Abstract
Naked mole rats (Heterocephalus glaber) are a unique rodent species originating in Africa and are increasingly being used in research. Their needs and characteristics differ from those of other rodents used in research. Unique housing systems are necessary to address the special macro- and microenvironmental requirements of NMRs. Naked mole rats are one of the 2 known eusocial mammalian species, are extremely long-living, are active burrowers, and are accustomed to a subterranean environment. Unlike typical rats and mice, naked mole rats need specific, unique housing systems that mimic their natural subterranean environment to support health and longevity. Here we provide an overview of naked mole rats and a housing method that can be used in research settings.
Abbreviations: NMR, naked mole rat; PVC, poly vinyl chloride; SOP, standard operating procedures
Introduction
Heterocephalus glaber, commonly known as the naked mole rat (NMR), is a subterranean rodent of the family Bathyergidae that inhabits areas in East Africa such as Ethiopia, Kenya, and Somalia. This burrowing species has evolved adaptations to successfully thrive in sub-Saharan African climate conditions.8 NMRs are poikilothermic and eusocial (that is, socially organized with a single female producing offspring and nonreproductive individuals caring for young).21,25 Their colonies range from 25 to over 300, with the hierarchical structure composed of a sole breeding female (the queen), 1 to 3 breeding males (pashas), reproductively suppressed male and female workers, juveniles, and neonates. Within the multigenerational colony, NMRs occupy different, overlapping roles, but alloparenting (that is, care provided by individuals other than parents) is exhibited by most workers to varying degrees.1-3,7 In addition, NMRs have maximum reported lifespan of 30 years, the longest of all known rodents.5,11 NMRs are increasingly used in biomedical research as study model in areas such as aging, bone elongation, cancer, cooperative behavior, hypoxia, innate immunity, pain insensitivity, reproduction, and somatosensory processing.6,10,12,15,16,19,34,41,48,51,57 NMRs require unique macro- and microenvironmental conditions compared with standard rodent species. To conduct research with NMRs, laboratory conditions must conform to their natural habitat, which includes providing a tunnel system and areas to forage and burrow.
Here, we describe our experience in developing a novel method for housing NMRs, provide an overview of NMRs, and offer additional suggestions for housing and care of these unique rodents in a research setting.
Taxonomy and Unique Features
Heterocephalus glaber are members of the order Rodentia, family Bathyergidae, which is further divided into solitary, social, and eusocial genera. NMRs and Darmaland mole-rats are the only reported eusocial mammals. Like all rodents, NMRs have upper and lower incisors that grow continuously. However, unlike hystricomorphs that possess a large infraorbital foramen for passage of a significant portion of the medial masseter muscle, Bathyergidaes, considered protogomorphous, retain a small infraorbital foramen, which alters the extension and attachment of masticatory musculature.14,28
NMRs have several unique features and characteristics. One that is of common interest is their eusocial behavior, similar to that of termites and bees.13,45 Although rare incidences of dual queening have been described, the social structure of NMR colonies entails the presence of primarily one dominant queen, her male reproductive partners, and the remaining members, which can range in age, life-stage, and duties.5,17,18,20 The queen reproduces until death and multiple generations within a colony can be from a single queen. Upon the death of the queen, another female will assume this role. In addition, in the research setting, a new breeding pair can be formed by removing a worker female and male and using them to create a new colony.8,9,37 This complex, hierarchical, ‘caste-like’ structure differs somewhat from colony to colony with varying roles and responsibilities among colony mates.21,31,37,38,52
Although they vary in size, NMRs are typically the approximate size of an adult mouse. These cylindrically shaped rodents lack a fur coat but have developed vibrissae over the body that are highly sensitive to touch and tactile stimulation.53 In addition, NMRs demonstrate several other dramatic differences compared with commonly used rat species. For example, NMRs have an average life span of approximately 30 y in captivity and 17 y in their natural habitat. As a result of their evolution in a subterranean habitat, NMRs have several physiologic adaptations for this hypoxic environment including smaller brains, lower cardiac function and heart rate, fewer neurons, lower metabolic rate, and the ability to tolerate lower levels of oxygen.10,11,22,23,30,39,40,42,44,49
NMRs have small eyes and limited sight, likely due to an extremely thin optic nerve.5,9 They use their prominent procumbent incisors for eating and as their primary tool for burrowing, defending territory, and displaying dominance.5,9,24 Compared with the common research rat, the NMR have less hearing capacity, perhaps diminished cochlear function.35,42 Males and females are sexually monomorphic and are one of the slowest growing mammals, with only the breeding female displaying rapid weight gain as compared with colony mates. Their gestation period ranges between 66 and 74 days, which is over 3 times longer than that of a common research rat such as a Sprauge–Dawley. The average litter size of NMRs average litter size is reported to be 12 pups.45,46
Housing and Husbandry
Natural habitat.
NMRs are native to eastern Africa and, although they live primarily in subterranean environments, recent publications describe the occasional dispersing above ground to relocate pups from one site to another.5 In addition, actively burrowed mole hills can often be found in certain regions of Kenya.32,46 Using radio-tracking technology, burrowing systems have been identified with a combined total length of up to 2,245 m (1.4 miles).47 Another unique characteristic of NMRs is that they are poikilotherms that have both endothermic and ectothermic capabilities. Behavioral thermoregulation includes huddling with colony-mates or moving between various chambers that are maintained at different temperatures, whereas physiologic thermoregulation includes mobilization of brown adipose tissue for heat and fuel as needed.5,36,54
The concentration of atmospheric gases in these extensive burrows is heavily dependent on the environment, and is influenced by season, soil, depth of the burrow, moisture (relative humidity), and types of microbes present.12 Although the gaseous composition in naturally occupied habitats is difficult to measure, NMRs are reported to be highly resilient to hypercapnic environments and able to tolerate concentrations of carbon dioxide as high as 80% for up to 5 h in laboratory settings.40 Depending on seasonal shifts, the temperature of the natural habitat may vary between 82 to 90 °F (27.8 to 32.2 °C), with relative humidity reported as high as 90%.45
Laboratory housing and handling.
Several reports have described housing for NMRs in research settings. Most describe constructed tunnel systems made with Plexiglas boxes, polycarbonate caging, or similar plastic material manipulated to fit cylindrical poly vinyl chloride (PVC) or acrylic tubes securely connected between boxes (recapitulating chambers connected by tunnel systems found in their natural burrows).1,9,26,32,50 These retrofitted tunnel systems are often placed in rooms without other species. NMRs can be housed on various types of bedding material ranging from aspen shavings to pelleted paper bedding; at our facility, NMRs are housed on pelleted paper bedding (Teklad 7084, Envigo, Indianaoplis, Indiana, USA) .
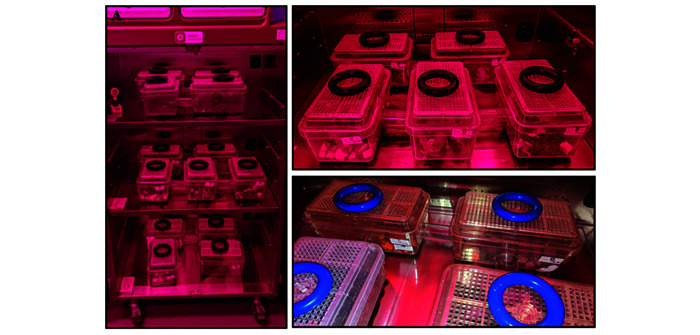
To provide optimal temperature and humidity conditions, supplemental heat and humidity sources are often required to effectively simulate natural settings. Standard recommended temperature and ventilation settings for research rodents, as described in the Guide for the Care and Use of Laboratory Animals, should not be used and can potentially have negative effects on the overall health and wellbeing of NMRs.9,33,55 This situation can present a logistical barrier to those interested in housing NMRs in an open room environment. In addition to fluctuations in temperature and humidity (the latter due to the frequency of air changes in a typical animal facility), NMRs are also sensitive to noise and especially to vibrations. To optimize breeding and colony acclimation, we use a temperature regulated unit that provides a constant source of heat at a temperature set point of 84 °F (28.9 °C) and serves as a secondary barrier between the macro and microenvironment. We use the Tecniplast Aria Ventilated Cabinet BIO-C36 (Tecniplast, Buguggiate, Italy), designed for bioexclusion and containment with built-in pressure mode settings, to provide a controlled environment including temperature, ventilation, and lighting. We adapted this cabinet for NMRs to serve as a primary macroenvironmental chamber and could house over 50 NMRs with up to 3 large colonies in a single unit (Figure 1).
Figure 1.

Tecniplast Aria Ventilated Cabinet BIO-36 in a housing room.
In accordance with IACUC-approved standard operating procedures (SOP) and the pertinent animal use protocols (IS00008428 and IS00008423), the units maintain the following set points for optimal breeding and maintenance: temperature, 85 ± 1 °F (29.4 ± 0.6 °C); humidity, approximately 50%; and light cycle, 12:12-h light-dark (because NMRs are functionally blind, a diurnal light cycle is of less concern than in other rodent species). The microenvironment consists of a tunnel system constructed of impact resistant polycarbonate tunnels (2.25 in. [5.7 cm] with a 2-in. [5.1 cm] ID; McMaster-Carr, Tampa, FL) connected to polycarbonate rodent cages (11½ in. L × 7½ in. W × 5 in. D [29.2 cm × 19.1 cm × 12.7 cm]) and, initially, with Low Profile Microisolation Filter Tops (Lab Products, Seaford Delaware, USA. The microisolation filter tops were later removed as the NMRs proved to be quite curious and often removed or damaged the integrity of the filter paper. Combinations and configurations of cages and tunnels vary in number and size depending on the population of each colony. A hand tap cutting tool was used to create specific fittings and tunnel connections for colony cages. To provide effective support to the tunnel-cage joints, tubing for the tunnels were rough cut on a band saw and then finished to size on an engine lathe. The tapped holes at each end of the tunnel were drilled on a manual mill and tapped by hand using a 4 to 40 hand tap. This approach has successfully prevented tunnel-cage disconnections (Figures 2–5).
Figure 3.
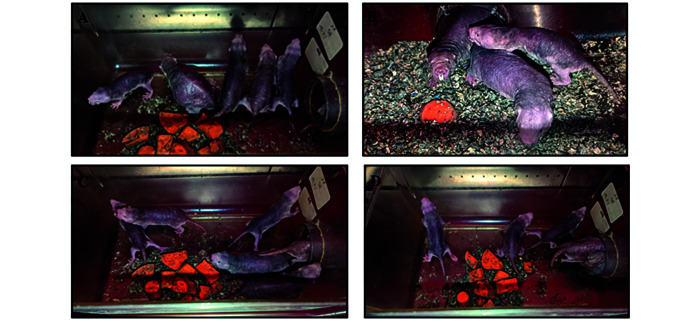
Naked mole rat colonies: A) Pregnant queen with male pashas and colony mates; B-D) Active colony mates.
Figure 4.
Tunnel systems: A) 3-cage tunnel system; B) 4-cage tunnel system; C-D) 5-cage tunnel system (multiple pictures provided to show the system from various angles).
Figure 2.
Tecniplast Aria Ventilated Cabinet BIO-C36 in a housing room. A) Closed cabinet; B) 2 Colony caging placed in temperature-controlled unit; C) Multiple colonies housed on 3 shelves in a ventilated cabinet.
NMRs in the wild primarily use tubers, such as potatoes and sweet potatoes, as a foundational component of their diet.2, In captivity, NMRs will eat cucumbers, grapes, dried fruit, kale, carrots, and corn.1,2,46 Some facilities adapt an infant cereal that can be mixed into the diet sparingly and at defined intervals to provide added nutrients to breeding queens and as a form of enrichment.1,24,56 Our institution provides fresh sweet potatoes daily and a rotation of rice cereal, dried vegetables, raisins, celery, grapes, carrots, apples, and corn on the cob.
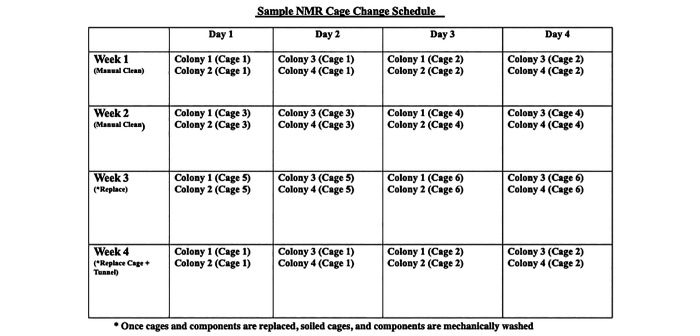
In contrast to standard cage change recommendations consistent with the Guide for the Care and Use of Laboratory Animals,33 NMR cages are changed on a schedule that determines which cages and tunnels are changed at any given time based on timing, density, and active breeding status. In this way, colony scent is always maintained (Figure 5), which in our experience reduces fighting and reduces cannibalism. Cages and tunnel systems are washed in a rack washer heated to 180 °F (82.2 °C; Steris 9500) at intervals defined by IACUC-approved SOPs. Each cage in the colony caging system is replaced with a sanitized cage or component (tube or T piece) at least monthly, except for the toilet chamber. The toilet compartment assists with the reestablishment of the colony scent, so its replacement is delayed by one week (Figure 6).
Figure 6.
Sample naked mole rat cage and caging components cleaning and replacement schedule.
Environmental parameters.
NMRs prefer warm and humid climates in their natural habitat, with reported temperatures ranging from 82.4 to 89.6 °F (28 to 32 °C) and humidity levels up to 90%.45 In captivity, humidity can fluctuate rapidly and the use of an external source, such as a humidifier, that can maintain a consistent, appropriate level is often required to keep rooms within the recommended range of 40% to 50%. Humidity higher than this can lead to moisture buildup at the room level, whereas humidity much lower than this can affect NMR skin health; therefore, low-humidity environments should be accompanied by high-moisture food.7 Due to the limited sight of NMRs, light cycles can be adjusted to red light or in a dimly lit area to mimic the natural subterranean environment. NMRs are also sensitive to vibrations.29 When considering housing in research settings, consideration must be given to placement near objects, animals, or equipment that may cause stress and colony disturbances. In our experience, housing away from autoclaves, tunnel washers, and large animals is beneficial to successful colony maintenance and reproduction. The added use of the Tecniplast Aria Ventilated Cabinet BIO-C36 units provide a consistent, quiet surrounding.
Enrichment can be provided in various forms that are not markedly different from other research rodents. The tunnel system allows unrestricted forward and backward movement and the constant company of colony mates. Fresh fruit and fresh and dried vegetables are provided daily. Bathyegids are normally docile but can be extremely territorial and potentially aggressive to any new animals introduced to the colony. As such, great care is necessary during cage changing or when moving rodents, and any escaped animals must be returned only to their native colony. Reintroduction, particularly after removal for prolonged periods, can be difficult.7 Cupping or gentle lifting is recommended for handling. If caretakers are to handle more than one cohort in a day, new gloves must be used to avoid the introduction of latent pheromones, which may increase inter- and intracage aggression. Due to their innate burrowing behavior, NMRs have been observed creating elaborate hills out of bedding and food, and bedding distribution is often disproportionately sparse in certain areas. These hills can be used to escape from the cage. Placing weights on top of the cage lids is recommended for avoiding escapes (Figure 2 and 4).
Figure 5.
Tunnel systems with weights on lids. A) Multiple colonies housed in Tecniplast Aria Ventilated Cabinet BIO-36; B) 5-tunnel caging system; C) 4-cage tunnel system.
Health surveillance.
NMRs have a unique innate immunity as compared with traditional rodents, including the absence of natural killer cells, the presence of a higher myeloid to lymphoid ratio, and higher proinflammatory cytokine production in macrophages.27,34 A few recent reports describe pathogens affecting NMRs.8,43 This apparent resistance could be due to their innate immune system or their body temperature, which may make them less susceptible to certain pathogens that are known to affect other rodent species.4,9 A spontaneous lethal enteric coronavirus infection was reported in one wild-caught founder population in Africa; the virus may have become virulent in association with inbreeding depression in the colony.43
As part of our standard surveillance procedures, we collect fecal pellets, pelt, and cage swabs at defined intervals and tested routinely for rodent pathogens based on our internal infectious agent exclusion lists. Our institution excludes Helicobacter spp., murine norovirus, Corynebacterium bovis, Corynebacterium HAC2, Syphacia spp., Aspiculuris tetraptera, parainfluenza virus type 1 (Sendai), coronavirus (mouse hepatitis virus), Mycoplasma pulmonis, paramyxovirus (pneumonia virus of mice), parvovirus (minute virus of mice and mouse parvovirus), poliovirus (Theiler murine encephalomyelitis virus strain GDVII), reovirus type 3, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, poxvirus (ectromelia virus), rotavirus (epizootic diarrhea of infant mice virus), papovavirus (polyoma virus), Hantaan virus, CAR bacillus, Clostridium piliforme (Tyzzer’s disease), and Encephalitozoon cuniculi. To date, we have not identified any infectious agents in our NMR population.
Conclusions
NMRs, one of 2 reported eusocial rodent species, are an exception to the rule when housing rodents in research settings. Unlike typical research rodents, NMRs require specific, unique housing systems that mimic their natural subterranean environment to support health and longevity. The use of temperature- and humidity-controlled housing units, along with adapted rodent caging and tunnels, allow these batheygids to be housed at various densities in constructed tunnel systems. These housing units provide consistent temperature, lighting, and ventilation and offer an alternative to commonly reported macroenvironmental housing modalities for NMRs and other rodent species.
Acknowledgments
We acknowledge Ana V. Garcia Almonte, Karah Alyse Sanders, Kiaylah Govan, and Selena Heard of the Division of Comparative Medicine (H Lee Moffitt Cancer Center and Research Institute and the University of South Florida) for their contributions. We also acknowledge Anthony Villicana from the College of Engineering at the University of South Florida for his contribution in fabricating the tunnel system for naked mole rat housing.
References
- 1.Ambar N, Eshar D, Shrader TC, Beaufrère H. 2020. Anesthetic Effects of Intramuscular Alfaxalone-Ketamine in Naked Mole Rats (Heterocephalus glaber). J Am Assoc Lab Anim Sci 59:539–545. 10.30802/AALAS-JAALAS-19-000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson LC, Fox JG, Otto GM, Pritchett-Corning KR, Whary M. T. 2015., 2.
- 3.Artwohl J, Hill T, Comer C, Park T. 2002. Naked mole-rats: unique opportunities and husbandry challenges. Lab Anim (NY) 31:32–36. [DOI] [PubMed] [Google Scholar]
- 4.Artwohl J, Ball-Kell S, Valyi-Nagy T, Wilson SP, Lu Y, Park TJ. 2009. Extreme susceptibility of African naked mole rats (Heterocephalus glaber) to experimental infection with herpes simplex virus type 1. Comp Med 59:83–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Braude S, Holtze S, Begall S, Brenmoehl J, Burda H, Dammann P, Del Marmol D, Gorshkova E, Henning Y, Hoeflich A, Höhn A, Jung T, Hamo D, Sahm A, Shebzukhov Y, Šumbera R, Miwa S, Vyssokikh MY, von Zglinicki T, Averina O, Hildebrandt TB. 2021. Surprisingly long survival of premature conclusions about naked mole-rat biology. Biol Rev Camb Philos Soc 96:376–393. 10.1111/brv.12660. [DOI] [PubMed] [Google Scholar]
- 6.Browe BM, Vice EN, Park TJ. 2020. Naked Mole-Rats: Blind, Naked, and Feeling No Pain. Anat Rec (Hoboken) 303:77–88. 10.1002/ar.23996. [DOI] [PubMed] [Google Scholar]
- 7.Buffenstein R, Park TJ, Holmes MM. 2021. The Extraordinary Biology of the Naked Mole-Rat. Cham, Switzerland: Springer. 10.1007/978-3-030-65943-1. [DOI] [Google Scholar]
- 8.Buffenstein R, Amoroso V, Andziak B, Avdieiev S, Azpurua J, Barker AJ. 2022. The naked truth: a comprehensive clarification and classification of current ‘myths’ in naked mole-rat biology. Biol Rev Camb Philos Soc 97:115–140. 10.1111/brv.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buffenstein R, Park T, Hanes M, Artwohl JE. 2012. Naked Mole Rat, p 1055–1074. In: Suckow MA, Stevens KA, Wilson RP, editors. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Waltham (MA): Academic Press. 10.1016/B978-0-12-380920-9.00045-6 [DOI] [Google Scholar]
- 10.Buffenstein R. 2008. Negligible senescence in the longest living rodent, the naked mole-rat: Insights from a successfully aging species. J Comp Physiol B 178:439–445. 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 11.Buffenstein R, Jarvis JUM. 2002. The naked mole rat - a new record for the oldest living rodent. Science of aging knowledge environment. Sci Aging Knowledge Environ 21:pe7. 10.1126/sageke.2002.21.pe7. [DOI] [PubMed] [Google Scholar]
- 12.Buffenstein R. 1996. Ecophysiological responses to a subterranean habitat; a Bathyergid perspective. Mammalia 60:591–606. 10.1515/mamm.1996.60.4.591. [DOI] [Google Scholar]
- 13.Cohn JP. 1992. Naked Mole-Rats. Bioscience 42:86–89. 10.2307/1311648. [DOI] [Google Scholar]
- 14.Cox PG, Faulkes CG. 2014. Digital dissection of the masticatory muscles of the naked mole-rat, Heterocephalus glaber (Mammalia, Rodentia). PeerJ 2:e448. 10.7717/peerj.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaney MA, Nagy L, Kinsel MJ, Treuting PM. 2013. Spontaneous Histologic Lesions of the Adult Naked Mole Rat (Heterocephalus glaber) A Retrospective Survey of Lesions in a Zoo Population. Vet Pathol 50:607–621. 10.1177/0300985812471543. [DOI] [PubMed] [Google Scholar]
- 16.Edrey YH, Medina DX, Gaczynska M, Osmulski PA, Oddo S, Caccamo A, Buffenstein R. 2013. Amyloid beta and the longest-lived rodent: the naked mole-rat as a model for natural protection from Alzheimer’s disease. Neurobiol Aging 34:2352–2360. 10.1016/j.neurobiolaging.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards PD, Mooney SJ, Bosson CO, Toor I, Palme R, Holmes MM, Boonstra R. 2020. The stress of being alone: Removal from the colony, but not social subordination, increases fecal cortisol metabolite levels in eusocial naked mole-rats. Horm Behav 121:104720. 10.1016/j.yhbeh.2020.104720. [DOI] [PubMed] [Google Scholar]
- 18.Faulkes CG, Bennett NC. 2021. Social Evolution in African Mole-Rats - A Comparative Overview. Adv Exp Med Biol 1319:1–33. 10.1007/978-3-030-65943-1_1. [DOI] [PubMed] [Google Scholar]
- 19.Faulkes CG, Davies KT, Rossiter SJ, Bennett NC. 2015. Molecular evolution of the hyaluronan synthase 2 gene in mammals: implications for adaptations to the subterranean niche and cancer resistance. Biol Lett 11:20150185. 10.1098/rsbl.2015.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faulkes CG, Bennett NC, Bruford MW, O’Brien HP, Aguilar GH, Jarvis JU. 1997. Ecological constraints drive social evolution in the African mole-rats. Proc Biol Sci 264:1619–1627. 10.1098/rspb.1997.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert JD, Rossiter SJ, Faulkes CG. 2020. The relationship between individual phenotype and the division of labour in naked molerats: It’s complicated. PeerJ 8:e9891. 10.7717/peerj.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimes KM, Voorhees A, Chiao YA, Han HC, Lindsey ML, Buffenstein R. 2014. Cardiac function of the naked mole-rat: ecophysiological responses to working underground. Am J Physiol Heart Circ Physiol 306:H730–H737. 10.1152/ajpheart.00831.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herculano-Houzel S, Ribeiro P, Campos L, Valotta da Silva A, Torres LB, Catania KC, Kaas JH. 2011. Updated neuronal scaling rules for the brains of Glires (rodents/lagomorphs). Brain Behav Evol 78:302–314. 10.1159/000330825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hite NJ, Germain C, Cain BW, Sheldon M, Perala S, Sarko DK. 2019. The Better to Eat You With: Bite Force in the Naked Mole-Rat (Heterocephalus glaber) Is Stronger Than Predicted Based on Body Size. Front Integr Neurosci 13:70. 10.3389/fnint.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes MM, Goldman BD. 2021. Social Behavior in Naked Mole-Rats: Individual Differences in Phenotype and Proximate Mechanisms of Mammalian Eusociality. Adv Exp Med Biol 1319:35–58. 10.1007/978-3-030-65943-1_2. [DOI] [PubMed] [Google Scholar]
- 26.Ke Z, Vaidya A, Ascher J, Seluanov A, Gorbunova V. 2014., J Am Assoc Lab Anim Sci 53:89–91. [PMC free article] [PubMed] [Google Scholar]
- 27.Lin T, Buffenstein R. 2021. The Unusual Immune System of the Naked Mole-Rat. Adv Exp Med Biol 1319:315–327. 10.1007/978-3-030-65943-1_12. [DOI] [PubMed] [Google Scholar]
- 28.Maier W, Schrenk F. 1987. The hystricomorphy of the Bathyergidae, as determined from ontogenetic evidence. Z Säugetierkunde 52:156–164. [Google Scholar]
- 29.Mason MJ, Narins PM. 2010. Seismic sensitivity and communication in subterranean mammals, p 121–139. In: O’Connell-Rodwell CE, editor. The Use of Vibrations in Communication: Properties, Mechanisms and Function across Taxa. Kerala: Transworld Research Network. [Google Scholar]
- 30.McNab BK. 1966. The Metabolism of Fossorial Rodents: A Study of Convergence. Ecology 47:712–733. 10.2307/1934259. [DOI] [Google Scholar]
- 31.Mooney SJ, Filice DC, Douglas NR, Holmes MM. 2015. Task specialization and task switching in eusocial mammals. Anim Behav 109:227–233. 10.1016/j.anbehav.2015.08.019. [DOI] [Google Scholar]
- 32.Mwobobia R, Abelson K, Kanui T. 2020. Housing behaviour of the naked mole rat (Heterocephalus glaber) under laboratory conditions. Scand J Lab Anim Sci 46:1. [Google Scholar]
- 33.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 34.Neff EP. 2020. The naked mole rat’s unique innate immunity. Lab Anim 49:43. 10.1038/s41684-020-0478-4. [DOI] [Google Scholar]
- 35.Okanoya K, Yosida S, Barone CM, Applegate DT, Brittan-Powell EF, Dooling RJ, Park TJ. 2018. Auditory-vocal coupling in the naked mole-rat, a mammal with poor auditory thresholds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 204:905–914. 10.1007/s00359-018-1287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oiwa Y, Oka K, Yasui H. 2020. Characterization of brown adipose tissue thermogenesis in the naked mole-rat (Heterocephalus glaber), a heterothermic mammal. Sci Rep 10:19488. 10.1038/s41598-020-74929-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Riain MJ, Jarvis JU, Alexander R, Buffenstein R, Peeters C. 2000. Morphological castes in a vertebrate. Proc Natl Acad Sci USA 97:13194–13197. 10.1073/pnas.97.24.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Riain MJ, Jarvis JU, Faulkes CG. 1996. A dispersive morph in the naked mole-rat. Nature 380:619–621. 10.1038/380619a0. [DOI] [PubMed] [Google Scholar]
- 39.Orr ME, Garbarino VR, Salinas A, Buffenstein R. 2016. Extended Postnatal Brain Development in the Longest-Lived Rodent: Prolonged Maintenance of Neotenous Traits in the Naked Mole-Rat Brain. Front Neurosci 10:504. 10.3389/fnins.2016.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park TJ, Smith E, Reznick J, Bennett NC, Applegate D, Larson J, Lewin GR. 2021. African Naked Mole-Rats Demonstrate Extreme Tolerance to Hypoxia and Hypercapnia. Adv Exp Med Biol 1319:255–269. 10.1007/978-3-030-65943-1_9. [DOI] [PubMed] [Google Scholar]
- 41.Petry H. 2003. Zur artgerechten Haltung von afrikanischen Nacktmullen (Heterocephalus glaber). [Husbandry appropriate to the species for African naked mole rats (Hetercephalus glaber)]. J Anim Physiol A Anim Nutr 87:421–432. [Article in German]. 10.1046/j.0931-2439.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 42.Pyott SJ, van Tuinen M, Screven LA, Schrode KM, Bai JP, Barone CM, Price SD, Lysakowski A, Sanderford M, Kumar S, Santos-Sacchi J, Lauer AM, Park TJ. 2020. Functional, Morphological, and Evolutionary Characterization of Hearing in Subterranean, Eusocial African Mole-Rats. Curr Biol 30:4329–4341. 10.1016/j.cub.2020.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross-Gillespie A, O’Riain MJ, Keller LF. 2007. Viral epizootic reveals inbreeding depression in a habitually inbreeding mammal. Evolution 61:2268–2273. 10.1111/j.1558-5646.2007.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze-Makuch D. 2019. The Naked Mole-Rat: An Unusual Organism with an Unexpected Latent Potential for Increased Intelligence? Life 9:76. 10.3390/life9030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schumacher L, Husson Z, Smith ES. 2015. The naked mole-rat as an animal model in biomedical research: current perspectives. Anim Physiol 7:137–148. 10.2147/OAAP.S50376. [DOI] [Google Scholar]
- 46.Suckow MA, Stevens KA, Wilson RP, editors. 2012. The Laboratory Rabbit, Guinea Pig, Hamster and Other Rodents. Waltham (MA): Academic Press. [Google Scholar]
- 47.Šumbera R, Mazoch V, Patzenhauerová H. 2012. Burrow architecture, family composition and habitat characteristics of the largest social African mole-rat: the giant mole-rat constructs really giant burrow systems. Acta Theriol (Warsz) 57:121–130. 10.1007/s13364-011-0059-4. [DOI] [Google Scholar]
- 48.Shepard A, Kissil JL. 2020. The use of non-traditional models in the study of cancer resistance-the case of the naked mole rat. Oncogene 39:5083–5097. 10.1038/s41388-020-1355-8. [DOI] [PubMed] [Google Scholar]
- 49.Sherman PW, Jarvis JUM, Alexander RD, editors. 2017. The biology of the naked mole-rat. Vol. 54. Princeton (NJ): Princeton University Press. 10.1515/9781400887132 [DOI] [Google Scholar]
- 50.Smith M, Buffenstein R. 2021. Managed Care of Naked Mole-Rats. Adv Exp Med Biol 1319:381–407. 10.1007/978-3-030-65943-1_16. [DOI] [PubMed] [Google Scholar]
- 51.Takasugi M, Firsanov D, Tombline G, Ning H, Ablaeva J, Seluanov A, Gorbunova V. 2020. Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nat Commun 11:2376. 10.1038/s41467-020-16050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toor I, Edwards PD, Kaka N, Whitney R, Ziolkowski D, Monks A, Holmes MM. 2020. Aggression and motivation to disperse in eusocial naked mole-rats, Heterocephalus glaber. Anim Behav 168:45–58. 10.1016/j.anbehav.2020.07.022. [DOI] [Google Scholar]
- 53.Vice EN, Lagestee S, Browe BM, Deb D, Smith ESJ, Park TJ. 2021. Sensory Systems of the African Naked Mole-Rat. Adv Exp Med Biol 1319:137–156. 10.1007/978-3-030-65943-1_5. [DOI] [PubMed] [Google Scholar]
- 54.Withers PC, Jarvis JUM. 1980. The effect of huddling on thermoregulation and oxygen consumption for the naked mole-rat. Comp Biochem Physiol 66:215–219. 10.1016/0300-9629(80)90154-1. [DOI] [Google Scholar]
- 55.Woodley R, Buffenstein R. 2002. Thermogenic changes with chronic cold exposure in the naked mole-rat (Heterocephalus glaber). Comp Biochem Physiol A Mol Integr Physiol 133:827–834. 10.1016/S1095-6433(02)00199-X. [DOI] [PubMed] [Google Scholar]
- 56.Yu C, Wang S, Yang G, Zhao S, Lin L, Yang W, Tang Q, Sun W, Cui S. 2017. Breeding and Rearing Naked Mole-Rats (Heterocephalus glaber) under Laboratory Conditions. J Am Assoc Lab Anim Sci 56:98–101. [PMC free article] [PubMed] [Google Scholar]
- 57.Zions M, Meehan EF, Kress ME, Thevalingam D, Jenkins EC, Kaila K, Puskarjov M, McCloskey DP. 2020. Nest Carbon Dioxide Masks GABA-Dependent Seizure Susceptibility in the Naked Mole-Rat. Curr Biol 30:2068–2077. 10.1016/j.cub.2020.03.071. [DOI] [PubMed] [Google Scholar]