Abstract
Cardiac amyloidosis (CA) is a highly underdiagnosed cause of heart failure. Amyloid light-chain (AL) and amyloid transthyretin (ATTR) cardiomyopathy are two major subtypes of cardiac amyloid. Amyloid fibril deposits cause cardiac dysfunction by mechanically infiltrating the myocardium or by direct cardiotoxicity. Achieving a timely diagnosis is important to initiate disease-modifying therapies and improve the survival of patients with CA. Therefore, physicians must be aware of “red flag symptoms” that increase suspicions for CA when assessing heart failure patients. Although endomyocardial biopsy is a definitive diagnostic tool, with recent advances in non-invasive imaging, non-biopsy diagnosis is feasible in ATTR CA. There have been major advances in treatments for both AL and ATTR CA, and survival of CA has improved. In addition to general management of heart failure, numerous treatment options are increasing for both AL and ATTR CA. Given the systemic nature of amyloids, multi-disciplined team approaches are crucial to management of CA. With recent development of diagnosis and treatment options for both AL and ATTR amyloidosis, it is no longer considered a non-treatable disease.
Keywords: Heart failure, Amyloidosis, Amyloid
INTRODUCTION
Cardiac amyloidosis (CA) is a rare disease but is likely a highly underdiagnosed cause of heart failure.1) According to data collected from Korea National Health Insurance, the age-standardized prevalence of amyloidosis was 0.93 persons per 100,000 persons in 2006 and 1.91 persons per 100,000 persons in 2015.2) Although incidences of amyloidosis have been increasing during past decades, the true prevalence of CA is uncertain. Recent studies suggested that the prevalence of wild-type amyloid transthyretin (wATTR) CA is substantially underestimated in older patients with heart failure, reporting 13% of wATTR incidence in heart failure with preserved ejection patients aged >60 years.3)
Up to 36 proteins are known to cause amyloid fibrils, and most CA is affected by either immunoglobulin amyloid light-chain (AL) or amyloid transthyretin (ATTR). With the introduction of a series of effective therapies for AL amyloidosis and promising new treatments for ATTR amyloidosis, awareness among cardiologists needs to be raised because these diagnoses are often delayed due to the various non-specific heart failure symptoms.
All types of CA share a common pathologic mechanism of extracellular deposition of amyloid in the heart, which results in restrictive cardiomyopathy. However, the pathophysiology, clinical presentation, and prognosis of AL and ATTR amyloidosis are different.
PATHOGENESIS OF CA
AL amyloidosis
AL amyloidosis is a hematologic disorder of plasma cells, mainly monoclonal gammopathy or multiple myeloma (10%), and (rarely) B cell lymphoma. It is caused by the proliferation of an abnormal clone of plasma cells as a monoclonal population of plasma-cell immunoglobulin light chains, which form beta-pleated sheets, ultimately polymerizing into amyloid fibrils.
In addition to the effects of infiltration of the myocardium, light chain cardiotoxicity was suggested from observation of clinical improvement after successful hematologic improvement as evidenced by lack of changes in echocardiographic parameters.4) Recent studies elucidated the mechanisms of light chain toxicity in cellular levels. Light chains directly damage cardiomyocytes by impairing the lysosomal function, which results in defective autophagy, increased reactive oxygen species, and eventually cellular death.5),6) Direct cardiac toxicity of light chains can be observed from more accelerated cardiac deteriorations and higher levels of cardiac biomarkers in AL CA compared to ATTR CA.
ATTR amyloidosis
Transthyretin (TTR) is a transport protein of thyroxine and retinol-binding protein/vitamin A complex and is mostly found in serum and cerebrospinal fluid.7) TTR is secreted predominantly by the liver (95%); a smaller portion (<5%) is produced by the choroid plexus and retinal pigment epithelium.8) TTR is composed of 4 beta-sheet-rich proteins. When TTR is destabilized from the tetramer by proteolytic cleavage into unstable monomers, misfolding of monomers results in aggregates of amyloid fibrils.9) The TTR gene is found on chromosome 18, and more than 120 single mutations in 127 amino acid sequences have been identified. TTR mutations in hereditary amyloid transthyretin (hATTR) increase the TTR amyloidogenic propensity by reducing tetramer stability or increasing the instability of monomers.10),11),12) The hATTR has autosomal-dominant patterns of inheritance, but penetrance is highly variable according to mutation. The variability of TTR mutations differs according to geographic and/or ethnic groups. In Korea, the aspartic acid to alanine substitution at position 38 (Asp38Ala) mutation is currently the most common.13) Among the variants of TTR predominantly involving the heart, the valine to isoleucine substitution at position 122 (V122I) is the most commonly detected mutation.14) In wATTR amyloidosis, the genes of TTR are normal, and age appears to be involved in the instability of TTR; however, the mechanisms are not yet clear. In contrast to AL CA, the mechanism of cardiac dysfunction is predominantly infiltration of extracellular amyloid deposits within the myocardium, leading to increased cardiac and vascular stiffness. There are some reports suggesting that pre-fibrillar protein aggregates exhibit proteotoxicity in ATTR.15)
Clinical features of CA
CA presents with symptoms of biventricular heart failure, arrhythmia, or sudden death. The symptom of dyspnea is due to left ventricular diastolic dysfunction and increased atrial stiffness, as reflected by prominent v waves in the pulmonary wedge pressure tracing in the absence of mitral regurgitation. Atrial involvement also causes thrombus formation, even in patients with sinus rhythm, which may cause systemic embolism. Peripheral edema, ascites, hepatomegaly, and raised jugular venous pressure are often observed. Some patients present with exertional syncope or pre-syncope likely due to low stroke volume, which is associated with poor prognosis.16) Microvascular angina due to perivascular infiltration is also frequently observed. Symptoms from involvement of other organs from patients in the Samsung Medical Center amyloid registry are described in Table 1 (unpublished data).
Table 1. Clinical presentation and involvement of organs according to subtype of amyloids.
| Organ involvement | All organs except central nervous system | Heart, peripheral and autonomous nerve system, ligaments | |
|---|---|---|---|
| AL amyloidosis (n=324) | hATTR amyloidosis (n=40) | wATTR (n=5) | |
| Heart | 75% | 63% | 100% |
| Kidney | 65% | 0% | - |
| Autonomic/peripheral nerve system | 10% | 56%/52% | 60% |
| Gastrointestinal tract and liver | 15% | 26% | - |
| Connective tissue/soft tissue | 15% | 22%/15% | - |
Unpublished data from the Samsung Medical Center amyloid registry.
AL = amyloid light-chain; hATTR = hereditary amyloid transthyretin; wATTR = wild-type amyloidosis transthyretin.
DIAGNOSTIC ALGORITHM
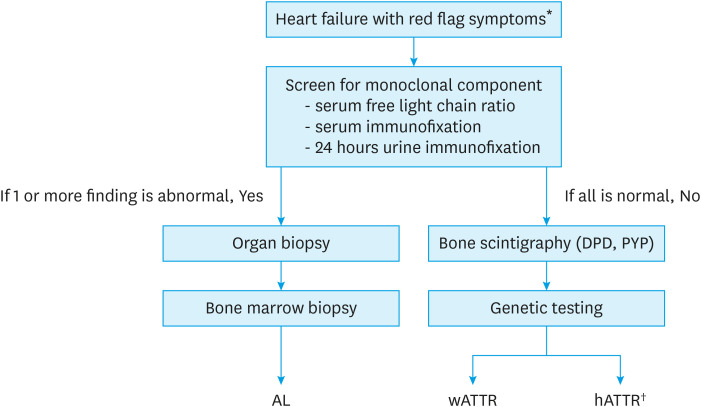
Early signs and symptoms of CA are non-specific and may mimic other diseases. Delayed diagnosis is still an important issue in clinical practice. More than 20% of AL CA patients experience more than two years of delayed diagnosis, and time delay is even longer in patients with ATTR CA.17),18) Table 2 summarizes red-flag signs that should raise suspicions for CA.19),20) The diagnostic algorithm of CA in patients presenting with symptoms of heart failure is summarized in Figure 1.21) In patients with suspected CA, screening for monoclonal gammopathy should be performed and includes serum-free kappa/lambda ratio and serum and urine immunofixation. Screening by electrophoresis is inadequate because 20% of patients with AL amyloidosis have only immunoglobulin light chains, with no intact immunoglobulin protein.22) In addition, both urine and serum must be studied by immunofixation because one-third of patients have negative immunofixation of the serum.23) Up to 40% of patients with ATTR CA can have monoclonal gammopathy of unknown significance, for which scintigraphy alone cannot ensure a proper diagnosis.24)
Table 2. Red flag symptoms for cardiac amyloidosis.
| Cardiac amyloidosis | |
|---|---|
| Echocardiography | - Unexplained increased in wall thickness (>12 mm) with non-dilated LV |
| - Thickening of RV free walls, valves, or interatrial septum | |
| - Small pericardial effusion | |
| - Reduced LV GLS with apical sparing pattern despite pEF | |
| ECG | - Low voltage* or pseudo infarct-pattern |
| Clinical features | - Intolerance to beta blocker, ACEi, or ARB |
| - Low normal blood pressure with previous history of hypertension | |
| Labs | - Mild increase in troponin level on repeated occasions |
| AL cardiac amyloidosis | - ATTR cardiac amyloidosis |
| MGUS | - AV block in presence of increased LV wall thickness |
| - Unexplained conduction block needing pacemaker | |
| Symptoms of HF pEF with nephrotic syndromes | - Autonomic signs and symptoms (orthostatic hypotension, alternating constipation/diarrhea, sweating abnormalities) associated with peripheral neuropathy |
| Macroglossia and periorbital purpura | - Carpal tunnel syndrome, particularly if bilateral |
| Peripheral neuropathy | - Newly diagnosed HCMP in elderly patients |
| - Newly diagnosed low flow, low gradient stenosis in elderly patients |
ACEi = angiotensin-converting-enzyme inhibitor; AL = amyloid light-chain; ARB = angiotensin II receptor blockers; ATTR = amyloid transthyretin; AV = atrioventricular; ECG = electrocardiography; GLS = global longitudinal strain; LV = left ventricle; MGUS = monoclonal gammopathy of undetermined significance; pEF = preserved ejection fraction; RV = right ventricle.
*Only 50% of AL cardiac amyloidosis and 30% of ATTR cardiac amyloidosis cases meet low-voltage ECG criteria of QRS amplitude <5 mm in limb leads or <10 mm in precordial leads.
Figure 1. Diagnostic algorithm of CA in patients with heart failure symptoms.
AL = amyloid light-chain; CA = cardiac amyloidosis; DPD = Tc-99m-3,3-diphosphono-1,2-propanodicarboxylic acid; hATTR = hereditary amyloid transthyretin; TTR = transthyretin; PYP = Tc-99m-pyrophosphate; wATTR = wild-type amyloid transthyretin.
*Refer to Table 2; †Currently, tissue confirmation of TTR amyloid is required in order to prescribe tafamidis in hATTR patients for reimbursement in Korea.
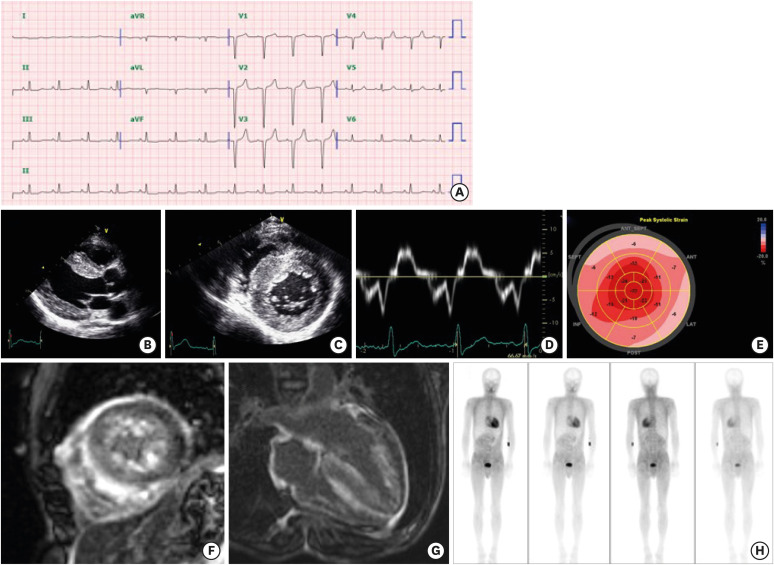
Classic imaging features of ATTR CA are described in Figure 2 and Table 3. Recent advances with nuclear imaging have enabled non-invasive diagnosis of ATTR CA without need for invasive cardiac biopsy. Three technetium-labeled radiotracers, Tc-99m-pyrophosphate (PYP), Tc-99m-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), and Tc-99m-hydroxymethylene diphosphonate (HDMP), are clinically used for identification of ATTR CA. The mechanisms for myocardial retention of radiotracers are not fully understood. From large-scale international studies, the sensitivity of grade 2 (cardiac uptake equal to that of bone) or grade 3 (cardiac uptake exceeding that of bone) tracer uptake for detecting TTR amyloid deposits was >99%, with a specificity of 86% in the absence of monoclonal protein in heart failure patients with suspicious features on echocardiography.25) Clinicians should be aware that about one-third of AL CA patients can show grade I or even higher myocardial retention.26),27)
Figure 2. Typical imaging features of ATTR CA. The patient (47/male) had an Asp58Val mutation with involvement of the heart, gastrointestinal tract, and peripheral nerve. (A) His electrocardiography showed a pseudo-infarct pattern. (B-E) Transthoracic echocardiography showed thickened myocardium (15 mm at septum) and decreased e′ velocity (4.2 cm/s) with an apical strain longitudinal strain pattern. (F and G) From cardiac magnetic resonance imaging, multifocal late gadolinium enhancement with subendocardial ring enhancement was noted. (H) A DPD scan showed grade 3 cardiac uptake with increased radioactive uptake in the gastrointestinal tract as well.
ATTR = amyloid transthyretin; CA = cardiac amyloidosis; DPD = Tc-99m-3,3-diphosphono-1,2-propanodicarboxylic acid.
Table 3. Imaging studies in diagnosis of ATTR CA.
| Advantages/limitations | Limitation | Typical features suggestive of ATTR CA | |
|---|---|---|---|
| Echocardiography | Cost-effective | Typical features may be variably present | Thickened myocardium (>12 mm), small LV cavity sized valve, restrictive diastolic function, thickened interatrial septum, pericardial effusion, and apical sparing regional longitudinal strain patterns |
| Commonly available | |||
| Can identify diastolic function | |||
| Cardiac MRI | Detailed information about cardiac function and tissue characterization | Limited in patients with renal dysfunction | Diffuse subendocardial or transmural late gadolinium enhancement, increased myocardial native T1, increased extracellular volume fraction, suboptimal myocardial nulling |
| Bone scintigraphy | High specificity for diagnosis with grade 2 or 3 uptake | Cannot rule out AL amyloidosis | Grade 2 or 3 tracer uptake in conjunction with no evidence of monoclonal gammopathy by serum/urine testing |
AL = amyloid light-chain; ATTR = amyloid transthyretin; CA = cardiac amyloidosis; LV = left ventricle; MRI = magnetic resonance imaging.
Presence of monoclonal protein is an important differential diagnostic clue for AL and ATTR CA. However, up to 40% of ATTR CA patients have monoclonal gammopathy of unknown significance.24) In such cases, a definitive diagnosis should be made by tissue biopsy from the affected organ. Abdominal fat pad biopsy by needle aspiration is reported to have 70–80% sensitivity for identifying amyloid deposition; however, false negatives and false positives may occur in less experienced centers.28),29),30) In our center, we prefer to perform endomyocardial biopsy because, in addition to tissue confirmation, we can also assess cardiac hemodynamics and coronary physiology.
TREATMENT
Therapy for CA includes optimal treatment of heart failure and specific therapies according to subtype of amyloid. Generally, standard heart failure medications, especially beta blockers, angiotensin-converting-enzyme inhibitors, and angiotensin II receptor blockers, are poorly tolerated. Diuretics are the mainstay of heart failure therapy. Digoxin toxicity could occur even in ranges of therapeutic levels because of altered binding properties.31) Atrial arrhythmia often accompanies heart failure, and anti-arrhythmic agents with negative inotropic or chronotropic effect should be avoided. Catheter ablations may be not be effective, likely due to the multifocal nature of the disease; high recurrence rates have been reported after catheter ablation.32) Sudden cardiac death in CA patients is mostly reported to be from pulseless electrical activity, and there is no evidence for benefit of prophylactic implantable defibrillator use.33) The role of a left ventricular assist device in CA patients with advanced heart failure is limited due to the small size of the cardiac chambers and right ventricular dysfunction. Cardiac transplant could be considered in selected patients with severe CA without significant involvement of other organs.34) For AL CA, patients with refractory heart failure after achieving complete hematologic responses (normalization of free light chain ratio) could benefit from heart transplantation. Some centers consider patients with severe AL CA limited to the heart and intolerant to chemotherapy because of cardiac function to be possible candidates for heart transplant with concomitant high-dose chemotherapy.
Specific treatments for AL and ATTR CA are summarized in Table 4.35) For ATTR amyloidosis, various pharmacological strategies are under development. A recent multi-center, randomized ATTR-ACT trial demonstrated the efficacy of tafamidis in ATTR CA patients. Tafamidis reduced mortality compared with placebo (29.5% vs. 42.9%; hazard ratio, 0.70; 95% confidence interval [CI], 0.51–0.96) and also reduced cardiovascular-related hospitalizations (0.48 vs. 0.70 per year; relative risk, 0.68; 95% CI, 0.56–0.81). Consistent improvements in mortality and hospitalizations were realized regardless of subtype of ATTR (hATTR vs. wATTR); however, patients with New York Heart Association Class III heart failure symptoms were observed to have a higher risk of cardiovascular-related hospitalization, suggesting that earlier treatment with tafamidis is likely to improve the prognosis in ATTR CA patients. The U.S. Food and Drug Administration (FDA) approved tafamidis for ATTR cardiomyopathy in 2019; it has approved patisiran and inotersen only for ATTR neuropathy not for ATTR cardiomyopathy. From a randomized trial investigating the efficacy of patisiran for ATTR polyneuropathy, patisiran significantly reduced N-terminal pro-brain natriuretic peptide level, LV wall thickness, and worsening of longitudinal strain in the ATTR CA subgroup.36)
Table 4. Treatment for AL and ATTR amyloidosis.
| Treatments | Compounds | Drugs | |
|---|---|---|---|
| AL | Anti-plasma cell therapies* | Alkylating agent | Mephalan, cyclophosphamide |
| Proteasome inhibitors | Bortezomib | ||
| Immunomodulator | Lenalidomide | ||
| Anti-CD38 monoclonal antibody | Daratumumab, Isatuximab | ||
| ATTR | TTR silencers | siRNA (Patisiran) | Approved for ATTR polyneuropathy |
| Limited data in ATTR-CA | |||
| ASO (Inotersen) | |||
| TTR stabilizers | Diflunisal | Effective in ATTR polyneuropathy | |
| Limited data in ATTR CA (off-label use) | |||
| Restricted to patients without significant renal dysfunction or thrombocytopenia | |||
| Significant bleeding may occur with concurrent use of anti-coagulant: consider administration with PPI | |||
| Tafamidis | FDA approval for ATTR-polyneuropathy and ATTR CA (both hATTR and wATTR) | ||
| TTR amyloid fibril disruption/extraction | Doxycycline/TUDCA | Being evaluated in a clinical trial | |
| PRX004 | A monoclonal antibody that binds to and potentially removes ATTR deposits (in development) |
This table is modified from Table 2, Cleve Clin J Med 2017;84:12-26.20)
AL = amyloid light-chain; ASO = anti-sense oligonucleotide; ATTR = amyloid transthyretin; CA = cardiac amyloidosis; hATTR = hereditary amyloid transthyretin; PPI = proton pump inhibitor; siRNA = small interfering RNA; TTR = transthyretin; TUDCA = tauro-deoxycholic acid; wATTR = wild-type amyloid transthyretin.
*High-dose chemotherapy with autologous stem cell transplantation is generally reserved for suitable CA cases; consultation from cardiology is important because stem cell collection is often accompanied by fluid retention, and atrial arrhythmia can follow chemotherapy.
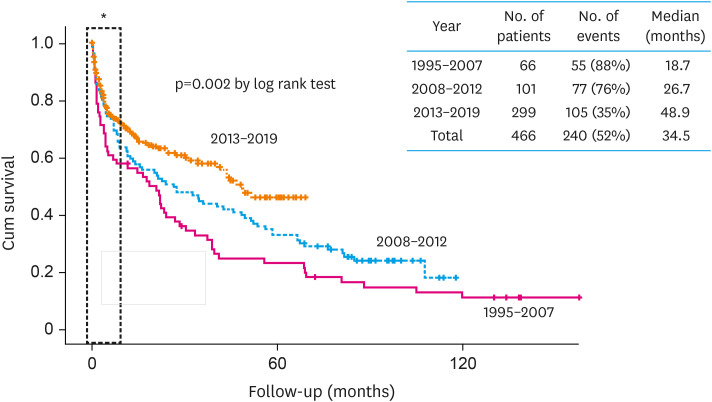
Regardless of amyloid subtype, because amyloid is a systemic disease and involves multiple organs, a multi-disciplinary team approach is important for improved survival of patients. Our center established an amyloid clinic in 2009 involving cardiologists, hematologists, neurologists, pathologists, nuclear medicine physicians, and gastroenterologists. The multi-disciplinary team approach significantly improved the survival of CA patients (Figure 3). Of note, as seen in Figure 3, early mortality reflected by 1-year survival did not change despite the multi-disciplinary team approach. This is because early mortality can be only reduced by early diagnosis. Therefore, the role of cardiologists in early diagnosis is extremely important to improve early morality even in a multi-disciplinary team setting.
Figure 3. Multi-disciplinary team approach and prognosis of CA patients.
Unpublished data from Samsung Medical Center amyloid registry (1995–2019). Presented as abstract at Heart Failure Seoul 2019.
CA = cardiac amyloidosis.
*Of note, early mortality (one-year mortality) is not affected by multi-disciplinary team approach.
CONCLUSIONS
CA affects a considerable proportion of patients presenting with heart failure. With advancements in contemporary imaging techniques, cardiologists now have the means to facilitate early diagnosis of CA and improve the prognosis of patients with CA. Despite major advances in diagnosis and treatments for both AL and ATTR CA, delayed diagnosis from lack of physician awareness is still a critical issue. Establishing a multi-disciplinary team-based amyloid clinic is of tremendous importance in successful diagnosis and treatment of CA patients. Hopefully, this review will contribute to raise awareness of physicians and increase the recognition that CA is no longer a non-treatable disease; it is now possibly becoming a curable condition.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim D, Kim SJ, Jeon ES.
- Writing - original draft: Jeon ES.
- Writing - review & editing: Kim D, Choi JO, Kim K, Kim SJ, Jeon ES.
References
- 1.Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135:1357–1377. doi: 10.1161/CIRCULATIONAHA.116.024438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo SR, Jang SY, Lee GY, et al. Prevalence of amyloidosis in Korea. Orphanet J Rare Dis. 2017;12:152. doi: 10.1186/s13023-017-0705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennani Smires Y, Victor G, Ribes D, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging. 2016;32:1403–1413. doi: 10.1007/s10554-016-0915-z. [DOI] [PubMed] [Google Scholar]
- 4.Dubrey S, Mendes L, Skinner M, Falk RH. Resolution of heart failure in patients with AL amyloidosis. Ann Intern Med. 1996;125:481–484. doi: 10.7326/0003-4819-125-6-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Mishra S, Guan J, Plovie E, et al. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am J Physiol Heart Circ Physiol. 2013;305:H95–103. doi: 10.1152/ajpheart.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner DA, Jain M, Pimentel DR, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 7.Raz A, Goodman DS. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem. 1969;244:3230–3237. [PubMed] [Google Scholar]
- 8.Kelly JW, Colon W, Lai Z, et al. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem. 1997;50:161–181. doi: 10.1016/s0065-3233(08)60321-6. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Buxbaum JN, Kelly JW. The V122I cardiomyopathy variant of transthyretin increases the velocity of rate-limiting tetramer dissociation, resulting in accelerated amyloidosis. Proc Natl Acad Sci U S A. 2001;98:14943–14948. doi: 10.1073/pnas.261419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekijima Y, Wiseman RL, Matteson J, et al. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121:73–85. doi: 10.1016/j.cell.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 11.Hammarström P, Jiang X, Hurshman AR, Powers ET, Kelly JW. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A. 2002;99 Suppl 4:16427–16432. doi: 10.1073/pnas.202495199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimtchuk ES, Prokaeva T, Frame NM, et al. Unusual duplication mutation in a surface loop of human transthyretin leads to an aggressive drug-resistant amyloid disease. Proc Natl Acad Sci U S A. 2018;115:E6428–36. doi: 10.1073/pnas.1802977115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi K, Seok JM, Kim BJ, et al. Characteristics of South Korean patients with hereditary transthyretin amyloidosis. J Clin Neurol. 2018;14:537–541. doi: 10.3988/jcn.2018.14.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 15.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamarthi B, Dubrey SW, Cha K, Skinner M, Falk RH. Features and prognosis of exertional syncope in light-chain associated AL cardiac amyloidosis. Am J Cardiol. 1997;80:1242–1245. doi: 10.1016/s0002-9149(97)00653-x. [DOI] [PubMed] [Google Scholar]
- 17.Vergaro G, Aimo A, Barison A, et al. Keys to early diagnosis of cardiac amyloidosis: red flags from clinical, laboratory and imaging findings. Eur J Prev Cardiol. 2019 doi: 10.1177/2047487319877708. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140:16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 19.Witteles RM, Bokhari S, Damy T, et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Heart Fail. 2019;7:709–716. doi: 10.1016/j.jchf.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly JP, Hanna M. Cardiac amyloidosis: An update on diagnosis and treatment. Cleve Clin J Med. 2017;84:12–26. doi: 10.3949/ccjm.84.s3.02. [DOI] [PubMed] [Google Scholar]
- 21.Ihne S, Morbach C, Obici L, Palladini G, Störk S. Amyloidosis in heart failure. Curr Heart Fail Rep. 2019;16:285–303. doi: 10.1007/s11897-019-00446-x. [DOI] [PubMed] [Google Scholar]
- 22.Gertz MA. Diagnosing primary amyloidosis. Mayo Clin Proc. 2002;77:1278–1279. doi: 10.4065/77.12.1278. [DOI] [PubMed] [Google Scholar]
- 23.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 24.Phull P, Sanchorawala V, Connors LH, et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR) Amyloid. 2018;25:62–67. doi: 10.1080/13506129.2018.1436048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 26.Kircher M, Ihne S, Brumberg J, et al. Detection of cardiac amyloidosis with 18F-florbetaben-PET/CT in comparison to echocardiography, cardiac MRI and DPD-scintigraphy. Eur J Nucl Med Mol Imaging. 2019;46:1407–1416. doi: 10.1007/s00259-019-04290-y. [DOI] [PubMed] [Google Scholar]
- 27.Quarta CC, Obici L, Guidalotti PL, et al. High 99mTc-DPD myocardial uptake in a patient with apolipoprotein AI-related amyloidotic cardiomyopathy. Amyloid. 2013;20:48–51. doi: 10.3109/13506129.2012.746938. [DOI] [PubMed] [Google Scholar]
- 28.Skinner M, Anderson J, Simms R, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine versus colchicine only. Am J Med. 1996;100:290–298. doi: 10.1016/s0002-9343(97)89487-9. [DOI] [PubMed] [Google Scholar]
- 29.Palladini G, Perfetti V, Obici L, et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–2938. doi: 10.1182/blood-2003-08-2788. [DOI] [PubMed] [Google Scholar]
- 30.Quarta CC, Gonzalez-Lopez E, Gilbertson JA, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38:1905–1908. doi: 10.1093/eurheartj/ehx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–1288. doi: 10.1161/01.cir.63.6.1285. [DOI] [PubMed] [Google Scholar]
- 32.Barbhaiya CR, Kumar S, Baldinger SH, et al. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm. 2016;13:383–390. doi: 10.1016/j.hrthm.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Kristen AV, Dengler TJ, Hegenbart U, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5:235–240. doi: 10.1016/j.hrthm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Varr BC, Liedtke M, Arai S, Lafayette RA, Schrier SL, Witteles RM. Heart transplantation and cardiac amyloidosis: approach to screening and novel management strategies. J Heart Lung Transplant. 2012;31:325–331. doi: 10.1016/j.healun.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Kastritis E, Dimopoulos MA. Recent advances in the management of AL Amyloidosis. Br J Haematol. 2016;172:170–186. doi: 10.1111/bjh.13805. [DOI] [PubMed] [Google Scholar]
- 36.Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]