4.5±0.5 | |
| Hemoglobin (g/dL) | 12.4±2.2 | 13.5±1.8 | - | |
| BNP (pg/mL) | 847 [428–1,721] | 348 [128–862] | - | |
| NT-proBNP (pg/mL) | 5,068 [2,386–12,526] | 1,608 [646–3,939] | 1,631 [885–3,154] for LCZ696 | |
| 1,594 [886–3,305] for enalapril | ||||
| HF treatments | ||||
| ACEI | 36.5% | 69% | 78% | |
| ARB | 40.1% | 21% | 23% | |
| BB | 57.3% | 90% | 93% | |
| MRA | 53.1% | 63% | 56% | |
| Loop diuretics | 78% | 96% | 80% | |
| ICD | 4% | 23% | 23% | |
| CRT | 2% | 18% | 7% | |
Continuous data presented as mean±standard deviation or median [interquartile range], as appropriate. Categorical data presented as proportions. Data adapted from reference 23.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker; BMI = body mass index; BNP = B-type natriuretic peptide; COPD = chronic obstructive pulmonary disease; CRT = cardiac resynchronization therapy; eGFR = estimated glomerular filtration rate; EORP = EURObservational Research Programme; ESC = European Society of Cardiology; HF = heart failure; HFA = Heart Failure Association; HF-LT = Heart Failure Long-Term; ICD = implantable cardioverter defibrillator; KorAHF = Korean Acute Heart Failure registry; LCZ696 = sacubitril/valsartan; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; PARADIGM-HF = Prospective Comparison of ARNi with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure.
On-treatment angiotensin converting enzyme inhibitor/angiotensin receptor blocker/enalapril equivalent daily dose
Table 3 showed the representative data for ACEI/ARB daily dose and switched enalapril equivalent daily dose at discharge. In the KorAHF registry, more ARBs (40.1%) were used for HFrEF patients than ACEIs (36.5%). The most commonly prescribed ACEI was perindopril (37.2%), followed by ramipril (35.6%) & captopril (18.3%) and the most commonly prescribed ARB was candesartan (35.6%), followed by losartan (30.1%) & valsartan (20.1%). The prescription rates of current American College of Cardiology/American Heart Association (ACC/AHA; captopril, enalapril, lisinopril, perindopril, ramipril) and ESC (captopril, enalapril, lisinopril, ramipril) HF guideline recommended ACEI use was 99.3% and 62.1%, respectively. Those for ARB were 85.8% for both ACC/AHA and ESC HF guideline (candesartan, valsartan, losartan). Based on the pre-specified criteria used in TITRATION trial (Table 1), we switched each ACEI/ARB dose to enalapril equivalent dose. This equivalent daily dose was similar in ACEI and ARB group in the KorAHF registry. More potent blood pressure-lowering ARBs (e.g. telmisartan, olmesartan) were used in higher enalapril equivalent daily dose.
Table 3. ACEI/ARB daily dose and enalapril equivalent daily dose at discharge.
| Medication | Prevalence | ACEI/ARB daily dose (mg) | Enalapril equivalent daily dose (mg) | |
|---|---|---|---|---|
| ACEIs | ||||
| Enalapril | 5.4 | 7.3±3.9 | 7.3±3.9 | |
| Captopril | 18.3 | 42.4±49.4 | 4.2±4.9 | |
| Cilazapril | 0.1 | 2.5±0.0 | 7.3±3.9 | |
| Imidapril | 0.6 | 8.3±4.9 | 8.3±4.9 | |
| Lisinopril | 2.8 | 8.5±3.7 | 8.5±3.7 | |
| Perindopril | 37.2 | 3.7±1.9 | 9.3±4.7 | |
| Ramipril | 35.6 | 3.8±2.5 | 7.7±5.1 | |
| ARBs | ||||
| Candesartan | 35.6 | 9.5±6.9 | 5.9±4.3 | |
| Eprosartan | 0.8 | 510.0±144.59 | 12.8±3.6 | |
| Fimasartan | 3.1 | 56.7±26.6 | 9.4±4.4 | |
| Irbesartan | 1.9 | 218.2±86.3 | 14.5±5.8 | |
| Losartan | 30.1 | 46.5±24.2 | 9.3±4.8 | |
| Olmesartan | 1.0 | 25.8±19.8 | 25.8±19.8 | |
| Telmisartan | 7.4 | 52.2±27.3 | 13.0±6.8 | |
| Valsartan | 20.1 | 88.9±61.4 | 5.6±3.8 | |
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker.
Eligibility for the inclusion criteria of PARADIGM-HF trial
Table 4 showed the eligibility for the inclusion criteria of PARADIGM-HF trial. Among the 2,491 patients with HFrEF, there were 80% symptomatic patients (NYHA class II–IV) at discharge. In overall, 11% patients had advanced chronic kidney disease (CKD, eGFR < 30 mL/min/1.73m2) and 9% patients had lower SBP (<95 mmHg) and only 1 patient had hyperkalemia (Serum K+ >5.4 mmol/L). However, about 60% patients did not take BB nor MRA and more than 65% patients were administered lower dose of ACEI/ARB (enalapril equivalent dose <10 mg/day). When combining these criteria, 9% were excluded from advanced CKD, 1% from hyperkalemia, 6% from lower SBP and 46% were excluded from lower dose of ACEI/ARB use. In addition, 6% and 5% were excluded from BB and MRA non-users, respectively. So, we changed the ACEI/ARB criteria from enalapril 10 to 5 mg/day and reanalyzed. Then, we could find the increase of eligible patients from 7% to 15% (with both BB and MRA use), from 12% to 30% (with only BB use), from 18% to 39% (without neither BB nor MRA use).
Table 4. Eligibility for the inclusion criteria of PARADIGM-HF trial.
| PARADIGM-HF criteria | Prevalence | |
|---|---|---|
| Single criteria | ||
| a. Age ≥18 years | 100% | |
| b. LVEF ≤40% | 100% | |
| c. NYHA class II–IV | 80% | |
| d. eGFR ≥30 mL/min/1.73m2 | 89% | |
| e. Serum K+ ≤5.4 mmol/L | 99% | |
| f. SBP, mmHg ≥95 mmHg | 91% | |
| g. Equivalent more than enalapril 10 mg/day | 34% | |
| h. BB treatment | 57% | |
| i. MRA treatment | 53% | |
| Combination criteria | ||
| a + b + c + d | 71% | |
| a + b + c + d + e | 70% | |
| a + b + c + d + e + f | 64% | |
| a + b + c + d + e + f + g | 18% | |
| a + b + c + d + e + f + g′ | 39% | |
| a + b + c + d + e + f + g + h | 12% | |
| a + b + c + d + e + f + g′ + h | 30% | |
| a + b + c + d + e + f + g + h + i | 7% | |
| a + b + c + d + e + f + g′ + h + i | 15% | |
| a + b + c + d + e + f + h + i | 23% | |
| a + b + c + d + e + g′ | 43% | |
Means equivalent more than enalapril 5 mg/day.
BB = beta-blocker; eGFR = estimated glomerular filtration rate; g′ = equivalent more than enalapril 5 mg/day; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; PARADIGM-HF = Prospective Comparison of ARNi with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure; SBP = systolic blood pressure.
Among the PARADIGM-HF inclusion criteria, we found that lower dose use for ACEI/ARB and lower SBP were clinical barriers to be ineligible so further analysed regarding these 2 factors. Firstly, we compared clinical characteristics between low-dose and high-dose ACEI/ARB inhibition strata based on current definition (Table 5). To be enrolled in the PARADIGM-HF trial, patients should be tolerated to, at least, enalapril 10 mg/day. So, we divided into 2 groups based on this daily dose of enalapril. Low-dose ACEI/ARB inhibition group showed higher age, eGFR and lower BMI, SBP, and LVEF compared with high-dose group. The prevalence of diabetes & hypertension was lower and the prescription rate of MRA and loop diuretics at discharge was higher in low-dose ACEI/ARB inhibition strata. Then we analyzed regarding on-treatment SBP during outpatient clinic (Table 6). Only when the patients' SBP was higher than 95 mmHg, they could be screened in the PARADIGM-HF trial. That is why we defined the SBP cut-off value as 95 mmHg. Lower SBP group showed higher eGFR and lower age, BMI, SBP, and LVEF compared to higher SBP group. The prevalence of diabetes and hypertension was lower and the prescription rate of MRA and loop diuretics at discharge was higher in low SBP group, but BB administration rate was lower in low SBP group.
Table 5. Clinical characteristics regarding on-treatment enalapril equivalent daily dose 10 mg (low-dose vs. high-dose ACEI/ARB inhibition strata).
| Characteristics | Enalapril ≤10 mg (n=1,575) | Enalapril >10 mg (n=820) | p value | |
|---|---|---|---|---|
| Male | 60.5% | 60.6% | 0.965 | |
| Age (years) | 66±14 | 65±15 | 0.014 | |
| BMI (kg/m2) | 23.1±3.6 | 24.0±4.1 | <0.001 | |
| Ischemic origin | 40.7% | 41.0% | 0.896 | |
| Diabetes | 38.7% | 44.0% | 0.012 | |
| Hypertension | 53.5% | 70.5% | <0.001 | |
| COPD | 10.5% | 10.7% | 0.889 | |
| SBP (mmHg) | 112±14 | 121±15 | <0.001 | |
| <95 | 11.5% | 4.6% | <0.001 | |
| NYHA class II/III | 71.9%/5.6% | 70.7%/7.8% | - | |
| LVEF (%) | 26.8±7.5 | 27.8±7.6 | 0.002 | |
| 31–35% | 85.4% | 82.1% | 0.038 | |
| 36–40% | 14.6% | 17.9% | - | |
| eGFR (mL/min/1.73m2) | 75.1±35.1 | 71.0±38.4 | 0.009 | |
| <30 | 7.1% | 13.4% | <0.001 | |
| Potassium (mmol/L) | 4.2±0.5 | 4.2±0.5 | 0.787 | |
| >5.4 | 1.1% | 0.9% | 0.672 | |
| Hemoglobin (g/dL) | 12.5±2.1 | 12.5±2.3 | 0.628 | |
| BNP (pg/mL) | 859 [438–1,663] | 730 [402–1,712] | 0.557 | |
| NT-proBNP (pg/mL) | 4,549 [2,081–10,532] | 4,839 [2,041–12,696] | 0.384 | |
| ACEI | 45.4% | 43.8% | 0.462 | |
| ARB | 47.9% | 51.6% | 0.093 | |
| BB | 59.7% | 63.7% | 0.064 | |
| MRA | 58.2% | 50.6% | <0.001 | |
| Loop diuretics | 81.4% | 76.1% | 0.009 | |
Continuous data presented as mean±standard deviation or median [interquartile range], as appropriate. Categorical data presented as proportions.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker; BMI = body mass index; BNP = B-type natriuretic peptide; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; SBP = systolic blood pressure.
Table 6. Clinical characteristics regarding on-treatment SBP.
| Characteristics | SBP <95 mmHg (n=262) | SBP ≥95 mmHg (n=2,675) | p value | |
|---|---|---|---|---|
| Male | 59.5% | 60.2% | 0.843 | |
| Age (years) | 64±15 | 66±15 | 0.011 | |
| BMI (kg/m2) | 22.1±3.6 | 23.3±3.8 | <0.001 | |
| Ischemic origin | 40.8% | 41.7% | 0.793 | |
| Diabetes | 30.5% | 42.3% | <0.001 | |
| Hypertension | 33.2% | 61.0% | <0.001 | |
| COPD | 9.2% | 10.5% | 0.595 | |
| SBP (mmHg) | 89±4 | 117±13 | <0.001 | |
| <95 | 11.5% | 4.6% | <0.001 | |
| NYHA class II/III | 66.7%/5.9% | 72.8%/6.5% | - | |
| LVEF (%) | 23.9±7.6 | 27.4±7.5 | <0.001 | |
| 31–35% | 90.8% | 83.1% | 0.001 | |
| 36–40% | 9.2% | 16.9% | - | |
| eGFR (mL/min/1.73m2) | 80.0±34.0 | 71.5±37.0 | <0.001 | |
| <30 | 5.3% | 11.5% | 0.002 | |
| Potassium (mmol/L) | 4.2±0.5 | 4.5±0.5 | 0.239 | |
| >5.4 | 0.8% | 1.0% | >0.999 | |
| Hemoglobin (g/dL) | 12.2±2.0 | 12.4±2.2 | 0.201 | |
| BNP (pg/mL) | 814 [438–1,964] | 851 [425–1,720] | 0.889 | |
| NT-proBNP (pg/mL) | 5,627 [2,469–12,502] | 4,977 [2,374–12,538] | 0.343 | |
| ACEI | 32.8% | 36.9% | 0.202 | |
| ARB | 46.6% | 39.4% | 0.029 | |
| BB | 46.9% | 58.3% | <0.001 | |
| MRA | 69.5% | 51.5% | <0.001 | |
| Loop diuretics | 84.0% | 77.5% | 0.045 | |
Continuous data presented as mean±standard deviation or median [interquartile range], as appropriate. Categorical data presented as proportions.
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; BB = beta-blocker; BMI = body mass index; BNP = B-type natriuretic peptide; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; SBP = systolic blood pressure.
DISCUSSION
Among the Korean acute HFrEF patients, 80% met KFDA label criteria, while only 12% met the inclusion criteria of PARADIGM-HF trial for SV if requiring ≥10 mg enalapril. Then we found that sub-optimal pharmacotherapy was the main reason for ineligible SV use based on the PARADIGM-HF criteria in Korea HF population.
SV is the first in class drug, called ARNI,1),2) and the PARADIGM-HF trial clearly demonstrated that SV reduced HF hospitalization and cardiovascular mortality compared to current ACEI/ARB.3) Following this study, up-to date both ACC/AHA and ESC HF guidelines strongly recommend SV use for HFrEF patients. However, the limited clinical application of SV even after current HF guidelines' recommendation has been documented in the Western population. The report from United States using data from Change the Management of Patients With Heart Failure (CHAMP-HF) registry showed that only 15% patients were administered SV (ACEI/ARB for 59%).8) They found that patients prescribed SV were younger, had lower LVEF, less likely to have CKD and more likely to have cardiac resynchronization therapy (CRT). Currently on-going prospective registry, Study of real-world treatment patterns and patients reported outcomes in heart failure patients in Korea (SPARK), can reveal the Korean real-world trend for SV use soon or later. It could inform useful clinical data for Asian HF population.
In general, the gap between randomized controlled trial (RCT) and real-world data is inevitable considering limited medical and economic resources for RCT. So many researchers have paid attention to real-world evidences following RCT evidences. Regarding SV eligibility, there have been many publications in Western population but there have been no reports in Asian population as far as we know.18),19),20),21),22),23) Recently-published large, European registry data showed that sub-optimal pharmacotherapy was the main reason for SV ineligibility for PARADIGM-HF criteria18) and it was consistent with our findings (only 12% met the PARADIGM-HF criteria and sub-optimal pharmacotherapy accounted for 52% ineligibility). In our study, only one third patients tolerated to enalapril 10 mg daily dose and very few patients could use target dose of ACEI/ARB (e.g. enalapril 40 mg daily in ESC HF guideline). In addition, previous studies also reported that GDMT for HF was related to improved clinical outcomes in both chronic and acute HFrEF patients in Korea.24),25),26) Therefore, our findings highlight that the need for better strategies to integrate GDMT in a real-world, especially for target medication dose in Korean population.
There has been known differences in clinical characteristics between Western and Asian HF patients. Our data (Table 2) showed comparable findings with previous studies, which found Asian HF patients had lower BMI, SBP, ischemic origin HF and higher use of ARB (rather than ACEI) than Western population.9),11),27),28) That could be why Asian HF patients are usually prone to lower dose of GDMT use like as finding from our study. In another point of view, HF patients with low SBP or low tolerated ACEI/ARB dose may be high risk patients. Current guidelines defined advanced heart failure as low SBP (< 100 mmHg), low dose of GDMT or cannot tolerated GDMT considering severe pump failure status. So, the patients who could not be enrolled in the PARADIGM-HF trial may be advanced HF patients. It suggests that we do not have enough clinical evidence for SV administration for advanced HF patients. However, recent report from the CHAMP-HF registry showed that the number of advanced practice providers was related with SV use after multivariate adjusting.8) It seems that physicians prescribe SV more for advanced HF although lack of clinical evidence. However, a recently-published Taiwan study demonstrated that in patients with baseline SBP lower than 100 mmHg, there were no significant differences in clinical outcomes between SV and ACEI/ARB groups.29) Therefore, the ongoing trial (EntrestoTM (LCZ696) In Advanced Heart Failure; LIFE, NCT02816736) which evaluate the clinical role of SV in advanced HF patients will uncover these unmet needs and further prospective study should be warranted to support the clinical evidence of SV use beyond inclusion criteria of the PARADIGM-HF trial.
The clinical evidence for GDMT for HF in CKD population was limited especially in patients with advanced CKD (stage 4 & 5, eGFR <30 mL/min/m2) and end stage of renal disease (ESRD). However, many patients have administered GDMT (e.g. ACEI or ARB) like as non-CKD HF patients in real world. In our study, 9% HF patients were ineligible for PARADIGM-HF criteria based on advanced CKD/ESRD (Table 4). Regarding the clinical evidence of SV in these population, the PARADIGM-HF trial could not answer these issues. So, the efficacy and safety of SV in these population remains uncertain. A recently published retrospective study demonstrated that in patients with advanced CKD (eGFR <30 mL/min/m2), SV treatment lowered cardiovascular death or HF hospitalizations by 28%.29) Therefore, further prospective study should be warranted to examine the effect and safety of SV in HFrEF patients with advanced CKD and ESRD.
Regarding LVEF, the ESC guideline recommends SV use in patients with LVEF ≤35%, but the PARADIGM-HF trial enrolled patients with LVEF ≤40%. In Korea, the label criteria for LVEF was 35% following the ESC guideline at the start of approval. Since September 2019, the Korean government widened the SV use criteria under insurance coverage, LVEF from 35% to 40%. In our study, there were 16% patients in LVEF group from 35% to 40% so these group can get benefit from SV treatments nowadays.
Our study does not go without limitations. Firstly, the patients in this study were not enrolled in outpatient clinic settings although enrolled prospectively and followed-up well. In addition, some laboratory parameters (e.g. eGFR, serum potassium) at discharge after HF hospitalization were used for this analysis. It can over or underestimate HF severity and risk factors because we can assume that patients in our study may be less stabilized than chronic stable HF patients. However, the recently published Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER-HF) study showed the clinical effectiveness and safety of SV in destabilized HFrEF patients following ADHF.30) So, our study population also have some clinical implication for widening the eligibility of SV in addition to chronic stable HFrEF patients like PIONEER-HF trial. And just 1–3 months after discharge from HF hospitalization, the patients have been known more fragile, so prone to decompensation and following rehospitalization.25) So, we have to consider this period as vulnerable phase of HF and pay more attention to GDMT. Therefore, our study can support the SV eligibility for this vulnerable period, too. Secondly, we could not adopt the inclusion criteria of PARADIGM-HF using natriuretic peptides in our analysis. As you can see in Table 2, the natriuretic peptide levels were relatively higher in the KorAHF registry compared to other registry because we had natriuretic peptide levels only at discharge after hospitalization (not at outpatient clinic at stabilized status). Thirdly, clinical evidences for optimal GDMT usually come from the trials of chronic stable HF patients, not from those of ADHF. So, there may be concerns for our conclusion that sub-optimal pharmacotherapy was the main reason for ineligible SV use based on the PARADIGM-HF criteria in Korean HF population. However, there has been no large data about the dosing of HF GDMT in chronic stable HF patients in Korea as far as we know. Therefore, this study has enough novelty for real-world evidences of HF in Korea even though inborn limitation of this study population. On-going SPARK study could be helpful to answer this question further. Fourthly, the KFDA criteria for SV has one more criterion as at least four weeks of stable prescription for ACEI/ARB and other standard HF medications. We could not have detailed prescription data for preadmission period so we could not adopt this criterion for our analysis. Fifthly, our study population was limited to Korean population. However, this is the first and largest study to show the SV eligibility in Asian HFrEF patients considering many studies have been published in Western population (e.g. Western Europe, Norther American). Larger prospective, long term follow-up study including RCT should be warranted in Asian population. Lastly, the patients were recruited only from tertiary hospital centers. So, our study is not free from selection bias that higher risk patients were included in this analysis.
In conclusion, among the Korean hospitalized HFrEF patients, 80% met the KFDA label criteria, while only 12% met the inclusion criteria of PARADIGM-HF trial for SV when we used the ACEI/ARB dose criteria as ≥10 mg enalapril equivalent daily dose. In addition, we found that sub-optimal pharmacotherapy could be the main reason for ineligible SV use based on the PARADIGM-HF criteria in Asian population for the first time. Therefore, our findings highlight that the need for better strategies to integrate GDMT in a real-world, especially for target medication dose in Asian population.
Footnotes
Funding: This work was supported by grants from Research of Korea Centers for Disease Control and Prevention (2010-E63003-00, 2011-E63002-00, 2012-E63005-00, 2013-E63003-00, 2014-E63003-01, 2015-E63003-02, 2016-ER6303-00, and 2017-ER6303-01).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Oh J, Choi DJ, Jeon ES, Cho MC, Oh BH, Kang SM.
- Data curation: Oh J, Lee SE.
- Formal analysis: Oh J.
- Funding acquisition: Choi DJ, Jeon ES, Cho MC, Oh BH, Kang SM.
- Investigation: Lee CJ, Choi DJ, Cho MC, Oh BH, Kang SM.
- Methodology: Oh J, Kang SM.
- Project administration: Lee CJ, Park JJ, Lee SE, Kim MS, Cho HJ, Choi JO, Lee HY, Hwang KK, Kim KH, Yoo BS, Choi DJ, Baek SH, Jeon ES, Kim JJ, Cho MC, Chae SC, Oh BH, Kang SM.
- Resources: Oh J.
- Writing - original draft: Oh J, Kang SM.
References
- 1.Braunwald E. The path to an angiotensin receptor antagonist-neprilysin inhibitor in the treatment of heart failure. J Am Coll Cardiol. 2015;65:1029–1041. doi: 10.1016/j.jacc.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Dewan P, Docherty KF, McMurray JJ. Sacubitril/valsartan in Asian patients with heart failure with reduced ejection fraction. Korean Circ J. 2019;49:469–484. doi: 10.4070/kcj.2019.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 6.Kim MS, Lee JH, Kim EJ, et al. Korean guidelines for diagnosis and management of chronic heart failure. Korean Circ J. 2017;47:555–643. doi: 10.4070/kcj.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez AL, Kittipibul V, Tang WH, Starling RC. Patients not meeting PARADIGM-HF enrollment criteria are eligible for sacubitril/valsartan on the basis of FDA approval: the need to close the gap. JACC Heart Fail. 2017;5:460–463. doi: 10.1016/j.jchf.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 8.DeVore AD, Hill CL, Thomas L, et al. Patient, provider, and practice characteristics associated with sacubitril/valsartan use in the United States. Circ Heart Fail. 2018;11:e005400. doi: 10.1161/CIRCHEARTFAILURE.118.005400. [DOI] [PubMed] [Google Scholar]
- 9.Choi DJ, Han S, Jeon ES, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean Heart Failure Registry. Korean Circ J. 2011;41:363–371. doi: 10.4070/kcj.2011.41.7.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam CS, Anand I, Zhang S, et al. Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry. Eur J Heart Fail. 2013;15:928–936. doi: 10.1093/eurjhf/hft045. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Cho HJ, Lee HY, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean Acute Heart Failure (KorAHF) registry. Eur J Heart Fail. 2014;16:700–708. doi: 10.1002/ejhf.91. [DOI] [PubMed] [Google Scholar]
- 12.Lee SE, Lee HY, Cho HJ, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean Acute Heart Failure Registry (KorAHF) Korean Circ J. 2017;47:341–353. doi: 10.4070/kcj.2016.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bank IE, Gijsberts CM, Teng TK, et al. Prevalence and clinical significance of diabetes in Asian versus White patients with heart failure. JACC Heart Fail. 2017;5:14–24. doi: 10.1016/j.jchf.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Balmforth C, Simpson J, Shen L, et al. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail. 2019;7:457–465. doi: 10.1016/j.jchf.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Oh J, Kang SM, Hong N, et al. The CKD-EPI is more accurate in clinical outcome prediction than MDRD equation in acute heart failure: data from the Korean Heart Failure (KorHF) registry. Int J Cardiol. 2013;167:1084–1087. doi: 10.1016/j.ijcard.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Senni M, McMurray JJ, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18:1193–1202. doi: 10.1002/ejhf.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SE, Lee HY, Cho HJ, et al. Reverse J-curve relationship between on-treatment blood pressure and mortality in patients with heart failure. JACC Heart Fail. 2017;5:810–819. doi: 10.1016/j.jchf.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Kapelios CJ, Lainscak M, Savarese G, et al. Sacubitril/valsartan eligibility and outcomes in the ESC-EORP-HFA Heart Failure Long-Term Registry: bridging between European Medicines Agency/Food and Drug Administration label, the PARADIGM-HF trial, ESC guidelines, and real world. Eur J Heart Fail. 2019 doi: 10.1002/ejhf.1532. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Pellicori P, Urbinati A, Shah P, et al. What proportion of patients with chronic heart failure are eligible for sacubitril-valsartan? Eur J Heart Fail. 2017;19:768–778. doi: 10.1002/ejhf.788. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues G, Tralhão A, Aguiar C, Freitas P, Ventosa A, Mendes M. Is the PARADIGM-HF cohort representative of the real-world heart failure patient population? Rev Port Cardiol. 2018;37:491–496. doi: 10.1016/j.repc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril-valsartan in a real-world heart failure population: a community-based single-centre study. ESC Heart Fail. 2018;5:337–343. doi: 10.1002/ehf2.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson J, Benson L, Jhund PS, Dahlström U, McMurray JJ, Lund LH. “Real World” eligibility for sacubitril/valsartan in unselected heart failure patients: data from the Swedish Heart Failure Registry. Cardiovasc Drugs Ther. 2019;33:315–322. doi: 10.1007/s10557-019-06873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinto G, Tondi L, Gemma M, et al. Real-life indications to sacubitril/valsartan treatment in patients with chronic systolic heart failure. J Cardiovasc Pharmacol. 2019;73:301–306. doi: 10.1097/FJC.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 24.Yoo BS, Oh J, Hong BK, et al. SUrvey of Guideline Adherence for Treatment of Systolic Heart Failure in Real World (SUGAR): a multi-center, retrospective, observational study. PLoS One. 2014;9:e86596. doi: 10.1371/journal.pone.0086596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn MS, Yoo BS, Yoon J, et al. Prognostic effect of guideline-directed therapy is more noticeable early in the course of heart failure. J Korean Med Sci. 2019;34:e133. doi: 10.3346/jkms.2019.34.e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn MS, Yoo BS, Yoon J, et al. Guideline-directed therapy at discharge in patients with heart failure and atrial fibrillation. Heart. 2019 doi: 10.1136/heartjnl-2019-315240. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 28.Atherton JJ, Hayward CS, Wan Ahmad WA, et al. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific) J Card Fail. 2012;18:82–88. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Chang HY, Feng AN, Fong MC, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction patients: real world experience on advanced chronic kidney disease, hypotension, and dose escalation. J Cardiol. 2019;74:372–380. doi: 10.1016/j.jjcc.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]