Abstract
Objectives
To assess whether data from 18F-fluorodeoxyglucose (FDG) PET should be incorporated into eligibility criteria for clinical trials in Takayasu’s arteritis (TAK).
Methods
The study was conducted in two parts. Part one was an international online survey among physicians with experience managing TAK to determine, using clinical vignettes, whether FDG-PET data influence decisions about enrolment in trials. Part two used patient data from an observational cohort study in TAK to assess agreement regarding decisions about enrolment into trials, based on clinical assessment with and without incorporation of FDG-PET data.
Results
In part one, 68 physicians responded to the survey. Most physicians had used FDG-PET to diagnose TAK (82%) or monitor disease activity (66%). In vignettes representing active clinical disease, FDG-PET findings increased physician confidence in disease assessment and reduced outlier assessments. The greatest variability in decisions regarding enrolment into trials was observed in vignettes representing constitutional symptoms alone and elevated acute-phase reactants. In these cases, FDG-PET findings influenced decisions about enrolment and improved physician confidence. In multivariable models, FDG-PET findings were 1.29 times more strongly associated with enrolment decisions compared with levels of acute-phase reactants. In part two, incorporation of FDG-PET data significantly improved agreement about enrolment decisions between raters [inter-rater reliability (IRR) = 0.68 (95% CI 0.67, 0.69) to IRR = 0.88 (95% CI 0.87, 0.89); P < 0.01].
Conclusions
Incorporation of FDG-PET data into assessment of TAK influences decisions about enrolment of patients into trials, improves physician confidence about clinical assessment and could help reduce variability in study populations. Future trials in TAK should consider incorporating FDG-PET data into eligibility criteria.
Keywords: Takayasu’s arteritis, positron emission tomography, clinical trials
Rheumatology key messages.
Physicians with experience managing Takayasu’s arteritis (TAK) are currently using 18F-fluorodeoxyglucose (FDG) PET.
The impact of FDG-PET on decisions about enrollment into trials varies according to clinical scenarios.
Future trials in TAK should consider incorporating FDG-PET data into the assessment of disease activity.
Introduction
Accurate assessment of disease activity status in determining eligibility for enrolment into randomized controlled trials (RCTs) in Takayasu’s arteritis (TAK) can be challenging, with physicians often relying on a combination of clinical symptoms, laboratory values and imaging findings [1]. Lack of standardized definitions of disease activity can lead to differences of opinion among physicians in the minimum requirements necessary to define active disease in TAK [1–3]. For example, non-specific constitutional symptoms and isolated laboratory abnormalities are commonly reported in TAK, but physicians may disagree as to whether these findings are sufficient to constitute active disease. Furthermore, the same specific clinical symptom in TAK (e.g. limb claudication) can signify ongoing vascular inflammation or reflect prior vascular damage, contributing to potential variability in clinical assessment.
Two prior RCTs have been conducted in TAK (AGATA, a North American study of abatacept, and TAKT, a Japanese study of tocilizumab) [4, 5]. Each trial used a combination of clinical features and laboratory abnormalities to define disease activity. Both trials relied on the subjective assessment of the physician-investigator to determine whether a particular clinical symptom represented active disease, damage or an unrelated problem. Definitions of disease activity were not fully uniform between the two RCTs. Specifically, in AGATA, patients with musculoskeletal symptoms or fatigue/malaise could be considered to have active disease if these symptoms occurred in combination with elevated acute-phase reactants [4]. In TAKT, patients with fatigue/malaise were considered to have active disease independent of acute-phase reactants, and patients with elevated acute-phase reactants without any clinical symptoms were also considered to have active disease [5].
18F-fluorodeoxyglucose (FDG) PET is used in the assessment of patients with large-vessel vasculitis [6–9]. Abnormal metabolic activity within the walls of large arteries due to activated immune cells has been proposed as a surrogate for vascular inflammation [10]. FDG-PET can complement the clinical assessment of disease activity in TAK and may help interpret to what extent an ongoing clinical symptom signifies active disease vs chronic damage. Whether there is consensus among physicians on how to incorporate FDG-PET into the assessment of disease activity and trial enrolment decisions in TAK is unclear. The two RCTs published to date in TAK did not incorporate FDG-PET data into decisions about trial eligibility.
The current study collected data from physicians with clinical experience managing TAK to assess whether data from FDG-PET should be incorporated into eligibility criteria in future RCTs in this disease.
Methods
The study was conducted in two parts. Part one was an online survey of an international group of physicians with experience managing patients with TAK, conducted to assess the use of FDG-PET in clinical practice and determine, via a series of clinical vignettes, whether FDG-PET data predictably influenced decisions about enrolment of patients into clinical trials. Part two was conducted within a single-centre observational cohort in TAK to assess agreement between two independent physicians about clinical trial enrolment decisions based on disease activity assessment using actual patient data with and without incorporation of FDG-PET findings.
Part one: international survey of physicians with experience managing patients with TAK
Survey participants
Physicians with clinical experience in TAK were identified based on publication records or participation in international projects related to large-vessel vasculitis [11, 12] and recruited to participate in an online survey. The survey was conducted in English and was open from 2 April 2021 through 23 April 2021. The survey designers did not participate in the survey.
Survey elements
Participants were asked a series of questions related to demographics, clinical experience evaluating patients with TAK, and personal experience with the use of FDG-PET in evaluating TAK. Eight clinical vignettes representing specific clinical scenarios in TAK were then presented. The vignettes were based on actual cases seen in an observational cohort of TAK at the National Institutes of Health (NIH) in Bethesda, MD, USA. The cases were specifically chosen to represent scenarios of concordance and discordance between clinical symptoms, acute-phase reactant values and vascular imaging findings. In section A of each vignette, clinical symptoms, physical examination findings, acute-phase reactant levels and angiographic data were presented. Participants were asked if there was sufficient evidence of ongoing active vasculitis to be eligible to enrol into a clinical trial studying a new treatment agent (i.e. does the patient have active disease that would prompt an increase or change in therapy?). Participants were also asked to rate their level of confidence about the assessment of the presence/absence of active vasculitis (scale of 0–100%, with 100% being the highest level of confidence). In section B of each vignette, detailed information from the same patient’s FDG-PET scan was presented. Participants were asked whether, after incorporating FDG-PET data into clinical assessment, there was sufficient evidence of active vasculitis to enrol into a treatment trial and to again rate their level of confidence about their assessment (scale of 0–100%), but now after considering the FDG-PET data (see Supplementary Materials, available at Rheumatology online, for the complete survey).
Part two: evaluation of NIH TAK cohort
Study population and assessment
Data from patients with TAK collected in a prospective, observational cohort at the NIH (NCT02257866) was evaluated in part two of this study. All patients fulfilled the 1990 ACR Classification Criteria for TAK [13]. Each patient underwent a standardized assessment of clinical symptoms, acute-phase reactants, non-invasive angiography, and an FDG-PET-CT scan during the initial study visit. Elevated acute-phase reactants were defined as CRP measurement above the laboratory normal limit and/or ESR level >40 mm in the first hour by the Westergren method. FDG-PET activity was assessed by a single reader (M.A.A.) with extensive experience in clinical vascular imaging interpretation, blinded to clinical data. Each FDG-PET scan was interpreted as active or inactive vasculitis based on subjective assessment of the reader, and the location of abnormalities was noted relative to specific arterial territories.
Rating of cases for trial enrolment
Blinded to FDG-PET findings, two physicians (K.A.Q., P.C.G.) independently rated whether each patient with TAK had sufficient evidence of ongoing active vasculitis to meet trial enrolment criteria from the previously published AGATA trial [4] based on clinical symptoms, physical examination findings, acute-phase reactant levels and angiographic data (comparable information to section A of the clinical vignettes in the online survey). One month later, the above information was re-presented along with information about each patient’s FDG-PET scan, and two physicians again rated whether there was sufficient evidence of active vasculitis to enrol into AGATA (comparable information to section B of the clinical vignettes in the online survey). Inter-rater reliability (IRR) about trial enrolment was calculated before and after the incorporation of FDG-PET scan information.
Statistical analysis
Mixed effects logistic regression, adjusting for individual respondent and case number as random effects, was used to evaluate associations between FDG-PET activity, acute-phase reactant levels and enrolment decisions using RStudio. IRR about trial enrolment was calculated before and after the incorporation of FDG-PET information using Fleiss’ Kappa.
Ethics and informed consent
The online survey was considered exempt from Institutional Review Board (IRB) review by the NIH Office of Human Subjects Research Protections. Patients with TAK seen at the NIH provided written informed consent, and the study was approved by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases IRB (NIAMS IRB Protocol: 14-AR-0200).
Results
Part one
Demographics of survey participants
Sixty-eight of 82 (83%) physicians responded to the survey and 61 of 68 (90%) physicians fully completed all survey questions (see Supplementary Materials, available at Rheumatology online for a list of survey participants). In cases where physicians only partially completed the survey, all available data were used.
The demographics of survey respondents are detailed in Table 1. The majority of physicians were from Europe (66%) or North America (24%). Most respondents were rheumatologists (79%) working in an academic practice setting (91%). Respondents were at varying stages in their careers, with the majority having completed specialty training >10 years ago (69%). Respondents reported a range of experience managing patients with TAK: <10 patients (22%), 10–40 patients (47%) and >40 patients (31%).
Table 1.
Demographics of survey participants
| Respondents (n = 68) | |
|---|---|
| Continent (currently practicing in), n (%) | |
| Europe | 45 (66.2) |
| North America | 16 (23.5) |
| Asia | 5 (7.4) |
| Other | 2 (2.9) |
| Area of specialization, n (%) | |
| Rheumatology | 54 (79.4) |
| Internal medicine | 5 (7.4) |
| Immunology | 3 (4.4) |
| Nephrology | 3 (4.4) |
| Other | 3 (4.4) |
| Practice setting, n (%) | |
| Academic | 62 (91.2) |
| Other | 6 (8.8) |
| Time since completion of specialty training, n (%) | |
| 0–5 years | 9 (13.2) |
| 6–10 years | 12 (17.7) |
| 11–20 years | 34 (50) |
| 21–30 years | 9 (13.2) |
| >30 years | 4 (5.9) |
| Experience managing patients with Takayasu’s arteritis (number of patients responsible for primarily managing), n (%) |
|
| <10 | 15 (22.1) |
| 10–19 | 20 (29.4) |
| 20–29 | 8 (11.8) |
| 30–39 | 4 (5.9) |
| >40 | 21 (30.9) |
Use of FDG-PET by survey respondents
Data about survey respondents’ use of FDG-PET in clinical practice is reported in Table 2. Most physicians had previously used FDG-PET to diagnose TAK (82%) or to monitor disease activity (66%). A similar proportion of physicians reported use of FDG-PET (82%), magnetic resonance angiography (91%) or CT angiogram (82%) to diagnose TAK, compared with ultrasonography (59%) or catheter-based angiography (9%). Respondents most commonly reported use of magnetic resonance angiography (90%) to monitor disease activity in TAK, followed by FDG-PET (66%), ultrasonography (52%), CT angiogram (46%) and catheter-based angiography (2%). Respondents reported varying levels of personal experience obtaining FDG-PET scans in TAK, with 72% having obtained more than five studies for diagnostic purposes and 61% having obtained more than five studies as part of disease activity assessment. Only 6% of participants reported never obtaining FDG-PET for clinical assessment purposes in TAK. Participants from Europe were more likely to sometimes (>5 but <20 studies) or frequently (≥20 studies) obtain FDG-PET scans for TAK compared with participants from North America (85% vs 38%, P < 0.01). The most common reasons provided for not frequently using FDG-PET to assess disease activity in TAK included preference for an alternative imaging modality (59%) and cost/inability to obtain insurance approval (50%). Most physicians believed there was moderate to high/very high level of evidence to support the use of FDG-PET for the diagnosis of TAK (87%) and to monitor disease activity in TAK (57%).
Table 2.
Use of FDG-PET to diagnose and to monitor disease activity over time in Takayasu’s Arteritis
| Response options | Diagnosis | Disease activity monitoring | |
|---|---|---|---|
| Imaging modalities used as part of assessment, n (%) | Catheter-based angiography | 6 (8.8) | 1 (1.5) |
| CTA | 56 (82.4) | 31 (45.6) | |
| FDG-PET | 56 (82.4) | 45 (66.2) | |
| MRA | 62 (91.2) | 61 (89.7) | |
| Ultrasonography | 40 (58.8) | 35 (51.5) | |
| Personal experience using FDG-PET, n (%) | Frequently (≥20 studies) | 26 (38.2) | 10 (14.7) |
| Sometimes (>5 but <20 studies) | 23 (33.8) | 32 (47.1) | |
| Occasionally (1–5 studies) | 15 (22.1) | 22 (32.3) | |
| Never (0 studies) | 4 (5.9) | 4 (5.9) | |
| Level of evidence to support use of FDG-PET, n (%) | Very high | 8 (11.8) | 1 (1.5) |
| High | 26 (38.2) | 10 (14.7) | |
| Moderate | 25 (36.8) | 28 (41.2) | |
| Low | 5 (7.3) | 19 (27.9) | |
| Very low | 3 (4.4) | 9 (13.2) | |
| I don’t know | 1 (1.4) | 1 (1.5) |
CTA: CT angiogram; FDG-PET: 18F-fluorodeoxyglucose PET; MRA: magnetic resonance angiography.
Decisions about enrolment into clinical trials from the clinical vignettes
Survey respondents were presented with a series of eight clinical vignettes with clinical characteristics of each case described in Table 3. In section A of the clinical vignettes, respondents were provided with clinical symptoms, physical examination findings, acute-phase reactant levels and angiographic data. The majority of participants agreed to enrol (case 1) or not enrol (cases 2, 3, 7 and 8) the patient in the section A assessment. The overall level of confidence about the presence/absence of active vasculitis in these cases ranged from 74% to 83%. Greater variability in trial enrolment decision was observed in cases featuring only constitutional symptoms (e.g. fatigue) and elevated levels of acute-phase reactants (cases 4–6), for which some respondents stated they would enrol the patient, while other respondents decided they would not enrol the patient. The overall level of confidence about the presence/absence of active vasculitis in these cases was 62–65% (Table 3).
Table 3.
Disease activity assessments from sections A and B of the clinical vignettes
| Case number | Clinical symptoms | Acute-phase reactants | Section A: enrolled (n, %) | Section A: not enrolled (n, %) | FDG-PET | Section B: enrolled (n, %) | Section B: not enrolled (n, %) | Level of confidence (section A → B) |
|---|---|---|---|---|---|---|---|---|
| 1 | Active symptoms (carotidynia, headache) | Elevated | 54 (84) | 10 (16) | Active | 63 (98) | 1 (2) | 79% → 93% |
| 2 | Symptoms of damage (stable arm claudication) | Normal | 1 (2) | 61 (98) | Active | 20 (33) | 41 (67) | 74% → 69% |
| 3 | Symptoms of damage (stable arm claudication) | Normal | 0 (0) | 61 (100) | Inactive | 1 (2) | 60 (98) | 82% → 91% |
| 4 | Fatigue | Elevated | 19 (31) | 42 (69) | Active | 54 (89) | 7 (11) | 62% → 80% |
| 5 | Fatigue | Elevated | 16 (26) | 45 (74) | Inactive | 9 (15) | 52 (85) | 64% → 75% |
| 6 | Fatigue | Elevated | 24 (39) | 37 (61) | Active | 55 (90) | 6 (10) | 65% → 80% |
| 7 | No symptoms | Normal | 1 (2) | 60 (98) | Active | 21 (34) | 40 (66) | 83% → 69% |
| 8 | No symptoms | Normal | 2 (3) | 59 (97) | Inactive | 3 (5) | 58 (95) | 83% → 93% |
FDG-PET: 18F-fluorodeoxyglucose PET.
In section B of the clinical vignettes, respondents were presented the same clinical information from section A in addition to detailed results from an FDG-PET obtained during initial clinical assessment. The FDG-PET results influenced trial enrolment decisions and level of confidence about the decision to enrol/not enrol. In cases where FDG-PET activity aligned with clinical assessment from section A [active FDG-PET and active symptoms (case 1) or inactive FDG-PET and no active symptoms (cases 3 and 8)], there was little change observed in the enrolment decision, and in certain cases outlier assessments decreased. The level of confidence about presence/absence of active vasculitis increased to >90% confidence in these cases. In cases where FDG-PET findings did not align with clinical assessment in section A [active FDG-PET and absent clinical symptoms (cases 2 and 7)], the degree of variability about whether to enrol/not enrol increased in section B, with only 2% of participants enrolling these patients based on information in section A, but 33–34% participants enrolling these patients once FDG-PET scan results were incorporated in section B. Level of confidence about enrolment decisions was 69%. In cases where there was the highest variability about enrolment in section A (cases 4–6), FDG-PET activity was associated with the decision of whether to enrol in section B. If there was FDG-PET activity, 89–90% of participants agreed to enrol these patients in section B (cases 4 and 6), and if there was not FDG-PET activity, 85% of participants agreed not to enrol the patient in section B (case 5). The level of confidence about the decision to enrol/not enrol was 75–80% in these cases (Table 3).
Mixed model to evaluate associations with enrolment decisions
Mixed effects logistic regression, adjusting for individual respondent and case number as random effects, showed that levels of acute-phase reactants independently contributed to trial enrolment decisions in section A of the clinical vignettes [effects estimate (95% CI) 4.61 (2.68–6.55), P < 0.001]. In section B, levels of acute-phase reactants also independently contributed to trial enrolment decision [effects estimate 3.58 (2.77–4.39), P < 0.001], but FDG-PET activity influenced trial enrolment decision 1.29 times more than acute-phase reactants [effects estimate 4.63 (3.62–5.65), P < 0.001] (Table 4).
Table 4.
Associations between FDG-PET activity, levels of acute-phase reactants, and decisions about enrolment
| Predictor variablea | Effects estimate (95% CI) | P-value | |
|---|---|---|---|
| Section A | Elevated acute-phase reactants (yes/no) | 4.61 (2.68, 6.55) | <0.001 |
| Section B | Elevated acute-phase reactants (yes/no) | 3.58 (2.77, 4.39) | <0.001 |
| FDG-PET activity (yes/no) | 4.63 (3.62, 5.65) | <0.001 |
All models adjusted for participant and case as random effects.
FDG-PET: 18F-fluorodeoxyglucose PET.
Part two
Clinical assessment and trial enrolment decisions for NIH cohort
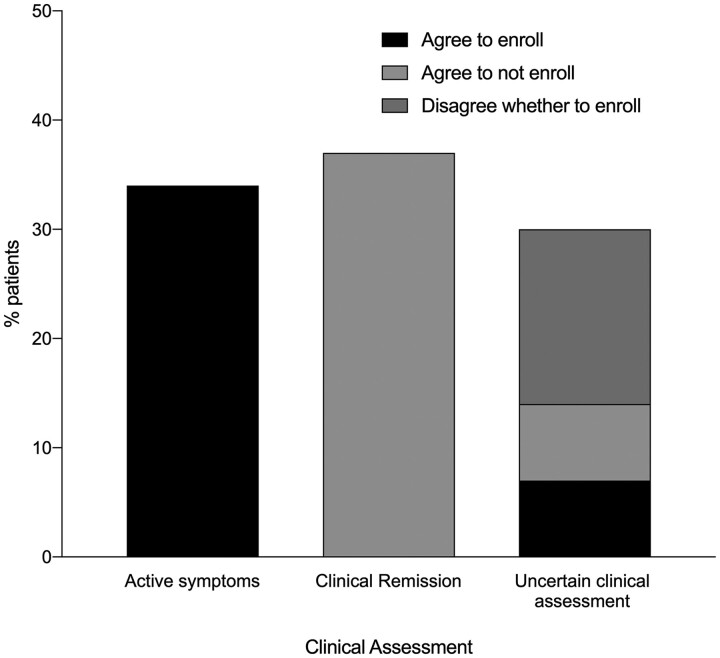
Seventy-six patients with TAK were evaluated. Twenty-six patients (34%) reported clinical symptoms felt by the physicians to be related to active disease (e.g. new onset carotidynia or vascular headache) and 12 (46%) of these patients had elevated acute-phase reactants. Both raters independently agreed to enrol all of these patients. Twenty-eight patients (37%) reported no clinical symptoms and two (7%) of these patients had elevated acute-phase reactants. Both raters agreed not to enrol any of these patients. Clinical assessment was uncertain in 22 patients (29%), which included 12 cases of non-specific constitutional symptoms alone (e.g. fatigue) and 10 cases of chronic symptoms without a clear pattern of improvement/worsening that could be interpreted as active disease or damage (e.g. limb claudication, positional lightheadedness). Forty-five percent of patients with uncertain clinical assessment had elevated acute-phase reactants. Variability about whether or not a patient met the AGATA trial enrolment criteria was observed in these cases [4]. Both raters agreed to enrol 5 patients (23%), agreed not to enrol 5 patients (23%) and disagreed as to whether to enrol/not enrol the remaining 12 patients (54%) (Fig. 1).
Fig. 1.
Decisions about enrolment into trials based on clinical assessment of disease activity
Patients were divided based on clinical assessment of disease activity by two physicians into three subgroups: active symptoms, clinical remission or uncertain clinical assessment, which included non-specific constitutional symptoms alone and symptoms that could be interpreted as active disease or damage. Both physicians independently rated whether each patient would fulfill the enrolment criteria for the AGATA trial. Both raters agreed to enrol all patients with active symptoms, agreed not to enrol all patients in clinical remission and disagreed about whether to enrol a subset of patients with uncertain clinical assessment. AGATA: A Randomized, Double-Blind Trial of Abatacept (CTLA-4Ig) for the Treatment of Takayasu Arteritis.
Incorporation of FDG-PET results in NIH cohort
When FDG-PET data was incorporated into assessment in the NIH cohort, 25/26 (96%) patients with active clinical symptoms had FDG-PET activity. Of patients with no symptoms, 12/76 (16%) had FDG-PET activity, suggestive of subclinical inflammation. FDG-PET activity was present in 16/22 (73%) patients with uncertain clinical assessment, including 8 patients who presented with fatigue alone (Fig. 2). Incorporation of FDG-PET scan data was associated with a significant increase in the IRR of agreement about enrolment decisions between raters [clinical assessment alone, IRR = 0.68 (95% CI 0.67, 0.69); clinical assessment + FDG-PET findings, IRR = 0.88 (95% CI 0.87, 0.89); P < 0.01].
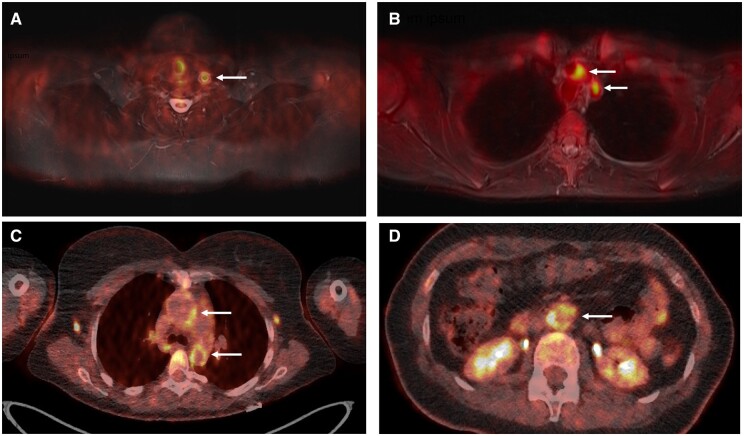
Fig. 2.
FDG-PET findings provide information about disease activity in TAK that may influence physician assessment in specific clinical scenarios
(A) A 15-year-old female with TAK with left carotidynia, frontal headaches, fatigue and elevated levels of acute-phase reactants. These clinical symptoms were thought to be related to active disease. FDG-PET demonstrated increased FDG uptake in the left common carotid artery (arrow), consistent with active vasculitis. (B) A 33-year-old female with TAK and persistent left arm claudication. There was uncertainty as to whether this symptom represented active disease or damage, as she did not have a clear pattern of improvement or worsening over the prior several months. FDG-PET showed increased FDG uptake in the aortic arch, proximal left subclavian artery and proximal left common carotid artery (arrows), suggesting that her ongoing symptoms were from active disease and not damage. Her symptoms subsequently improved with increased treatment. (C and D) A 38-year-old female with fatigue but no other symptoms and mild elevations in levels of acute-phase reactants. On clinical assessment alone, there was disagreement whether the degree of fatigue and laboratory abnormalities represented active vasculitis. FDG-PET showed high FDG uptake in the ascending and descending thoracic aorta (C, arrows), and the abdominal aorta (D, arrow). FDG: 18F-fluorodeoxyglucose; TAK: Takayasu’s arteritis.
Discussion
The conducting of RCTs in TAK has been challenging, in part because assessing disease activity is difficult. Even specific inclusion criteria typically rely heavily on physician-based subjective interpretation of whether an individual symptom represents active disease or not. As a result, physicians may disagree about which patients with TAK should be enrolled into a treatment trial. This study highlights how incorporation of FDG-PET data into clinical assessment can influence decisions about trial enrolment, improve physician confidence about disease activity assessment and be used to reduce variability of study populations to potentially improve the success of future clinical trials in TAK.
The present study details that physicians with experience managing TAK are using FDG-PET in clinical practice. Among physicians surveyed, most respondents believed the use of FDG-PET in TAK was evidence-based, with a high-to-very high level of evidence supporting use of FDG-PET to diagnose TAK and a moderate-to-high level of evidence supporting use of FDG-PET to monitor disease activity. Although the frequency with which FDG-PET was reportedly used to diagnose and monitor patients varied among respondents, the majority of surveyed physicians reported previous use of FDG-PET both to diagnose TAK and to monitor disease activity over time. Only 6% of respondents reported never having used FDG-PET to assess at least one patient with TAK.
The impact of FDG-PET data on decisions by survey respondents regarding trial enrolment varied according to specific clinical scenarios. In cases in which patients had symptoms highly suggestive of active disease (e.g. carotidynia) most physicians agreed to enrol these patients into an RCT independent of FDG-PET results. However, incorporation of FDG-PET data decreased outlier assessments and improved confidence about enrolment decisions. In the cases in which patients presented with non-specific constitutional symptoms alone (e.g. fatigue) or isolated levels of elevated acute-phase reactants, there was great variability among physicians about decisions regarding trial enrolment. In these scenarios, the corresponding vascular FDG-PET findings heavily influenced decision making.
Data from the NIH cohort highlights that non-specific constitutional symptoms alone or isolated levels of elevated acute-phase reactants is a common clinical scenario in TAK, representing 16% of the cohort. Similarly, fatigue/malaise and elevated levels of acute-phase reactants were the most prevalent baseline disease features among the AGATA trial participants [4]. Determining whether these clinical findings represent active disease is often challenging for investigators because some common symptoms, such as fatigue, may be secondary to active TAK, side-effects of treatment or other comorbidities; elevated levels of acute-phase reactants are not specific for active vasculitis [14, 15]. Incorporation of FDG-PET data could help streamline decisions about whether to enrol patients with this clinical presentation. For example, future RCT enrolment criteria could specify that to be enrolled into a treatment trial in TAK, patients with isolated constitutional symptoms must have evidence of active vasculitis on a corresponding FDG-PET scan.
In the clinical vignettes in which patients presented with no symptoms or only with symptoms suggestive of vascular damage, most physicians agreed not to enrol these patients into an RCT. Consideration of concomitant FDG-PET scan information often confirmed clinical impressions and improved physicians’ confidence in cases where FDG-PET scans were inactive; however, FDG-PET also identified a subset of patients with findings suggestive of ongoing active vasculitis despite clinical remission (i.e. ‘subclinical inflammation’). Subclinical inflammation demonstrated by FDG-PET is not an infrequent occurrence in TAK [16, 17] and was observed in 16% of patients in the NIH cohort where every patient underwent an FDG-PET scan per study protocol regardless of clinical presentation. Autopsy studies performed on patients with TAK during periods of apparent clinical remission have shown widespread active vasculitic lesions on histology, and ‘silent’ angiographic progression of disease can occur during periods of apparent clinical remission [1, 18]. Furthermore, prior RCTs in TAK have considered subclinical inflammation as evidence of disease activity. In AGATA, a new vascular stenosis/aneurysm on angiography was considered a feature of active disease, even in cases where corresponding clinical symptoms were absent [4]. FDG-PET activity in the absence of other clinical symptoms poses unique challenges for trial enrolment, as this may represent subclinical vasculitis or increased metabolic activity due to vascular damage/remodelling. In the clinical vignettes, physicians disagreed as to whether to enrol patients who had active vasculitis on FDG-PET who were otherwise in clinical remission. Approximately one-third of respondents chose to enrol these patients, while the remainder opted not to enrol. If FDG-PET is incorporated at the time of trial enrolment in future RCTs, specific consideration will need to be given as to how to proceed with cases of subclinical inflammation, and whether a specific trial should enrol or exclude this subset of patients.
There are several strengths of this study. This study addressed an area of active discussion in the field of rheumatology and an unmet research need. In part one, many physicians from a broad geographic distribution were surveyed. Their responses document a common belief that use of FDG-PET in TAK is evidence-based and provides some of the only information to date detailing how FDG-PET is currently being incorporated into clinical management of patients with TAK. The clinical vignettes and NIH cohort data revealed how FDG-PET can be useful in different specific clinical scenarios to facilitate recruitment into clinical trials and reduce variability in clinical assessment among physicians.
Several limitations of this study should also be noted. Although the cases in the clinical vignettes were specifically chosen to represent a range of clinical scenarios in TAK, a relatively small number of cases were presented in part one. Additionally, the written clinical vignette survey format could have resulted in possible loss of key clinical information obtainable from personal interaction with patients and may not have represented a substantive gradation of clinical descriptors and PET data. Most physicians who completed this survey were academic rheumatologists and responses could be influenced by selection bias; however, TAK is a complex disease that is primarily managed by these kinds of physicians. In part two, the two physician raters were at the same academic institution with similar experience with use of FDG-PET in TAK, so these data may not be fully generalizable to all investigators. However, the influence of PET data on trial enrolment decisions in scenarios of clinical uncertainty was similar in parts one and two of the study.
This study provides guidance for the design of future RCTs in TAK. While FDG-PET is increasingly being adopted into clinical practice in TAK, RCTs should consider incorporating FDG-PET data into the assessment of disease activity. Incorporation of FDG-PET data into the clinical assessment of TAK may improve agreement among physicians about disease activity status thus leading to a more homogeneous trial population, increase feasibility of trial recruitment to include more patients and enrich study populations with patients who are most likely to have disease that could be modified by treatment. While this study shows that physicians incorporate FDG-PET findings into medical decision making in TAK in predictable ways, there are still many challenges regarding use of FDG-PET in clinical studies in TAK related to cost, radiation exposure, institutional expertise, acquisition of imaging studies in a timely manner, and standardization of imaging protocols and nuclear medicine interpretation. In the absence of a histologic gold standard, uncertainty will also always remain about to what degree vascular FDG-PET abnormalities represent active vasculitis. Despite these challenges, multi-modal assessment of disease activity will likely be a key aspect of the conduct of future clinical trials in TAK.
Funding: This work was supported by the Intramural Research Program at the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary Material
Contributor Information
Kaitlin A Quinn, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA.
Hugh D Alessi, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA.
Cristina Ponte, Rheumatology Department, Hospital de Santa Maria, Centro Hospitalar Universitario Lisboa Norte EPE; Rheumatology Research Unit, Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal.
Emily Rose, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA.
Mark A Ahlman, Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD, USA.
Christopher Redmond, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA.
Yiming Luo, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA.
Ertugrul Cagri Bolek, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA; Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Hacettepe University, Ankara, Turkey.
Carol A Langford, Department of Rheumatic and Immunologic Diseases, Cleveland Clinic, Cleveland, OH, USA.
Peter A Merkel, Division of Rheumatology, Department of Medicine, and Division of Epidemiology, Department of Biostatistics, Epidemiology, and Informatics, University of Pennsylvania, Philadelphia, PA, USA.
Peter C Grayson, Systemic Autoimmunity Branch, National Institutes of Health, NIAMS, Bethesda, MD, USA.
Data availability statement
All data relevant to this study are included in the article or uploaded and in its online supplementary material, available at Rheumatology online.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Kerr GS, Hallahan CW, Giordano J. et al. Takayasu arteritis. Ann Intern Med 1994;120:919–29. [DOI] [PubMed] [Google Scholar]
- 2. Aydin SZ, Yilmaz N, Akar S. et al. Assessment of disease activity and progression in Takayasu's arteritis with Disease Extent Index-Takayasu. Rheumatology (Oxford) 2010;49:1889–93. [DOI] [PubMed] [Google Scholar]
- 3. Misra R, Danda D, Rajappa SM. et al. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology (Oxford) 2013;52:1795–801. [DOI] [PubMed] [Google Scholar]
- 4. Langford CA, Cuthbertson D, Ytterberg SR. et al. ; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of Takayasu arteritis. Arthritis Rheumatol 2017;69:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakaoka Y, Isobe M, Takei S. et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018;77:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blockmans D, Bley T, Schmidt W.. Imaging for large-vessel vasculitis. Curr Opin Rheumatol 2009;21:19–28. [DOI] [PubMed] [Google Scholar]
- 7. Cimmino MA, Camellino D.. Large vessel vasculitis: which imaging method? Swiss Med Wkly 2017;147:w14405. [DOI] [PubMed] [Google Scholar]
- 8. Prieto-González S, Depetris M, García-Martínez A. et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014;73:1388–92. [DOI] [PubMed] [Google Scholar]
- 9. Lee YH, Choi SJ, Ji JD, Song GG.. Diagnostic accuracy of 18F-FDG PET or PET/CT for large vessel vasculitis: a meta-analysis. Z Rheumatol 2016;75:924–31. [DOI] [PubMed] [Google Scholar]
- 10. Slart R, Writing G, Reviewer G. et al. ; EANM Committee Coordinator. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging 2018;45:1250–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Craven A, Robson J, Ponte C. et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol 2013;17:619–21. [DOI] [PubMed] [Google Scholar]
- 12. Aydin SZ, Robson JC, Sreih AG. et al. Update on outcome measure development in large-vessel vasculitis: report from OMERACT 2018. J Rheumatol 2019;46:1198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arend WP, Michel BA, Bloch DA. et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 14. Maksimowicz-McKinnon K, Clark TM, Hoffman GS.. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum 2007;56:1000–9. [DOI] [PubMed] [Google Scholar]
- 15. Quinn KA, Gribbons KB, Carette S. et al. Patterns of clinical presentation in Takayasu's arteritis. Semin Arthritis Rheum 2020;50:576–81. [DOI] [PubMed] [Google Scholar]
- 16. Grayson PC, Alehashemi S, Bagheri AA, Civelek AC. et al. 18F-fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol 2018;70:439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quinn KA, Ahlman MA, Malayeri AA, Marko J. et al. Comparison of magnetic resonance angiography and (18)F-fluorodeoxyglucose positron emission tomography in large-vessel vasculitis. Ann Rheum Dis 2018;77:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostberg G. Morphological changes in the large arteries in polymyalgia arteritica. Acta Med Scand Suppl 1972;533:135–59. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this study are included in the article or uploaded and in its online supplementary material, available at Rheumatology online.