Abstract
With great interest, our independent groups of scientists located in Korea and Germany recognized the use of a very similar methodologic approach to quantify the uptake of radioactive glucose (18F-FDG) at the cellular level. The focus of our investigations was to disentangle microglial 18F-FDG uptake. To do so, CD11b immunomagnetic cell sorting was applied to isolate microglia cells after in vivo 18F-FDG injection, to allow simple quantification via a γ-counter. Importantly, this technique reveals a snapshot of cellular glucose uptake in living mice at the time of injection since 18F-FDG is trapped by hexokinase phosphorylation without a further opportunity to be metabolized. Both studies indicated high 18F-FDG uptake of single CD11b-positive microglia cells and a significant increase in microglial 18F-FDG uptake when this cell type is activated in the presence of amyloid pathology. Furthermore, another study noticed that immunomagnetic cell sorting after tracer injection facilitated determination of high 18F-FDG uptake in myeloid cells in a range of tumor models. Here, we aim to discuss the rationale for single-cell radiotracer allocation via immunomagnetic cell sorting (scRadiotracing) by providing examples of promising applications of this innovative technology in neuroscience, oncology, and radiochemistry.
Keywords: scRadiotracing, cell sorting, 18F-FDG, PET, cellular resolution, tracer uptake
The following sections discuss knowledge gaps of cellular tracer uptake and potential applications of scRadiotracing in different fields of molecular imaging.
POTENTIAL APPLICATIONS OF scRADIOTRACING IN NEUROSCIENCE
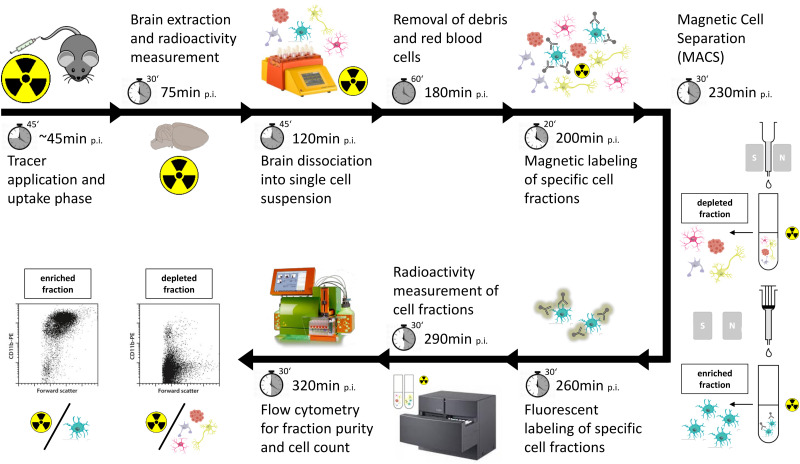
Spatiotemporal alterations in the 18F-FDG PET signal comprise a well-established readout in the diagnostic workup of patients with neurologic disorders (1–3). However, the method lacks the cellular resolution to distinguish respective contributions of different cell types to the 18F-FDG uptake. Most earlier studies claimed that neuronal activity and neuronal 18F-FDG uptake dominate glucose uptake and consumption in the mammalian brain (4). However, several recent studies highlighted a significant contribution of glial cells to the energy metabolism of the brain (5–7), questioning 18F-FDG PET as a pure biomarker of neuronal activation (8). We applied immunomagnetic cell sorting after in vivo radiotracer injection and brain extraction, followed by subsequent measurement of γ-emission and cell count in enriched cell fractions to calculate radiotracer uptake per specific single cell (scRadiotracing; Fig. 1 shows the workflow). Subsequently, our 2 recent studies exploring scRadiotracing technology identified high microglial 18F-FDG uptake in mouse models with amyloid pathology (7,9). Furthermore, microglial 18F-FDG uptake was the most likely reason for elevated 18F-FDG PET signals in these mice (7,9). However, many remaining questions about altered 18F-FDG PET signals may also be addressed by an analysis of glucose uptake at cellular resolution. Recently, we discovered a reduction in the 18F-FDG PET signal in a progranulin knockout mouse model with hyperactivated microglia and in a mouse model with homeostatic microglia (Trem2 knockout) (10). Although this finding speaks for reduced brain function in both genotypes, it still remains unclear whether progranulin knockout microglia have lower 18F-FDG uptake or whether the net signal is driven by reduced neuronal activity despite higher 18F-FDG uptake by activated microglia (7,9). This example illustrates the need to study glucose uptake at cellular resolution. Given the growing evidence for an astrocyte–neuron lactate shuttle (11,12), it will be of interest to reveal whether increasing neuronal activity (13) can stimulate glial 18F-FDG uptake, which could be addressed using scRadiotracing. The presence of such shuttle systems questions the cellular glucose uptake regardless of the individual glucose consumption of different cell types, which is undeniably high in active neurons (14). In this regard, there could be an imbalance between the cellular localizations of glucose uptake and energy consumption. Importantly, scRadiotracing after 18F-FDG injection could be specifically used to allocate glucose uptake, whereas other tracers could be used to track metabolites of aerobic and anaerobic glycolysis at the cellular level. Furthermore, uptake mechanisms during acute stimulation of glucose consumption by physiologic (15) or pharmacologic interventions could be deciphered by scRadiotracing. To this end, task-related stimuli could be applied together with resurgent functional PET methodology (16) and scRadiotracing in experimental models. scRadiotracing could be applied after brain extraction at the time of maximal stimulation, determined by the functional PET readout, to track the changes in cellular 18F-FDG uptake in contrast to unstimulated conditions.
FIGURE 1.
Workflow of scRadiotracing to determine microglial 18F-FDG uptake in brain at cellular resolution. After tracer injection into tail vein, brain is removed during tracer-specific uptake period. After generation of single-cell suspension, immunomagnetic cell separation is used to separate fractions of enriched cells from their depleted counterparts, which contain bound radioactivity. Fluorescent labeling, γ-counting, and flow cytometry are used to calculate radioactivity per cell as primary readout. Time to complete each step is indicated, together with summed time during workflow. CD11b is used to detect microglia. p.i. = after injection; S/N = south and north pole of the magnet. (Courtesy of Miltenyi Biotec B.V. & Co. KG. All rights reserved. Copyright © 2022.)
As another example in the field of neuroimaging, tau PET tracers emerged as valuable biomarkers for the differentiation of tauopathies from controls (17). However, the translation of in vitro tau PET tracer binding to an in vivo signal is still under debate, and the detailed cellular sources of autoradiography and tau PET signal elevations remain unclear. Hence, our novel scRadiotracing approach could be used to calculate tau PET tracer uptake at cellular resolution of single neurons and astrocytes in models of tauopathies in order to close this knowledge gap. Brain subregion analyses by scRadiotracing are also feasible as long as the product of the cellular yield and the tracer abundance exceed the detection limit of the γ-counter. In this regard, we successfully dissected the hippocampus to study region-specific 18F-FDG uptake in mice with amyloid pathology (9). In tauopathies, this feature could be used to compare single-cell radiotracer uptake of regions with high and low tau abundance.
POTENTIAL APPLICATIONS OF scRADIOTRACING IN NEUROONCOLOGY AND ONCOLOGY
The novel combination of tracer injection and immunomagnetic cell sorting could also facilitate dedicated analysis of tumor cells in experimental models of brain tumors together with analysis of specific immune cell fractions and tumor surrounding cells such as neurons and ependymal cells. This ability could be highly valuable since the target of several tracers for glioblastoma imaging, including the 18-kDa translocator protein (TSPO), is not restricted to a single cell type. Determining the radiotracer uptake of TSPO ligands at cellular resolution in the brain is of general interest since the target is expressed not only by microglia cells but also by tumor cells, astrocytes, endothelial cells, and neurons (18). Since blood–brain barrier disruption is often questioned as a strong influencer of PET tracer signals in brain tumor imaging, scRadiotracing could also act as a proof of cellular radiotracer allocation. Here, the magnitude of radiotracer uptake per cell could be multiplied by respective absolute cell numbers to investigate whether the entire PET signal is explained by cellular sources.
Apart from brain tumors, the aforementioned oncologic investigation (19) already applied scRadiotracing in a wide range of tumor models, including renal cell carcinoma, colorectal carcinoma (CT26, MC38), and breast cancer. Importantly, the authors used not only 18F-FDG but also 18F-labeled glutamine to disentangle its metabolism in tumor cells and the tumor microenvironment (19). This use shows that scRadiotracing is not limited to 18F-FDG and highlights the broad range of potential scRadiotracing applications in experimental oncology. For example, prostate-specific membrane antigen radiotracers are preferred for targeting of prostate cancer cells because they exhibit very low glucose consumption and therefore cannot be detected by 18F-FDG PET. However, prostate-specific membrane antigen is expressed not only in prostate cells but also in several other tissues, such as nonprostatic epithelial cells, other neoplastic cells, and tumor-associated neovasculature. Prostate-specific membrane antigen uptake was also associated with inflammatory and infectious processes (20). Thus, scRadiotracing could be used to differentiate prostate-specific membrane antigen uptake in prostatic cancer cells from others and to avoid pitfalls in prostate cancer diagnostics. The same applies to the diagnostics of well-differentiated neuroendocrine tumors such as gastroenteropancreatic neuroendocrine tumors and meningiomas using radiolabeled somatostatin receptor (SSTR) ligands. The importance of neuroendocrine tumor diagnostics and therapy is underpinned by an interventional multicenter phase III clinical trial (NETTER) to compare peptide receptor radionuclide therapy using 177Lu-DOTA0-Tyr3-octreotate with high-dose octreotide LAR therapy in patients with metastasized or locally advanced, inoperable, SSTR-positive, midgut carcinoid tumors. However, SSTR expression is not exclusive to neuroendocrine tumor or meningioma cells. Tracer uptake was also observed by inflammatory pathologies such as cardiovascular disease and ischemia and by various other benign and malignant tumors (21). scRadiotracing has the potential to resolve such inconclusive results by use of back translation in experimental models. This may help to determine off-target sources and prevents false-positive findings. In cases with borderline SSTR expression, dual scRadiotracing may also be suitable to elucidate whether SSTR radioligands or 18F-FDG is better suited for follow-up PET imaging of the individual patient.
POTENTIAL APPLICATIONS OF scRADIOTRACING IN RADIOCHEMISTRY AND RADIOTRACER DEVELOPMENT
scRadiotracing might also be a versatile tool in tracer development to investigate cell-specific uptake of acutely isolated cells when compared with cell cultures, in which the metabolic activity of cells may be altered. In general, ex vivo radiopharmaceutical research methods rely mostly on macroscopic samples for quantification in a γ-counter or on autoradiography blocking experiments, including correlation studies with immunohistochemistry staining. However, such techniques do not investigate tracer enrichment on the cellular level. Nevertheless, cellular-level investigations are of particular interest when the specificity of a novel tracer has to be explored or when the radioactivity distribution is to be assigned to specific cell types. In neuroinflammatory tissue, the discrimination of tracer accumulation in different microglia phenotypes could be of eminent importance. Such approaches could support the development of specific ligands for homeostatic and disease-associated microglia, which would facilitate monitoring of therapeutic agents that modulate distinct microglia phenotypes. In principle, scRadiotracing could be applied to any radiotracer binding to an intracellular target. However, it is questionable whether ligands of membrane-bound targets on the surface of cells also qualify for scRadiotracing analysis. Upstream cell processing promises a gentle mechanical and enzymatic dissociation preserving cell integrity and surface epitopes, but it has to be proven whether high-affinity binders withstand hydrolytic treatment. This also applies to subsequent downstream applications beyond quantification in a γ-counter. In this respect, ligands showing a high internalization rate may most likely be applicable to scRadiotracing analysis.
METHODOLOGIC LIMITATIONS AND CONSIDERATIONS
First, we note the difficulty in quantifying the uptake of a whole cell population via scRadiotracing. The procedure of cell dissection and harvesting may over- or underestimate the proportion of viable cells in the brain or in specific regions (22), hampering extrapolation to absolute cell numbers. Cell proportions can also be influenced by proliferation and cell loss, which lead to subsequent alterations in cell density. Thus, scRadiotracing facilitates robust calculation of radiotracer uptake per cell, but extrapolation to the whole fraction is erroneous. A full allocation model of radiotracer uptake per cell type and fraction could be established by simultaneous light sheet microscopy (23). Light sheet microscopy offers the possibility to quantify the absolute number of cells per cell type in a 3-dimensional and analysis of a subsample allows combination of cellular tracer uptake with absolute cell numbers.
Unlike well-established nearly irreversibly bound 18F-FDG, all non–18F-FDG radioligands may suffer from higher instability during the scRadiotracing procedure. For instance, the binding stability of a TSPO ligand to the TSPO complex at the mitochondrial membrane could decrease during the scRadiotracing procedure. This decreased binding stability also accounts for current tau ligands such as 18F-PI-2620, which is characterized by a decrease in target-bound radiotracer over time in 4-repeat tauopathies (24).
Loss of processes and synapses potentially impacts quantitative results in scRadiotracing. 18F-FDG in neuronal synapses comprises one important example of missed radiotracer in the scRadiotracing workflow (7,9). Optimization of the dissociation procedure has the potential to further enhance the accuracy of scRadiotracing. In a similar regard, consideration of live and dead cells may stabilize scRadiotracing results since cells with leaky membranes can be excluded.
Cell separation after TSPO tracer injection was also proposed by a recent study using a similar technique of fluorescence-activated cell sorting (FACS) to determine the cellular source of a 125I-labeled SPECT TSPO ligand (125I-CLINDE) (25). Here, the long half-life of 125I offered the opportunity to conduct the experiments with less of a time constraint. In a comparison of the two approaches, immunomagnetic cell sorting systems can be installed relatively simply within a radiation protection controlled area, and the higher cell yield is another advantage of immunomagnetic cell sorting over FACS, which allows even proteomic analyses (26). On the other hand, FACS offers the advantage of direct separation of genetically determined fluorescent cells (i.e., green fluorescent protein), which cannot be achieved via immunomagnetic cell sorting. Furthermore, specific cell populations, such as homeostatic or disease-associated microglia, can be selected via FACS gating, when discriminative antibodies are used. Considering the stability of radiotracer binding once more, there is a need to investigate the impact of emitted energy during cell sorting via FACS, which could be of high relevance for tracers bound at voltage channels. Head-to-head comparisons of both approaches will be required to allow recommendations on whether immunomagnetic cell sorting or FACS systems are to be preferred for scRadiotracing.
scRadiotracing in human tissue after in vivo or in vitro tracer application comprises another promising methodologic variant (25). In vitro application of radiotracer to tissue allows investigation of small amounts of tissue that do not yield a sufficient signal-to-noise ratio when the tracer is applied in vivo (i.e., before surgery on tumors). However, scRadiotracing after tracer application in vivo could be used to validate in vitro scRadiotracing in the same tissue when amounts are large enough. Furthermore, blocking with cold ligands could be performed to test for the specificity of cellular radiotracer binding using in vitro scRadiotracing. As a limitation, we note that the extensive workflow (Fig. 1) likely restricts scRadiotracing to an experimental setting and prevents it from use in clinical routine at the current stage.
CONCLUSION
We highlighted some of the broad range of highly demanded applications for the novel scRadiotracing workflow to elucidate tracer uptake mechanisms and their underlying cell biology.
DISCLOSURE
Matthias Brendel was funded by the Deutsche Forschungsgemeinschaft (DFG) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198), and Philipp Beumers and Nathalie L. Albert were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project number 421887978. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260:2744–2753. [DOI] [PubMed] [Google Scholar]
- 2. Jack CR, Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guedj E, Varrone A, Boellaard R, et al. EANM procedure guidelines for brain PET imaging using [18F]FDG, version 3. Eur J Nucl Med Mol Imaging. 2022;49:632–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. [DOI] [PubMed] [Google Scholar]
- 5. Zimmer ER, Parent MJ, Souza DG, et al. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci. 2017;20:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ulland TK, Song WM, Huang SC-C, et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 2017;170:649–663.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiang X, Wind K, Wiedemann T, et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. 2021;13:eabe5640. [DOI] [PubMed] [Google Scholar]
- 8. Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. [DOI] [PubMed] [Google Scholar]
- 9. Choi H, Choi Y, Lee EJ, et al. Hippocampal glucose uptake as a surrogate of metabolic change of microglia in Alzheimer’s disease. J Neuroinflammation. 2021;18:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Götzl JK, Brendel M, Werner G, et al. Opposite microglial activation stages upon loss of PGRN or TREM2 result in reduced cerebral glucose metabolism. EMBO Mol Med. 2019;11:e9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel AB, Lai JC, Chowdhury GM, et al. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci USA. 2014;111:5385–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adachi K, Cruz NF, Sokoloff L, Dienel GA. Labeling of metabolic pools by [6-14C]glucose during K(+)-induced stimulation of glucose utilization in rat brain. J Cereb Blood Flow Metab. 1995;15:97–110. [DOI] [PubMed] [Google Scholar]
- 14. Vergara RC, Jaramillo-Riveri S, Luarte A, et al. The energy homeostasis principle: neuronal energy regulation drives local network dynamics generating behavior. Front Comput Neurosci. 2019;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rischka L, Gryglewski G, Pfaff S, et al. Reduced task durations in functional PET imaging with [18F]FDG approaching that of functional MRI. Neuroimage. 2018;181:323–330. [DOI] [PubMed] [Google Scholar]
- 16. Verger A, Guedj E. The renaissance of functional 18F-FDG PET brain activation imaging. Eur J Nucl Med Mol Imaging. 2018;45:2338–2341. [DOI] [PubMed] [Google Scholar]
- 17. Beyer L, Brendel M. Imaging of tau pathology in neurodegenerative diseases: an update. Semin Nucl Med. 2021;51:253–263. [DOI] [PubMed] [Google Scholar]
- 18. Nutma E, Ceyzeriat K, Amor S, et al. Cellular sources of TSPO expression in healthy and diseased brain. Eur J Nucl Med Mol Imaging. 2021;49:146–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinfeld BI, Madden MZ, Wolf MM, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Galiza Barbosa F, Queiroz MA, Nunes RF, et al. Nonprostatic diseases on PSMA PET imaging: a spectrum of benign and malignant findings. Cancer Imaging. 2020;20:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helgebostad R, Revheim ME, Johnsrud K, Amlie K, Alavi A, Connelly JP. Clinical applications of somatostatin receptor (agonist) PET tracers beyond neuroendocrine tumors. Diagnostics (Basel). 2022;12:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keller D, Ero C, Markram H. Cell densities in the mouse brain: a systematic review. Front Neuroanat. 2018;12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stelzer EHK, Strobl F, Chang B-J, et al. Light sheet fluorescence microscopy. Nature Reviews Methods Primers. 2021;1:73. [Google Scholar]
- 24. Song M, Beyer L, Kaiser L, et al. Binding characteristics of [18F]PI-2620 distinguish the clinically predicted tau isoform in different tauopathies by PET. J Cereb Blood Flow Metab. 2021;41:2957–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tournier BB, Tsartsalis S, Ceyzeriat K, et al. Fluorescence-activated cell sorting to reveal the cell origin of radioligand binding. J Cereb Blood Flow Metab. 2020;40:1242–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sebastian Monasor L, Muller SA, Colombo AV, et al. Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. eLife. 2020;:9:e54083. [DOI] [PMC free article] [PubMed] [Google Scholar]