Abstract
Background and Objectives
Acute pulmonary embolism(PE) has high mortality and morbidity. Although reperfusion therapies can be used in high-risk PE patients, a few patients remain in a highly hemodynamically unstable state. In these patients, extracorporeal membrane oxygenation (ECMO) can be used to restore tissue oxygenation and improve their hemodynamic status. We retrospectively assessed the outcomes of ECMO in patients with high-risk PE.
Methods
We retrospectively screened all acute PE patients from January 2010 to December 2019 in 5 university hospitals in Korea. We reviewed their medical records and clinical outcomes.
Results
During the study period, we screened total 3,572 patients with PE and found 33 high-risk PE patients with ECMO (17 women, 58.3±14.7 years old) whose data were analyzed. Common causes of acute PE included limited mobility (8, 24.2%), a recent operation (6, 18.2%) and a recent hospitalization for medical diseases (3, 9.1%). Among the patients, 25 (75.0%) had a history of cardiopulmonary resuscitation. Nineteen patients had received primary therapy (intravenous thrombolysis in 10, thrombectomy in 8 and catheter-based thrombolysis in 1). The mean duration of ECMO was 5.0 days (range, 1–23 days). The in-hospital mortality rate was 51.5%. Twenty-two patients (66.7%) had ECMO related complications (15 [46.9%] had bleeding, 10 [31.3%] had an infection, and 5 [15.6%] had vascular complications). Of 15 cases with bleeding, 13 of them had mild bleeding associated with catheter insertion, and 2 had moderate multiorgan bleeding.
Conclusions
ECMO can be used as an additional or alternative circulatory support method in high-risk PE patients. However, physicians should keep in mind a high incidence of complications related to ECMO.
Keywords: Shock, cardiogenic; Extracorporeal membrane oxygenation; Pulmonary embolism
INTRODUCTION
Pulmonary embolism (PE) is the third most frequent cardiovascular disease and can be a life-threatening cardiovascular condition.1) Clinical presentations of acute PE range from asymptomatic to sudden death2),3); and about 34% of patients with venous thromboembolism had a sudden fatal PE in an epidemiologic study conducted in Europe.4)
Acute high-risk PE includes cardiac arrest (a need for cardiopulmonary resuscitation [CPR]), obstructive shock (systolic blood pressure [SBP] <90 mmHg or vasopressors required to achieve a SBP level more than 90 mmHg with evidence of hypoperfusion to end-organs) and persistent hypotension (SBP <90 mmHg or an SBP drop more than 40 mmHg, lasting longer than 15 minutes without other causes including new-onset arrhythmia, hypovolemia, or sepsis).5) Because high-risk PE is associated with high mortality, thrombolytic therapy or mechanical thrombectomy should be applied to save the patient's life.
Recently, extracorporeal membrane oxygenation (ECMO) has become an option for patients with acute high-risk PE as a bridge therapy, which helps the right ventricle to recover during exogenous or endogenous thrombolytic processes.6) Recent treatment guidelines of acute PE include ECMO as a treatment option.5) In this study, we investigated the presentation, management, and in-hospital outcomes of consecutive patients with high-risk PE who were treated with ECMO in 5 tertiary hospitals in Korea.
METHODS
Enrollment of patients and data collection
We retrospectively collected all consecutive patients with high-risk PE who were treated with ECMO in 5 tertiary university hospitals (Chonnam National University Hospital in Gwangju, Chungbuk National University Hospital in Cheongju, Chungnam National University Hospital in Daejeon, Pusan National University Yangsan Hospital in Yangsan, and Pusan National University Hospital in Busan) from January 2010 to December 2019.
Patients' data were obtained by reviewing their medical records and included the initial presentation, demographics, past medical history, risk factors of PE, vital signs, presence of CPR, initial management, and in-hospital outcomes.
In-hospital outcomes included treatments (anticoagulation, systemic intravenous thrombolysis, catheter-based thrombectomy, open surgical thromboembolectomy, placement of inferior vena cava filter, and application of ECMO) and outcomes (including all-cause mortality and complications associated with ECMO). This study complied with the Declaration of Helsinki principles. The study protocol of this study was approved by the Institutional Review Board (IRB) of each hospital. The IRBs waved the need for a written informed consent from the study patients.
Statistical analysis
We presented continuous variables as mean±standard deviations and categorical variables as frequencies with proportions. The data were analyzed using SPSS version 24 (IBM Corp., Chicago, IL, USA).
RESULTS
Characteristics of the study population
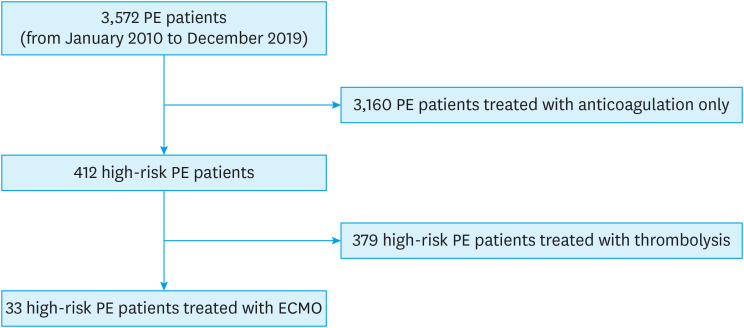
During the study period, we screened 3,572 patients with PE, and analyzed 33 high-risk PE patients with ECMO (17 women, 58.3±14.7 years old, Figure 1). Their baseline characteristics are summarized in Table 1. Hypertension was the most common cardiovascular risk factor, and 5 had the previous history of venous thromboembolism. Causes of acute PE were limited mobility (n=8, 24.2%), a recent operation (n=6, 18.2%), a recent hospitalization for a medical condition (n=3, 9.1%), trauma (n=2, 6.1%), malignancy (n=1, 3.0%), thrombophilia (n=3, 9.1%), pregnancy (n=1, 3.0%), myxoma of the right atrium (n=1, 3.0%), and unknown (n=8, 24.2%). After presentation with PE, 25 (75.0%) underwent CPR. Nineteen patients received primary therapy (intravenous thrombolysis in 10, thrombectomy in 8 and catheter-based thrombolysis in 1), and 31 patients (93.9%) had secondary therapy with heparin (2 of them died just after thrombolytic therapy).
Figure 1. Study flow diagram.
ECMO = extracorporeal membrane oxygenation; PE = pulmonary embolism.
Table 1. Baseline characteristics and pulmonary embolism risk factors.
| Characteristics | Values (n=33) | |
|---|---|---|
| Age (years) | 58.3±14.7 (range, 30–86) | |
| Female (sex) | 17 (51.5) | |
| Body mass index (kg/m2) | 25.9±7.1 (range, 18.8–56.7) | |
| Cardiovascular risk factors | ||
| Hypertension | 10 (30.3) | |
| Diabetes | 9 (27.3) | |
| Dyslipidemia | 7 (21.2) | |
| Smoking | 8 (24.2) | |
| History of venous thromboembolism | 5 (15.2) | |
| Cause of pulmonary embolism | ||
| Thrombophilia | 3 (9.1) | |
| Recent trauma (<4 weeks) | 2 (6.1) | |
| Recent operation or invasive procedure (<4 weeks) | 6 (18.2) | |
| Limited mobility | 8 (24.2) | |
| Recent hospitalization due to medical illness (<4 weeks) | 3 (9.1) | |
| Malignancy | 1 (3.0) | |
| Myxoma | 1 (3.0) | |
| Pregnancy | 1 (3.0) | |
| Unknown | 8 (24.2) | |
| Presence of cardiopulmonary resuscitation | 25 (75.8) | |
| Primary therapy | 19 (57.6) | |
| Thrombectomy | 8 (24.2) | |
| Catheter-based thrombolysis | 1 (3.0) | |
| Intravenous thrombolysis | 10 (30.3) | |
| Use of heparin | 31 (93.9) | |
| Insertion of inferior vena cava filter | 6 (18.2) | |
Data are shown as mean±standard deviation or number (%).
We described a representative case with an episode of cardiac arrest to demonstrate the successful use of ECMO in a patient with high-risk PE.
Representative case
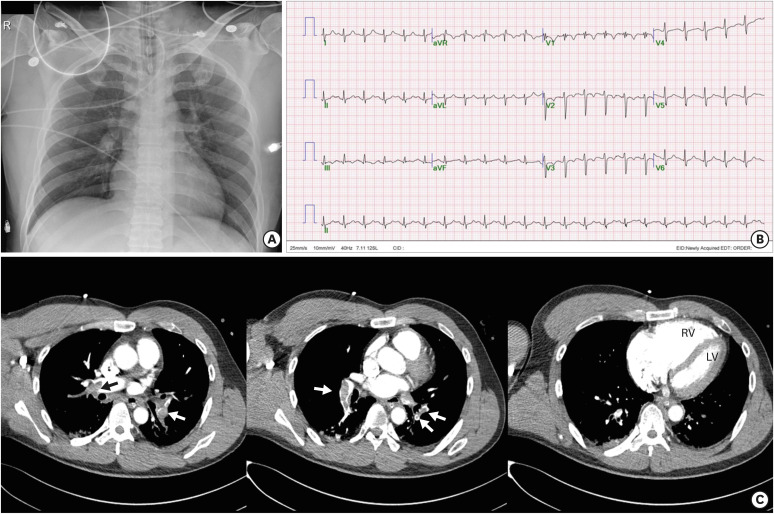
A 45-year-old man with sudden syncope was transferred to the emergency department (ED) by his colleagues. He had injured his right lower leg during a soccer game about 20 days ago, and he reported swelling of his leg for 3 days. The initial chest X-ray showed a normal cardiac size (Figure 2A). Because the patient was in shock and exhibited no spontaneous breathing, the attending physician performed CPR for 15 minutes. After the CPR, the patient had a return of spontaneous circulation. His vital signs at that time were blood pressure 60/40mmHg, heart rate 130/min, respiratory rate 32/min and body temperature 36.0°C. The electrocardiogram showed sinus tachycardia with a heart rate of 133/min with inversion of the T wave in leads V1–V3 (Figure 2B). The echocardiogram performed at the ED demonstrated severely reduced right ventricular function with D-shaped left ventricle suggesting acute PE with high right ventricular systolic pressure. His blood pressure remained at 80/40 mmHg after a full infusion of normal saline and administration of norepinephrine. The attending physician inserted a veno-arterial (VA) type ECMO and applied conventional heparinization. Contrast-enhanced chest computerized tomography showed obstruction of both pulmonary arteries by thrombi (arrows, Figure 2C) and increased right ventricular size. His blood pressure increased up to 124/78 mmHg, and his heart rate decreased to 96/min with ECMO use. After 3 days of ECMO and ventilatory support, the patient was successfully weaned. There were no complications associated with ECMO use.
Figure 2. Chest X-ray shows a normal cardiac size (A). Electrocardiogram after cardiopulmonary resuscitation demonstrates sinus tachycardia with a heart rate of 133/min with a T wave inversion in leads V1–V3 (B). Contrast-enhanced chest computerized tomography reveals multiple sites of pulmonary arterial obstruction (arrows) with an increased right ventricular size. The ratio of the RV to the LV (RV/LV ratio) is over 1.0, suggesting a poor prognosis (C).
RV = right ventricle; LV = left ventricle.
Parameters of ECMO and its outcome
All ECMO procedures were done via femoral approaches using percutaneous methods under fluoroscopic guidance. The mean duration of ECMO was 5.0 days (range, 1–23days). Characteristics of ECMO and the patients' clinical outcomes are summarized in Table 2. The in-hospital mortality rate was 51.5%, and 2 patients who survived the acute events had hypoxic brain injury. Twenty-two patients (66.7%) had ECMO related complications (15 [46.9%] had bleeding, 10 [31.3%] had an infection, and 5 [15.6%] had vascular complications). Bleeding complications were assessed by the Global Utilization of Streptokinase and TPA for Occluded arteries (GUSTO) classification more than mild was found in 15 patients. We found 13 patients had mild GUSTO classification bleeding associated with the catheter insertion, and 2 patients had moderate GUSTO classification multiorgan bleeding that may have been associated with coagulopathy. For the infection complications, 8 patients had a skin infection associated with catheter insertion, and 2 had disseminated intravascular coagulation features associated with pneumonia and acute respiratory distress syndrome. Vascular complications included 3 patients with pseudoaneurysms and 2 with peripheral limb ischemia. There were no statistically significant parameters associated with mortality in the univariate analysis. Table 3 lists the outcomes of the patients.
Table 2. Characteristics of extra-corporeal membrane oxygenation and its outcome.
| Characteristics | Values (n=33) | |
|---|---|---|
| Type of ECMO | ||
| Veno-atrial type | 31 (93.9) | |
| Veno-venous type | 2 (6.1) | |
| Duration of ECMO (day) | 5.0±4.9 (range, 1–23) | |
| Complications associated with ECMO | 22 (66.7) | |
| Bleeding | 15 (46.9) | |
| Vascular complication | 5 (15.6) | |
| Infection | 10 (31.3) | |
| In-hospital mortality | 17 (51.5) | |
| Cardiovascular death | 13 (39.4) | |
| Non-cardiovascular death | 4 (12.1) | |
Data are shown as mean±standard deviation or number (%).
ECMO = extracorporeal membrane oxygenation.
Table 3. Characteristics of patients and their outcomes.
| Age | Sex | Risk factor | Cause of PE | CPR | Primary therapy | ECMO | In-hospital outcome | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Duration (day) | Complication | ||||||||
| 52 | Male | DL, Previous DVT | Thrombophilia | No | Thrombectomy | VA | 13 | Bleeding | Survived | CTEPH, Pulmonary artery endarterectomy |
| 67 | Male | DL | Unknown | Yes, 2 times | Thrombectomy | VA | 5 | Bleeding, Infection | Survived | IVC filter |
| 41 | Female | HT, Diabetes | Malignancy | Yes | Thrombectomy | VA | 1 | None | Survived | Hypoxic brain damage |
| 63 | Male | HT, Diabetes, Smoking | Operation | Yes, 4 times | None | VA | 6 | Bleeding, Infection | Survived | Spine operation, IVC filter |
| 48 | Male | Diabetes, DL, Smoking | Recent hospitalization | Yes, 4 times | None | VA | 5 | None | Survived | Diabetic ketoacidosis |
| 45 | Male | None | Trauma | Yes | None | VA | 3 | None | Survived | Lower leg injury |
| 35 | Female | None | Operation | Yes | None | VA | 14 | Bleeding, Infection | Survived | C-sec delivery, Hypoxic brain injury |
| 78 | Female | Previous DVT | Unknown | Yes | Thrombolysis | VA | 3 | Infection | Survived | |
| 38 | Male | Smoking, Previous DVT | Unknown | Yes | Thrombolysis | VA | 7 | Infection | Survived | |
| 68 | Female | None | Unknown | Yes | None | VA | 2 | Infection | Survived | |
| 62 | Female | None | Limited mobility | Yes | Thrombolysis | VA | 4 | Vascular | Survived | |
| 52 | Female | None | Limited mobility | Yes | Thrombolysis | VA | 3 | Bleeding | Survived | Prolonged sitting |
| 30 | Female | None | Operation | No | None | VA | 4 | Bleeding | Survived | C-sec delivery, IVC filter |
| 65 | Female | HT, DL, Diabetes | Trauma | No | None | VA | 3 | Bleeding | Survived | Severe burn |
| 66 | Male | Diabetes, Smoking | Recent hospitalization | No | None | VV | 6 | None | Survived | Community acquired pneumonia |
| 47 | Male | None | Limited mobility | No | None | VA | 3 | Infection | Survived | Ankle problem |
| 61 | Female | None | Limited mobility | Yes | Thrombectomy | VA | 2 | None | Died | Knee joint problem, IVC filter |
| 61 | Female | HT | Limited mobility | Yes, 2 times | Catheter based fragmentation | VA | 15 | Bleeding, Vascular, Infection | Died | Knee joint problem, IVC filter |
| 36 | Female | None | Pregnancy | Yes, 2 times | Thrombectomy | VA | 9 | Bleeding | Died | |
| 63 | Female | None | Thrombophilia | Yes, 4 times | Thrombectomy | VA | 23 | Infection | Died | Acute PE on CTEPH, Died due to ICH |
| 68 | Female | None | Unknown | Yes, 2 times | Thrombectomy | VA | 2 | None | Died | Died due to sepsis |
| 50 | Male | Diabetes, DL, Smoking | Myxoma | Yes | Thrombectomy | VA | 6 | Infection | Died | PE due to RA myxoma |
| 53 | Male | HT | Operation | Yes, 2 times | Thrombolysis | VA | 5 | Bleeding, Vascular | Died | |
| 69 | Male | Diabetes | Operation | Yes, 5 times | None | VA | 1 | Bleeding, Vascular | Died | Spine operation, Died due to multiorgan failure |
| 34 | Male | Previous DVT | Thrombophilia | Yes | None | VA | 3 | Bleeding | Died | Acute PE on CTEPH |
| 70 | Female | None | Recent hospitalization | Yes | Thrombolysis | VA | 1 | Bleeding | Died | Aspiration pneumonia |
| 76 | Female | HT | Limited mobility | Yes | Thrombolysis | VA | 1 | Bleeding | Died | Prolonged bedrest due to spinal problem |
| 52 | Male | HT | Operation | Yes | None | VA | 1 | None | Died | Stomach cancer operation |
| 60 | Male | None | Unknown | Yes | Thrombolysis | VA | 1 | None | Died | |
| 74 | Female | HT, DL, Smoking, Previous DVT | Limited mobility | No | Thrombolysis | VA | 1 | None | Died | Use of hypnotics |
| 86 | Female | HT | Unknown | No | Thrombolysis | VA | 2 | None | Died | |
| 70 | Male | HT, DL, Diabetes, Smoking | Limited mobility | No | None | VV | 7 | Bleeding, Infection | Died | Stroke |
| 83 | Male | Smoking, Diabetes | Unknown | Yes, 1 time | None | VA | 3 | None | Died | |
CPR = cardiopulmonary resuscitation; CTEPH = chronic thromboembolic pulmonary hypertension; C-sec = cesarean section; DL = dyslipidemia; DVT = deep vein thrombosis; ECMO = extracorporeal membrane oxygenation; HT = hypertension; ICH = intracranial hemorrhage; IVC = inferior vena cava; PE = pulmonary embolism; RA = right atrium; VA = veno-atrial; VV = veno-venous.
DISCUSSION
In this study, we analyzed the clinical data of 33 patients with acute high-risk PE who were treated with ECMO. Most of them were treated with VA type ECMO, and 22 patients (66.7%) developed ECMO related complications. The in-hospital mortality rate was 51.5% in our study cohort, and 2 of our survivors had hypoxic brain injuries.
The major component of the management of high-risk PE is reducing the thrombus burden by mechanical thrombectomy or intravenous thrombolysis as primary therapy.2) Other therapies include anticoagulation, providing an oxygen supply, and hemodynamic support. Many inotropic agents are used to maintain cardiac output. However, the results are often insufficient. Mechanical support with ECMO can be used in these cases. Although there has been no randomized controlled trials testing the efficacy and safety of ECMO in high-risk PE patients, ECMO is recommended as a treatment option in the latest European Society of Cardiology practice guideline for acute PE.5) However, the latest practice guideline by the American Heart Association did not include the use of ECMO in the management of massive PE.7)
The recommendation of ECMO was based on only on several case reports and case series. Kjaergaard et al.8) showed a beneficial effect of using circulatory support in experimental animals with a massive PE model induced by injecting numerous thrombi into the right atrium. Also, there are several cases reported with favorable results of using ECMO in patients with massive PE.9),10) In one registry including 38 patients with massive PE, 22 were treated with ECMO and 12 of them (54.5%) survived.11) Another retrospective analysis include 32 patients with massive PE treated with ECMO and found that 17 (53.1%) survived during the index hospitalization.12) An additional study of 13 massive PE patients had an in-hospital survival rate of 46.2% (6 of 13 patients).13) Corsi et al.14) reported that the 9-day survival rate was 47.0% among 17 patients with massive PE treated with ECMO. In our study cohort, the in-hospital mortality rate was 51.5%, and 2 of the survivors had hypoxic brain injuries.
ECMO should be considered as a bridge therapy to recovery in patients with severe right ventricular failure or respiratory failure, applied at the same time as primary treatment begins. Potential indications for ECMO use in acute high-risk PE may be included cardiac arrest, severe hemodynamic compromise without cardiac arrest, contraindications to systemic thrombolysis or thrombectomy, failed systemic thrombolysis or catheter-based therapy, and severe hypoxemia.15) In our cohort, 25 patients (75.8%) had history of CPR and were treated with ECMO as a bridge therapy to recover.
However, when applying ECMO, care must be taken because of ECMO-related complications, especially bleeding. Because anticoagulation should be used in patients with PE without contraindication to anticoagulation,2) bleeding complications are common. In one study with 17 patients with massive PE treated with ECMO, there were 15 (88%) patients who suffered severe hemorrhage during their stay in the intensive care unit.14) In our study, 22 patients (66.7%) had complications including 15 bleeding complications (46.9%) associated with ECMO use.
This study has several limitations. First, this study was a retrospective study. Many parameters were gathered by reviewing medical records. Moreover, some of the patients died early in their course, before a full evaluation of the cause of the PE could be conducted. Thus, the etiology of PE remained unknown in some patients. Second, 5 tertiary university hospitals participated in this study. Treatment pattern at each hospital for PE may vary slightly, usually in regards to a preference for surgical thrombectomy rather than using intravenous thrombolysis. However, physicians usually follow the latest guideline from the European Society of Cardiology, and this study results could represent the current status of ECMO use in Korea.
In conclusions, ECMO could be used as a circulatory support in hemodynamically unstable patients with high-risk PE. The in-hospital mortality rate was 51.5% and 2 of the survivors had hypoxic brain injury. The use of ECMO was associated with high incidence of complications. Thus, physicians should take into account complications associated with ECMO use.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Data curation: Choi JH, Lee SY, Park YH, Park JH, Kim KH.
- Formal analysis: Park YH, Park JH.
- Methodology: Park JH.
- Supervision: Park JH.
- Writing - original draft: Choi JH, Park JH.
- Writing - review & editing: Choi JH, Park JH, Kim KH.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. 3069a–3069k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 3.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 6.Meneveau N, Guillon B, Planquette B, et al. Outcomes after extracorporeal membrane oxygenation for the treatment of high-risk pulmonary embolism: a multicentre series of 52 cases. Eur Heart J. 2018;39:4196–4204. doi: 10.1093/eurheartj/ehy464. [DOI] [PubMed] [Google Scholar]
- 7.Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 8.Kjærgaard B, Rasmussen BS, de Neergaard S, Rasmussen LH, Kristensen SR. Extracorporeal cardiopulmonary support may be an efficient rescue of patients after massive pulmonary embolism. An experimental porcine study. Thromb Res. 2012;129:e147–51. doi: 10.1016/j.thromres.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Delnoij TS, Accord RE, Weerwind PW, Donker DW. Atrial trans-septal thrombus in massive pulmonary embolism salvaged by prolonged extracorporeal life support after thrombo-embolectomy. A bridge to right-sided cardiovascular adaptation. Acute Card Care. 2012;14:138–140. doi: 10.3109/17482941.2012.741247. [DOI] [PubMed] [Google Scholar]
- 10.Badheka A, Bangalore Prakash P, Allareddy V. Successful use of extracorporeal membrane oxygenation in a child with obstructive shock due to massive bilateral pulmonary embolism. Perfusion. 2018;33:323–325. doi: 10.1177/0267659117736380. [DOI] [PubMed] [Google Scholar]
- 11.Kjaergaard B, Kristensen JH, Sindby JE, de Neergaard S, Rasmussen BS. Extracorporeal membrane oxygenation in life-threatening massive pulmonary embolism. Perfusion. 2019;34:467–474. doi: 10.1177/0267659119830014. [DOI] [PubMed] [Google Scholar]
- 12.George B, Parazino M, Omar HR, et al. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation. 2018;122:1–5. doi: 10.1016/j.resuscitation.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Al-Bawardy R, Rosenfield K, Borges J, et al. Extracorporeal membrane oxygenation in acute massive pulmonary embolism: a case series and review of the literature. Perfusion. 2019;34:22–28. doi: 10.1177/0267659118786830. [DOI] [PubMed] [Google Scholar]
- 14.Corsi F, Lebreton G, Bréchot N, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21:76. doi: 10.1186/s13054-017-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberg A, Tapson VF, Ramzy D. Massive pulmonary embolism: extracorporeal membrane oxygenation and surgical pulmonary embolectomy. Semin Respir Crit Care Med. 2017;38:66–72. doi: 10.1055/s-0036-1597559. [DOI] [PubMed] [Google Scholar]