Abstract
Saccharomyces cerevisiae transcribes two genes, ARE1 and ARE2, that contribute disproportionately to the esterification of sterols. Are2p is the major enzyme isoform in a wild-type cell growing aerobically. This likely results from a combination of differential transcription initiation and transcript stability. By using ARE1 and ARE2 promoter fusions to lacZ reporters, we demonstrated that transcriptional initiation from the ARE1 promoter is significantly reduced compared to that from the ARE2 promoter. Furthermore, the half-life of the ARE2 mRNA is approximately 12 times as long as that of the ARE1 transcript. We present evidence that the primary role of the minor sterol esterification isoform encoded by ARE1 is to esterify sterol intermediates, whereas the role of the ARE2 enzyme is to esterify ergosterol, the end product of the pathway. Accordingly, the ARE1 promoter is upregulated in strains that accumulate ergosterol precursors. Furthermore, ARE1 and ARE2 are oppositely regulated by heme. Under heme-deficient growth conditions, ARE1 was upregulated fivefold while ARE2 was down-regulated. ARE2 requires the HAP1 transcription factor for optimal expression, and both ARE genes are derepressed in a rox1 (repressor of oxygen) mutant genetic background. We further report that the ARE genes are not subject to end product inhibition; neither ARE1 nor ARE2 transcription is altered in an are mutant background, nor does overexpression of either ARE gene alter the response of the ARE-lacZ reporter constructs. Our observations are consistent with an important physiological role for Are1p during anaerobic growth when heme is limiting and sterol precursors may accumulate. Conversely, Are2p is optimally required during aerobiosis when ergosterol is plentiful.
The conjugation of sterols and fatty acids is a critical homeostatic response by all eukaryotic cells to an excess of either resource. This intracellular esterification reaction is mediated by enzymes known collectively as O-acyltransferases and provides an important storage depot and detoxification process by which to overcome the membrane perturbations that accrue from elevated sterol or free fatty acid levels. Thus, the uptake, synthesis, and conjugation of these metabolites are subject to multiple levels of regulation. In mammalian cells, sterol and fatty acid biosynthesis and receptor-mediated lipoprotein uptake are controlled primarily at the transcriptional level by the sterol regulatory element binding protein, a positive transcription factor which is inactive when sterols and fatty acids exceed cellular requirements (7). Sterol biosynthesis is further regulated posttranslationally, by phosphorylation and proteasomal degradation (18). Each mechanism causes metabolic down-regulation in response to excess cholesterol or fatty acids. By contrast, the sterol esterification reaction is up-regulated by elevated cellular cholesterol or fatty acids (14). The major mode of regulation of the mammalian acyl coenzyme A (CoA):cholesterol acyltransferases arises from allosteric binding of the sterol substrates, particularly cholesterol and oxysterol (11, 12, 15). However, they are also regulated transcriptionally (36, 40, 47).
ACAT1 is the founding member of the O-acyltransferase gene family that now extends to multiple organisms (17, 43). In the model eukaryote Saccharomyces cerevisiae, a paradigm has been identified whereby, within the same cell, more than one form of the enzymes are expressed (42, 49). The ACAT-related enzymes of yeast encoded by the ARE1 and ARE2 genes differentially determine the sterol ester pools of the cell. Deletion of both genes is required to produce a cell lacking sterol esterification activity (49, 51). However, under normal growth conditions, the approximate contributions of the ARE1 and ARE2 gene products to the total sterol ester mass are 25 and 75%, respectively, as assessed by the phenotypes produced by single ARE gene disruptions (2, 49). In mammals, two ACAT genes exist, ACAT1 and ACAT2 (1, 10, 35). In induced-mutant mouse models, sterol esterification is determined by ACAT1 in all tissues except the liver and intestine, where ACAT2-mediated activity predominates (8, 10, 31). In humans, ACAT2 is expressed primarily in hepatocytes and enterocytes while ACAT1 is ubiquitous (34). ACAT1 accounts for the majority of sterol esterification in most human cells, with the exception of those of intestinal origin, where ACAT2 appears to be the major contributor (13). Thus, the paradigm persists that in hepatocytes and enterocytes, ACAT1 and ACAT2 are both expressed in the same cell and yet contribute differentially to the esterification of sterols.
The expression of multiple genes for the sterol esterification reaction in a single cell must confer a selective advantage, given its retention throughout evolution. This could reflect differences in subcellular localizations, responses to the environment, or substrate specificity. In yeast, the Are proteins are both localized to the endoplasmic reticulum but exhibit marked substrate preferences (48, 53). In this study, we confirm that, in terms of contribution to the sterol ester mass in yeast, the ARE1 gene product primarily esterifies intermediates in the sterol biosynthetic pathway such as lanosterol, whereas ARE2 is responsible for esterification of the end product ergosterol. Furthermore, we demonstrate that the ARE genes are differentially regulated in response to alterations in sterol metabolism. The ARE1 gene is up-regulated by the accumulation of pathway intermediates and heme deficiency, whereas in the latter case, ARE2 is repressed. This would be physiologically relevant under anaerobic growth conditions. We propose that the regulated removal of biosynthetic pathway intermediates before they either become toxic or participate further in the production of the end product represents a novel form of sterol homeostasis that may be common to all eukaryotic cells.
MATERIALS AND METHODS
Strains, growth conditions, ARE expression constructs, and transformations.
Yeast strains (Table 1) were grown at 30°C in a mixture of yeast extract, peptone, and 2% glucose (YEPD) or complete synthetic medium (0.67% yeast nitrogen base, 2% glucose; CSM [3, 9]) with appropriate nutrients omitted as required for plasmid selection. Supplementation with adenine at 40 mg/liter was done when adenine auxotrophic strains were used for analysis of β-galactosidase activity. Yeast strains and Escherichia coli strain DH5α were transformed and maintained as previously described (3, 23). The ergosterol biosynthesis inhibitor fenpropimorph was added to the growth media at 0.5 μM at the time of culture inoculation, reducing the growth rate by 50%. Once the culture reached a density of 5 × 106 cells/ml, fenpropimorph was added at a final concentration of 25 μM and the culture was incubated for an additional 18 h before harvesting. Expression plasmids for ARE1 (YEp3-16 and pADH5-36) and ARE2 (YEpARE2 and pS5-ARE2) have been described elsewhere (20, 49).
TABLE 1.
Strains used for analysis of ARE1 and ARE2 regulation
| Strain | Genotype | Source |
|---|---|---|
| BWG1-7a | MATaade1-100 his4-519 leu2-112 ura3-52 | L. Guarente |
| BWG/erg2 | MATaade1-100 his4-519 leu2-112 ura3-52 erg2Δ::LEU2 | M. Bard |
| BWG/erg3 | MATaade1-100 his4-519 leu2-112 ura3-52 erg3Δ::LEU2 | M. Bard |
| BWG/erg6 | MATaade1-100 his4-519 leu2-112 ura3-52 erg6Δ::LEU2 | M. Bard |
| JR527 | MATaura3-52 his3Δ200 ade2-101 lys2-801 met | J. Rine |
| JR1159 | MATaura3-52 his3Δ200 ade2-101 lys2-801 met hmg1::LYS2 | J. Rine |
| JR1160 | MATaura3-52 his3Δ200 ade2-101 lys2-801 met hmg2::HIS3 | J. Rine |
| TKY22 | MATaleu2-3,112 ura3-52 ade1 trp1::hisG hem1Δ | T. Keng |
| LPY22 | MATaade1-100 his4-519 leu2-112 ura3-52 hap1Δ::LEU2 | L. Guarente |
| RZ53-6 | MATα trp1-289 leu2-3,112 ura3-52 ade1-100 | R. Zitomer |
| RZ53-6/rox1 | MATα trp1-289 leu2-3,112 ura3-52 ade1-100 rox1Δ::LEU2 | R. Zitomer |
| SCY059 | MATα his3-11,15 leu2-3,112 trp1-1 ura3-1 kan1-100 ade2 met14Δ are1::HIS3 are2::LEU2 | S. Sturley |
| SCY060 | MATα his3-11,15 leu2-3,112 trp1-1 ura3-1 kan1-100 ade2-1 are1::HIS3 | S. Sturley |
| SCY061 | MATa his3-11,15 leu2-3,112 trp1-1 ura3-1 kan1-100 met14Δ are2::LEU2 | S. Sturley |
| SCY062 | MATa his3-11,15 leu2-3,112 trp1-1 ura3-1 kan1-100 | S. Sturley |
| SCY983 | MATa ura3-52 leu2-3 rpb1-1 | M. Culbertson |
Sterol extraction and analysis.
Total sterols (free and esterified) were extracted and quantified from yeast cells as previously described (5, 32). Yeast cells were grown in 100 ml of minimal medium for 36 h in a 30°C water bath with shaking at 250 rpm. Nitric acid-washed glass beads (425 to 600 μm) were added at 0.5 volume, the tubes were vortexed twice for 30 s, and this was followed by sequential additions of 4 ml of methanol, 2 ml of chloroform, and 2 ml of 0.9% (wt/vol) NaCl with frequent vortexing. The chloroform phase containing extracted sterols was removed, dried under nitrogen, and resuspended in a small volume of methylene chloride and streaked on F254 precoated silica gel thin-layer chromatography (TLC) plates (E. Merck, Darmstadt, Germany). TLC was performed in methylene chloride (CHCl2), and the lipids were visualized by using 0.01% (wt/vol) berberine. Free sterols and steryl esters were scraped from the TLC plate, and the sterols were separated from the silica by resuspension in methylene chloride and vacuum filtration. Steryl esters were saponified overnight, at room temperature in the dark, in 1 ml of 6% (wt/vol) KOH in methanol. Hydrolyzed esters were extracted in n-heptane. Free sterol and ester fractions were quantified by gas chromatography. Sterols were separated on a Hewlett-Packard 5890 series II gas chromatograph with a capillary column (15 m by 0.25 mm by 0.25 μm [film thickness]; Hewlett-Packard HP5) programmed from 195 to 300°C. The temperature was initially 195°C for 3 min; it was then increased at 5.5°C/min to a final temperature of 300°C, at which it was held for 4 min. The linear velocity was 30 cm/s, nitrogen was used as the carrier gas, and injections were run in the splitless mode. Sterol fractions were resuspended in 1 ml of n-heptane, and 1 μl was coinjected with 0.1 μg of cholesterol, which was used as an internal standard. The area of each peak was compared to the area of the cholesterol peak to determine the amount of each sterol present. Each sample was injected twice, and the value reported was the average of the two injections. The dry weight was measured prior to extraction, so the total amount of sterol per gram of cells was determined. Samples of 1 to 3 μl, dissolved in n-heptane, were injected, and sterol composition was determined on the basis of retention times relative to the retention times of known sterol standards.
Construction of promoter-lacZ fusion plasmids
ARE1 and ARE2 promoter regions were amplified by PCR using genomic DNA from strain W303 as the template and sequence-specific primers KP-3 (5′ GGGGGGAATTCCGTCCATGGTCACACCGTCC 3′) and KP-4 (5′ GGGGGGATCCATTCTTGCAATCTGTTTTGG 3′) for ARE1 and KP-1 (5′ GGGGGGAATTCGGTACCCAAAATTCAAGCCTT 3′) and KP-5 (5′ GGGGGGATCCATGGTTGTGTTTGTTATTGT 3′) for ARE2. Each set of primers was designed with EcoRI and BamHI recognition sequences to facilitate cloning into the lacZ reporter plasmid pYLZ-6 (22) to yield pARE1-lacZ (pIU1107) and pARE2-lacZ (pIU1113). Plasmids pIU1107 and pIU1113 were used to construct targeted chromosomal integrations. To create an integrating plasmid from pIU1113, the CEN6 region was deleted by digestion with ScaI and replaced with the ScaI sequence from pRS306 (41), yielding pIU1116. The presence of a ScaI site in the promoter of ARE1 prevented the use of this strategy, so its integration was accomplished by removing the ARE2 promoter from pIU1116 and replacing it with the ARE1 promoter sequence from pIU1107, generating pIU1115. Deletion plasmids were made from the integrating vectors pIU1115 and pIU1116. A truncated ARE1 promoter was made by digestion with EcoRI and EcoRV, deleting 500 bp of promoter sequence from pIU1115. The resulting fusion plasmid, pIU1160, contained 500 bp of ARE1 sequence proximal to the ATG start codon. Part of the promoter region of ARE1 (220 bp upstream of the start codon) was amplified by PCR from pIU1115 using Pfu polymerase and the primers KP-4 and Are1-1 (5′ GGGGGGAATTCGTATGTGCTGCTCATCTC 3′). The amplified fragment was digested with EcoRI and BamHI, purified, and ligated into EcoRI- and BamHI-digested pIU1115, to which the promoter sequence had been removed, yielding pIU1146. The deletion of 589 bp of ARE2 distal promoter sequence was done by restriction digestion of pIU1116 with EcoRI and BglII. The EcoRI cohesive ends were blunted by using Klenow fragment and religated, generating pIU1141 containing 411 bp of ARE2 promoter sequence proximal to the ATG codon. Each plasmid used in this study was sequenced by using primers YLZ6-1 (5′ CAATACGCAAACCGCCTG 3′) and YLZ6-2 (5′ AGGCGATTAAGTTGGGTA 3′).
RNA hybridizations and measurements of mRNA decay.
Total RNA was prepared from yeast by using a hot acidic phenol extraction method (3). Equal amounts (15 μg) of total RNA were resolved in 1.2% formaldehyde agarose gels and transferred to nylon membranes by using conventional procedures (3). DNA probes specific for ARE1 and ARE2 were chosen close to the 5′ terminus, since this is the region least conserved between the genes. A 518-bp probe for ARE1 (nucleotides 45 to 564) was made by digesting the construct ARE1/pGEX-3X with BamHI (Z. Guo and S. L. Sturley, unpublished data). A 498-bp probe for ARE2 (nucleotides 45 to 542) was made by digesting the construct ARE2/pGEX-3X with BamHI and EcoRI (20). The probes were radiolabeled with [32P]dCTP (Prime-it; Stratagene) and used in hybridizations at 65°C in ExpressHyb buffer (Clontech). The membrane was washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at room temperature and in 0.1% SSC–0.1% SDS at 60°C. mRNA decay rates were measured by using strain SCY983 (a gift of M.Culbertson), which carries a temperature-sensitive mutation in RNA polymerase II (33). Briefly, SCY983 was grown in 100-ml cultures (0.5 × 107 cells) and the temperature of the culture was abruptly adjusted from 24 to 36°C by adding an equal volume of YEPD medium at 48°C and then transferring the culture flask to a shaker bath at 36°C. Aliquots of the culture were removed after 0 to 100 min, and cell pellets were frozen on dry ice. Total cellular RNA was extracted from the frozen cells, suspended in sterile water, and stored at −80°C.
Protein electrophoresis and immunoblotting.
Microsomes from various yeast strains were prepared and resolved by denaturing gel electrophoresis (5 μg of total protein per lane) by using 10% polyacrylamide in the presence of 0.1% SDS. The proteins were electroblotted to nitrocellulose, blocked in 5% nonfat milk in 20 mM Tris-HCl–137 mM NaCl–0.1% Tween 20 (TBST), and probed at 3.4 μg/ml with chicken immunoglobulin Y (IgY) antibody generated against the NH2-terminal 180 residues of Are2p (20) in TBST–1% nonfat milk for 1 h. Detection of the immune complexes was attained by using horseradish peroxidase-conjugated secondary anti-chicken IgY antibody (Promega) and the ECL Western blotting detection reagent (Amersham).
β-Galactosidase enzyme assays
Strains to be assayed were transformed with the described plasmids linearized at StuI to target integration at the endogenous URA3 locus. In each case, two independent transformants were grown overnight in CSM lacking uracil. Cultures were harvested at an optical density at 600 nm of 0.7 to 0.8 (≈1.5 × 107 cells/ml). β-Galactosidase assays from the promoter-lacZ fusion genes (39) were performed on total protein extracts prepared from duplicate colonies in three independent experiments. Protein concentrations were assessed by using the Bradford dye-binding assay (6).
RESULTS
Transcriptional activity of ARE1 and ARE2 promoters
We and others have demonstrated a marked imbalance in the contribution of the yeast ARE gene products to total sterol esterification within the cell (50, 51, 53). To assess whether this reflects expression differences at the transcriptional level, we quantified the promoter activity of ARE1 and ARE2 in wild-type cells by using promoter-lacZ fusions. We constructed several fusions with various 5′ untranslated regions from each of the genes to determine the minimal sizes of the promoters that exhibit optimal activity (Table 2). The sequences of the lacZ fusion plasmids pIU1115, pIU1160, and pIU1146 (ARE1) and pIU1116 and pIU1141 (ARE2) were confirmed at the nucleotide level relative to the sequences in the Saccharomyces genome database (http://genome-www.stanford.edu/Saccharomyces/). To minimize differences in plasmid copy number or stability, we digested each fusion plasmid with StuI for integration at the URA3 locus. β-Galactosidase activity was assessed by conventional methods. We confirmed that the patterns of expression for each fusion were similar in multiple integrants and in cells carrying fusions expressed autosomally (data not shown).
TABLE 2.
β-Galactosidase activities conferred by ARE1-lacZ and ARE2-lacZ fusions on wild-type strain BWG 1-7a
| Plasmidb (promoter length, bp) | β-Galactosidase activity in BWG1-7aa
|
|
|---|---|---|
| ARE1-lacZ | ARE2-lacZ | |
| pIU1116 (1,000) | 87 + 3 | |
| pIU1141 (411) | 167 + 18 | |
| pIU1115 (1,000) | 1.2 + 0.2 | |
| pIU1160 (500) | 1.7 + 0.2 | |
| pIU1146 (220) | 1.7 + .3 | |
β-Galactosidase activity is expressed as nanomoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein and is the average of two independent transformants assayed in duplicate over 3 days.
The control plasmid lacking an ARE promoter insert typically gave a β-galactosidase activity of 0.5 U or less (21).
ARE2-driven β-galactosidase expression increased in the integrants containing the minimal promoter region, suggesting the presence of transcription-repressing sequences between positions −411 and −1000 of the ARE2 gene. This repressive effect may reflect the overlap of this construct with the 3′ region of the neighboring open reading frame YNR018w. The 411-bp promoter fusion (in pIU1141) that exhibited the greatest activity was thus chosen for subsequent experiments. In promoter deletion experiments for ARE1, all of the promoter lengths tested (220 bp to 1 kb) conferred comparable β-galactosidase activities. The 220-bp promoter fusion from pIU1146 was used for all subsequent experiments because it did not contain DNA sequence from other genes on chromosome III. The β-galactosidase activity of the ARE1 promoter construct was consistently and significantly lower than that conferred by the ARE2 promoter. This is in accordance with the 15 to 25% contribution to the esterification activity of a wild-type strain that can be accounted for by the Are1 enzyme.
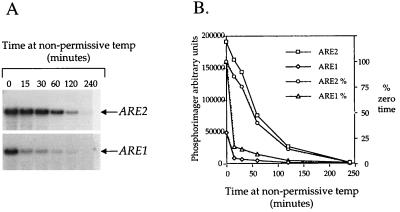
To confirm the fidelity of the reporter gene fusions, we also assessed the steady-state levels of ARE gene transcripts in wild-type cells by Northern RNA hybridizations by using probes labeled to similar specific activity and by comparison to expression of the ACT1 gene encoding actin. ARE1 transcripts were consistently observed at significantly lower levels than the ARE2 transcript (Fig. 1, zero time point). Furthermore, the ARE1 gene transcript was significantly less stable than the ARE2 transcript (Fig. 1). By using a temperature-sensitive mutation in the largest subunit of RNA polymerase II (rpb1-1), we studied the decay of the ARE transcripts over 4 h after a rapid shift to the nonpermissive temperature. The ARE1 transcript displayed a half-life of approximately 5 min, whereas the half-life of ARE2 was approximately 60 min. Thus, the differences in ARE-mediated sterol esterification activity under normal aerobic growth conditions can most simply be explained by the relative abundance of the specific transcripts. This results from the combined effects of differences in transcription initiation and transcript stability for each gene.
FIG. 1.
Stability measurements of ARE1 and ARE2 transcripts. Strain SCY983 (temperature sensitive for RNA polymerase II [33]) was grown to mid-logarithmic phase at 24°C and rapidly shifted to the nonpermissive temperature (36°C). Total RNA was extracted from samples withdrawn at the indicated times and processed for hybridization analysis with a ARE1- or ARE2-specific probe. (A) Autoradiograph of RNA blot hybridization. (B) Graphical representation of data collected from a phosphorimager of panel A. Data are presented as total counts (arbitrary phosphorimager units) or as a percentage of the zero time point value.
Overexpression of Are1p and Are2p and steryl ester quantification.
To assess whether the ARE-encoded enzymes differentially contribute to sterol esterification at the posttranscriptional level, the contribution of transcription was minimized by overexpressing the ARE1 and ARE2 genes by using the multicopy vectors Yep3-16 (ARE1) and Yep352Are2 (ARE2) in an are1Δ are2Δ deletion strain. We first addressed whether yeast can overexpress ARE1 and ARE2 and accumulate steryl esters and whether the enzymes esterify the same or different sterols. Free sterols and steryl esters were extracted and quantified from 100-ml cultures grown in CSM containing additional uracil for 36 h. Free sterol levels were not statistically significantly different in are1Δ are2Δ mutants, regardless of which gene was expressed (Tables 3 and 4). Overexpression of either gene in are1Δ are2Δ mutants showed little difference in the total amount of steryl ester accumulated. It is possible that the accumulation of steryl ester is regulated by substrate supply. Quantitative sterol analysis was then performed with an are1Δ are2Δ mutant strain (SCY059) overexpressing either ARE1 (Table 3) or ARE2 (Table 4) to quantify the sterol type accumulating in the free and ester fractions. Cells were grown in 100-ml cultures of CSM containing additional uracil for 36 h and harvested. Free sterol and steryl ester amounts were determined as percentages of the dry weight. Overexpression of ARE1 or ARE2 had no significant effect on free sterol composition; ergosterol was the major sterol produced in the free sterol fraction. By contrast, the ester fraction of cells overexpressing ARE1 show a marked accumulation of sterol intermediates, specifically lanosterol and, to a lesser extent, zymosterol. Lanosterol represents 15.5% of the total ester fraction, whereas ergosterol represents only 19% when ARE1 is overexpressed. In this genetic background or in a wild-type background, lanosterol is converted to the end product ergosterol or other sterol intermediates since it is not esterified to levels greater than 1% of the total esters. Cells overexpressing ARE2 favor esterification of ergosterol (39% of the total ester fraction), while lanosterol esterification is again minor (1% of the ester fraction). Although Are1p and Are2p clearly esterify all of the sterol types analyzed, these data suggest possible substrate preferences in vivo. Are1p preferentially esterified sterol intermediates, especially lanosterol, whereas ergosterol was the preferred substrate for Are2p
TABLE 3.
Quantification of sterols and sterol esters during overexpression of ARE1 in YEp3-16 (strain SCY059 [are1Δ are2Δ])
| Compound(s) | Free sterolsa (% of free compounds) | Estersb (% of total esters) | SE/FS ratioc |
|---|---|---|---|
| Lanosterol | 7.6 ± 14 (1.2) | 484 ± 159 (15.5) | 63 |
| Zymosterol | 4.1 ± 8.6 (0.6) | 1,321 ± 284 (42.3) | 322 |
| Fecosterol | 63 ± 9.2 (10.2) | 329 ± 70 (10.5) | 5 |
| Episterol | 36 ± 9 (5.8) | 396 ± 120 (12.6) | 11 |
| Ergosterol | 504 ± 125 (82.2) | 598 ± 199 (19.1) | 1 |
| Total sterols | 614 ± 138 (100) | 3,128 ± 798 (100) | 5 |
Sterols (micrograms per gram [wet weight] of cells ± the standard deviation) from three separate cultures were extracted, and the average value is reported.
Mass of sterols (micrograms per gram [wet weight] of cells ± the standard deviation) in the esterified form after saponification (three separate cultures were extracted, and the average value is reported).
The SE/FS ratio represents the proportion of steryl ester (SE) relative to that remaining unesterfied (FS).
TABLE 4.
Quantification of sterols and steryl esters during overexpression of ARE2 in Yep352Are2 (strain SCY059 [are1Δ are2Δ])
| Compound(s) | Free sterolsa (% of free compounds) | Estersb (% of total esters) | SE/FS ratioc |
|---|---|---|---|
| Lanosterol | 1.8 ± 4.9 (0.3) | 22.9 ± 8.6 (0.8) | 13 |
| Zymosterol | 24 ± 27 (4.7) | 1,029 ± 249 (34.9) | 43 |
| Fecosterol | 56.4 ± 14 (11.1) | 467 ± 143 (15.8) | 8 |
| Episterol | 47 ± 24 (9.2) | 289 ± 96 (9.8) | 6 |
| Ergosterol | 380 ± 198 (74.7) | 1,140 ± 302 (38.7) | 3 |
| Total sterols | 509 ± 251 (100) | 2,948 ± 714 (100) | 6 |
Sterols (micrograms per gram [wet weight] of cells ± the standard deviation) from three separate cultures were extracted, and the average value is reported.
Mass of sterols (micrograms per gram [wet weight] of cells ± the standard deviation) in the esterified form after saponification (three separate cultures were extracted, and the average value is reported).
The SE/FS ratio represents the proportion of steryl ester (SE) relative to that remaining unesterfied (FS).
Regulation of ARE gene expression in response to impaired ergosterol biosynthesis.
The observation that overexpression of ARE1 and ARE2 causes accumulation of different steryl esters led us to question whether these genes may also respond to different sterol signals. We were interested in determining whether early or late sterol pathway mutants, and thus, the accumulation of intermediates in these strains, would affect the transcription of ARE1 and ARE2. To assess the effects of sterol intermediate accumulation in late sterol pathway mutants, an isogenic panel of ergosterol biosynthetic deletion mutants transformed with linearized plasmids pIU1141 and pIU1146 was assayed for β-galactosidase activity under aerobic conditions (Table 5). To assess the effects of a lesion in the essential gene ERG24, the ergosterol biosynthesis inhibitor fenpropimorph was used. Fenpropimorph targets both the sterol C14 reductase (ERG24) and the C8 sterol isomerase (ERG2), but the C14 reductase is before the isomerase in the pathway.
TABLE 5.
β-Galactosidase activity conferred by ARE1-lacZ and ARE2-lacZ promoter fusions in response to lesions or pharmacological inhibition of the ergosterol biosynthetic pathway
| Strain or condition | β-Galactosidase activity (fold change relative to wild type)d
|
|
|---|---|---|
| ARE1-lacZ | ARE2-lacZ | |
| Wild-type BWG 1-7aa | 1.6 ± 0.2 (1.0) | 186 ± 17 (1.0) |
| erg2 mutant | 6.0 ± 0.9 (3.8) | 184 ± 30 (1.0) |
| erg3 mutant | 6.5 ± 0.5 (4.1) | 246 ± 25 (1.3) |
| erg6 mutant | 5.2 ± 0.9 (3.3) | 199 ± 30 (1.1) |
| Fenpropimorph addedb | 3.7 ± 0.3 (2.3) | 339 ± 30 (1.8) |
| Wild-type JR 527c | 1.7 ± 0.3 (1.0) | 80 ± 11 (1.0) |
| hmg1 mutant | 4.2 ± 0.5 (2.5) | 156 ± 20 (2.0) |
| hmg2 mutant | 1.5 ± 0.2 (0.9) | 103 ± 16 (1.3) |
The erg mutants were created in the BWG 1-7a background.
Fenpropimorph was added to strain BWG 1-7a at 0.5 μM, the cells were grown to 50% and then inhibited by the addition of 25 μM fenpropimorph, and the cells grew for an additional 18 h.
JR527 is the wild-type strain isogenic to the hmg1 and hmg2 mutations.
β-Galactosidase activity is reported as nanomoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein and is the average of two independent transformants assayed in duplicate over 3 days.
ARE1 promoter activity increased in the erg2Δ (3.8-fold), erg3Δ (4.1-fold), and erg6Δ (3.3-fold) late pathway mutants, suggesting that ARE1 expression is modulated in response to accumulation of ergosterol intermediates (Table 5). ARE2 did not display significant transcriptional changes in response to the same mutations, suggesting that accumulation of sterol intermediates is not a regulatory signal for ARE2 expression. Both ARE1 and ARE2 expression increased in BWG 1-7a cells inhibited with fenpropimorph. Yeast cells inhibited with fenpropimorph accumulate ignosterol (4), an intermediate in sterol biosynthesis. These results were confirmed in Northern hybridizations (not shown).
We were also interested in the transcription of ARE1 and ARE2 in strains lacking the HMG1 and HMG2 genes encoding isoforms of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. HMG1 encodes the major isoform for this activity, which is rate limiting for isoprenoid biosynthesis (16) and is oxygen regulated. HMG2p activity is increased during oxygen limitation (21). Expression of both ARE1- and ARE2-lacZ fusions in an hmg1 mutant increased twofold over the wild-type level, while no significant changes were observed in the hmg2 mutant (Table 5).
Transcription of ARE1 and ARE2 due to changes in esterification of sterol.
To determine whether the ARE1 and ARE2 genes are subject to end product regulation, isogenic strains SCY059 (are1Δ are2Δ), SCY060 (are1Δ), SCY061 (are2Δ), and SCY062 (wild type) were transformed with pIU1141 and pIU1146 and assayed for β-galactosidase activity. Plasmids pADH (vector), pADH5-36 (ARE1), and pS5ARE2 were transformed into SCY059 (are1Δ are2Δ) to test whether overexpression of the ARE genes would alter transcription. These plasmids use the alcohol dehydrogenase (ADH1) promoter in place of the endogenous promoter to drive expression of ARE1 and ARE2. The ADH1 promoter is constitutively active and should not be subject to regulation. No significant differences in transcription of ARE1 or ARE2 were observed in the are mutant strains or when either gene was overexpressed (Table 6). The unchanged gene expression in response to mutations in the ARE genes, in addition to the lack of response when esters are overproduced, suggests that ARE1 and ARE2 are not subject to end product feedback inhibition at the level of transcription.
TABLE 6.
β-Galactosidase activity conferred by ARE1-lacZ and ARE2-lacZ fusions on strains either bearing deletions in the endogenous ARE genes or overexpressing ARE1 or ARE2
| Genotype | β-Galactosidase activity (fold change relative to wild type)a
|
|
|---|---|---|
| ARE1-lacZ | ARE2-lacZ | |
| ARE1 ARE2 (wild type) | 2.1 ± 0.3 (1.0) | 140 ± 16 (1.0) |
| are1Δ are2Δ | 2.0 ± 0.2 (0.95) | 158 ± 18 (1.1) |
| ARE1 are2Δ | 2.0 ± 0.3 (0.95) | 177 ± 36 (1.3) |
| are1Δ ARE2 | 2.1 ± 0.3 (1.0) | 154 ± 28 (1.1) |
| ARE1 ARE2/pS5 | 1.6 ± 0.3 (1.0) | 126 ± 13 (1.0) |
| ARE1 ARE2/pADH5-36 | 2.0 ± 0.3 (1.3) | |
| ARE1 ARE2/pS5ARE2 | 150 ± 14 (1.2) | |
β-Galactosidase activity is reported as nanomoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein and is the average of two independent transformants assayed in duplicate over 3 days.
Divergent effects of heme and oxygen on ARE1 and ARE2 expression.
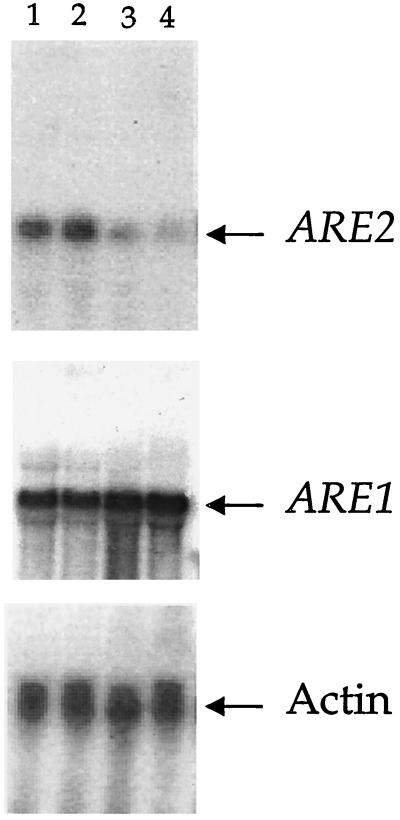
Heme is required for sterol synthesis and as a cofactor for the cytochrome P450 enzymes lanosterol 14α-demethylase and sterol C-22 desaturase and is associated as a cytochrome b5 cofactor with the C-5 desaturase and C-24 sterol methyl oxidase. Heme may also have a role in sterol esterification since δ-aminolevulinic acid (δ-ALA) synthase mutants (hem1) supplemented with δ-ALA displayed a fourfold increase in steryl ester synthase activity compared to heme depleted cells (24). In strains lacking the HEM1 gene (TKY22 [25]), supplementation with 50 μg of δ-ALA, the enzymatic product of Hem1p, per ml simulates a wild-type HEM1 sterol profile. By contrast, the addition of 0.5 μg of δ-ALA per ml allows slow growth, low levels of ergosterol, and elevated amounts of lanosterol (45). TKY22 cells supplemented with 50 μg of δ-ALA per ml were compared to those exhibiting the hem1 mutant sterol phenotype (0.5 μg of δ-ALA per ml). While ARE2 was repressed by growth under δ-ALA-limiting conditions, the ARE1 gene was clearly induced (Fig. 2). These results were verified by the introduction of the ARE1 and ARE2 reporter constructs into strain TKY22. ARE2-mediated expression was decreased ninefold in a hem1 δ-ALA-deficient background, whereas ARE1-lacZ activity increased over fivefold in the same situation (Table 7). TKY22 cells grown in 50 μg of δ-ALA per ml have a wild-type sterol profile, accumulating ergosterol as the major sterol (45.6%). Strain TKY22 grown in 0.5 μg of δ-ALA per ml accumulates 16% lanosterol and markedly decreased levels of ergosterol in the total sterols (4.2%). Increased lanosterol esterification could reflect the up-regulation of ARE1 in the hem1 strain.
FIG. 2.
RNA blot hybridization analysis of ARE1 and ARE2 gene expression in hem1 mutants. Strain TKY22 was grown under heme-sufficient (50 μg of δ-ALA per ml; lanes 1 and 2) or heme-depleted (0.5 μg of δ-ALA per ml; lanes 3 and 4) conditions. Total RNA was extracted and analyzed by RNA blot hybridization with an ARE1- or ARE2-specific probe. The message from the ACT1 gene encoding actin was used as a loading control.
TABLE 7.
β-Galactosidase activity conferred by ARE1-lacZ and ARE2-lacZ fusions during changes in heme or due to mutations in transcription factors HAP1 and ROX1
| Strain (condition or genotype) | β-Galactosidase activity (fold change relative to wild type)c
|
|
|---|---|---|
| ARE1-lacZ | ARE2-lacZ | |
| TKY22 (50 μg of δ-ALA/ml) | 1.8 ± 0.1 (1.0) | 85 ± 17 (1.0) |
| TKY22 (0.5 μg of δ-ALA/ml) | 9.3 ± 0.2 (5.2) | 9.0 ± 1.5 (0.11) |
| BWG 1-7aa | 1.9 ± 0.2 (1.0) | 155 ± 19 (1.0) |
| LPY22 (hap1) | 1.7 ± 0.3 (0.9) | 25 ± 5.0 (0.16) |
| RZ53-6b | 0.91 ± 0.17 (1.0) | 43 ± 4.7 (1.0) |
| RZ53-6/rox1 | 4.6 ± 0.87 (5.1) | 130 ± 20 (3.0) |
The hap1 mutant was created in the BWG 1-7a background.
RZ53-6 is the wild-type strain isogenic to the rox1 mutant.
β-Galactosidase activity is reported as nanomoles of o-nitrophenyl-β-d-galatopyranoside hydrolyzed per minute per milligram of protein and is the average of two independent transformants assayed in duplicate over 3 days.
Heme is also required for activation of the transcriptional activator Hap1p (heme-activated protein) in response to oxygen (19). Hap1p regulates many genes involved in oxygen-requiring processes such as cytochrome synthesis, sterol biosynthesis, fatty acid biosynthesis, and oxidative stress response (52). Heme also mediates repression of hypoxic genes during aerobic growth by increased expression of the ROX1 transcriptional repressor (46). Transcription of ROX1 is regulated by the Hap1 activator. During aerobic growth, Rox1p binds to promoters of hypoxic genes, repressing their transcription. During anaerobiosis, expression of ROX1 is decreased and hypoxic gene transcription is derepressed. To test the role of these transcription factors in the regulation of the ARE genes, we measured the activity of the promoter fusions in a strain lacking HAP1 or ROX1. Strains BWG1-7a (wild type) and LPY22 (hap1Δ) are isogenic and were used to measure ARE transcription in a hap1 deletion strain (37), while the effects of ROX1 on the ARE genes were measured in the isogenic strains RZ53-6 (wild type) and RZ53-6/rox1 (rox1Δ) (30). The effects of HAP1 and ROX1 on transcription of ARE1 and ARE2 are consistent with different effects of heme on ARE1 and ARE2 expression (Table 7). ARE2 expression decreased sixfold in the hap1 mutant. ARE1 transcription was unchanged by the hap1 mutation, consistent with the lack of a Hap1p consensus sequence in its promoter. Both ARE1 and ARE2 are upregulated by the absence of the transcriptional repressor rox1 (fivefold and threefold, respectively).
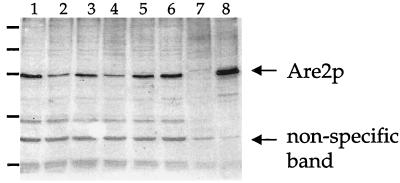
We wished to confirm the effects of these alterations in gene transcription at the protein level and focused on the Are2 protein since it demonstrated striking changes in transcription profiles. By using a polyclonal antibody to the NH2-terminal 180 residues of Are2p (20), we confirmed that the changes in transcript abundance produce a corresponding change in microsomal Are2 protein levels. The Are2 protein was more abundant in the presence of normal HAP1 function and δ-ALA levels but was upregulated in the absence of the ROX1 gene (Fig. 3).
FIG. 3.
Regulation of Are2p due to alterations in heme metabolism or mutations in the HAP1 and ROX1 transcription factors. Microsomes from wild-type (BWG, lane 1; RZ253–6, lane 5), hap1 (lane 2), rox1 (lane 6), or hem1 (lanes 3 and 4 with 50 or 0.5 μg of δ-ALA per ml, respectively) cells grown to mid-logarithmic phase were prepared and resolved by denaturing gel electrophoresis (5 μg of total protein per lane). The proteins were transferred to nitrocellulose and probed with chicken IgY antibody generated against the NH2-terminal 180 residues of Are2p (20). Detection of the immune complexes was attained by using a horseradish peroxidase-conjugated secondary anti-chicken IgY antibody (Promega) and the ECL Western blotting detection reagent (Amersham). Lanes 7 and 8 represent microsomes from are1Δ are2Δ cells carrying a vector control or ARE2 on YEpARE2. The indicated nonspecific band serves as a loading control.
DISCUSSION
Our results confirm and extend previous analyses indicating that the two yeast ARE genes have different physiological functions, especially in response to oxygen (46, 51). The most fundamental question is why yeast contains two sterol esterification genes and yet neither is essential for survival. Previous work using are1 and are2 strains indicate that the role of Are1p is to esterify sterol intermediates, principally lanosterol, the first sterol in the pathway, whereas Are2p principally esterifies the end product ergosterol (38, 48, 53). Our results are similar in that we overexpressed ARE1- and ARE2-containing plasmids in an are1 are2 double deletion strain. Under the conditions reported here, the esterified-to-free ratio of lanosterol was fivefold greater in an are1 are2 strain overexpressing ARE1 relative to the same strain overexpressing ARE2. However, the esterified-to-free ratio of zymosterol in an are1 are2 double mutant overexpressing ARE1 relative to the same strain overexpressing ARE2 was only one-fourth. These results suggest that the role of ARE1 under aerobic growth conditions is to limit conversion of lanosterol to zymosterol, thereby interrupting the pathway such that ergosterol precursors can be stored in microlipid droplets and mobilized to free sterols as required (28)
The role of ARE1 in esterifying sterol intermediates is confirmed by our analysis indicating that accumulation of sterol intermediates in erg2, erg3, and erg6 strains results in significant increases in ARE1 expression (Table 5). erg2 mutants accumulate sterol intermediates containing only Δ-8 sterols, erg3 strains are unable to desaturate sterols at the C-5 position, and erg6 mutants are unable to methylate the sterol side chain. All three strains are viable even though the end product ergosterol is not made. Although the accumulation of ergosterol intermediates gives rise to changes in ARE gene expression, a reduction in ergosterol, as seen in an HMG1 mutant, also results in modest increases in both ARE1 and ARE2 expression. A mutation in the minor isoform of HMG-CoA reductase, HMG2, essentially does not affect ARE transcription.
Although an interruption in ergosterol biosynthesis or decreased cellular levels of ergosterol affect ARE gene transcription, mutations in the two ARE genes themselves have no effect on transcription. We found that ARE1-driven expression of the lacZ construct was unaltered in a wild-type, are2Δ, or are1Δ are2Δ strain or in a wild-type strain overexpressing ARE1. Similar results were observed for ARE2 expression. Neither deletion nor overexpression of ARE2 altered the expression of the ARE2-lacZ construct. The genes are thus not subject to end product regulation. Sterol synthesis is intimately dependent upon the cell's being heme competent. Both ERG11 and ERG5 encode cytochrome P450 enzymes required for C-14 demethylation and C-22 desaturation, respectively, and ERG3 requires the cofactor cytochrome b5 for desaturation at the C-5 position in the sterol molecule. The HMG-CoA reductase genes HMG1 and HMG2 are positively and negatively regulated by heme (45). It appears that a similar situation of contraregulation exists for ARE1 and ARE2, which are induced or repressed by heme (Table 7). We demonstrated a sixfold decrease in ARE2 expression in a hap1 strain, and Thorsness et al. (45) demonstrated a 23-fold decrease in HMG1 expression in a hap1 strain. However, the promoter for neither gene predicts canonical HAP1 DNA binding motifs (5′ CGGNNNTANCGG 3′ [27]). Similarly, the ERG9 promoter lacks a HAP1 recognition motif and yet a twofold decrease in ERG9-lacZ expression is seen in a hap1 background (26). These results suggest either that the effects of the HAP1 mutations are indirect or that novel HAP1 binding domains exist in ergosterol biosynthetic genes. Lastly, our results indicate an increase in ARE1 expression during anaerobiosis. This is consistent with data reported by Valachovic et al. (48) and also with a DNA microarray analysis of anaerobically growing cells which revealed an eightfold increase in ARE1 message levels (44). We demonstrated that in a rox1 mutant background, both ARE1 and ARE2 expression increased. The ARE1 promoter does contain a canonical ROX1 recognition sequence (3′ GCTATTGTTCGC 5′ [29]) located on the antisense strand at −135 bp upstream of the ATG. However, it is unclear how ROX1 exerts an effect on the transcription of ARE2, although similar results were seen in an ERG9-lacZ fusion, which also lacks a ROX1 recognition motif (26).
This investigation elucidates an important physiological role for Are1p during anaerobic growth when heme is limiting, as well as under conditions under which ergosterol is either not synthesized or made at less than wild-type levels. Conversely, we show that Are2p is optimally required during aerobiosis when ergosterol is plentiful.
ACKNOWLEDGMENTS
This work represents equal contributions from the Sturley and Bard laboratories.
This work was supported in part by NIH grants DK54320 to S.L.S. and GM62104 to M.B. S.L.S. also acknowledges grants from the Ara Parseghian Medical Research Foundation and the Hirschl/Weil-Caulier Trust. S.L.S. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Anderson R A, Joyce C, Davis M, Reagan J W, Clark M, Shelness G S, Rudel L L. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 2.Arthington-Skaggs B A, Crowell D N, Yang H, Sturley S L, Bard M. Positive and negative regulation of a sterol biosynthetic gene (ERG3) in the post-squalene portion of the yeast ergosterol pathway. FEBS Lett. 1996;392:161–165. doi: 10.1016/0014-5793(96)00807-1. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1998. [Google Scholar]
- 4.Baloch R I, Mercer E I, Wiggins T E, Baldwin B C. Inhibition of ergosterol biosynthesis in Saccharomyces cerevisiae and Ustilago maydis by tridemorph, fenpropiomorph, and fenpropidin. Phytochemistry. 1984;23:2219–2226. [Google Scholar]
- 5.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brown M S, Goldstein J L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buhman K K, Accad M, Novak S, Choi R S, Wong J S, Hamilton R L, Turley S, Farese R V., Jr Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 9.Burke D, Dawson D, Stearns T. Methods in yeast genetics, 2000 edition. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 10.Cases S, Novak S, Zheng Y W, Myers H M, Lear S R, Sande E, Welch C B, Lusis A J, Spencer T A, Krause B R, Erickson S K, Farese R V., Jr ACAT-2, A second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 11.Chang C C, Chen J, Thomas M A, Cheng D, Del Priore V A, Newton R S, Pape M E, Chang T Y. Regulation and immunolocalization of acyl-coenzyme A:cholesterol acyltransferase in mammalian cells as studied with specific antibodies. J Biol Chem. 1995;270:29532–29540. doi: 10.1074/jbc.270.49.29532. [DOI] [PubMed] [Google Scholar]
- 12.Chang C C, Lee C Y, Chang E T, Cruz J C, Levesque M C, Chang T Y. Recombinant acyl-CoA:cholesterol acyltransferase-1 (ACAT-1) purified to essential homogeneity utilizes cholesterol in mixed micelles or in vesicles in a highly cooperative manner. J Biol Chem. 1998;273:35132–35141. doi: 10.1074/jbc.273.52.35132. [DOI] [PubMed] [Google Scholar]
- 13.Chang C C, Sakashita N, Ornvold K, Lee O, Chang E T, Dong R, Lin S, Lee C Y, Strom S C, Kashyap R, Fung J J, Farese R V, Jr, Patoiseau J F, Delhon A, Chang T Y. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem. 2000;275:28083–28092. doi: 10.1074/jbc.M003927200. [DOI] [PubMed] [Google Scholar]
- 14.Chang T Y, Chang C C, Cheng D. Acyl-coenzyme A:cholesterol acyltransferase. Annu Rev Biochem. 1997;66:613–638. doi: 10.1146/annurev.biochem.66.1.613. [DOI] [PubMed] [Google Scholar]
- 15.Cheng D, Chang C C, Qu X, Chang T Y. Activation of acyl-coenzyme A:cholesterol acyltransferase by cholesterol or by oxysterol in a cell-free system. J Biol Chem. 1995;270:685–695. doi: 10.1074/jbc.270.2.685. [DOI] [PubMed] [Google Scholar]
- 16.Dimster-Denk D, Thorsness M K, Rine J. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in Saccharomyces cerevisiae. Mol Biol Cell. 1994;5:655–665. doi: 10.1091/mbc.5.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farese R V., Jr Acyl CoA:cholesterol acyltransferase genes and knockout mice. Curr Opin Lipidol. 1998;9:119–123. doi: 10.1097/00041433-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein J L, Brown M S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 19.Guarente L, Lalonde B, Gifford P, Alani E. Distinctly regulated tandem upstream activation sites mediate catabolite repression of the CYC1 gene of S. cerevisiae. Cell. 1984;36:503–511. doi: 10.1016/0092-8674(84)90243-5. [DOI] [PubMed] [Google Scholar]
- 20.Guo, Z., D. Cromley, J. T. Billheimer, and S. L. Sturley. Identification of potential substrate binding sites in yeast and human acyl-CoA sterol acyltransferases by mutagenesis of conserved sequences. J. Lipid Res., in press. [PubMed]
- 21.Hampton R Y, Rine J. Regulated degradation of HMG-CoA reductase, an integral membrane protein of the endoplasmic reticulum, in yeast. J Cell Biol. 1994;125:299–312. doi: 10.1083/jcb.125.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermann H, Hacker U, Bandlow W, Magdolen V. pYLZ vectors: Saccharomyces cerevisiae/Escherichia coli shuttle plasmids to analyze yeast promoters. Gene. 1992;119:137–141. doi: 10.1016/0378-1119(92)90079-5. [DOI] [PubMed] [Google Scholar]
- 23.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keesler G A, Casey W M, Parks L W. Stimulation by heme of steryl ester synthase and aerobic sterol exclusion in the yeast Saccharomyces cerevisiae. Arch Biochem Biophys. 1992;296:474–481. doi: 10.1016/0003-9861(92)90600-2. [DOI] [PubMed] [Google Scholar]
- 25.Keng T. HAP1 and ROX1 form a regulatory pathway in the repression of HEM13 transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:2616–2623. doi: 10.1128/mcb.12.6.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy M A, Barbuch R, Bard M. Transcriptional regulation of the squalene synthase gene (ERG9) in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1999;1445:110–122. doi: 10.1016/s0167-4781(99)00035-4. [DOI] [PubMed] [Google Scholar]
- 27.King D A, Zhang L, Guarente L, Marmorstein R. Structure of HAP1-18-DNA implicates direct allosteric effect of protein-DNA interactions on transcriptional activation. Nat Struct Biol. 1999;6:22–27. doi: 10.1038/4893. [DOI] [PubMed] [Google Scholar]
- 28.Leber R, Zinser E, Hrastnik C, Paltauf F, Daum G. Export of steryl esters from lipid particles and release of free sterols in the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1995;1234:119–126. doi: 10.1016/0005-2736(94)00270-y. [DOI] [PubMed] [Google Scholar]
- 29.Lowry C V, Cerdán M E, Zitomer R S. A hypoxic consensus operator and a constitutive activation region regulate the ANB1 gene of Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5921–5926. doi: 10.1128/mcb.10.11.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowry C V, Zitomer R S. ROX1 encodes a heme-induced repression factor regulating ANB1 and CYC7 of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:4651–4658. doi: 10.1128/mcb.8.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meiner V M, Cases S, Myers H, Sande E R, Bellosta S, Schambelan M, Pitas R E, McGuire J, Herz J, Farese R V. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci USA. 1996;93:14041–14046. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molzahn S W, Woods R A. Polyene resistance and the isolation of sterol mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1972;72:339–348. doi: 10.1099/00221287-72-2-339. [DOI] [PubMed] [Google Scholar]
- 33.Nonet M, Scafe C, Sexton J, Young R. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987;7:1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oelkers P, Behari A, Cromley D, Billheimer J T, Sturley S L. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J Biol Chem. 1998;273:26765–26771. doi: 10.1074/jbc.273.41.26765. [DOI] [PubMed] [Google Scholar]
- 35.Oelkers P, Sturley S L, Tinkelenberg A. Sterol esterification and homeostasis in a model eukaryote. In: Freeman D A, Chang T-Y, editors. Intracellular cholesterol transport. Norwell, Mass: Kluwer Academic Publishers; 1998. pp. 43–51. [Google Scholar]
- 36.Pape M E, Schultz P A, Rea T J, DeMattos R B, Kieft K, Bisgaier C L, Newton R S, Krause B R. Tissue specific changes in acyl-CoA:cholesterol acyltransferase (ACAT) mRNA levels in rabbits. J Lipid Res. 1995;36:823–838. [PubMed] [Google Scholar]
- 37.Pfeifer K, Prezant T, Guarente L. Yeast HAP1 activator binds to two upstream activation sites of different sequence. Cell. 1987;49:19–27. doi: 10.1016/0092-8674(87)90751-3. [DOI] [PubMed] [Google Scholar]
- 38.Polakowski T, Bastl R, Stahl U, Lang C. Enhanced sterol-acyl transferase activity promotes sterol accumulation in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1999;53:30–35. doi: 10.1007/s002530051610. [DOI] [PubMed] [Google Scholar]
- 39.Rose M, Botstein D. Construction and use of gene fusions to lacZ (beta-galactosidase) that are expressed in yeast. Methods Enzymol. 1983;101:167–180. doi: 10.1016/0076-6879(83)01012-5. [DOI] [PubMed] [Google Scholar]
- 40.Seo T, Oelkers P M, Giattina M R, Worgall T S, Sturley S L, Deckelbaum R J. Differential modulation of ACAT1 and ACAT2 transcription and activity by long chain free fatty acids in cultured cells. Biochemistry. 2001;40:4756–4762. doi: 10.1021/bi0022947. [DOI] [PubMed] [Google Scholar]
- 41.Sikorski R S, Boeke J D. In vitro mutagenesis and plasmid shuffling: from clone gene to mutant yeast. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 42.Sturley S L. A molecular approach to understanding human sterol metabolism using yeast genetics. Curr Opin Lipidol. 1998;9:85–91. doi: 10.1097/00041433-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Sturley S L. Molecular aspects of intracellular sterol esterification: the acyl coenzyme A:cholesterol acyltransferase (ACAT) reaction. Curr Opin Lipidol. 1997;8:167–173. doi: 10.1097/00041433-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 44.ter Linde J J, Liang H, Davis R W, Steensma H Y, van Dijken J P, Pronk J T. Genome-wide transcriptional analysis of aerobic and anaerobic chemostat cultures of Saccharomyces cerevisiae. J Bacteriol. 1999;181:7409–7413. doi: 10.1128/jb.181.24.7409-7413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorsness M, Schafer W, D'Ari L, Rine J. Positive and negative transcriptional control by heme of genes encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:5702–5712. doi: 10.1128/mcb.9.12.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trueblood C E, Wright R M, Poyton R O. Differential regulation of the two genes encoding Saccharomyces cerevisiae cytochrome c oxidase subunit V by heme and the HAP2 and REO1 genes. Mol Cell Biol. 1988;8:4537–4540. doi: 10.1128/mcb.8.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uelman P J, Oka K, Sullivan M, Chang C C Y, Chang T Y, Chan L. Tissue-specific expression and cholesterol regulation of acyl coenzyme A:cholesterol acyltransferase (ACAT) in mice. Molecular cloning of mouse ACAT cDNA, chromosomal localization, and regulation of ACAT in vivo and in vitro. J Biol Chem. 1995;270:26192–26201. doi: 10.1074/jbc.270.44.26192. [DOI] [PubMed] [Google Scholar]
- 48.Valachovic M, Hronská L, Hapala I. Anaerobiosis induces complex changes in sterol esterification pattern in the yeast Saccharomyces cerevisiae. FEMS Microbiol Lett. 2001;9844:1–5. doi: 10.1111/j.1574-6968.2001.tb10580.x. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Bard M, Bruner D A, Gleeson A, Deckelbaum R J, Aljinovic G, Pohl T, Rothstein R, Sturley S L. Sterol esterification in yeast: a two gene process. Science. 1996;272:1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- 50.Yang H, Cromley D, Wang H, Billheimer J T, Sturley S L. Functional expression of a cDNA to human acyl-CoA:cholesterol acyltransferase (ACAT) in yeast: species-dependent substrate specificity and inhibitor sensitivity. J Biol Chem. 1997;272:3980–3985. doi: 10.1074/jbc.272.7.3980. [DOI] [PubMed] [Google Scholar]
- 51.Yu C, Kennedy N J, Chang C C Y, Rothblatt J A. Molecular cloning and characterization of two isoforms of Saccharomyces cerevisiae acyl-CoA:sterol acyltransferase. J Biol Chem. 1996;271:24157–24163. doi: 10.1074/jbc.271.39.24157. [DOI] [PubMed] [Google Scholar]
- 52.Zitomer R S, Carrico P, Deckert J. Regulation of hypoxic gene expression in yeast. Kidney Int. 1997;51:507–513. doi: 10.1038/ki.1997.71. [DOI] [PubMed] [Google Scholar]
- 53.Zweytick D, Leitner E, Kohlwein S D, Yu C, Rothblatt J, Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur J Biochem. 2000;267:1075–1082. doi: 10.1046/j.1432-1327.2000.01103.x. [DOI] [PubMed] [Google Scholar]