Abstract
Over the past three decades, pharmacological treatment of heart failure (HF) with reduced ejection fraction (HFrEF) has witnessed a significant progress with the introduction of multiple disease-modifying therapies with a proven benefit on morbidity, mortality and quality of life. Recently, several novel medications (sacubitril/valsartan, sodium-glucose contransporter-2 [SGLT2] inhibitors, vericiguat and omecamtiv mecarbil) have shown to provide further improvement in outcomes in patients already receiving standard therapy for HFrEF. Available evidence suggests that sacubitril/valsartan and SGLT2 inhibitors (dapagliflozin and empagliflozin) are beneficial and well-tolerated in the majority inpatients and could be the mainstay treatment of HFrEF. Another group of medications (vericiguat and omecamtiv mecarbil) has shown promising results in reducing the risk of the composite of HF hospitalisation or cardiovascular mortality in patients with the more severe or advanced HF requiring recent hospitalisation. Therefore, these medications may be considered for the treatment of select group of patients with HFrEF with persisting or worsening symptoms despite optimal treatment. In addition, advances in pharmacological management of comorbidities frequently seen in HFrEF patients (diabetes, iron deficiency/anaemia, hyperkalaemia) provide further opportunities to improve outcomes. Given the increasing complexity of evidence-based therapies for HFrEF, there is a growing need to provide a practical perspective to their use. The purpose of this review is to summarise scientific evidence on the efficacy and safety of new and emerging medical therapies in HFrEF, with a focus on the clinical perspective of their use.
Keywords: Heart failure, Treatment, Sacubitril/valsartan, Dapagliflozin, Empagliflozin, Sotagliflozin, Vericiguat, Omecamtiv mecarbil, clinical trials, Outcomes
INTRODUCTION
Medical treatment of heart failure (HF) with reduced ejection fraction (HFrEF) has significantly advanced with an introduction of disease-modifying therapies with a proven benefit on survival, morbidity and functional limitations. Most recently, an incremental improvement in outcomes has been documented with several novel medications (sacubitril/valsartan, sodium-glucose contransporter-2 [SGLT2] inhibitors, vericiguat and omecamtiv mecarbil) in patients already receiving contemporary standard of care. Given the increasing complexity of evidence-based therapies for HFrEF, there is a growing need to provide a clinical perspective to use of the available medications, taking into the account evidence on patient eligibility and clinical characteristic, as well as the efficacy, safety and tolerability of these new medications. The purpose of this review is to summarise scientific evidence on the efficacy and safety of new and emerging medical therapies in HFrEF, with a focus on the clinical perspective of their use.
TOWARDS A HOLISTIC APPROACH TO THE MANAGEMENT OF HFREF
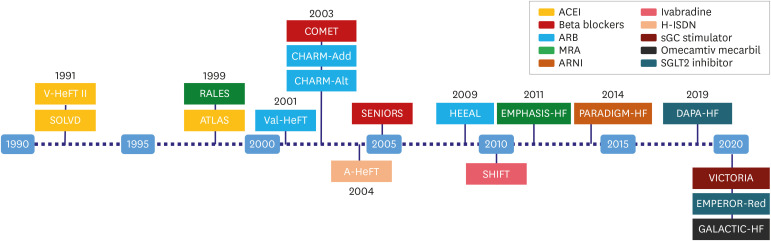
HFrEF is a complex clinical syndrome, characterised by the compensatory activation of neurohormonal axes in response to the fall in cardiac output.1) In the long-run neurohormonal activation leads to maladaptive changes in the heart, kidneys and the vasculature, which underly disease progression and contribute to end-organ damage. This concept has been put to the test in the landmark clinical trials, which have conclusively demonstrated beneficial effects of neurohormonal inhibition with angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers (ARB), beta-blockers and mineralocorticoid receptor antagonists (MRA) (Figure 1). Despite the use of these disease-modifying and life-prolonging interventions, many of the HFrEF patients still suffer a residual risk of mortality, HF hospitalisation and disease progression. This risk could be attributed to the severity of HF, underlying aetiology, advanced age, comorbidities, frailty, limited implementation and inappropriate dosing of guideline-directed medical therapies (GDMT). However, in the recent clinical trials of novel medications in HFrEF, the residual risk remained high despite optimal GDMT use. Following the positive results of these trials, pharmacological options in HFrEF treatment has extended beyond neurohormonal blockade to include innovative therapeutic principles such as haemodynamic and metabolic benefits of SGLT2 inhibitors, improved cardiac and vascular nitric-oxide bioavailability and endothelial function with vericiguat, and increased cardiac contractility with omecamtiv mecarbil.
Figure 1. Development pathway of medical treatment in heart failure with reduced ejection fraction.
ACEI = angiotensin converting enzyme inhibitors; ARB = angiotensin receptor blockers; MRA = mineralocorticoid receptor antagonists; ARNI = angiotensin receptor-neprilysin inhibitor; SGLT2 = sodium-glucose contransporter-2; HF = heart failure.
The complexity of clinical care for patients with HFrEF is further underlined by the high burden of comorbidities and frailty, especially among the older individuals and women. According to a recent review, the most prevalent comorbidities in clinical trials of HFrEF have been hypertension (89%), dyslipidaemia (61%), ischaemic heart disease (39%), chronic kidney disease (34%), atrial fibrillation (29%), diabetes mellitus (28%), investigator-reported depression (27%) and anaemia (12.5%), while temporal trends have shown a rise in the prevalence of several comorbidities.2) Clinical trial data also suggest that frailty is frequent (63%) and associated with worse symptoms and outcomes in HFrEF.3) Comorbidities and frailty often limit the possibilities for implementation and up-titration of GDMT due to hypotension, renal dysfunction, hyperkalaemia, poor adherence and tolerability. Nevertheless, a holistic approach to the management of HFrEF offers a possibility to improve outcomes and quality of life with the appropriate treatment of comorbidities.
PUTTING CLINICAL TRIAL EVIDENCE INTO THE CLINICAL PERSPECTIVE
In the contemporary era of expanding treatment options for HFrEF, the appropriate use of multiple therapies has become more complex. New and emerging therapies have been evaluated on top of the conventional HF treatments, yet there was no suggestion of an interaction between novel drugs and background therapies in terms of efficacy. However, only a minority of patients in trials of SGLT2 inhibitors, vericiguat and omecamtiv mecarbil received sacubitril/valsartan. SGLT2 inhibitors were sporadically used in trials of vericiguat and omecamtiv mecarbil, because of an overlap in periods when the three drug classes were assessed. Also, evidence is lacking on the direct efficacy comparisons between new medications and the sequence in which they should be introduced. Despite these limitations, thorough understanding of the study eligibility criteria, patient characteristics, efficacy and safety profile of novel drugs could provide directions to the practical perspective of their use.
SACUBITRIL/VALSARTAN
Sacubitril/valsartan is a first-in-class angiotensin-receptor neprilysin inhibitor (ARNI), which combines the positive effects of neprilysin inhibition and renin-angiotensin system blockade with an ARB. Neprilysin could not be combined with an ACE inhibitor due to an excess risk of angioedema, which provides a rationale for a 36-hour washout period when switching a patient from an ACE inhibitor to sacubitril/valsartan.
The long-term efficacy and safety of sacubitril/valsartan was evaluated in 8,399 HFrEF patients with left ventricular ejection fraction (LVEF) ≤40% in the PARADIGM-HF trial4) against an active comparator, enalapril. The inclusion criteria and major patient characteristics are presented in Table 1. Patients with a history of angioedema, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, systolic blood pressure <100 mmHg, or decompensated HF were not included. The trial was prematurely terminated after a median of 27 months because of a significant risk reduction in the primary composite endpoint of cardiovascular death or HF hospitalization with sacubitril/valsartan (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.73–0.87; p<0.001, number needed to treat, NNT=21). Both components of the primary endpoint were significantly reduced, as was the risk of all-cause mortality (Table 2). The beneficial effects with sacubitril/valsartan were observed early after randomisation, and no evidence of heterogeneity in efficacy was reported according to age, sex or background GDMT. Hypotensive episodes occurred more frequently with sacubitril/valsartan (14% vs 9.2%, p<0.001), but deterioration of renal function and severe hyperkalaemia occurred less frequently. Of note, sub-analyses of the PARADIGM-HF trial have shown a reduction in sudden cardiac death and death due to worsening HF with sacubitril/valsartan,5) as well as a lower risk of worsening HF, including the requirement for therapy intensification, emergency department visits, hospitalisation in intensive care unit, all-cause and cardiovascular hospitalisations.6) Patients in the sacubitril/valsartan arm experienced greater reductions in haemoglobin A1c levels and less frequently required insulin initiation for diabetes treatment compared with enalapril.7)
Table 1. Baseline clinical characteristic of patients included in recent HFrEF trials.
| Variables | PARADIGM-HF (n=8,399) | PIONEER-HF (n=881) | DAPA-HF (n=4,744) | EMPEROR-Reduced (n=3,730) | VICTORIA (n=5,050) | GALACTIC-HF (n=8,256) | |
|---|---|---|---|---|---|---|---|
| Inclusion criteria | |||||||
| LVEF (%) | ≤40 (≤35 after year 2010) | ≤40 | ≤40 | ≤40 | <45 | ≤35 | |
| Natriuretic peptides, pg/mL – sinus rhythm | If no HF hospitalizations in past 12 months: BNP ≥150 or NT-proBNP ≥600 | BNP ≥400 or NT-proBNP ≥1,600 | If no HF hospitalizations in past 12 months: NT-proBNP ≥600 pg/mL | EF ≥36% to ≤40%: NT-proBNP ≥2,500; EF ≥31% to ≤35%: NT-proBNP ≥1,000; EF≤ 30%: ≥600; EF ≤40% and HF hospitalization in past 12 months: NT-proBNP ≥600 | BNP≥300 or NT-proBNP ≥1,000 | BNP≥125 or NT-proBNP ≥400 | |
| If a HF hospitalization in past 12 months: BNP ≥100 or NT-proBNP ≥400 | If HF hospitalizations in past 12 months or NT-proBNP ≥400 pg/mL | ||||||
| Natriuretic peptides, pg/mL – AF/F | Not specified | Not specified | NT-proBNP ≥900 | EF ≥36% to ≤40%: ≥5,000; EF ≥31% to ≤35%: NT-proBNP ≥2,000; EF ≤30%: NT-proBNP ≥1,200; EF ≤40% and HF hospitalization in past 12 months: NT-proBNP ≥1,200 | BNP ≥500 or NT-proBNP ≥1,600 | BNP ≥375 or NT-proBNP ≥1,200 | |
| SBP (mmHg) | ≥95 | >100 | ≥95 | >100 | ≥100 | ≥85 | |
| eGFR (mL/min/1.73 m2) | ≥30 | ≥30 | ≥30 | >20 | ≥15 | ≥20 | |
| Prior HF hosp. (or equiv.) required? | No | No | No | No | Yes (100%) | Yes (100%) | |
| In-patients included? | No | Yes | No | No | Yes | Yes (25%) | |
| Baseline patient characteristics | |||||||
| Age (years) | 63.8±11.5 | 61.0±14.0 | 66.2±11.0 | 67.2±10.8 | 67.5±12.2 | 64.5±11.3 | |
| Female, % | 21.0 | 25.7 | 23.8 | 23.5 | 24.0 | 21.2 | |
| NYHA class III/IV, % | 23.9 | 73.1 | 32.3 | 24.9 | 41.4 | 46.7 | |
| LVEF, % | 29.6±6.1 | 24.0±6.0 | 31.2±6.7 | 27.7±6.0 | 29.0±8.3 | 26.6±6.3 | |
| Prior HF hosp. (or equiv.), % | 62.3 | 67.7 | 47.4 | 31.0 | 100.0 | 100.0 | |
| NT-proBNP, pg/mL median (IQR) | 1,631 (885–3,154) | 4,821 (3,109–8,767) | 1,428 (857–2,655) | 1,887 (1,077–3,429) | 2,803.5 (1,572–5,380) | 1,977 (980–4,061) | |
| Atrial fibrillation, % | 36.2 | 33.4 | 38.6 | 35.6 | 43.5 | 27.8 | |
| Hypertension, % | 70.9 | 87.3 | Not listed | 72.4 | 48.6 | Not listed | |
| Diabetes, % | 34.7 | 18.0 | 41.8 | 49.8 | 48.6 | 40.1 | |
| Myocardial infarction, % | 43.4 | 6.1 | Not listed | Not listed | Not listed | Not listed | |
| Ischaemic cardiomyopathy, % | 59.9 | Not listed | 55.5 | 52.8 | 59.8 | 53.2 | |
| Stroke, % | 8.5 | 10.0 | Not listed | Not listed | 11.2 | Not listed | |
| eGFR, mL/min/1.73 m2 (SD or IQR) | 68.0 | 58.4 (47.5–71.5) | 66.0±19.6 | 61.8±21.7 | 61.3±27 | 58.8 (44.3–74.3) | |
| Beta-blocker, % | 94.0 | 59.5 | 96.0 | 94.7 | 93.0 | 94.2 | |
| ACEI, % | 78.0 | 47.3* | 56.1 | 70.5* | 73.3* | 87.0† | |
| ARB, % | 22.2 | - | 28.4 | - | - | - | |
| MRA, % | 54.2 | 10.9 | 71.0 | 70.1 | 69.3 | 77.6 | |
| ARNI, % | Not applicable | Not applicable | 10.5 | 18.3 | 14.3 | 19.9 | |
| Diuretic, % | 80.3 | 59.5 | 93.4 | Not listed | Not listed | Not listed | |
| Digitalis, % | 29.2 | 9.3 | 18.8 | Not listed | Not listed | Not listed | |
Medical history and comorbidities data refers to investigated drug group; *ACEI or ARB, †ACEI or ARB or ARNI.
LVEF = left ventricular ejection fraction; HF = heart failure; BNP = brain natriuretic peptide; NT-proBNP = N-terminal pro-brain natriuretic peptide; AF/F = atrial fibrillation/flutter; SBP = systolic blood pressure; eGFR = estimated glomerular filtration rate; NYHA = New York Heart Association; IQR = interquartile range; SD = standard deviation; ACEI = angiotensin converting enzyme inhibitors; ARB = angiotensin receptor blockers; MRA = mineralocorticoid receptor antagonists; ARNI = angiotensin receptor-neprilysin inhibitor.
Table 2. Primary and secondary outcomes in clinical trials of sacubitril/valsartan, dapagliflozin, empagliflozin, vericiguat and omecamtiv mecarbil.
| Variables | PARADIGM-HF | PIONEER-HF | DAPA-HF | EMPEROR-Reduced | VICTORIA | GALACTIC-HF | |
|---|---|---|---|---|---|---|---|
| Trial characteristics | |||||||
| Investigated medication | Sacubitril/valsartan | Sacubitril/valsartan | Dapagliflozin | Empaglflozin | Vericiguat | Omecamtiv mecarbil | |
| Comparator | Enalapril | Enalapril | Placebo | Placebo | Placebo | Placebo | |
| No. of patients enrolled | 8,442 | 881 | 4,744 | 3,730 | 5,050 | 8,256 | |
| Median follow up | 27 months | 8 weeks | 18.2 months | 16 months | 10.8 months | 21.8 months | |
| Primary outcome | |||||||
| Death from CV causes or hospitalization for HF | Time-averaged proportional change in NT-proBNP | WHF (hospitalization or an urgent visit resulting in intravenous therapy for HF) or CV death | CV death or hospitalization for WHF | Death from CV causes or first hospitalization for HF | First HF event (hospitalization or urgent visit for HF) or death from CV causes | ||
| HR, 0.80; 95% CI, 0.73–0.87; p<0.001 | - | HR, 0.74; 95% CI, 0.65–0.85; p<0.001 | HR, 0.75; 95% CI,0.65–0.86; p<0.001 | HR, 0.90; 95% CI, 0.82–0.98; p=0.02 | HR, 0.92; 95% CI, 0.86–0.99; p=0.03 | ||
| Secondary outcomes | |||||||
| Death from CV causes | HR, 0.79; 95% CI, 0.71–0.89; p<0.001 | - | HR, 0.82; 95% CI, 0.69–0.98; p=0.03 | HR, 0.92; 95% CI, 0.75–1.12; p<0.001 | HR, 0.93; 95% CI, 0.81–1.06 | HR, 1.01; 95% CI, 0.92–1.11 | |
| First HF hospitalization | HR, 0.79; 95% CI, 0.71–0.89; p<0.001 | HR, 0.56; 95% CI, 0.37–0.84 | HR, 0.70; 95% CI, 0.59–0.83; p<0.001 | HR, 0.70; 95% CI, 0.58–0.85, p<0.001) | HR, 0.90; 95% CI, 0.81–1.00 | HR, 0.95; 95% CI, 0.87–1.03 | |
| Death from any cause | HR, 0.84; 95% CI, 0.76–0.93; p<0.001 | HR, 0.66, 95% CI, 0.30–1.48 | HR, 0.83; 95% CI, 0.71–0.97; p=0.02 | HR, 0.92; 95% CI, 0.77–1.10 | HR, 0.95; 95% CI, 0.84–1.07; p=0.38 | HR, 1.00; 95% CI, 0.92–1.09 | |
| Change in KCCQ* | At 8 months; 1.64 (0.63–2.65), p=0.001 | - | At 8 months; 1.18 (1.11–1.26), p<0.001 | At 13 months;1.7 (0.5–3.0) | - | At 3 months; inpatients: 2.5 (0.5–4.5), outpatients: −0.5 (−1.4, 0.5), p=0.03 by joint omnibus F-testing | |
| Worsening renal function | †HR, 0.86; 95% CI, 0.65–1.13; p=0.28 | †RR, 0.93; 95% CI, 0.67–1.28 | †HR, 0.71; 95% CI, 0.44–1.16; p=0.17 | ‡HR, 0.50; 95% CI, 0.32–0.77 | - | - | |
CV = cardiovascular; HF = heart failure; NT-proBNP = N-terminal pro-brain natriuretic peptide; WHF = worsening heart failure; HR = hazard ratio; CI = confidence interval; KCCQ = The Kansas City Cardiomyopathy Questionnaire ; eGFR = estimated glomerular filtration rate; GFR = glomerular filtration rate.
*Between group difference; †Decline in renal function defined as end-stage renal disease or 50% eGFR decline from randomization or decrease of eGFR of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2; ‡The composite renal outcome includes chronic dialysis or renal transplantation or a sustained reduction of 40% or more in the estimated GFR or a sustained estimated GFR of less than 15 mL/min/ 1.73 m2 in patients with a baseline estimated GFR of 30 mL/min/1.73 m2 or more or a sustained estimated GFR of less than 10 mL/min/1.73 m2 in those with a baseline estimated GFR of less than 30 mL/min/1.73m2.
The PROVE-HF trial provides evidence for reverse left-ventricular (LV) remodelling with sacubitril/valsartan in HFrEF, as demonstrated by an increase in LVEF (from 28.2% to 37.8%, difference 9.4%; 95% CI, 8.8–9.9%; p<0.001) and a reduction in LV volumes after 12 months of treatment, including patients with de novo HF.8) Further support is provided by the EVALUATE trial in which an early improvement in echocardiographic parameters occurred after 12 weeks of therapy with sacubitril/valsartan compared with enalapril,9) as well as by the PRIME study which suggested an improvement in functional mitral regurgitation following 12 months of sacubitril/valsartan treatment.10)
The PIONEER-HF study investigated the effect of an 8-week sacubitril/valsartan treatment vs. enalapril on NT-proBNP concentration in 881 patients with an LVEF ≤40%, hospitalised for acute decompensated HF (see Table 1 for eligibility criteria and baseline characteristics).11) The initiation of sacubitril/valsartan after haemodynamic stabilisation resulted in a greater reduction in NT-proBNP levels compared with enalapril, as early as one week after treatment initiation. The rates of worsening renal function, hyperkalaemia, symptomatic hypotension, and angioedema were comparable between the two groups. Further analysis of the PIONEER-HF trial demonstrated a significantly lower risk of an adjudicated composite outcome of death, HF rehospitalization, LV assist device implantation, and listing for cardiac transplantation in the sacubitril/valsartan arm (HR, 0.58; 95% CI, 0.40–0.85; p=0.005), Table 2.12) The TRANSITION trial assessed safety and tolerability of sacubitril/valsartan initiation at different time points after haemodynamic stabilisation in patients with acute decompensated HF and LVEF ≤40%.13) The results point to a similar tolerability regardless whether the treatment commenced before or within two weeks after hospital discharge, whilst discontinuation rates due to adverse events remained low in both groups. Moreover, TRANSITION trial data support tolerability of sacubitril/valsartan in patients with de novo HF, previously naïve to ACE inhibitor/ARB therapy.14) Accordingly, 29% of the trial population had de novo HF, and compared to those with prior HFrEF, they were more likely to obtain the target dose and were less likely to discontinue treatment due to adverse events.
SGLT2 INHIBITORS
The first trial to evaluate a role of an SGLT2 inhibitor in the treatment of HFrEF was DAPA-HF, which assessed the effect of dapagliflozin vs. placebo on the risk of cardiovascular mortality or worsening HF (defined as HF hospitalisation or urgent outpatient visit for HF treatment).15) The trial enrolled 4,744 patients with HFrEF and LVEF ≤40%, with and without diabetes (eligibility criteria and major baseline patient characteristics are presented in Table 1). Patients with type 1 diabetes mellitus, systolic blood pressure <95 mmHg, eGFR <30 mL/min/1.73 m2 and those intolerant to SGLT2 inhibitors were excluded. The results have demonstrated a significantly lower risk of the primary endpoint (HR, 0.74; 95% CI, 0.65–0.85; p<0.001; NNT=21), as well as a significant reduction in cardiovascular mortality and worsening HF events with dapagliflozin vs placebo (Table 2). The benefits occurred early after randomisation and were observed irrespective of the background GDMT, including sacubitril/valsartan (~11% of the trial patients). Importantly, the efficacy of dapagliflozin was similar in patients with and without diabetes. This was further explored in a sub-analysis which has shown consistent treatment effects across a spectrum of haemoglobin A1c.16) There was no evidence of heterogeneity in dapagliflozin efficacy among the predefined subgroups, except possibly for a lesser efficacy in patients with the New York Heart Association functional class III-IV compared with class II. However, no heterogeneity was observed among patients with lower LVEF or higher NT-proBNP, which suggests that dapagliflozin is similarly effective regardless of the severity of HF.15) Dapagliflozin treatment was safe and well tolerated and no excess in serious adverse events was noted, including diabetic ketoacidosis.
The EMPEROR-Reduced trial assessed the efficacy and safety of empagliflozin vs. placebo in 3,730 patients with HFrEF and LVEF ≤40%, with and without diabetes.17) Patients with recent acute coronary syndrome, myocardial revascularisation or stroke, acute HF, systolic blood pressure <100 mmHg, intolerance to SGLT2 inhibitors and eGFR <20 mL/min/1.73m2 were excluded. The majority of the trial population had LVEF <30% and NT-proBNP >1000 pg/mL (73% and 79%, respectively) and almost a half of the patients had renal dysfunction (eGFR ≥20 to 60 mL/min/1.73m2) (Table 1). After a median follow-up of 16 months, empagliflozin treatment was associated with a lower risk of the composite primary outcome of cardiovascular mortality or HF hospitalisation (HR, 0.75; 95% CI, 0.65–0.86, p<0.001).17) The beneficial effects of empagliflozin occurred early and were similar in patients with and without diabetes. There was no heterogeneity in treatment effects according to age, sex, and background therapy, including sacubitril/valsartan (~20% of the trial population). The effect on the primary outcome was primarily attributed to a lower risk of HF hospitalisation with empagliflozin (HR, 0.69; 95% CI, 0.59–0.81; p<0.001). There was also a reduction in the total number of HF hospitalisations (first and recurrent; HR, 0.70; 95% CI, 0.58–0.85; p<0.001) as well as a slower decline in renal function (a mean slope of change in eGFR mL/min/1.73 m2 per year) with empagliflozin, whereas the rate of all-cause mortality was similar with placebo (Table 2). Serious adverse events (hypoglycaemia, lower limb amputation, bone fracture and diabetic ketoacidosis) were rare and comparable between empagliflozin and placebo.
More recently, the SOLOIST-WHF trial has assessed the effect of sotagliflozin (combined SGTL2 and SGLT1 inhibitor) vs. placebo on the primary endpoint of the total number of cardiovascular deaths, hospitalisations and urgent visits for HF treatment (first and recurrent) in 1,222 patients with type 2 diabetes and recent hospitalisation for worsening HF.18) Patients with end-stageHF, recent acute coronary syndrome, stroke, or myocardial revascularisation, or eGFR <30 mL/min/1.73 m2 were excluded, The trial population mostly comprised individuals with HF and mid-range or reduced LVEF (~79%), with a median LVEF of 35%, but the SOLOIST-WHF also included diabetic patients with HF and preserved LVEF ≥50% (HFpEF). The trial ended prematurely (due to the lack of funding). After a median of 9 months of follow-up, patients randomised to sotagliflozin demonstrated a significantly lower risk of the primary outcome (HR, 0.67; 96% CI, 0.52–0.85; p<0.001). This finding was consistent across several prespecified subgroups, including stratification according to the timing of the first dose of the study medications (before or 3 days after hospital discharge) and LVEF <50% or ≥50%. Also, the first secondary endpoint of the total number of HF hospitalisations was significantly reduced with sotagliflozin, whilst the rates of cardiovascular and total mortality were comparable between the study arms. These results suggest that an early introduction of sotagliflozin after stabilisation of decompensated HF may be safe, with similar benefits in patients with HFrEF and HFpEF. However, given the premature termination and a small sample size, these observations should be considered as hypothesis generating.
A meta-analysis of the DAPA-HF and EMPEROR-Reduced trials has confirmand a significant risk reduction of CV mortality or HF hospitalisation with SGLT2 inhibition (HR, 0.74; 95% CI, 0.68–0.82, p<0.0001) and a consistent effect of dapagliflozin and empagliflozin on risk reduction of cardiovascular (HR, 0.86; 95% CI, 0.76–0.98, p=0.027) and all-cause mortality (HR, 0.87; 95% CI, 0.77–0.98, p=0.018), as well as of worsening renal function (HR, 0.62; 95% CI, 0.43–0.90, p=0.013).19) There was no excess in serious adverse events, including volume depletion, renal dysfunction, bone fractures, lower limb amputations or diabetic ketoacidosis.
VERICIGUAT
Vericiguat, a soluble guanylate cyclase stimulator acts to increase myocardial and vascular NO bioavailability, with an effect on the improvement of endothelial function, myocardial remodelling and diastolic relaxation.20) The VICTORIA trial assessed the effects of vericiguat vs. placebo on the primary outcome of death from cardiovascular cause or first HF hospitalization in 5050 patients with HF, an LVEF <45% and a recent HF hospitalisation or urgent HF treatment.21) Exclusion criteria were a systolic blood pressure <100 mmHg; concomitant use of long-acting nitrates, soluble guanylate cyclase stimulators, or phosphodiesterase type 5 inhibitors; and use of intravenous inotropes or implantable left ventricular assist devices. The trial population comprised high-risk patients with a mean LVEF of 29%, a median NT-proBNP of 2,816 pg/mL and a recent (<3 months) hospitalisation for HF in 67% (Table 1). Over a median of 10.8 months, vericiguat treatment vs. placebo reduced the primary outcome (HR, 0.90; 95% CI, 0.83–0.98; p=0.02, NNT 24) as well as total number of HF hospitalisations and death from any cause or first HF hospitalisation. The difference favouring vericiguat became apparent after approximately 3 months from randomisation and was comparable in patients receiving (15% of the trial population) vs. those not receiving sacubitril/valsartan. Cardiovascular and all-cause mortality rates were similar between vericiguat and placebo (Table 2). An analysis of the treatment effects across prespecified subgroups indicated that the benefit of vericiguat may be attenuated in patients with severe or advanced HF, including those with the highest quartile of NT-proBNP values (>5,314 pg/mL) as well in patients older than 75 years and in those with significant renal dysfunction (eGFR <30 mL/min/1.73m2). The overall incidence of adverse events was comparable between vericiguat and placebo, although symptomatic hypotension and syncope were numerically more frequent with vericiguat.21)
OMECAMTIV-MECARBIL
The effect of a selective cardiac myosin activator, omecamtiv mecarbil, on cardiovascular mortality or hospitalisation for HF was assessed in the GALACTIC-HF trial, which randomised 8256 patients with HF and an LVEF ≤35% to receive omecamtiv mecarbil or placebo, in addition to GDMT.22) The trial included 25% of patients currently hospitalised for acute HF (inpatients), as well as those with a hospitalisation or urgent visit for HF treatment within 1 year before screening (outpatients) (Table 1). Haemodynamically unstable patients, those with a systolic blood pressure <85 mmHg, an eGFR <20 mL/min/1.73 m2, and a recent acute coronary syndrome or cardiovascular procedure were excluded. During a median of 21.8 months, patients in the omecamtiv mecarbil group experienced a reduction in the primary composite outcome of a first HF event (hospitalization or urgent visit for HF) or death from cardiovascular causes compared with the placebo group (HR, 0.92; 95% CI, 0.86–0.99; p=0.03). The treatment effects were consistent across predefined subgroups (including inpatients and outpatients), apart from a potentially greater efficacy of omecamtiv mecarbil in patients with LVEF <28%. A secondary outcome of cardiovascular mortality was not significantly reduced (Table 2). Major cardiac ischaemic events and ventricular arrhythmias occurred at similar rates between the study arms. The study medications were discontinued at rates similar between the omecamtiv mecarbil and placebo arms (20.6% and 21.9%), mostly due to adverse events.22)
TREATMENT OF COMORBIDITIES TO IMPROVE OUTCOMES IN HFREF
Diabetes mellitus
In patients with type 2 diabetes and established cardiovascular disease or with multiple risk factors, several cardiovascular outcome trials have demonstrated a consistent reduction in the risk of HF hospitalisation with SGLT2 inhibitors (empagliflozin, canagliflozin, dapagliflozin and ertugliflozin).23),24),25),26) Furthermore, the use of empagliflozin was associated with a reduction in cardiovascular mortality in the EMPA-REG-Outcome trial,25) and there was a consistent effect on renal protection with SGLT2 inhibitors in patients with diabetes.27),28),29) The DAPA-HF and EMPEROR-Reduced trials have shown no signal of heterogeneity in the efficacy of SGLT2 inhibition in reducing the risk of cardiovascular mortality or HF hospitalisation in patients with or without diabetes.15),17) This is further supported by the SOLOIST-WHF trial, which has suggested that an early introduction of sotagliflozin in type 2 diabetes patients stabilised after an episode of worsening HF may be safe and effective in the prevention of recurrent hospitalisations or cardiovascular mortality.18)
Considering other glucose-lowering medications, clinical trial data suggest a significant increase in the risk of HF hospitalisation in type 2 diabetes patients receiving thiazolidinediones (rosiglitazone and pioglitazone),30),31),32) and a dipeptidyl peptidase-4 inhibitor, saxagliptin.33) In addition, two small trials of patients with HFrEF (with and without diabetes) suggested a signal of harm with a glucagon-like peptide-1 receptor agonist, liraglutide.34),35) Clinical trial data on the safety of metformin in patients with HFrEF are missing but accumulated clinical experience and observational data suggest that metformin is safe and associated with lesser risk of cardiovascular mortality or HF, compared with sulphonylurea agents or insulin.36),37),38) Although data on the effect of insulin on the risk of mortality or worsening HF remains ambiguous,39),40) many patients require insulin to control diabetes and it is often a part of the combined glucose-lowering regiments. Importantly, cardiovascular outcome trials have not shown an interaction between insulin treatment and the reduction of cardiovascular outcomes in patients receiving SGLT2 inhibitors.
Iron deficiency/anaemia
Iron deficiency (serum ferritin concentration <100 ng/mL or 100–299 ng/mL with transferrin saturation <20%) and anaemia (haemoglobin level <120 g/L in females and <130 g/L in males) are common in HFrEF.41) They are independently associated with reduced exercise tolerance and a higher risk of HF hospitalisation, cardiovascular and all-cause cause mortality.41) Several clinical trials (FAIR-HF, CONFIRM-HF and EFFECT-HF) have documented the efficacy of intravenous ferric carboxymaltose to improve symptoms, exercise capacity and quality of life in patients with HFrEF.42),43),44) Prospective evaluation of the effects of parenteral iron supplementation on HF hospitalisation and mortality in HFrEF is currently underway.
Hyperkalaemia
Hyperkalaemia is often the reason for under-prescription, under-dosing or discontinuation of renin-angiotensin-aldosterone inhibitors, in particular MRAs, although sub-analyses of the RALES and EMPHASIS-HF trials (with spironolactone and eplerenone in HFrEF, respectively) have not documented an attenuation of therapeutic effects of MRAs in patients with serum potassium >5.5–6.0 momL/L.45),46) Potassium binding-agents, patiromer and zirconium cyclosilicate have shown promising results in controlling hyperkalaemia, which may allow optimisation of HFrEF treatment. The PEARL-HF trial included 105 patients with HF, an eGFR of <60 mL/min or a documented history of renin-angiotensin-aldosterone inhibitors discontinuation due to hyperkalaemia within 6 months.47) Following 28 days of treatment, patients randomised to patiromer had a lower serum potassium concentration, lesser incidence of hyperkalaemia and a greater proportion of patients achieved target dose of spironolactone. Significant hypokalaemia (<3.5 mmol/L) was numerically more frequent with patiromer, whilst other adverse events were mainly gastrointestinal.47)
CLINICAL PERSPECTIVE OF NOVEL PHARMACOTHERAPEUTIC OPTIONS IN HFREF
Accumulating data suggests that sacubitril/valsartan4) and SGLT2 inhibitors (dapagliflozin and empagliflozin)15),17) are beneficial and well-tolerated in the majority of patients with HFrEF and therefore could be considered as the mainstay treatment. Sacubitril/valsartan has been proven to reduce the risk of death, worsening HF, cardiac arrhythmia, and to improve LV reverse remodelling and quality of life in HFrEF.48) It can be safely initiated in patients with de novo HF and during a vulnerable phase following an episode of acute HF. Therefore, this drug could be preferred to ACE inhibitors/ARBs in the majority of HFrEF patients.49) A caution is needed in patients with a lower systolic blood pressure, and in those hospitalised for acute HF due to a higher risk of hypotension.11) A lower starting dose (24/26 mg twice daily), careful up-titration and a reduction in the dose of loop diuretics should be considered in those individuals.50) Renal function and serum potassium should be checked within 1 to 2 weeks following an initiation of sacubitril/valsartan, and data is lacking on its safety and efficacy in severe renal impairment (eGFR <30 mL/min/1.73 m2).
A growing body of evidence supports the role of SGLT2 inhibitors (dapagliflozin and empagliflozin) as a novel class of medications with beneficial cardiovascular and renal effects in the majority of HFrEF patients, regardless of diabetes status. These medications may be introduced early in the sequence of GDMT initiation, before obtaining target doses of other medications since clinical trial data suggest their complementary therapeutic value.51) The use of SGLT2 inhibitors is further facilitated by a favourable safety profile, good tolerability and by the lack of requirement for up-titration. A reduction in the dose of loop diuretics may be needed due to a mild/transient diuretic/natriuretic effect of SGLT2 inhibitors. Caution is advised in patients with severe renal dysfunction, and an adjustment of antidiabetic medications may be needed in patients with diabetes.
The evidence base for an early introduction of sacubitril/valsartan (instead of an ACE inhibitor/ARB) and an SGLT2 inhibitor in patients with HFrEF is solidified by a recent cross-trial analysis in HFrEF, which has suggested that an early comprehensive disease-modifying therapy with sacubitril/valsartan, a beta-blocker, an MRA and an SGLT2 inhibitor can afford 2.7 additional years (for an 80-year-old) to 8.3 additional years (for a 55-year-old) free from cardiovascular death or first HF hospitalisation and 1.4 additional years (for an 80-year-old) to 6.3 additional years (for a 55-year-old) of survival compared with conventional therapy (ACE inhibitors/ARB and beta-blockers).52) This concept has been endorsed by recent expert practice recommendations for the treatment of HFrEF.49),53),54)
Vericiguat and omecamtiv mecarbil has been assessed in the more selective populations in the context of greater symptom burden, higher natriuretic peptide levels and recent hospitalisation for worsening HF despite standard treatment.20),22) These medications may not be needed in all HFrEF patients but may be considered as a treatment effective in specific populations of HFrEF patients who remain symptomatic and/or experience worsening symptoms despite optimal treatment.
Considering the treatment of comorbidities in HFrEF, recent clinical trials solidify the evidence that a holistic approach to the management of HFrEF can significantly improve outcomes. In the general population of patients with type 2 diabetes, SGLT2 inhibitors should be the first-line treatment to prevent or delay HF hospitalisation, whilst in patients with known HFrEF and diabetes, these medications should be the preferred treatment to improve clinical outcomes. Furthermore, intravenous iron supplementation in individuals with iron deficiency/anaemia provides an opportunity to improve functional status and quality of life, whilst novel therapeutic options for hyperkalaemia (potassium binders), demonstrate promising results in enabling the maintenance and up-titration of GDMT. Another practical tip from the PARADIGM-HF trial is that substitution of an ACE inhibitor with sacubitril/valsartan may be associated with a lower risk of hyperkalaemia, which may allow more space for the optimisation of GDMT in HFrEF.
CONCLUSIONS
Pharmacological management of HFrEF has witnessed major breakthroughs over the past decades and contemporary drug therapy offers a possibility to alter the natural course, prolong lives and decrease the burden of morbidity and disability in the affected patients. Accumulating evidence indicates that selecting sacubitril/valsartan (instead of an ACE inhibitor/ARB) and introducing an SGLT2 inhibitor early in the treatment pathway, along with an evidence-based beta-blocker and an MRA, is feasible and associated with an incremental prognostic benefit in the majority of patients with HFrEF. Even more, in high-risk individuals with severe or advanced HFrEF, vericiguat or omecamtiv mecarbil may be a valuable, emerging option to improve outcomes in addition to standard care. Appropriate selection of devices, surgical therapies and targeted treatment of comorbidities complete the holistic approach to the management of HFrEF. Future developments will further broaden the spectrum of emerging therapies, provide new insights into the optimal sequencing of available drugs and will lead the way to treatment pathways tailored to the individual patient’s requirements.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Seferovic PB, Polovina M, Milinkovic I, Coats AJS.
- Supervision: Seferovic PB, Rosano G.
- Validation: Seferovic PB.
- Visualization: Milinkovic I.
- Writing - original draft: Seferovic PB, Polovina M, Milinkovic I, Anker SD, Rosano G, Coats AJS.
- Writing - review & editing: Seferovic PB, Polovina M, Milinkovic I, Anker SD, Rosano G, Coats AJS.
References
- 1.Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. doi: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MS, Samman Tahhan A, Vaduganathan M, et al. Trends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail. 2020;22:1032–1042. doi: 10.1002/ejhf.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewan P, Jackson A, Jhund PS, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction - an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail. 2020;22:2123–2133. doi: 10.1002/ejhf.1832. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
- 7.Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5:333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Januzzi JL, Jr, Prescott MF, Butler J, et al. Association of change in N-Terminal pro-B-Type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–1095. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1077–1084. doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–1365. doi: 10.1161/CIRCULATIONAHA.118.037077. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF trial. Circulation. 2019;139:2285–2288. doi: 10.1161/CIRCULATIONAHA.118.039331. [DOI] [PubMed] [Google Scholar]
- 13.Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21:998–1007. doi: 10.1002/ejhf.1498. [DOI] [PubMed] [Google Scholar]
- 14.Senni M, Wachter R, Witte KK, et al. Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail. 2020;22:303–312. doi: 10.1002/ejhf.1670. [DOI] [PubMed] [Google Scholar]
- 15.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 16.Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 19.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong PW, Roessig L, Patel MJ, et al. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail. 2018;6:96–104. doi: 10.1016/j.jchf.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 22.Teerlink JR, Diaz R, Felker GM, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–116. doi: 10.1056/NEJMoa2025797. [DOI] [PubMed] [Google Scholar]
- 23.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 24.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 25.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 26.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 27.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 28.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 29.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 30.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 31.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 32.Komajda M, McMurray JJ, Beck-Nielsen H, et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824–831. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 34.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 35.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–508. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah DD, Fonarow GC, Horwich TB. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J Card Fail. 2010;16:200–206. doi: 10.1016/j.cardfail.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail. 2011;4:53–58. doi: 10.1161/CIRCHEARTFAILURE.110.952556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eurich DT, Weir DL, Majumdar SR, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 39.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 40.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. 2019;7:36–46. doi: 10.1016/j.jchf.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 43.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Veldhuisen DJ, Ponikowski P, van der Meer P, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–1383. doi: 10.1161/CIRCULATIONAHA.117.027497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) Circ Heart Fail. 2014;7:51–58. doi: 10.1161/CIRCHEARTFAILURE.113.000792. [DOI] [PubMed] [Google Scholar]
- 46.Vardeny O, Claggett B, Anand I, et al. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7:573–579. doi: 10.1161/CIRCHEARTFAILURE.114.001104. [DOI] [PubMed] [Google Scholar]
- 47.Pitt B, Anker SD, Bushinsky DA, et al. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khariton Y, Fonarow GC, Arnold SV, et al. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7:933–941. doi: 10.1016/j.jchf.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maddox TM, Januzzi JL, Jr, Allen LA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Senni M, McMurray JJ, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18:1193–1202. doi: 10.1002/ejhf.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Docherty KF, Jhund PS, Inzucchi SE, et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020;41:2379–2392. doi: 10.1093/eurheartj/ehaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396:121–128. doi: 10.1016/S0140-6736(20)30748-0. [DOI] [PubMed] [Google Scholar]
- 53.Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–1186. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 54.Seferović PM, Fragasso G, Petrie M, et al. Heart Failure Association of the European Society of Cardiology update on sodium-glucose co-transporter 2 inhibitors in heart failure. Eur J Heart Fail. 2020;22:1984–1986. doi: 10.1002/ejhf.2026. [DOI] [PubMed] [Google Scholar]