Abstract
Background and Objectives
Donor heart utilization patterns in Korea are not well known. We aimed to analyze the current utilization rate of donor heart and to evaluate the reason for non-utilization in Korea.
Methods
Brain death donors were identified from the Korean Network for Organ Sharing registry from November 2009 to December 2019. Baseline characteristics and heart transplant related parameters were compared between transplanted and discarded donor hearts.
Results
Among 4,401 donors, 1,383 (31.4%) hearts were utilized. Hearts from younger, male donors without history of hypertension or diabetes mellitus were utilized more frequently. The most common blood type in the transplanted group was O, while blood type A was the most common blood type in the discarded group. The ejection fraction in the discarded group were significantly lower (57.0 ± 13.5% vs. 61.7 ± 7.0%, p<0.001). Reasons for non-utilization were as follows: medical condition (34.4%), old age (31.8%), refused by all candidates (22.5%), refused to donate (11.0%), and others (0.2%). The most common cause of brain death was cerebrovascular disease in both the transplanted and discarded groups, but the incidence was significantly higher in the discarded group (46.8% vs. 37.5%, p<0.001). In multivariate analysis, old age, small body surface area, rapid heart rate, blood type other than O, abnormal echocardiography findings were independent predictors for non-utilization.
Conclusions
Average donor heart utilization rate was 31.4% and it improved to 42.9% in 2019. However, substantial numbers of donor hearts are still discarded. A strategic approach to improve donor heart utilization is necessary.
Keywords: Heart transplantation, Donor selection, Korea
INTRODUCTION
Donor shortage is a universal problem in solid organ transplantation. The issue is especially important in heart transplantation due to the urgency of the recipient's condition and the unavailability of living donors. While optimal utilization of the available donor heart is critical, fewer than 50% of potential heart donors become actual donor worldwide.1),2),3)
According to previous reports, younger donors, absence of cardiac abnormalities, no inotropic dependency, acceptable body size mismatches (20–30% of height and weight), and negative underlying diseases are conditions that characterize an optimal donor.4) Hearts from older donors and decreased left ventricular ejection fractions were the most important reasons for non-utilization in previous studies.5) To expand the donor pool, an extended criteria donor (ECD) heart has been suggested. Transplantation centers that match higher risk recipients with higher risk donors now use ECD hearts with acceptable outcomes.4)
In Korea, the number of heart transplantations has been gradually increasing, but more rapidly increasing advanced heart failure patients on the wait list have resulted in longer wait times for these high-risk patients. It is important to investigate the current status of donor heart utilization and the reasons for non-acceptance in Korea to search for better ways to improve the acceptance rate of the proper donor hearts. The aim of this study was to analyze the current utilization rate of donor heart in Korea and to evaluate predictors of donor heart non-utilization.
METHODS
Study population
Brain death donors were consecutively identified from the Korean Network for Organ Sharing (KONOS) registry between November 2009 and December 2019. Donor heart utilization rate was evaluated from the number of heart transplantations divided by the number of brain death donors during the period. Reasons for non-utilization were classified as refused to donate, unacceptable due to old age, unacceptable due to medical causes, refused by all candidates and others. Baseline characteristics and heart transplantation-related parameters were compared between transplanted donor hearts and discarded donor hearts. When all the candidate refused to accept the donor, it was classified to ‘refused by all candidates’. The classification to ‘refused to donate’ was the modification of decision to donate the organ by the donor side (probably because of the donor condition or decision changed by the family).
Causes of brain death were categorized into cerebrovascular disease, trauma, brain hypoxia, and others.
Temporal trend analysis of donor heart utilization
Donor heart acceptance rates were evaluated on a yearly basis during the study period. Acceptance rates and reasons for non-utilization were compared between the early (January 2010–December 2014) and late (January 2015–December 2019) periods.
Donor heart allocation system in Korea
The Korea Organ Donation Agency (KODA) covers the process of organ donation from brain-death donors. KONOS is involved in organ allocation based on the national wait list and controls associate organizations related to organ transplantation. When an available brain-death donor is detected, the KODA manages the process for donor procurement. According to the wait list, recipients for each organ are allocated under the control of KONOS considering urgency, wait time, blood type, body size, and etc.4),6)
Statistical analysis
Continuous variables are presented as means ± standard deviations and were compared using an independent t-test. Categorical variables were compared using the chi-squared test or Fisher's exact test, as appropriate. Donor characteristics, laboratory findings and electrocardiographic and echocardiographic abnormalities were compared between transplanted and discarded hearts. Logistic regression analysis was used to identify independent predictors of donor heart non-utilization. For multivariate analysis, age, male sex, body surface area (BSA), pulse rate, smoking, alcohol, hypertension, diabetes mellitus, blood type O, cerebrovascular cause of brain death, use of inotropes, trauma history, positive findings of anti-hepatitis B core antibody (anti-HBcAb), cytomegalovirus (CMV) immunoglobulin G (IgG), Epstein-Barr virus (EBV) IgG, abnormal findings on electrocardiography (ECG), and abnormal findings on transthoracic echocardiography (TTE) were used as variables. p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS v. 18.0 (SPSS Inc., Chicago, IL, USA) and R package version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics of transplanted and discarded donor hearts
A total of 4,401 heart transplantation donors were identified in the study period. Among them, 1,383 (31.4%) hearts were utilized. Table 1 shows the clinical characteristics of donors in transplanted vs. discarded hearts. Hearts from younger, male donors without a history of hypertension or diabetes mellitus were utilized more often. Donors who had higher heart rate with inotropes and showing abnormalities on ECGs or TTEs were utilized less. The ejection fractions of the left ventricles in the discarded group were significantly lower. Table 2 compares the laboratory findings of the donors in transplanted and discarded hearts. Donors with discarded hearts had worse laboratory findings showing lower platelet count, serum albumin, and arterial blood oxygen saturation, and higher creatinine, glucose, and amylase levels. The most common donor blood type in the transplanted group was O (34.9% in the transplanted group), while, the most common blood type in the discarded group was A (34.1% in the discarded group). Blood type AB was least transplanted in both groups (6% in the transplanted group and 14.8% in the discarded group). The positive ratios of viral markers (anti-HBc IgG, CMV IgG, EBV IgG) were also higher in discarded donor hearts.
Table 1. Comparison for clinical characteristics of transplanted hearts and discarded hearts.
| Variables | Transplanted (n=1,383) | Discarded (n=3,018) | p value |
|---|---|---|---|
| Age (years) | 37.0±13.1 | 50.5±15.2 | <0.001 |
| Sex (male) | 968 (70.0) | 2,007 (66.5) | 0.022 |
| Alcohol | 809 (58.5) | 1,561 (51.7) | <0.001 |
| Tobacco | 655 (47.4) | 1,268 (42.0) | 0.003 |
| Hypertension | 174 (12.6) | 911 (30.2) | <0.001 |
| Diabetes mellitus | 67 (4.8) | 418 (13.9) | <0.001 |
| Height (cm) | 167.1±14.9 | 164.5±14.9 | <0.001 |
| Weight (kg) | 65.5±14.9 | 63.8±14.7 | 0.001 |
| BSA (m2) | 1.74±0.26 | 1.70±0.26 | <0.001 |
| Systolic BP (mmHg) | 124.6±19.8 | 123.9±21.9 | 0.313 |
| Diastolic BP (mmHg) | 74.1±15.0 | 73.4±15.1 | 0.119 |
| Pulse rate | 90.0±21.0 | 96.1±25.3 | <0.001 |
| PR over 100 bpm | 411 (30.2) | 1,225 (41.2) | <0.001 |
| Body temperature (°C) | 36.50±0.77 | 36.56±0.82 | 0.012 |
| Daily input (cc) | 5,043.8±3,624.5 | 5,114.5±2,988.1 | 0.622 |
| Daily output (cc) | 3,580.8±2,171.3 | 3,483.8±2,367.8 | 0.348 |
| Central venous pressure (mmHg) | 9.5±4.1 | 9.9±5.4 | 0.071 |
| History of trauma | 253 (18.3) | 362 (12.0) | <0.001 |
| History of surgery | 586 (42.4) | 1,329 (44.1) | 0.293 |
| CPR | 615 (44.5) | 1,394 (46.2) | 0.287 |
| CRRT | 76 (5.5) | 190 (6.3) | 0.301 |
| Use of inotropes | 1,258 (91.0) | 2,856 (94.6) | <0.001 |
| Use of vasopressin | 282 (20.4) | 516 (17.1) | 0.008 |
| ECG abnormality | 266 (60.0) | 403 (70.0) | 0.001 |
| TTE abnormality | 88 (24.6) | 223 (50.0) | <0.001 |
| Ejection fraction (%) | 61.7±7.0 | 57.0±13.5 | <0.001 |
Data are shown as mean ± standard deviation or number (%).
BSA = body surface area; BP = blood pressure; bpm, beat per minute; CPR = cardiopulmonary resuscitation; CRRT = continuous renal replacement therapy; ECG = electrocardiography; PR = pulse rate; TTE = transthoracic echocardiography.
Table 2. Comparison for laboratory findings of transplanted hearts and discarded hearts.
| Variables | Transplanted (n=1,383) | Discarded (n=3,018) | p value | |
|---|---|---|---|---|
| WBC (/μL) | 13,309±9,610 | 13,716±12,461 | 0.241 | |
| Hemoglobin (g/dL) | 10.0±1.8 | 10.1±1.9 | 0.142 | |
| Platelet (/μL) | 145,704±108,781 | 128,313±98,410 | <0.001 | |
| INR | 1.30±0.33 | 1.35±0.48 | <0.001 | |
| Na (mmol/L) | 147.6±10.8 | 148.0±10.8 | 0.230 | |
| K (mmol/L) | 3.86±0.77 | 3.94±0.86 | 0.002 | |
| Cl (mmol/L) | 114.3±11.5 | 115.0±11.9 | 0.081 | |
| BUN (mg/dL) | 24.8±20.0 | 28.4±20.8 | <0.001 | |
| Cr (mg/dL) | 1.46±1.31 | 1.74±1.52 | <0.001 | |
| Glucose (mg/dL) | 169.3±76.0 | 193.4±111.9 | <0.001 | |
| Amylase (IU/L) | 132.5±192.0 | 195.5±359.6 | <0.001 | |
| Lipase (IU/L) | 116.5±410.9 | 91.3±180.7 | 0.131 | |
| Protein (g/dL) | 5.18±0.74 | 5.01±0.78 | <0.001 | |
| Albumin (g/dL) | 2.79±0.45 | 2.69±0.48 | <0.001 | |
| AST (IU/L) | 109.7±276.6 | 141.4±434.3 | 0.005 | |
| ALT (IU/L) | 81.5±154.5 | 88.0±231.4 | 0.288 | |
| T-bilirubin (mg/dL) | 1.40±2.10 | 1.48±2.11 | 0.258 | |
| CRP (mg/dL) | 20.4±19.0 | 23.3±33.9 | 0.084 | |
| CK (IU/L) | 848±1,490 | 1,072±1,956 | 0.020 | |
| CK-MB (ng/mL) | 21.8±165.2 | 23.3±70.9 | 0.837 | |
| pH | 7.40±0.08 | 7.38±0.10 | <0.001 | |
| pO2 (mmHg) | 247.0±164.4 | 177.7±126.2 | <0.001 | |
| pCO2 (mmHg) | 37.53±8.65 | 37.57±8.37 | 0.887 | |
| O2sat (%) | 70.7±28.8 | 66.4±26.9 | 0.047 | |
| FiO2 | 61.7±7.0 | 57.0±13.5 | <0.001 | |
| ABO typing | <0.001 | |||
| A | 436 (31.5) | 1,029 (34.1) | ||
| B | 382 (27.6) | 836 (27.7) | ||
| O | 482 (34.9) | 705 (23.4) | ||
| AB | 83 (6.0) | 448 (14.8) | ||
| VDRL | 9 (0.7) | 22 (0.8) | 0.747 | |
| HBsAg | 16 (1.8) | 59 (3.2) | 0.042 | |
| Anti-HBsAb | 562 (63.8) | 1,176 (62.9) | 0.817 | |
| HBeAg | 3 (0.4) | 7 (0.5) | 0.922 | |
| Anti-HBeAb | 100 (14.2) | 266 (20.3) | 0.004 | |
| Anti-HBc IgM | 11 (1.0) | 8 (0.3) | 0.034 | |
| Anti-HBc IgG | 353 (32.2) | 1,220 (51.7) | <0.001 | |
| Anti-HCV | 2 (0.2) | 13 (0.7) | 0.166 | |
| CMV IgM | 7 (0.7) | 26 (1.1) | 0.317 | |
| CMV IgG | 1,030 (94.7) | 2,251 (97.7) | <0.001 | |
| EBV IgM | 29 (4.3) | 46 (3.3) | 0.111 | |
| EBV IgG | 612 (87.4) | 1,306 (91.2) | 0.013 | |
Data are shown as mean ± standard deviation or number (%).
ALT = alanine aminotransferase; Anti-HBc = anti-hepatitis B core; Anti-HBeAb = anti-hepatitis B e-antibody; Anti-HBsAb = anti-hepatitis B surface antibody; AST = aspartate aminotransferase; BUN = blood urea nitrogen; CK = creatine phosphokinase; CK-MB = creatine kinase MB fraction; CMV = cytomegalovirus; CRP = c-reactive protein; EBV = Epstein-Barr virus; HBeAg = hepatitis B e-antigen; HBsAg = hepatitis B surface antigen; HCV = hepatitis C virus; IgG = immunoglobulin G; IgM = immunoglobulin M; INR = international normalized ratio; VDRL = venereal disease research laboratory; WBC = white blood cell.
Reason for non-utilization of donor hearts and brain death causes
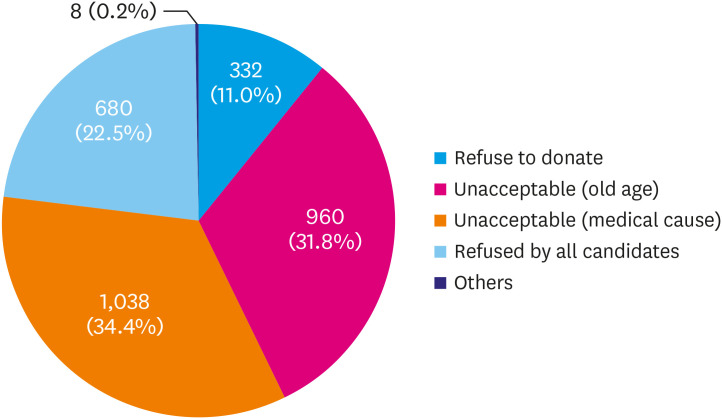
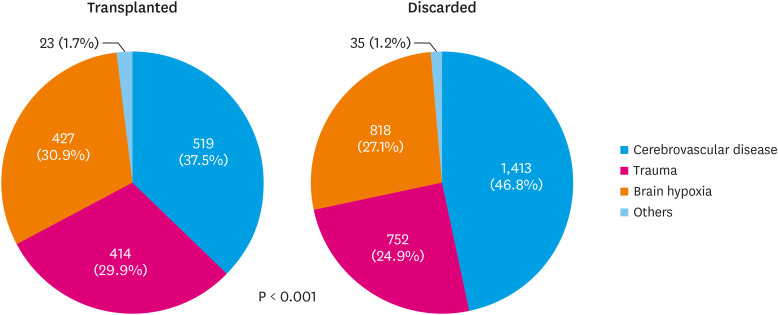
Reasons for non-utilization were as follows: unacceptable due to the medical condition (34.4%), unacceptable due to old age (31.8%), refused by all candidates (22.5%), refused to donate (11.0%), and others (0.2%) (Figure 1). The most common cause of brain death was cerebrovascular disease in both the transplanted and discarded groups, but the incidence was significantly higher in the discarded group (46.8% vs. 37.5%, p<0.001) (Figure 2).
Figure 1. Reasons for non-utilization of donor hearts.
Figure 2. Comparisons for brain death causes of transplanted hearts and discarded hearts.
Temporal trends of donor heart utilization and non-utilization rates
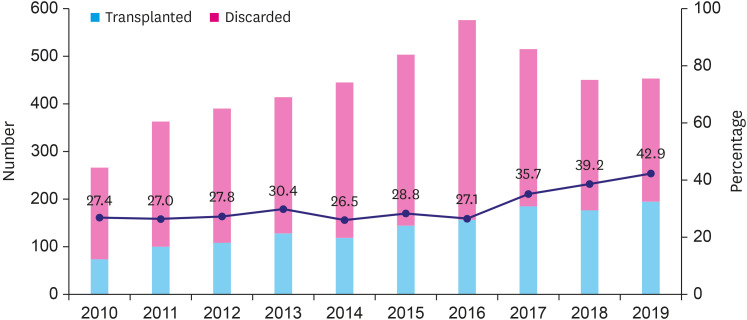
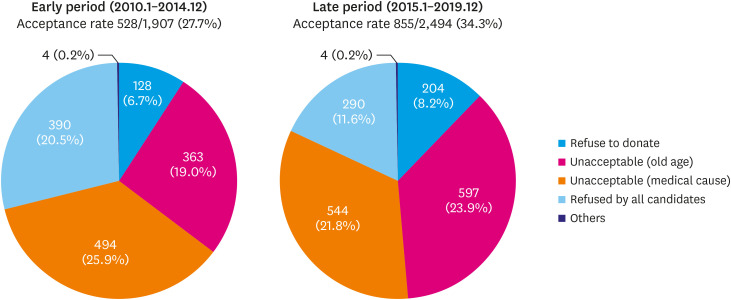
Heart donations gradually increased until 2016 and decreased after 2017. However, the absolute number of transplants has grown continuously until 2019 due to the remarkable increase in donor heart utilization in 2018 and 2019 (39.2% and 42.9% respectively) (Figure 3). The reasons for donor heart non-utilization were compared in the early (January 2010–December 2014) and late periods (January 2015–December 2019). Donations ‘unacceptable due to medical causes’ was the most common reason (25.9%) for non-utilization in the early period, while ‘unacceptable due to old age’ (23.9%) was the most common reason in the late period (Figure 4).
Figure 3. Temporal trends of donor heart utilization from January 2010 to December 2019.
Figure 4. Reasons for non-utilization of donor hearts in early (January 2010–December 2014) and late (January 2015–December 2019) period.
Independent predictors for donor heart non-utilization
Old age, male sex, low BSA, rapid pulse rate, smoking, alcohol, hypertension, diabetes mellitus, blood type other than O, cerebrovascular cause of brain death, use of inotropes, trauma history, anti-HBcAb positive, CMV IgG positive, EMB IgG positive, abnormal ECG, and abnormal TTE were univariate predictors of donor heart non-utilization. In multivariate analysis, older age, low BSA, rapid pulse rate, blood type other than O, and abnormal TTE findings were independent predictors for non-utilization of donor hearts (Table 3).
Table 3. Univariate and multivariate analysis of predictors for donor heart non-utilization.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Exp (B) | CI | p value | Exp (B) | CI | p value | |
| Age | 0.943 | 0.938–0.947 | <0.001 | 0.948 | 0.910–0.986 | 0.009 |
| Sex (male) | 1.175 | 1.024–1.348 | 0.022 | 1.635 | 0.600–4.461 | 0.337 |
| BSA | 1.745 | 1.347–2.261 | <0.001 | 12.110 | 2.139–68.546 | 0.005 |
| Pulse rate | 0.989 | 0.986–0.992 | <0.001 | 0.969 | 0.954–0.985 | <0.001 |
| Tobacco | 1.242 | 1.093–1.411 | 0.001 | 1.007 | 0.377–2.694 | 0.989 |
| Alcohol | 1.316 | 1.157–1.496 | <0.001 | 1.294 | 0.483–3.471 | 0.608 |
| HTN | 0.333 | 0.279–0.397 | <0.001 | 0.359 | 0.118–1.096 | 0.072 |
| DM | 0.317 | 0.243–0.413 | <0.001 | 0.728 | 0.164–3.242 | 0.678 |
| Blood type O | 1.755 | 1.527–2.017 | <0.001 | 3.207 | 1.252–8.215 | 0.015 |
| Cerebrovascular cause of brain death | 0.682 | 0.599–0.777 | <0.001 | 0.987 | 0.430–2.263 | 0.975 |
| Use of inotropes | 0.571 | 0.448–0.728 | <0.001 | 0.517 | 0.040–6.731 | 0.614 |
| History of trauma | 1.641 | 1.378–1.955 | <0.001 | 1.060 | 0.269–4.179 | 0.934 |
| Anti-HBcAb | 0.441 | 0.380–0.513 | <0.001 | 0.865 | 0.365–2.049 | 0.741 |
| CMV IgG | 0.418 | 0.286–0.611 | <0.001 | 0.538 | 0.080–3.638 | 0.525 |
| EBV IgG | 0.671 | 0.503–0.896 | 0.007 | 0.460 | 0.139–1.524 | 0.204 |
| Abnormal ECG | 0.645 | 0.497–0.837 | 0.001 | 0.409 | 0.167–0.998 | 0.050 |
| Abnormal TTE | 0.327 | 0.241–0.443 | <0.001 | 0.406 | 0.175–0.943 | 0.036 |
Anti-HBcAb = anti-hepatitis B core antibody; BSA = body surface area; CI = confidence interval; CMV = cytomegalovirus; DM = diabetes mellitus; EBV = Epstein-Barr virus; ECG = electrocardiography; HTN = hypertension; IgG = immunoglobulin G; TTE = transthoracic echocardiography.
DISCUSSION
Since the first human heart transplantation in 1967, and with the development of multiple immunosuppressive drugs to reduce rejection after the 1980s, the number of heart transplants has reached more than 5,500 cases worldwide annually.7) After the initial increase in cases during the 1980s and early 1990s, the number of heart transplantations plateaued because of donor shortages. Therefore, the number of candidates on wait lists far exceeded that of available donor organs.4),8)
Optimal donor heart utilization is an important issue especially in heart transplantation due to the urgency of the recipient's condition and the unavailability of living donors. However, previous data from the United Network of Organ Sharing (UNOS) database showed only 29% of donor hearts were utilized. Even though the temporal trend of the acceptance rate can be variable, globally fewer than 50% of potential heart donors become actual donors.1),2),3)
In Korea, after the first heart transplantation in 1992, the annual number of surgeries performed has been increasing. The number has increased to more than 50 cases between 2000 and 2007 and reached 191 in 2019.4),9) However, heart transplantations in critically ill patients using extracorporeal membrane oxygenation (ECMO) has increased in recent years (16% in 2014–2015 to 33% in 2016–2017), resulting in longer wait time for status 1 patients on chronic inotropic therapy or left ventricular assist devices.10),11)
Selection of a proper donor heart requires thorough review of the patient's history, cause of death, past medical history, ABO typing, donor height and weight, and clinical course considering vital signs, urine output, and basic laboratory studies (complete blood count, metabolic panel, and blood gas analysis). Other laboratory findings of viral serologies such as hepatitis B and C, human immunodeficiency virus, human T-cell leukemia virus, EBV, and CMV were also evaluated. Additional studies including chest radiographies, 12-lead ECGs, and TTEs are also considered in the decision for acceptance.4),12) Favorable donor characteristics include: ≤ 55 years of age without inotropic dependence, acceptable donor-recipient body size match, no significant structural heart disease shown on TTE, negative serologic markers for viral infections, no active malignancies and no severe systemic infections.4) However, marginal donors can still be used depending on the recipient's condition and their outcomes have been reported as comparable.2) Among the parameters predicting donor heart utilization, younger age, higher BSA, lower pulse rate, blood type O, and normal TTEs remained significant in multivariate analysis. It is understandable that younger and structurally normal hearts with blood type O are preferred for acceptance. Larger hearts are usually well tolerated when compared to smaller ones. Tachycardia is a sign of increased catecholamine release, and high-dose inotrope usage may suggest an improper donor condition, both of which result in a lower rate of donor heart utilization. Although there are conflicting results on optimal vasoactive drugs for brain-death donors in the previous reports, vasopressin showed positive physiologic effects.13),14),15),16) Brain-death donor is known to be a physiologically vasopressin deficiency state, which results in the decrease of blood pressure (BP). Vasopressin, not catecholamine, can maintain the optimal BP without further stimulation of sympathetic nervous system. Vasopressin usage was related with more frequent donor heart utilization in this study.
The number of heart donors have gradually increased until 2016 but decreased thereafter. This was due to the public misconception regarding organ donations during that period and in part by the suspension of the end-of-life care act.17) Nevertheless, in virtue of the remarkable increase in donor heart utilizations after 2017, the total number of heart transplantations did not decline. Cerebrovascular causes of brain death were the most common in both transplanted and discarded hearts; however, there were fewer heart transplant donors with cerebrovascular disease. Donors with cerebrovascular disease tend to have more risk factors and co-morbidities that can result in decreased donor heart function.7)
ECD heart utilization is an appropriate method to decrease the discard rate of donor hearts. A previous consensus statement described that ECD hearts are considered when one of the following risk factors is present: donor age >40 years; a history of chest trauma; prolonged hospitalization; prolonged cardiopulmonary resuscitation or downtime; a history of diabetes, tobacco, or illicit drug use; transient reversible hypotension; short-term, high-dose catecholamine administration; and a substantially smaller donor weight compared to the recipient.2) Accepting ECD hearts might be a reasonable solution for high-risk candidates on ECMOs or for older patients.
Appropriate management of donors with brain-death can also increase the usage of potentially available donor hearts.13),18) It is important to keep the organ condition in an optimal state for successful heart transplantations. Most centers have their own protocol to manage donors with brain-death, but more systemic efforts to adopt a universal donor management protocol including appropriate usage of vasopressin and to maintain the best standard is needed. Thorough examination of donor organs is also important.19) Initially decreased donor heart function can be improved on follow-up echocardiography. If needed, coronary angiography might be considered.20) Coordination with hospital organ procurement organizations is required to minimize non-utilization of donor hearts which are potentially acceptable.3),17)
Recently, the concept of donation after circulatory death (DCD) has been successfully utilized for heart transplantation in line with an effort to expand the donor pool. In DCD, retrieval of organs for the purpose of transplantation occurs from patients whose death is diagnosed and confirmed using cardiorespiratory criteria. Asystole must be confirmed for at least 5 minutes to declare death, but this standoff time varies in different countries. The recent developments in organ perfusion and retrieval techniques have shown that this valuable resource may provide an answer to the global shortage of suitable donor hearts.21),22) To minimize ischemic injury in organs from DCD donors, the Portable Organ Care System (OCS™; TransMedics, Andover, MA, USA) can extend donor pools in this regard.4) This device could be a solution to improve donor heart utilization by reducing cold ischemic time while providing additional donor heart assessment options for physicians.23)
This is a retrospective study, and donor parameters including echocardiographic data are limited. The recipient's data were not fully available. However, we could extensively analyze currently available heart transplantation data and could evaluate temporal trends, the current status for donor heart utilization, and reasons for non-utilization.
Currently, only 4 major categories are collected (refused to donate, unacceptable due to old age, unacceptable due to medical causes, and no final candidate) for non-acceptance reasons in the KONOS system which appears insufficient in understanding different situations during the allocation process. In the UNOS system, detailed classifications for non-acceptance enabled in-depth analyses regarding donor heart utilization.24) More structured categories need to be adopted for reasons of non-acceptance to investigate ways to improve donor heart management systems and to increase donor heart utilization in Korea. Further prospective studies including both donor and recipient data using structured classifications for non-utilization are necessary.
In conclusion, according to the KONOS registry, a substantial number of donor hearts were discarded. While average donor heart utilization rate was 31.4% in Korea, it improved to 42.9% in 2019. Multivariate analysis revealed that old age, small BSAs, rapid heart rates, blood types other than O, and abnormal TTE findings were independent predictors for non-utilization of donor hearts. A strategic approach to improve donor heart utilization is necessary.
ACKNOWLEDGEMENTS
We would like to express our sincere gratitude to KONOS for their efforts to collect and provide datasets regarding donor heart utilization for this work.
Footnotes
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0575) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1C1B6005448). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest: The author has no financial conflicts of interest.
- Conceptualization: Kim IC, Youn JC, Lee SE, Jung SH, Kim JJ.
- Data curation: Kim IC, Jung SH.
- Formal analysis: Kim IC.
- Investigation: Kim IC, Youn JC, Jung SH, Kim JJ.
- Methodology: Kim IC, Youn JC, Jung SH, Kim JJ.
- Project administration: Kim IC, Youn JC, Lee SE, Jung SH, Kim JJ.
- Resources: Kim IC, Youn JC, Jung SH, Kim JJ.
- Supervision: Youn JC, Lee SE, Jung SH.
- Validation: Youn JC, Lee SE, Jung SH.
- Visualization: Kim IC, Youn JC.
- Writing - original draft: Kim IC, Youn JC.
- Writing - review & editing: Kim IC, Youn JC, Lee SE, Jung SH, Kim JJ.
References
- 1.Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am J Transplant. 2015;15:642–649. doi: 10.1111/ajt.13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobashigawa J, Khush K, Colvin M, et al. Report from the American Society of Transplantation conference on donor heart selection in adult cardiac transplantation in the United States. Am J Transplant. 2017;17:2559–2566. doi: 10.1111/ajt.14354. [DOI] [PubMed] [Google Scholar]
- 3.Cha MJ, Lee HY, Cho HJ, Hwang HY, Kim KB, Oh BH. Under-utilization of donor hearts in the initial era of the heart transplant program in Korea- review of 13 years' experience from the Korea national registry. Circ J. 2013;77:2056–2063. doi: 10.1253/circj.cj-12-1269. [DOI] [PubMed] [Google Scholar]
- 4.Kim IC, Youn JC, Kobashigawa JA. The past, present and future of heart transplantation. Korean Circ J. 2018;48:565–590. doi: 10.4070/kcj.2018.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guglin M, Omar HR, Wright C. Underutilization of donor hearts: an observational study. Transplant Proc. 2018;50:3698–3704. doi: 10.1016/j.transproceed.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Cho HJ, Kim MS, et al. Focused update of 2016 Korean Society of Heart Failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019;1:4–24. doi: 10.36628/ijhf.2019.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khush KK, Cherikh WS, Chambers DC, et al. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018;37:1155–1168. doi: 10.1016/j.healun.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Choi HM, Park MS, Youn JC. Update on heart failure management and future directions. Korean J Intern Med. 2019;34:11–43. doi: 10.3904/kjim.2018.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim IC, Youn JC. Understanding the current status of Korean Heart Transplantation based on initial KOTRY report. Korean Circ J. 2017;47:858–860. doi: 10.4070/kcj.2017.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim D, Choi JO, Oh J, et al. The Korean Organ Transplant Registry (KOTRY): second official adult heart transplant report. Korean Circ J. 2019;49:724–737. doi: 10.4070/kcj.2018.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youn JC, Kim IC, Park NH, Kim H. Increased risk with older donor age and more frequent pre-transplant ECMO: the second official KOTRY report. Korean Circ J. 2019;49:738–741. doi: 10.4070/kcj.2019.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kittleson MM, Kobashigawa JA. Cardiac transplantation: current outcomes and contemporary controversies. JACC Heart Fail. 2017;5:857–868. doi: 10.1016/j.jchf.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Anwar AS, Lee JM. Medical management of brain-dead organ donors. Acute Crit Care. 2019;34:14–29. doi: 10.4266/acc.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plurad DS, Bricker S, Neville A, Bongard F, Putnam B. Arginine vasopressin significantly increases the rate of successful organ procurement in potential donors. Am J Surg. 2012;204:856–860. doi: 10.1016/j.amjsurg.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Pennefather SH, Bullock RE, Mantle D, Dark JH. Use of low dose arginine vasopressin to support brain-dead organ donors. Transplantation. 1995;59:58–62. doi: 10.1097/00007890-199501150-00011. [DOI] [PubMed] [Google Scholar]
- 16.Chen JM, Cullinane S, Spanier TB, et al. Vasopressin deficiency and pressor hypersensitivity in hemodynamically unstable organ donors. Circulation. 1999;100:II244–6. doi: 10.1161/01.cir.100.suppl_2.ii-244. [DOI] [PubMed] [Google Scholar]
- 17.Cho WH. Organ donation in Korea in 2018 and an introduction of the Korea national organ donation system. Korean J Transplant. 2019;33:83–97. doi: 10.4285/jkstn.2019.33.4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powner DJ, Darby JM, Kellum JA. Proposed treatment guidelines for donor care. Prog Transplant. 2004;14:16–26. doi: 10.1177/152692480401400103. [DOI] [PubMed] [Google Scholar]
- 19.Nair N, Gongora E. Role of cardiovascular imaging in selection of donor hearts. World J Transplant. 2015;5:348–353. doi: 10.5500/wjt.v5.i4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravi Y, Campagna R, Rosas PC, et al. Successful heart transplantation using a donor heart afflicted by takotsubo cardiomyopathy. Proc Bayl Univ Med Cent. 2016;29:73–74. doi: 10.1080/08998280.2016.11929367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchana-Sato V, Ledoux D, Detry O, et al. Successful clinical transplantation of hearts donated after circulatory death using normothermic regional perfusion. J Heart Lung Transplant. 2019;38:593–598. doi: 10.1016/j.healun.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Page A, Messer S, Large SR. Heart transplantation from donation after circulatory determined death. Ann Cardiothorac Surg. 2018;7:75–81. doi: 10.21037/acs.2018.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunjaya AF, Sunjaya AP. Combating donor organ shortage: organ care system prolonging organ storage time and improving the outcome of heart transplantations. Cardiovasc Ther. 2019;2019:9482797. doi: 10.1155/2019/9482797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi JR, Cheng A, Gallo M, Schumer EM, Massey HT, Slaughter MS. Predictors of donor heart utilization for transplantation in United States. Ann Thorac Surg. 2017;103:1900–1906. doi: 10.1016/j.athoracsur.2016.08.101. [DOI] [PubMed] [Google Scholar]