Abstract
Whereas traditional understanding of left ventricular afterload was focused on a steady-state circulation model with continuous pressures and flow, a more realistic concept is emerging, taking the pulsatile nature of the heart and the arterial system into account. The most simple measure of pulsatility is brachial pulse pressure, representing the pulsatility fluctuating around the mean blood pressure level. Brachial pulse pressure is widely available, fundamentally associated with the development and treatment of heart failure (HF), but its analysis is often confounded in patients with established HF. The next step of analysis consists of arterial stiffness, central (rather than brachial) pressures, and of wave reflections. The latter are closely related to left ventricular late systolic afterload, ventricular remodeling, diastolic dysfunction, exercise capacity, and, in the long term, the risk of new-onset HF. Wave reflection may also evolve as a suitable therapeutic target for HF with preserved and reduced ejection fraction. A full understanding of ventricular-arterial coupling, however, requires dedicated analysis of time-resolved pressure and flow signals. This review provides a summary of current understanding of pulsatile hemodynamics in HF.
Keywords: Hemodynamics, Pulse pressure, Heart failure
INTRODUCTION
Heart failure (HF) is an increasing health problem globally and regionally,1) with high rates of hospitalization and mortality.2) An increased brachial systolic blood pressure (bSBP) and brachial diastolic blood pressure (bDBP), starting at levels as low as 115 and 75 mm Hg, respectively, predict incident HF across all adult age groups.3) Arterial hypertension is an important predictor of both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF).4) 75% of patients hospitalized for decompensated HF have a history of hypertension.5) Consequently, reduction of incident HF was the most pronounced benefit of intensive BP lowering in the Systolic Blood Pressure Intervention Trial (SPRINT).6) In a recent meta-analysis, antihypertensive drug treatment in middle-aged patients7) and in elderly patients with isolated systolic hypertension8) lowered the risk for HF by almost 50%, in elderly patients with a history of myocardial infarction by 80%.
bSBP and bDBP, introduced over a century ago by Riva-Rocci et al.,9) are among the most widely performed measurements in clinical medicine. Due to the pulsatile nature of the pump (i.e., the heart), BP is a curve rather than 2 extremes (systolic BP [SBP], diastolic BP [DBP]), with a certain amount of pressure (pulse pressure; PP) fluctuating around a mean value (mean arterial pressure; MAP); the curve contains features that provide insights into arterial function, cardiac function, and their interplay.10) Patients with identical brachial BPs may have substantially different afterload patterns and/or differences in the blood flow generated by the left ventricle (LV). LV afterload (arterial load) cannot be estimated without knowledge of both pressure and flow. Conceptually, arterial load has 2 components: steady (or “resistive”) load, and pulsatile load. “Steady” load (total peripheral resistance [TPR] or systemic vascular resistance [SVR]) is largely determined by the radius of small arteries and arterioles, hence small vessel tone (and density). Together with cardiac output (CO), TPR determines MAP [MAP = CO × TPR]. In contrast, or rather complimentary, pulsatile afterload is influenced by multiple mechanical properties of the aorta, large and also small arteries (aortic stiffness and geometry, timing and magnitude of arterial wave reflections). The aim of this review is to provide a comprehensive overview of the role of the pulsatile component of cardiac afterload, its assessment, prognostic value, and therapeutic consequences.
WHICH MEASUREMENTS ARE AVAILABLE, AND WHAT IS THEIR PHYSIOLOGICAL BACKGROUND?
PP
Brachial PP (bPP) is a simple and widely used pulsatile hemodynamic index. It can be easily calculated as SBP minus DBP and is thus available with every BP measurement. It results from left ventricular ejection, interacting with the arterial tree. Therefore, the main determinants are cardiac (stroke volume [SV], forward flow) as well as arterial (aortic characteristic impedance, aortic and large artery stiffness, aortic and large artery size, wave reflections). Among the latter group, wave reflection seems to be prominent.11) When LV function is preserved and significant aortic valve disease (particularly aortic regirgitation) is absent, a high PP is a marker of increased pulsatile afterload. In contrast, in HFrEF, PP is directly related to measures of LV function, such as ejection fraction (EF), SV, CO, left ventricular dp/dt, and LV longitudinal axis shortening.12) In other words, in HFrEF, a lower PP is often a consequence of a worse LV function. This needs to be considered, when the prognostic value of PP is investigated. Several epidemiological studies have investigated the prognostic role of bPP, most of them demonstrating that a high bPP is associated with a poor prognosis. According to European Guidelines on Hypertension,13),14) a bPP value ≥60 mmHg in elderly individuals reflects asymptomatic damage (stiffening) of the large arteries.
As the BP wave travels from central aorta to peripheral sites (e.g. brachial artery), MAP drops only by 2 mm Hg, and DBP is roughly equivalent, whereas SBP and PP can increase markedly10) (SBP and PP amplification [PPA]), more pronounced in young individuals. The PPA15) ratio (peripheral PP/central PP [cPP]) is determined by many factors, including LV contractility and ejection duration, heart rate, arterial stiffness, arterial caliber (and taper), the timing and amplitude of wave reflections, and arteriolar tone (TPR). cPP is different from bPP (cPP is lower than bPP) and cannot be calculated directly from bPP. One needs dedicated instruments that record pressure waveforms at the carotid artery or in more peripheral locations. In the absence of obstructive atherosclerotic carotid disease, the carotid pressure waveform is considered a reasonable “direct” surrogate of the aortic pressure waveform, whereas more peripheral waveform recordings (brachial or radial) require mathematical algorithms, mainly so-called transfer functions, to “rebuild” the aortic pressure waveform. In a first step, the obtained peripheral pressure waveform is calibrated with brachial cuff BP. There are basically 2 options for calibrating the waveforms (bSBP and DBP versus MAP and DBP). Although the first method (SBP/DBP) is more popular, the second method (MAP/DBP) seems to be more accurate, particularly when MAP is directly determined by oscillometry.16),17) Further technical details are beyond the scope of this manuscript and can be found in dedicated reviews.18)
Wave reflections in the arterial tree
Left ventricular ejection generates a forward-traveling wave (incident or forward wave). The wave travels at a given speed (pulse wave velocity [PWV], up to 5–15 m/sec in humans) along the wall of the aorta and more distal conduit arteries, and is partially reflected at sites of impedance mismatch (branching points, lumen diameter tapering, change in local stiffness).19) Reflected pressure waves add to forward pressure, whereas reflected flow subtracts from forward flow.19) Myriads of reflections from all over the body are transmitted back towards the heart, summarizing in the ascending aorta as one “net” reflected wave. In young adults, aortic PWV is low, and the bulk of reflected waves arrive at the aortic root during diastole. With advancing age, PWV increases, and reflected waves arrive at the heart during mid-to-late systole.20),21) Under these conditions, wave reflections exert important unfavorable effects,10) including: (i) an increase in mid-to-late systolic load (relative to early systolic load); (ii) an increase in aortic SBP, although the degree of pressure augmentation vs. flow reduction depends on LV function; (iii) a decrease in DBP, including the area under the pressure waveform (pressure-time integral) in diastole, which is a key determinant of coronary blood flow.22) Importantly, reflected waves also re-reflect at the heart, contributing to an increase in the amplitude of the forward pressure wave, above and beyond the influence of the aortic root load and flow requirements.23) Wave reflections can be quantified in humans through several methods (Figure 1).24),25),26)
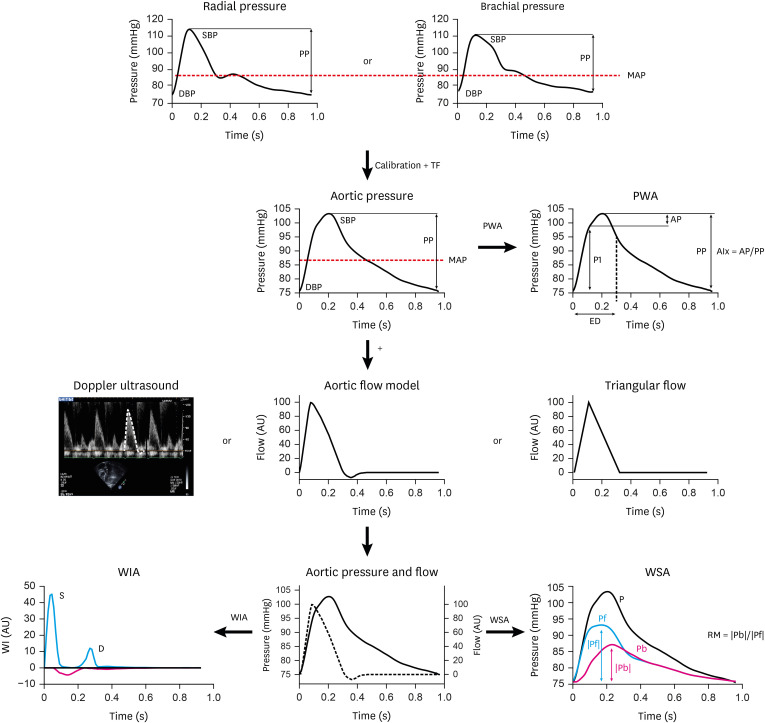
Figure 1. Assessment of pulsatile hemodynamics - overview. Top line: Recording of signal-averaged radial or brachial pressure waveforms with tonometry or brachial cuff. Second line: Following calibration with brachial pressures, aortic waveforms are calculated with a TF. Pulse waveform analysis, based on pressure signals alone, yields measures of the first (P1) and second (P2) systolic peaks for computation of augmented pressure and augmentation index (AP, AIx). Third line: Flow waveforms are obtained, either with Doppler recording of LV outflow (which equals aortic inflow), or as model-deriven flow or triangular flow as a proxy. Bottom line: Combined and time-aligned analysis of a pressure-flow pair is used for wave separation analysis, wave intensity analysis, and other analytical approaches.
Modified from Parragh et al.26) and Hametner et al.,24) and reprinted with permission from Weber and Chirinos.25)
AIx = augmentation index; AP = augmented pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; Pb = amplitude backward wave; Pf = amplitude forward wave; PP = pulse pressure; PWA = pulse waveform analysis; RM = reflection magnitude; SBP = systolic blood pressure; TF = transfer function; WIA = wave intensity analysis; WSA = wave separation analysis.
Pulse waveform analysis (PWA): With this method, information about wave reflections is derived from analysis of the pressure waveform. The rationale behind is that analysis of the entire waveform might unravel information about the arterial system, which is additive to conventional BP (systolic and diastolic are the extreme points of the pressure curve). The principle of PWA is the following: the reflected wave causes a visible notch (inflection point) on the pressure curve, which occurs at the time of maximum flow.19) Augmented pressure (AP), expressed in mmHg, is the difference between the second and first systolic peak on the pressure waveform. Augmentation index (AIx) is the ratio between AP and (central) PP (AIx=AP/cPP), typically expressed as a percentage. Thus, AP and AIx can be seen as the effect of wave reflection on the aortic pressure waveform. Both AIx and AP are higher with increasing age, lower heart rate (a relatively longer systolic period enables reflected waves to exert greater pressure augmentation during systole), smaller body height (shorter travel distance), female sex, and are lower following food ingestion and exercise.27) PWA-derived indexes are dependent not only on the magnitude, but also on the timing of wave reflection. To overcome this potential limitation and focus on the amount of wave reflection only, wave separation analysis (WSA) can be used, which requires simultaneously acquired pressure and flow waves at the same location to separate the pressure wave into its forward (Pf) and backward (Pb) components.28) As flow curves are much more laborious to aquire, compared to pressure curves, a validated simplification29) uses aortic flow curves, which are estimated from pressure curves, based on 3 element Windkessel models and complex mathematical modeling. Reflection magnitude (RM) is the ratio of amplitudes of Pb/Pf. A more recent development is wave intensity analysis (WIA),30) in which BP and flow velocity measured at the same arterial site are considered and a separation into forward and backward travelling wavefronts can be achieved. Waves can originate either from the proximal (forward-traveling) or distal (backward-traveling) end of the circulation and can be either a compression (“pushing”) or decompression (“sucking”) wave. A compression wave will accelerate or decelerate blood flow depending on its origin: if it arises proximal to the site of measurement, it will increase pressure and accelerate flow, but compression waves of distal origin will increase pressure and decelerate blood flow.31) WIA is a useful approach that complements WSA, but it overemphasizes high-frequency components of the pulse (i.e., rapid changes in pressure and flow waves), and thus tends to under-represent reflected waves (which are rich in low-frequency content). A key advantage of WIA may be related to the study of cardiac-derived compression and suction waves (rather than wave reflection per se): the early systolic S-compression wave peak is related to the maximum derivative of left ventricular pressure increase in early systole, while the D-late systolic forward traveling suction wave peak is related to the time constant of pressure decay in late systole/early diastole.32) Both may, therefore, provide insights into ventricular function24) and LV-arterial coupling.
PWV, characteristic impedance, total arterial compliance (TAC)
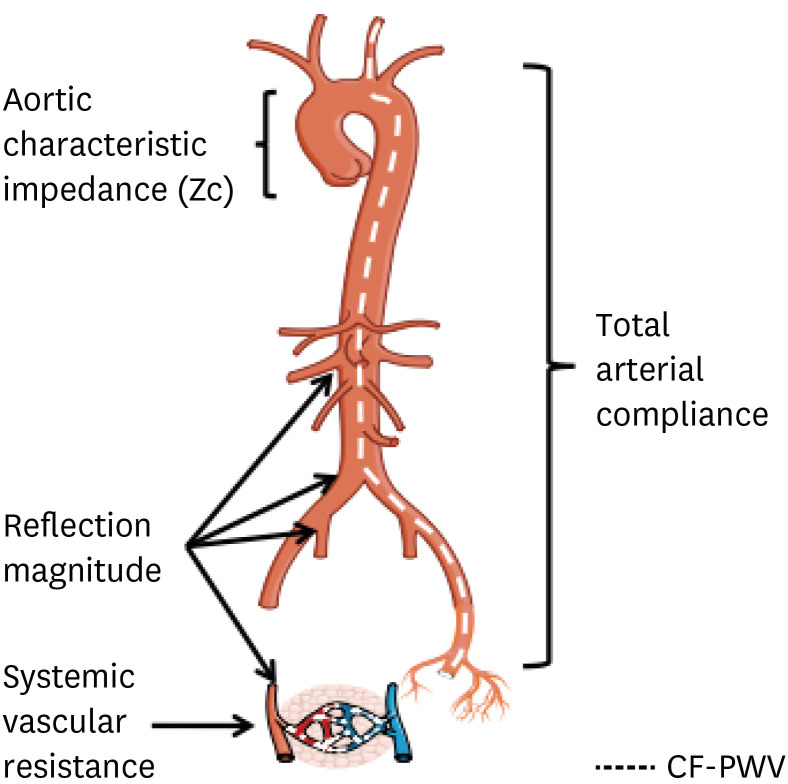
PWV is the travel distance divided by transit time of the pulse between two recording sites. Whereas mainly pressure waves are recorded for PWV measurement with tonometric probes or piezoelectric sensors,10) also volume waves, recorded with impedance cardiography, can be used.33) Carotid-femoral PWV (cfPWV) is currently considered the gold-standard metric of (regional) aortic stiffness,34) and is more popular in Europe. In Asian countries, brachial-ankle PWV (baPWV),35) recorded with cuffs at the arms and legs, is more common. PWV is not a direct measure of ventricular afterload, but is informative of arterial wall properties, and has far-reaching prognostic implications.10),34),36) Proximal aortic impedance (Zc) is the slope of the pressure-flow relation in the absence of wave reflections, and represents the pulsatile load imposed by the proximal aorta. It is highly dependent on proximal aortic size and also dependent on its stiffness. TAC represents the lumped compliance provided by the arterial tree. In the systemic circulation, it is largely determined by conduit arteries (including the aorta and more distal muscular conduit arteries). Arterial functional parameters used to describe pulsatile hemodynamics and arterial stiffness are summarized in Table 1. The anatomic conceptualization of the various measurements of LV afterload25) is depicted in Figure 2.
Table 1. Arterial functional parameters used to describe pulsatile hemodynamics and arterial stiffness.
| Measure | Advantage | Disadvantage | Measurement technique | Prognostic value for heart failure |
|---|---|---|---|---|
| Brachial PP | Simple | Depends on cardiac and arterial function | Brachial cuff | +++ (direct or inverse) |
| Central PP | The PP “seen” by the heart and central organs | Depends on cardiac and arterial function | Radial/brachial waveforms (tonometry or cuff), calibrated with brachial BP, and TF → central pressure waveform | + |
| PWA: AIx, AP | Physiological rationale | Depends on cardiac and arterial function, heart rate, sex, height, etc. | Automated analysis of the central pressure waveform | + |
| WSA: Pb, RM | Physiological rationale | Needs pressure and flow waveforms | Automated analysis of the central pressure and flow waveform | ++ |
| WIA | Physiological rationale | Needs pressure and flow waveforms; sensitive to waveform quality | Automated analysis of the central pressure and flow waveform | − |
| cfPWV | Relatively robust measurement | Depends on actual BP; determination of travel distance on body surface only an estimate | Tonometry, piezo-electronic sensors, cuffs | ++ |
| baPWV | Relatively robust measurement | Depends on actual BP; determination of travel distance is “virtual” | Cuffs | + |
AIx = augmentation Index; AP = augmented pressure; baPWV = brachial-ankle pulse wave velocity; BP = blood pressure; cfPWV = carotid-femoral pulse wave velocity; Pb = amplitude backward wave; PP = pulse pressure; PWA = pulse waveform analysis; RM = reflection magnitude; TF = transfer function; WIA = wave intensity analysis; WSA = wave separation analysis.
Figure 2. Anatomic origin of arterial properties that impact LV afterload. Although arterial load results from complex interaction between various arterial segments, in general, specific loading patterns can be attributed to anatomic sites. cfPWV, a measure of large artery wall stiffness, is also shown, although this is not a measure of LV load per se.
Modified from Chirinos and Segers.40) and reprinted with permission from Weber and Chirinos.25)
cfPWV = carotid-femoral pulse wave velocity; LV = left ventricle.
THE RELATIONSHIP BETWEEN CARDIAC AND ARTERIAL FUNCTION (“VENTRICULO-ARTERIAL (VA) COUPLING”)
Traditionally, the invasive assessment of pressure and volume in the LV has been used to study VA coupling.37) By connecting all end-systolic points of pressure-volume loops obtained during various loading conditions, the so-called ‘end-systolic pressure volume relation’ (ESPVR) line is retrieved. This relation is roughly linear within physiologic ranges, sensitive to inotropic changes, and insensitive to afterload, and the respective line slope has been termed as end-systolic elastance (Ees).38) The intersection between the ESPVR (upper left-hand corner of the P-V loop) and a line drawn from the end-diastolic volume on the horizontal axis identifies a second line. The respective slope represents the end-systolic pressure to SV ratio, named effective arterial elastance (Ea). Stroke work generation is maximal when the Ea/Ees ratio equals 1, while maximal cardiac efficiency is achieved when the Ea/Ees ratio equals 0.5.39)
However, arterial load is time-varying (i.e. not stable across the cardiac cycle), complex and cannot be expressed as a single number.19),25),40) Ea is not a true elastance (i.e., the inverse of a compliance) and is almost exclusively dependent on TPR and heart rate,41),42) but demonstrates weak, inconsistent and in some cases, erratic/paradoxical relationships with gold-standard measures of pulsatile load.41) Importantly, Ea is not related to aortic wall stiffness41); therefore, an increase in Ea should not be interpreted as arterial “stiffening” and, by extension, and parallel increase in Ea and Ees should not be interpreted as a state of “ventricular-arterial stiffening”.25) As a consequence, Ea did not predict incident HF in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort,43) whereas wave reflections (RM) and late systolic load were strong predictors.44),45)
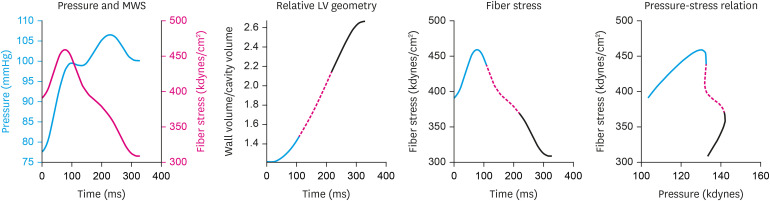
A more comprehensive approach to investigate VA coupling takes time-varying changes (i.e., the changes that occur during the cardiac cycle) of LV and arterial function and their interplay into account (Figure 3). LV afterload can be defined as the hydraulic load imposed by the systemic circulation (i.e., relationship between pressure and flow as discussed above), whereas myocardial afterload is best defined as the myocardial wall stress (MWS) required to generate fiber shortening.25) The time-varying LV geometry during ejection is dependent on: (1) LV volume at the beginning of LV contraction (i.e., end-diastolic volume), which in turn is determined by chronic LV remodeling and preload; (2) the interaction between myocardial contraction, LV geometry and arterial load throughout ejection.25) In accordance with Laplace's law of the heart, MWS is lower for any given LV pressure, as the ratio of LV chamber volume to LV wall volume decreases. This is true throughout the whole ejection period. Among normotensive and hypertensive adults with a normal LVEF, peak MWS typically occurs in early systole, when quasi-diastolic geometry coexists with systolic pressure.46),47),48). This is followed by a marked change in the relationship between LV pressure and MWS during mid-systole, which determines a lower MWS for any given LV (and aortic) pressure.47) This phenomenon appears ideal to protect cardiomyocytes against excessive load in mid-to-late systole,47),49) and depends on the dynamic reduction of LV chamber size relative to wall volume, and its magnitude is highly variable between individuals.47) Subjects with lower EF,47) concentric remodeling,47) or those who demonstrate poor early systolic contraction (and ejection)50) demonstrate less pronounced shifts in the pressure-stress relation and are, thus, more vulnerable to increases in late systolic load.
Figure 3. Time-resolved MWS. The first panel shows the ejection-phase aortic pressure and MWS profiles. The second panel shows the time-resolved relative myocardial geometry (ratio of wall volume to cavity volume) that correlates with wall stress via the Laplace law; the first, second and last thirds of systole are shown in blue, dotted red and black lines, respectively. The third panel shows the ejection-phase MWS, and the 4th panel shows pressure-MWS relation. It can be seen that MWS peaks in early systole and subsequently decreases, even in the context of increasing pressure. This is due to a mid-systolic shift in the pressure-stress relation, which favors lower MWS for any given pressure. This shift is due to the geometric reconfiguration of the LV (decreased cavity volume relative to LV wall volume), and is impaired in the presence of reductions in LV ejection fraction, concentric geometric remodeling, and reduced early systolic ejection (reduced early-phase ejection fraction).
Reprinted with permission from Weber and Chirinos.25)
LV = left ventricle; MWS = myocardial wall stress.
With respect to arterial function, an increase in late systolic load apparently has the worst impact on cardiac function.51) Epidemiologically, HFpEF is most prevalent among elderly women, most of them have hypertension, diabetes, or both, and often coronary artery disease. These comorbidities exactly resemble conditions with increased arterial stiffness/wave reflections.51) Functionally, an increase in late systolic pressure leads to an impairment of diastolic function in experimental animals52) and in humans.53),54) In detail, in a study comprising 336 patients undergoing invasive coronary angiography,53) early diastolic velocity (E′), as assessed by tissue Doppler echocardiography (TDE), showed a strong, negative correlation with AP, PWV, and Zc. Higher filling pressures of the LV were associated with increased wave reflections (AIx, AP) and arterial stiffness (PWV, Zc).
To summarize the chapter, there is an important interaction between myocardial geometry, the myocardial contraction pattern, and the effect of wave reflections on LV hydraulic load. Wave reflections tend to increase mid-to-late systolic LV load and MWS,46),47) but the time course of LV contraction impacts the degree to which cardiomyocytes are “exposed” to the ill effects of wave reflections in mid-to-late systole (a period in which there appears to be particular vulnerability to the deleterious effects of increased afterload).49),52),55),56),57) In other words, mid-to-late systolic load on the LV, imposed by increased wave reflections, is deleterious and may lead to HF,45) particularly in a susceptible LV with delayed early systolic contraction, which can be observed in hypertensive patients with left ventricular hypertrophy (LVH) and/or diastolic dysfunction.50)
Differences in the effects of arterial load in HFpEF and HFrEF
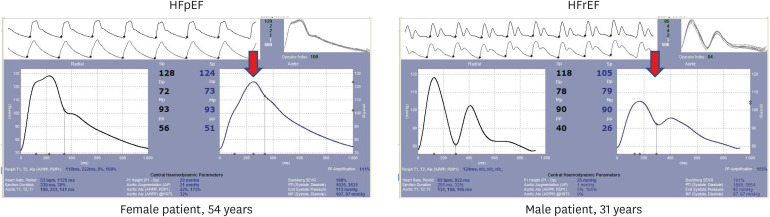
When LV pump function is preserved, the reflected wave typically induces a late systolic pressure peak in the pressure waveform, augmenting aortic pressure in mid-to-late systole (Figure 4). These features are prominent in patients with HFpEF,53),58),59),60) and may be useful in the diagnostic workup of the condition58): measures of pulsatile arterial function (including bPP, but favoring central hemodynamics) were as good as TDE in separating patients with HFpEF from those without the condition in a population of patients with exertional dyspnea. When LV pump function is reduced, however, wave reflection may exert more pronounced effects to decrease flow, initially with no apparent alteration in the appearance of the pressure waveform (when the latter is analyzed in isolation). In patients with severe LV systolic dysfunction (LVEF ≤30%), wave reflections truncate flow, reduce SV and induce a shortening of ejection duration.26),61),62) In these patients, PWA-derived measures of wave reflection (AIx, AP) are typically very low (Figure 4).12),63) In addition, forward waves are also altered: in patients with severely reduced EF (mean value 27.8%), WIA derived ratio of first to second systolic peak (S/D ratio) is reduced,64) as compared to individuals with normal EF, and could be used to divide patients with HFrEF from controls with normal EF with an area under the curve of 0.879.24)
Figure 4. Pressure waveforms in HFpEF versus HFrEF. Radial pressure waves are shown as obtained, aortic pressure waves were derived with a valid transfer function. Note the striking differences in waveforms, which cannot be attributed to the small differences in brachial BP (128/72 mm Hg in HFpEF versus 118/78 mm Hg in HFrEF). In HFpEF, a striking late-systolic peak is visible, which is completely missing in HFrEF (arrows). Corresponding aortic AIx, normalized for heart rate 75, is 32% in HFpEF vs. 0% in HFrEF, respectively.
AIx = augmentation index; BP = blood pressure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
WHICH ARE THE CONSEQUENCES OF INCREASED ARTERIAL STIFFNESS AND CENTRAL HEMODYNAMICS?
LV hypertrophy
LVH is a marker of asymptomatic organ damage in hypertension13) and an important intermediate step from hypertension to HF.65) In animal models, an increase in aortic stiffness without any change in TPR leads to LVH.66) Moreover, late systolic loading resulted in much more prominent hypertrophy than early systolic loading in rats.67) Patients with isolated systolic hypertension, a condition characterized by increased aortic stiffness, show higher LV mass than those with systolic-diastolic hypertension. Moreover, LV mass is more strongly related to PP than to MAP, underlining the importance of pulsatile phenomena.68) This relationship is stronger for cPP,69),70) in particular when measured over 24-hours.71) The relationship between LV mass and arterial stiffness/wave reflections can be seen even in adolescents and young adults.72) In a recent analysis from the population-based MESA, including more than 4,000 adults, the contribution of steady state (SVR) and pulsatile hemodynamics (TAC, Pf, Pb) on LV mass and geometry was investigated.73) In multivariable models, SVR, TAC, and Pb were directly, and Pf was inversely associated with LVM, with wave reflection (Pb) demonstrating the strongest relationship, and SVR demonstrating a relatively weak relationship. In a longitudinal study in a family-based population sample, progression to LV concentric remodeling pattern over 4.7 years was independently associated with higher baseline cfPWV.74) Moreover, in women, higher cPP at baseline predicted the longitudinal increase in LV mass. Reductions in LV mass, which have proven prognostic benefit, are more closely associated with reductions in wave reflection than with reductions in brachial BP.75),76) When different drugs (ACE-inhibitor-diuretic combination versus a beta-blocker) were compared, those favourably affecting pulsatile hemodynamics (reducing cSBP and cPP) were superior in reducing LV mass,77) whereas both therapeutic regimens did not differ regarding steady state hemodynamics (CO and SVR).
Impaired exercise capacity
Consistent with the important role of pulsatile arterial load on the myocardium, pulsatile arterial properties have been shown to be associated with exercise capacity, both in the general population and in patients with HF (HFrEF and HFpEF).
An inverse relationship between late systolic pressure augmentation and a reduced aerobic capacity in the general population was described in the Baltimore Longitudinal Study of Aging,78) in healthy men,79) in athletes,80) and in patients undergoing cardiac rehabilitation.81) More recently, Broufa et al.82) demonstrated that pulsatile hemodynamic indices (including Pb and AIx) are independently related to peak oxygen uptake (peak VO2) in adults with exertional dyspnea and preserved LV systolic function.
Studies in HFrEF patients also demonstrate a significant relationship between arterial pulsatile hemodynamics and exercise capacity.83) Specifically, in patients with dilated cardiomyopathy, PWV is inversely and independently associated with peak VO2.84),85) Patients with HFrEF due to idiopathic dilated cardiomyopathy demonstrate an impairment in the reduction in wave reflections during exercise, with a smaller reduction in wave reflections for any given reduction in SVR, compared to normal controls.86) This could increase myocardial work during exercise and contribute to exercise intolerance.
Hundley et al.87) first demonstrated that patients with HFpEF exhibit increased aortic stiffness, which was in turn strongly associated with a lower aerobic capacity. Patients with HFpEF have higher large artery stiffness at rest, which is inversely related to exercise capacity.88) Measurements taken at rest may underestimate the impact of pulsatile hemodynamics.89) Indeed, HFpEF patients also show an abnormal increase of wave reflections,89),90) carotid stiffness,91) aortic PWV91) and an abnormal decrease of TAC89) during exercise. The combination of increased pulsatile load and impaired vasodilatory reserve seems to be associated with impaired SV and CO reserve, and with increased LV filling pressures during exercise.89),90),91)
Risk of incident HF
In the Framingham study and in the East Boston Senior Health project, bPP (and bSBP) were stronger predictors than DBP for congestive HF (CHF) in middle-aged men and women (Table 2).45),92),93),94),95),96),97),98) In 5,690 participants from MESA, wave reflection (RM) was strongly and independently predictive of new-onset HF.45) In particular, RM compared favorably to other risk factors for CHF in various statistical measures of clinical utility, and predicted CHF even in patients with normal BP. Along the same lines of evidence, late systolic hypertension was strongly associated with incident HF.44) In contrast, SVR, TAC and EA did not predict HF, indicating the importance of pulsatile over steady state hemodynamics and of late systolic load.43) In the Framingham Heart study, after adjustments for standard risk factors including MAP, cfPWV was independently associated with incident clinical HF94) after a follow-up of 10.1 years. Moreover, a higher cfPWV was associated with both HFpEF and HFrEF. In 2,602 patients with chronic kidney disease (mean GFR 45 mL/min/1.73m2), after a mean follow up of 3.5 years, cfPWV as well as bSBP, cSBP and PP predicted hospitalized HF, with cfPWV showing the best relationship.95) In another community-based cohort of 2,290 older adults (mean age 74 years),96) cfPWV was associated with overall HF and HFrEF only in unadjusted analysis and, with respect to overall HF, only in partially, but not in fully adjusted models. Finally, in asymptomatic patients at risk for HF, worsening of arterial stiffness (increase in baPWV) within 5 years was associated with increased risk of incident HF.97) In summary, available evidence supports a relationship between arterial stiffness and measures of wave reflection/late systolic load, and the risk of incident HF in the community.
Table 2. Prognostic value of arterial parameters for incident HF.
| Study acronym | Setting/population | First author | Sample size | Mean age years | FU duration years | Arterial measurement | Study endpoint |
|---|---|---|---|---|---|---|---|
| Framingham | General population | Haider et al.92) | 2,024 | 61 | 17.4 | bPP | CHF |
| East Boston Senior health project | General population | Chae et al.93) | 1,621 | 77.9 | 3.8 | bPP | CHF |
| MESA | General population | Chirinos et al.44) | 5,960 | 62 | 7.6 | RM | CHF; hard CVEs |
| CRIC | Chronic Kidney disease (eGFR 45) | Chirinos et al.95) | 2,602 | 59.9 | 3.5 | cfPWV | Hospitalized HF |
| Framingham | General population | Tsao et al.94) | 2,539 | 64 | 10.1 | cfPWV | HF |
| Health ABC | Older adults | Pandey et al.96) | 2,290 | 73.5 | 11.4 | cfPWV | Hospitalized HF* |
| - | Asymptomatic with HF risk factors | Aisu et al.97) | 456 | - | 4.9 | baPWV | Hospitalized HF |
| - | Acute STEMI patients | Feistritzer et al.98) | 160 | 62 | 1.2 | PWV (MRI) | CVE including CHF |
bPP = brachial pulse pressure; baPWV = brachial-ankle pulse wave velocity; cfPWV = carotid-femoral pulse wave velocity; CHF = congestive heart failure; CRIC = Chronic Renal Insufficiency Cohort; CVE = cardiovascular event; FU = follow-up; eGFR = estimated glomerular filtration rate; Health ABC = Health, Aging, and Body Composition; HF = heart failure; MESA = Multi-Ethnic Study of Atherosclerosis; MRI = magnetic resonance imaging; PWV = pulse wave velocity; RM = reflection magnitude; STEMI = ST elevation myocardial infarction.
*Not significant result following multivariable adjustment.
Risk in established HF
Due to the ease of assessment, most of the evidence available is related to bPP (Table 3).99),100),101),102),103),104),105),106),107),108),109),110),111),112),113),114),115),116) In advanced HFrEF, a low bPP is due to poor LV function, and, in term, associated with a worse prognosis.99),100),101),102),103),104),105),106),107),108) In patients with less severe HFrEF, which can be indicated by higher bPP or higher SBP, the relationship may become direct (i.e. a higher bPP being associated with a worse prognosis).109),110),111) In these patients, a higher PP is due to increased pulsatile afterload. In HFpEF, the relationship between bPP and outcomes tends to be direct.103),112) In some studies, however, particularly in acute HFpEF, patients with the lowest bPPs also demonstrate a worse prognosis.106),112) These patients may have pronounced concentric remodeling with lower SVs, despite a preserved EF (which does not prove preserved myocardial contractility in HFpEF).117)
Table 3. Prognostic value of measures of pulsatile hemodynamics in established HF.
| Study | First author | Setting/population | Sample size | Age | EF | Brachial SBP/DBP/PP mmHg | Relationship with outcome | |
|---|---|---|---|---|---|---|---|---|
| Brachial PP | ||||||||
| - | Fagard et al.99) | Advanced chronic HF | 284 | 51.5 | 25.5 | 114/75,739 | Inverse (all-cause mortality) | |
| PRIME | Voors et al.100) | Advanced chronic HF NYHA III and IV | 1,901 | 65 | 26 | 107/73 and 133/77; (above/below median PP) mean PP: 47 | Inverse (all-cause mortality) | |
| VMAC | Aronson and Burger101) | Hospitalized decompensated HF | 489 | 59/60/67 (PP Tertiles) | 21/26/34 (PP Tertiles) | 105/70/34; 119/68/51; 141/65/75 (PP tertiles) | Inverse (all-cause mortality) | |
| HIJC-HF | Kawashiro et al.102) | Hospitalized congestive HF | 3,169 | 69.8 | 42 | NA | Inverse (all-cause mortality increased with PP ≤30 mmHg) | |
| MAGGIC meta-analysis | Jackson et al.103) | HFrEF | 22,038 | 60–69 (PP quintiles) | 27–34 (PP quintiles) | 106–156/75–79 (PP quintiles) mean PP: 52 | Inverse (all-cause mortality) consistent findings in acute and chronic HFrEF | |
| - | Yildiran et al.104) | HF NYHA I–IV | 225 | 56.5 | 25–35 | 98–127/66–83/31–46 | Inverse (CV death) | |
| CAPRICORN | Petrie et al.105) | LV dysfunction (EF ≤40) after acute MI | 1,995 | 63 | 33 | 121/74/47 | Inverse (all-cause mortality) | |
| DIG | Maeder and Kaye106) | Stable HF | 6,792 (HFrEF) | 63 | 29 | 126/75/51 | Inverse (all-cause mortality) | |
| EPHESUS | Regnault et al.107) | 3–14 days after acute MI with LF dysfunction (EF ≤40) and HF | 6,613 | 64 | 33 | 119/72/47 | Inverse (all-cause mortality, CV death, CV death + hosp) | |
| - | Schillaci et al.108) | Outpatients with congestive HF | 8,660 | 64 | <30: 20% | 132/79/53 | Inverse (all-cause mortality, CV mortality) | |
| >30: 41% | ||||||||
| NA: 39% | ||||||||
| SAVE | Mitchell et al.109) | Impaired EF following MI (no overt HF) | 2,231 | 59.5 | 31 | 112–113/70/42–43 | Direct (all-cause mortality; recurrent MI) | |
| SOLVD | Domanski et al.110) | LV dysfunction (EF ≤35); asymptomatic or symptomatic (NYHA 1.7) | 6,781 | 55–64 | 26–28 | 109–144/77–78/32–67 | Direct (all-cause mortality, CV mortality) | |
| DCH cohort | Lip et al.111) | Incident HF | 2,159 | 58.9 | NA | 149/86.5/62.6 | Non-significant direct (stroke) | |
| GWTG-HF | Laskey et al.112) | Hospitalized HFrEF (EF <40) | 40,421 | 80 | 35–53 (PP quartiles) | 139/73 | U-shaped (inverse at PP <50 mmHg, direct at PP ≥50 mmHg) (all-cause mortality) | |
| GWTG-HF | Laskey et al.112) | Hospitalized HFpEF (EF ≥40) | Direct (all cause mortality) | |||||
| DIG | Maeder and Kaye106) | Stable HF | 988 (HFpEF) | 67 | 55 | 138/77/61 | J-shaped (all-cause mortality; HF hospitalization) | |
| MAGGIC metaanalysis | Jackson et al.103) | HFpEF | 5,008 | 65–73 (PP quintiles) | 58–60 (PP quintiles) | 115–172/77–80 (PP quintiles) mean PP: 62 | Direct (only unadjusted analysis) | |
| Inverse (subgroup with acute HFpEF) | ||||||||
| - | Tokitsu et al.116) | Hospitalized HFpEF | 512 | 71.7 | 63 | 134/73/61 | U-shaped (total CV events, HF events) | |
| Wave reflections | ||||||||
| - | Sung et al.113) | Acute heart failure NYHA III and IV | 120 | 72 | 41.2/42.9 | 141/81/60; 149/80/69 | Direct (cPP, Pb, AP) (HF hospitalization, MI, stroke, death); consistent effect in HFrEF and HFpEF | |
| Pulse wave velocity | ||||||||
| EPHESUS | Regnault et al.107) | 3–14 days after acute MI with LF dysfunction (EF ≤40) and HF | 306 | 61 | 34 | 118/74/89 | Direct (cfPWV) (all-cause mortality, CV mortality) | |
| - | Bonapace et al.115) | HF and EF ≤45% | 156 | Direct (aortic PWV) | ||||
| - | Meguro et al.114) | Stable HF NYHA II–III | 72 | 68 | 53 | 129/75/54 | Direct (baPWV) (HF hospitalisation, cardiac death) | |
AP = augmented pressure; baPWV = brachial-ankle pulse wave velocity; CAPRICORN = Carvedilol Post-Infarct Survival Control in LV Dysfunction; cfPWV = carotid-femoral pulse wave velocity; cPP = central pulse pressure; DBP = diastolic blood pressure; DCH = Diet, Cancer and Health; DIG = Digitalis Investigator Group; EF = ejection fraction; EPHESUS = Eplerenone Post–Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; GWTG-HF = Get With The Guidelines-Heart Failure; other abbreviations see Tables 1,2 HIJC-HF = Heart Institute of Japan–Department of Cardiology Heart Failure; LV = left ventricle; MAGGIC = Meta-Analysis Global Group in Chronic Heart Failure; MI = myocardial infarction; Pb = amplitude backward wave; PP = pulse pressure; PRIME = Prospective Randomized study of Ibopamine on Mortality and Efficacy; SAVE = Survival and Ventricular Enlargement; SBP = systolic blood pressure; SOLVD = Studies on Left Ventricular Dysfunction; VMAC = Vasodilation in the Management of Acute Congestive HF.
Given the fact that PP depends on both arterial and LV function, direct measurements of arterial properties are likely to be more informative. One single-center study demonstrated the adverse prognostic value of wave reflections in patients with acute decompensated HF.113) Likewise, cfPWV, a direct measure of arterial stiffness, was directly related to prognosis (HF hospitalization, CV and all-cause mortality) in HFrEF and HFpEF.107),113),114),115)
THERAPEUTIC IMPLICATIONS
HFrEF
In HFrEF, guideline-directed pharmacologic therapy2) may substantially improve arterial properties and wave reflections. However, study results have been somewhat inconsistent so far. Whereas ACE-inhibitors have been shown to improve arterial stiffness (PWV) additive to the effect of BP lowering in hypertensives,118) such data are not available (yet ?) in HF patients. Omapatrilat, a composite ACE-inhibitor/neprilysin inhibitor, has been shown to reduce Zc, as compared to enalapril, in patients with isolated systolic hypertension.119) The successor drug, sacubitril-valsartan, was compared against enalapril in 464 patients with HFrEF (< 40%), but the primary outcome, a change in Zc, was not significantly different between both drugs.120)
A small randomized study of arterial pressure waveform-guided therapy for HFrEF (aimed at reducing AIx) was performed in a total of 50 patients (EF 38–39%, predominantly NYHA II, brachial BP in the lower normal range). The main therapeutic changes in the active group, based on a prespecified algorithm, were the more frequent use of spironolactone, nitrates, and hydralazine, in addition to guideline-based treatment with ACE-inhibitors/angiotensin-receptor-blockers and beta-blockers. The outcome was a greater improvement in peak VO2 in the active group, compared to standard care, while AIx was reduced in both groups without a significant difference.121) Higher measures of pulsatile hemodynamics and wave reflections (cPP, AP, Pf, Pb) at baseline, and their larger decrease during treatment were associated with functional improvement, defined as increase in 6 minute walk test.122) These findings were not apparent from brachial BP. Thus, central aortic waveform analysis may allow an individualized treatment regimen for patients with HFrEF.37)
Another relatively novel drug class which shows promise in the prevention and treatment of HF are sodium glucose cotransporter 2 inhibitors.123) Recently, the effect of dapagliflozin was tested against placebo in 4,744 patients with HFrEF (EF roughly 31%).124) It was observed that the risk of worsening HF or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes. The results were confirmed in a quite similar setting, but somewhat more advanced HF, for empagliflozin.125) In the context of this review article it is of interest, that empagliflozin has been shown to improve pulsatile vascular function (cSBP, cPP, reflected wave amplitude) in a double-blind cross-over study,126) as compared to placebo, suggesting a possible mechanistic explanation for the clinical benefit.
HFpEF
No treatment has yet been shown, convincingly, to reduce morbidity or mortality in patients with HFpEF.2) Although it has been repeatedly shown51),58),127) that increased arterial stiffness and increased pulsatile hemodynamics are hallmarks of HFpEF, results of treatment trials based on the improvement of vascular properties have been mixed so far.
Despite the well-documented acute effect on nitroglycerin and other organic nitrates on wave reflection,128) isosorbide dinitrate, alone or in combination with hydralazine, administered chronically (24 weeks) in a small study did neither reduce wave reflection (RM) nor increase exercise tolerance (6-minute walk test) in patients with HFpEF.129) This may be due to tolerance associated with long-term use.130) Following the same lines, treatment with isosorbide mononitrate did not improve exercise capacity in patients with HFpEF, as compared to placebo.131) In addition to organic nitrates, inorganic nitrates and nitrite have been investigated as treatments for HFpEF as well. These agents take advantage of the endogenous nitrate-nitrite-NO pathway, in which inorganic nitrate (derived from dietary ingestion or from the oxidation of endogenous NO) undergoes a regulated 2-step reduction process to NO (nitrate → nitrite → NO). In addition to the well-known hypoxia/acidosis-dependent microvascular reduction of nitrite to NO (which favors microvascular vasodilation during exercise), a normoxia-dependent reduction pathway that operates in the wall of conduit muscular systemic arteries has recently been described.132) Normoxia-dependent activation accounts for the high selectivity of inorganic nitrate and nitrite for conduit muscular arteries, and the recently described effect of exogenously administered inorganic nitrate/nitrite on arterial wave reflection.59),89),132),133) In a small study in 17 patients with HFpEF, a single dose of NO3− rich beetroot juice reduced late systolic LV load (AIx) and increased exercise capacity.59) Also, acute sodium nitrite infusion improves exercise hemodynamics in HFpEF.134) Accordingly, one week of daily dosing with beetroot juice (6.1 mmol inorganic nitrate) significantly improved submaximal aerobic endurance in elderly HFPEF patients.135) Unlike organic nitrates, these effects were achieved without significantly reducing MAP or cerebrovascular resistance and without increasing pulsatile power penetration into the cerebrovascular circulation.133),136) Inhaled sodium nitrite, on the other hand, has a very short half (<40 minutes) and its intermittent administration results in pronounced circulating nitrite level fluctuations, which are unlikely to exert sustained therapeutic effects throughout the day. Accordingly, inhaled inorganic nitrite was not effective in improving exercise capacity in patients with HFpEF.137)
Regarding large morbidity/mortality trials, current evidence is largely limited to bPP. In 2 recent phase II trials, Aldosterone Receptor Blockade in Diastolic Heart Failure (ALDO-DHF)trial138) and Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) trial139) a substantial decrease in bPP was achieved in the active intervention arm (Spironolactone or Sacubitril-Valsartan, respectively), associated with an improvement in filling pressures or natriuretic peptides. In clinical endpoint trials,140),141),142) baseline bPP has generally been <60 mmHg, the cutoff defined by European Hypertension Guidelines,13) suggesting that enrolled populations exhibited a relative paucity (or already successful treatment at baseline) of pulsatile hemodynamic abnormalities, which are otherwise typical for HFpEF patients.58) Moreover, in most clinical endpoint trials in HFpEF, bPP was not substantially reduced. For instance in one of the the largest studies (Irbesartan in Heart Failure with Preserved Ejection Fraction Study140)), which showed a neutral outcome, bPP was lowered by only 1.7 mm Hg by Irbesartan (and unchanged with placebo).
CONCLUSION
Arterial stiffness and central hemodynamics contributed significantly to our current understanding of HF, both HFrEF and HFpEF. Arterial pulsatile hemodynamics are key to comprehensively describe VA interactions, and have clinically relevant prognostic implications. What is currently missing in the concept of translational medicine are widely available drugs for reduction of pulsatile load and arterial destiffening. This would undoubtedly translate into clinical benefits in patients with HF.
Footnotes
Funding: T.W. received a research grant for a multicenter study from i.e.m., Stolberg, Germany.
Conflict of Interest: The author has no financial conflicts of interest.
References
- 1.Park JJ, Choi DJ. Current status of heart failure: global and Korea. Korean J Intern Med. 2020;35:487–497. doi: 10.3904/kjim.2020.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 3.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Hypertension. 2015;65:1372–1407. doi: 10.1161/HYP.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 5.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–2295. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 8.Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 9.Riva-Rocci S, Zanchetti A, Mancia G. A new sphygmomanometer. Sphygmomanometric technique. J Hypertens. 1996;14:1–12. [PubMed] [Google Scholar]
- 10.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namasivayam M, McDonnell BJ, McEniery CM, O'Rourke MF Anglo-Cardiff Collaborative Trial Study Investigators. Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension. 2009;53:979–985. doi: 10.1161/HYPERTENSIONAHA.108.125179. [DOI] [PubMed] [Google Scholar]
- 12.Parragh S, Hametner B, Bachler M, et al. Determinants and covariates of central pressures and wave reflections in systolic heart failure. Int J Cardiol. 2015;190:308–314. doi: 10.1016/j.ijcard.2015.04.183. [DOI] [PubMed] [Google Scholar]
- 13.Williams B, Mancia G, Spiering W, et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36:2284–2309. doi: 10.1097/HJH.0000000000001961. [DOI] [PubMed] [Google Scholar]
- 14.Weber T, Arbeiter K, Ardelt F, et al. Austrian consensus on high blood pressure 2019. Wien Klin Wochenschr. 2019;131:489–590. doi: 10.1007/s00508-019-01565-0. [DOI] [PubMed] [Google Scholar]
- 15.Avolio AP, Van Bortel LM, Boutouyrie P, et al. Role of pulse pressure amplification in arterial hypertension: experts' opinion and review of the data. Hypertension. 2009;54:375–383. doi: 10.1161/HYPERTENSIONAHA.109.134379. [DOI] [PubMed] [Google Scholar]
- 16.Papaioannou TG, Karageorgopoulou TD, Sergentanis TN, et al. Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J Hypertens. 2016;34:1237–1248. doi: 10.1097/HJH.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 17.Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–832. doi: 10.1161/HYPERTENSIONAHA.111.176313. [DOI] [PubMed] [Google Scholar]
- 18.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719–1725. doi: 10.1093/eurheartj/eht565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols WW, O'Rourke MF, Vlachopoulos C. McDonald's blood flow in arteries. London: Hodder Arnold; 2011. [Google Scholar]
- 20.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 21.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Weber T, Lang I, Zweiker R, et al. Hypertension and coronary artery disease: epidemiology, physiology, effects of treatment, and recommendations : a joint scientific statement from the Austrian Society of Cardiology and the Austrian Society of Hypertension. Wien Klin Wochenschr. 2016;128:467–479. doi: 10.1007/s00508-016-0998-5. [DOI] [PubMed] [Google Scholar]
- 23.Phan TS, Li JK, Segers P, Chirinos JA. Misinterpretation of the determinants of elevated forward wave amplitude inflates the role of the proximal aorta. J Am Heart Assoc. 2016;5:e003069. doi: 10.1161/JAHA.115.003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hametner B, Parragh S, Weber T, Wassertheurer S. Wave intensity of aortic root pressure as diagnostic marker of left ventricular systolic dysfunction. PLoS One. 2017;12:e0179938. doi: 10.1371/journal.pone.0179938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber T, Chirinos JA. Pulsatile arterial haemodynamics in heart failure. Eur Heart J. 2018;39:3847–3854. doi: 10.1093/eurheartj/ehy346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parragh S, Hametner B, Bachler M, Weber T, Eber B, Wassertheurer S. Non-invasive wave reflection quantification in patients with reduced ejection fraction. Physiol Meas. 2015;36:179–190. doi: 10.1088/0967-3334/36/2/179. [DOI] [PubMed] [Google Scholar]
- 27.O'Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–522. doi: 10.1046/j.0306-5251.2001.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 29.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- 30.Parker KH, Jones CJ. Forward and backward running waves in the arteries: analysis using the method of characteristics. J Biomech Eng. 1990;112:322–326. doi: 10.1115/1.2891191. [DOI] [PubMed] [Google Scholar]
- 31.Manisty CH, Zambanini A, Parker KH, et al. Differences in the magnitude of wave reflection account for differential effects of amlodipine- versus atenolol-based regimens on central blood pressure: an Anglo-Scandinavian Cardiac Outcome Trial substudy. Hypertension. 2009;54:724–730. doi: 10.1161/HYPERTENSIONAHA.108.125740. [DOI] [PubMed] [Google Scholar]
- 32.Ohte N, Narita H, Sugawara M, et al. Clinical usefulness of carotid arterial wave intensity in assessing left ventricular systolic and early diastolic performance. Heart Vessels. 2003;18:107–111. doi: 10.1007/s00380-003-0700-5. [DOI] [PubMed] [Google Scholar]
- 33.Skrabal F, Weber T, Skrabal K, et al. Measurement of aortofemoral volume wave velocity during the routine 12-channel ECG: relation to age, physiological hemoglobin A 1C, triglycerides and SBP in healthy individuals. J Hypertens. 2020;38:1989–1999. doi: 10.1097/HJH.0000000000002493. [DOI] [PubMed] [Google Scholar]
- 34.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 35.Kim HL, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Independent association between brachial-ankle pulse wave velocity and global longitudinal strain of left ventricle. Int J Cardiovasc Imaging. 2015;31:1563–1570. doi: 10.1007/s10554-015-0744-5. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikonomidis I, Aboyans V, Blacher J, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail. 2019;21:402–424. doi: 10.1002/ejhf.1436. [DOI] [PubMed] [Google Scholar]
- 38.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974;35:117–126. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 39.Chirinos JA. Ventricular-arterial coupling: invasive and non-invasive assessment. Artery Res. 2013;7:2–14. doi: 10.1016/j.artres.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56:563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 41.Chirinos JA, Rietzschel ER, Shiva-Kumar P, et al. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension. 2014;64:1022–1031. doi: 10.1161/HYPERTENSIONAHA.114.03696. [DOI] [PubMed] [Google Scholar]
- 42.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002;282:H1041–6. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 43.Zamani P, Lilly SM, Segers P, et al. Pulsatile load components, resistive load and incident heart failure: the Multi-Ethnic Study of Atherosclerosis (MESA) J Card Fail. 2016;22:988–995. doi: 10.1016/j.cardfail.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chirinos JA, Segers P, Duprez DA, et al. Late systolic central hypertension as a predictor of incident heart failure: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015;4:e001335. doi: 10.1161/JAHA.114.001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chirinos JA, Kips JG, Jacobs DR, Jr, et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chirinos JA, Segers P, Gillebert TC, et al. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012;60:64–70. doi: 10.1161/HYPERTENSIONAHA.112.190710. [DOI] [PubMed] [Google Scholar]
- 47.Chirinos JA, Segers P, Gupta AK, et al. Time-varying myocardial stress and systolic pressure-stress relationship: role in myocardial-arterial coupling in hypertension. Circulation. 2009;119:2798–2807. doi: 10.1161/CIRCULATIONAHA.108.829366. [DOI] [PubMed] [Google Scholar]
- 48.Chirinos JA, Segers P, Rietzschel ER, et al. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 49.Shah SJ, Wasserstrom JA. Increased arterial wave reflection magnitude: a novel form of stage B heart failure? J Am Coll Cardiol. 2012;60:2178–2181. doi: 10.1016/j.jacc.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 50.Gu H, Li Y, Fok H, et al. Reduced first-phase ejection fraction and sustained myocardial wall stress in hypertensive patients with diastolic dysfunction: a manifestation of impaired shortening deactivation that links systolic to diastolic dysfunction and preserves systolic ejection fraction. Hypertension. 2017;69:633–640. doi: 10.1161/HYPERTENSIONAHA.116.08545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weber T. Systolic and diastolic function as related to arterial stiffness. Artery Res. 2010;4:122–127. [Google Scholar]
- 52.Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–13. doi: 10.1152/ajpheart.1991.261.3.H805. [DOI] [PubMed] [Google Scholar]
- 53.Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]
- 54.Kim HL, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Association between invasively measured central aortic pressure and left ventricular diastolic function in patients undergoing coronary angiography. Am J Hypertens. 2015;28:393–400. doi: 10.1093/ajh/hpu146. [DOI] [PubMed] [Google Scholar]
- 55.Eichhorn EJ, Willard JE, Alvarez L, et al. Are contraction and relaxation coupled in patients with and without congestive heart failure? Circulation. 1992;85:2132–2139. doi: 10.1161/01.cir.85.6.2132. [DOI] [PubMed] [Google Scholar]
- 56.Hori M, Inoue M, Kitakaze M, et al. Loading sequence is a major determinant of afterload-dependent relaxation in intact canine heart. Am J Physiol. 1985;249:H747–54. doi: 10.1152/ajpheart.1985.249.4.H747. [DOI] [PubMed] [Google Scholar]
- 57.Yano M, Kohno M, Kobayashi S, et al. Influence of timing and magnitude of arterial wave reflection on left ventricular relaxation. Am J Physiol Heart Circ Physiol. 2001;280:H1846–H1852. doi: 10.1152/ajpheart.2001.280.4.H1846. [DOI] [PubMed] [Google Scholar]
- 58.Weber T, Wassertheurer S, O'Rourke MF, et al. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–1883. doi: 10.1016/j.jacc.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 59.Zamani P, Rawat D, Shiva-Kumar P, et al. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber T, Auer J, O'Rourke MF, Punzengruber C, Kvas E, Eber B. Prolonged mechanical systole and increased arterial wave reflections in diastolic dysfunction. Heart. 2006;92:1616–1622. doi: 10.1136/hrt.2005.084145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paglia A, Sasso L, Pirozzi F, et al. Arterial wave reflections and ventricular-vascular interaction in patients with left ventricular systolic dysfunction. Int Heart J. 2014;55:526–532. doi: 10.1536/ihj.14-159. [DOI] [PubMed] [Google Scholar]
- 62.Denardo SJ, Nandyala R, Freeman GL, Pierce GL, Nichols WW. Pulse wave analysis of the aortic pressure waveform in severe left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:149–156. doi: 10.1161/CIRCHEARTFAILURE.109.862383. [DOI] [PubMed] [Google Scholar]
- 63.Weber T, Auer J, Lamm G, O'Rourke MF, Eber B. Arterial stiffness, central blood pressures, and wave reflections in cardiomyopathy-implications for risk stratification. J Card Fail. 2007;13:353–359. doi: 10.1016/j.cardfail.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Curtis SL, Zambanini A, Mayet J, et al. Reduced systolic wave generation and increased peripheral wave reflection in chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H557–62. doi: 10.1152/ajpheart.01095.2006. [DOI] [PubMed] [Google Scholar]
- 65.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5:543–551. doi: 10.1016/j.jchf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Ioannou CV, Morel DR, Katsamouris AN, et al. Left ventricular hypertrophy induced by reduced aortic compliance. J Vasc Res. 2009;46:417–425. doi: 10.1159/000194272. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi S, Yano M, Kohno M, et al. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 68.Pini R, Cavallini MC, Bencini F, et al. Cardiovascular remodeling is greater in isolated systolic hypertension than in diastolic hypertension in older adults: the Insufficienza Cardiaca negli Anziani Residenti (ICARE) a Dicomano Study. J Am Coll Cardiol. 2002;40:1283–1289. doi: 10.1016/s0735-1097(02)02159-9. [DOI] [PubMed] [Google Scholar]
- 69.Sharman JE, Fang ZY, Haluska B, Stowasser M, Prins JB, Marwick TH. Left ventricular mass in patients with type 2 diabetes is independently associated with central but not peripheral pulse pressure. Diabetes Care. 2005;28:937–939. doi: 10.2337/diacare.28.4.937. [DOI] [PubMed] [Google Scholar]
- 70.Norton GR, Majane OH, Maseko MJ, et al. Brachial blood pressure-independent relations between radial late systolic shoulder-derived aortic pressures and target organ changes. Hypertension. 2012;59:885–892. doi: 10.1161/HYPERTENSIONAHA.111.187062. [DOI] [PubMed] [Google Scholar]
- 71.Weber T, Wassertheurer S, Schmidt-Trucksäss A, et al. Relationship between 24-hour ambulatory central systolic blood pressure and left ventricular mass: a prospective multicenter study. Hypertension. 2017;70:1157–1164. doi: 10.1161/HYPERTENSIONAHA.117.09917. [DOI] [PubMed] [Google Scholar]
- 72.Urbina EM, Dolan LM, McCoy CE, Khoury PR, Daniels SR, Kimball TR. Relationship between elevated arterial stiffness and increased left ventricular mass in adolescents and young adults. J Pediatr. 2011;158:715–721. doi: 10.1016/j.jpeds.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zamani P, Bluemke DA, Jacobs DR, Jr, et al. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: the multi-ethnic study of atherosclerosis. Hypertension. 2015;65:85–92. doi: 10.1161/HYPERTENSIONAHA.114.04333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cauwenberghs N, Knez J, D'hooge J, et al. Longitudinal changes in LV structure and diastolic function in relation to arterial properties in general population. JACC Cardiovasc Imaging. 2017;10:1307–1316. doi: 10.1016/j.jcmg.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Hashimoto J, Imai Y, O'Rourke MF. Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens. 2007;20:378–384. doi: 10.1016/j.amjhyper.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 77.de Luca N, Asmar RG, London GM, O'Rourke MF, Safar ME REASON Project Investigators. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J Hypertens. 2004;22:1623–1630. doi: 10.1097/01.hjh.0000125448.28861.fc. [DOI] [PubMed] [Google Scholar]
- 78.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 79.Binder J, Bailey KR, Seward JB, et al. Aortic augmentation index is inversely associated with cardiorespiratory fitness in men without known coronary heart disease. Am J Hypertens. 2006;19:1019–1024. doi: 10.1016/j.amjhyper.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Edwards DG, Lang JT. Augmentation index and systolic load are lower in competitive endurance athletes. Am J Hypertens. 2005;18:679–683. doi: 10.1016/j.amjhyper.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 81.Pfob M, Mürzl N, Müller E, Eber B, Weber T. Ambulatory cardiac rehabilitation improves pulsatile arterial hemodynamics: a pilot trial. Wien Med Wochenschr. 2014;164:220–227. doi: 10.1007/s10354-014-0284-y. [DOI] [PubMed] [Google Scholar]
- 82.Broufa M, Wassertheurer S, Hametner B, Zweiker R, Binder RK, Weber T. Pulsatile hemodynamics are associated with exercise capacity in patients with Exertional dyspnea and preserved left ventricular ejection fraction. Am J Hypertens. 2018;31:574–581. doi: 10.1093/ajh/hpy001. [DOI] [PubMed] [Google Scholar]
- 83.Rerkpattanapipat P, Hundley WG, Link KM, et al. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90:1221–1225. doi: 10.1016/s0002-9149(02)02838-2. [DOI] [PubMed] [Google Scholar]
- 84.Bonapace S, Rossi A, Cicoira M, et al. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation. 2003;107:1603–1608. doi: 10.1161/01.CIR.0000051458.39176.43. [DOI] [PubMed] [Google Scholar]
- 85.Patrianakos AP, Parthenakis FI, Karakitsos D, Nyktari E, Vardas PE. Proximal aortic stiffness is related to left ventricular function and exercise capacity in patients with dilated cardiomyopathy. Eur J Echocardiogr. 2009;10:425–432. doi: 10.1093/ejechocard/jen304. [DOI] [PubMed] [Google Scholar]
- 86.Laskey WK, Kussmaul WG. Arterial wave reflection in heart failure. Circulation. 1987;75:711–722. doi: 10.1161/01.cir.75.4.711. [DOI] [PubMed] [Google Scholar]
- 87.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 88.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reddy YN, Andersen MJ, Obokata M, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70:136–148. doi: 10.1016/j.jacc.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holland DJ, Sacre JW, Leano RL, Marwick TH, Sharman JE. Contribution of abnormal central blood pressure to left ventricular filling pressure during exercise in patients with heart failure and preserved ejection fraction. J Hypertens. 2011;29:1422–1430. doi: 10.1097/HJH.0b013e3283480ddc. [DOI] [PubMed] [Google Scholar]
- 91.Tartière-Kesri L, Tartière JM, Logeart D, Beauvais F, Cohen Solal A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;59:455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 92.Haider AW, Larson MG, Franklin SS, Levy D Framingham Heart Study. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 93.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 94.Tsao CW, Lyass A, Larson MG, et al. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:e002189. doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chirinos JA, Khan A, Bansal N, et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail. 2014;7:709–716. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pandey A, Khan H, Newman AB, et al. Arterial stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the Health ABC study (Health, Aging, and Body Composition) Hypertension. 2017;69:267–274. doi: 10.1161/HYPERTENSIONAHA.116.08327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aisu H, Saito M, Inaba S, et al. Association of worsening arterial stiffness with incident heart failure in asymptomatic patients with cardiovascular risk factors. Hypertens Res. 2017;40:173–180. doi: 10.1038/hr.2016.116. [DOI] [PubMed] [Google Scholar]
- 98.Feistritzer HJ, Klug G, Reinstadler SJ, et al. Prognostic value of aortic stiffness in patients after ST-elevation myocardial infarction. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.117.005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fagard RH, Pardaens K, Vanhaecke J. Is the predictive power of a low-pulse pressure independent of peak oxygen uptake in advanced chronic heart failure? J Hum Hypertens. 2008;22:57–59. doi: 10.1038/sj.jhh.1002258. [DOI] [PubMed] [Google Scholar]
- 100.Voors AA, Petrie CJ, Petrie MC, et al. Low pulse pressure is independently related to elevated natriuretic peptides and increased mortality in advanced chronic heart failure. Eur Heart J. 2005;26:1759–1764. doi: 10.1093/eurheartj/ehi270. [DOI] [PubMed] [Google Scholar]
- 101.Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol. 2004;93:785–788. doi: 10.1016/j.amjcard.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 102.Kawashiro N, Kasanuki H, Ogawa H, Matsuda N, Hagiwara N Heart Institute of Japan--Department of Cardiology (HIJC) Investigators. Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC-HF registry. Circ J. 2008;72:2015–2020. doi: 10.1253/circj.cj-08-0323. [DOI] [PubMed] [Google Scholar]
- 103.Jackson CE, Castagno D, Maggioni AP, et al. Differing prognostic value of pulse pressure in patients with heart failure with reduced or preserved ejection fraction: results from the MAGGIC individual patient meta-analysis. Eur Heart J. 2015;36:1106–1114. doi: 10.1093/eurheartj/ehu490. [DOI] [PubMed] [Google Scholar]
- 104.Yildiran T, Koc M, Bozkurt A, Sahin DY, Unal I, Acarturk E. Low pulse pressure as a predictor of death in patients with mild to advanced heart failure. Tex Heart Inst J. 2010;37:284–290. [PMC free article] [PubMed] [Google Scholar]
- 105.Petrie CJ, Voors AA, Robertson M, van Veldhuisen DJ, Dargie HJ. A low pulse pressure predicts mortality in subjects with heart failure after an acute myocardial infarction: a post-hoc analysis of the CAPRICORN study. Clin Res Cardiol. 2012;101:29–35. doi: 10.1007/s00392-011-0360-x. [DOI] [PubMed] [Google Scholar]
- 106.Maeder MT, Kaye DM. Differential impact of heart rate and blood pressure on outcome in patients with heart failure with reduced versus preserved left ventricular ejection fraction. Int J Cardiol. 2012;155:249–256. doi: 10.1016/j.ijcard.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 107.Regnault V, Lagrange J, Pizard A, et al. Opposite predictive value of pulse pressure and aortic pulse wave velocity on heart failure with reduced left ventricular ejection fraction: insights from an Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) substudy. Hypertension. 2014;63:105–111. doi: 10.1161/HYPERTENSIONAHA.113.02046. [DOI] [PubMed] [Google Scholar]
- 108.Schillaci G, Di Luzio S, Coluccini M, et al. A low pulse pressure is an independent predictor of mortality in heart failure: data from a large nationwide cardiology database (IN-CHF Registry) Ital Heart J. 2004;5:892–898. [PubMed] [Google Scholar]
- 109.Mitchell GF, Moyé LA, Braunwald E, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 110.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33:951–958. doi: 10.1016/s0735-1097(98)00679-2. [DOI] [PubMed] [Google Scholar]
- 111.Lip GY, Skjøth F, Overvad K, Rasmussen LH, Larsen TB. Blood pressure and prognosis in patients with incident heart failure: the Diet, Cancer and Health (DCH) cohort study. Clin Res Cardiol. 2015;104:1088–1096. doi: 10.1007/s00392-015-0878-4. [DOI] [PubMed] [Google Scholar]
- 112.Laskey WK, Wu J, Schulte PJ, et al. Association of arterial pulse pressure with long-term clinical outcomes in patients with heart failure. JACC Heart Fail. 2016;4:42–49. doi: 10.1016/j.jchf.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 113.Sung SH, Yu WC, Cheng HM, et al. Pulsatile hemodynamics and clinical outcomes in acute heart failure. Am J Hypertens. 2011;24:775–782. doi: 10.1038/ajh.2011.26. [DOI] [PubMed] [Google Scholar]
- 114.Meguro T, Nagatomo Y, Nagae A, et al. Elevated arterial stiffness evaluated by brachial-ankle pulse wave velocity is deleterious for the prognosis of patients with heart failure. Circ J. 2009;73:673–680. doi: 10.1253/circj.cj-08-0350. [DOI] [PubMed] [Google Scholar]
- 115.Bonapace S, Rossi A, Cicoira M, et al. Increased aortic pulse wave velocity as measured by echocardiography is strongly associated with poor prognosis in patients with heart failure. J Am Soc Echocardiogr. 2013;26:714–720. doi: 10.1016/j.echo.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 116.Tokitsu T, Yamamoto E, Hirata Y, et al. Clinical significance of pulse pressure in patients with heart failure with preserved left ventricular ejection fraction. Eur J Heart Fail. 2016;18:1353–1361. doi: 10.1002/ejhf.559. [DOI] [PubMed] [Google Scholar]
- 117.Brutsaert DL, De Keulenaer GW. Diastolic heart failure: a myth. Curr Opin Cardiol. 2006;21:240–248. doi: 10.1097/01.hco.0000221587.02114.da. [DOI] [PubMed] [Google Scholar]
- 118.Ait-Oufella H, Collin C, Bozec E, et al. Long-term reduction in aortic stiffness: a 5.3-year follow-up in routine clinical practice. J Hypertens. 2010;28:2336–2341. doi: 10.1097/HJH.0b013e32833da2b2. [DOI] [PubMed] [Google Scholar]
- 119.Mitchell GF, Izzo JL, Jr, Lacourcière Y, et al. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 120.Desai AS, Solomon SD, Shah AM, et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1–10. doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Borlaug BA, Olson TP, Abdelmoneim SS, et al. A randomized pilot study of aortic waveform guided therapy in chronic heart failure. J Am Heart Assoc. 2014;3:e000745. doi: 10.1161/JAHA.113.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wohlfahrt P, Melenovsky V, Redfield MM, et al. Aortic waveform analysis to individualize treatment in heart failure. Circ Heart Fail. 2017;10:10. doi: 10.1161/CIRCHEARTFAILURE.116.003516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Butler J, Packer M, Greene SJ, et al. Heart failure end points in cardiovascular outcome trials of sodium glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a critical evaluation of clinical and regulatory issues. Circulation. 2019;140:2108–2118. doi: 10.1161/CIRCULATIONAHA.119.042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 125.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 126.Striepe K, Jumar A, Ott C, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation. 2017;136:1167–1169. doi: 10.1161/CIRCULATIONAHA.117.029529. [DOI] [PubMed] [Google Scholar]
- 127.Park KT, Kim HL, Oh S, et al. Association between reduced arterial stiffness and preserved diastolic function of the left ventricle in middle-aged and elderly patients. J Clin Hypertens (Greenwich) 2017;19:620–626. doi: 10.1111/jch.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pauca AL, Kon ND, O'Rourke MF. Benefit of glyceryl trinitrate on arterial stiffness is directly due to effects on peripheral arteries. Heart. 2005;91:1428–1432. doi: 10.1136/hrt.2004.057356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zamani P, Akers S, Soto-Calderon H, et al. Isosorbide dinitrate, with or without hydralazine, does not reduce wave reflections, left ventricular hypertrophy, or myocardial fibrosis in patients with heart failure with preserved ejection fraction. J Am Heart Assoc. 2017;6:e004262. doi: 10.1161/JAHA.116.004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Omar SA, Artime E, Webb AJ. A comparison of organic and inorganic nitrates/nitrites. Nitric Oxide. 2012;26:229–240. doi: 10.1016/j.niox.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373:2314–2324. doi: 10.1056/NEJMoa1510774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Omar SA, Fok H, Tilgner KD, et al. Paradoxical normoxia-dependent selective actions of inorganic nitrite in human muscular conduit arteries and related selective actions on central blood pressures. Circulation. 2015;131:381–389. doi: 10.1161/CIRCULATIONAHA.114.009554. [DOI] [PubMed] [Google Scholar]
- 133.Chirinos JA, Londono-Hoyos F, Zamani P, et al. Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;19:1507–1515. doi: 10.1002/ejhf.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66:1672–1682. doi: 10.1016/j.jacc.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 135.Eggebeen J, Kim-Shapiro DB, Haykowsky M, et al. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2016;4:428–437. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Londono-Hoyos F, Zamani P, Beraun M, Vasim I, Segers P, Chirinos JA. Effect of organic and inorganic nitrates on cerebrovascular pulsatile power transmission in patients with heart failure and preserved ejection fraction. Physiol Meas. 2018;39:044001. doi: 10.1088/1361-6579/aab2ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Borlaug BA, Anstrom KJ, Lewis GD, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA. 2018;320:1764–1773. doi: 10.1001/jama.2018.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 139.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 140.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 141.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 142.Solomon SD, McMurray JJ, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]