Abstract
The ChAdOx1 nCoV-19 adenoviral vector-based vaccine was the first to be approved against COVID-19 in India. Vaccine induced thrombotic thrombocytopenia (VITT) is a rare but serious adverse effect of the ChAdOx1 nCoV-19 vaccine, causing widespread thrombosis and thrombocytopenia. A 22-year-old patient, who had undergone mechanical mitral valve replacement, presented to us one week after administration of 1st dose of the ChAdOx1 nCoV-19 Coronavirus vaccine with acute prosthetic heart valve thrombosis (PVT). Laboratory investigations revealed thrombocytopenia and high D-Dimer levels, suggestive of probable VITT. In this report, we highlight the importance of identification of VITT as a cause of PVT and discuss management options.
Keywords: Coronavirus, Heart valve diseases, Vaccines, Thrombosis
1. Introduction
The Coronavirus 2019 (COVID-19) pandemic was brought under some control due to universal, widespread vaccination programmes instituted by countries across the world. The ChAdOx1 nCoV-19 adenoviral vector vaccine (Oxford/AstraZeneca, England/Serum institute, India) was the first to be approved in India and Europe. As of July 2022, over 62% of the world population are fully vaccinated.1 This vaccination programme has brought about reductions in death, hospitalisations and severity of the disease.
The ChAdOx1 nCoV-19 adenoviral vaccine is a recombinant, chimpanzee adenoviral vector vaccine against the spike glycoprotein of the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2).2 While efficacious, easy to store and administer, various vaccine related major problems – demyelination, neuropathies, encephalitis and vaccine-induced immune thrombocytopenia and thrombosis (VITT) after ChAdOx1 nCoV-19 vaccination have been reported in the literature.3 , 4
VITT is similar in presentation and pathogenesis to heparin-induced thrombocytopenia (HIT) but occurs without exposure to heparin or other polyanions. It presents 5–20 days after exposure to the ChAdOx1 nCoV-19 vaccine and is characterised by thrombocytopenia and extensive arterial and venous thrombosis. In the original series of VITT, the mortality rate was 22%.4
In this report, we describe a case of VITT following ChAdOx1 nCoV-19 vaccination, presenting with mechanical prosthetic valve thrombosis (PVT).
2. Case presentation
A 22-year-old, previously well male patient presented with sudden onset severe dyspnea, tachycardia and hypotension. He had received the first dose of ChAdOx1 nCoV-19 vaccine one week prior to this presentation. Post vaccination, he had developed fever and myalgia which had recovered with oral paracetamol. At presentation, he was diaphoretic, in shock, and had very shallow respiratory efforts. Immediately after arrival to the hospital, he was intubated and mechanically ventilated.
The patient had significant past medical history of Lutembacher syndrome (Rheumatic Heart Disease (RHD), severe mitral stenosis and a coexisting atrial septal defect (ASD)). In 2014, 8 years prior to this presentation, he had undergone an open mitral valvotomy (OMV) and ASD closure. Subsequently he developed severe mitral restenosis, pulmonary hypertension (PH) and secondary tricuspid regurgitation (TR). In 2021, 1 year prior to the current presentation, he underwent mitral valve replacement with a 27mm SJM bileaflet valve (Abbot medical, USA) and tricuspid valvuloplasty with a 26mm CE ring (Edwards medical, USA). He had made a good recovery post operatively. Post procedure echo had showed good valve function and regression of TR and PH. He was compliant with medications, had regular follow up visits and excellent time over therapeutic ranges (TTR) of his prothrombin time (PT)/international normalised values (INR). His last follow up was 2 weeks prior to this presentation wherein he was clinically normal.
Soon after admission, a bedside transthoracic echocardiogram (TTE) was performed. It revealed the presence of thrombogenic material on the valve leaflets, poor valve mobility, increased transmitral gradients and PH (Fig. 1, Fig. 2 Video 1). The laboratory tests are presented in Table 1 . It showed thrombocytopenia, elevated d-dimer, INR and activated partial thromboplastin time (aPTT). Arterial blood gas showed metabolic acidosis with incomplete respiratory compensation. Tests of renal were deranged, and he had acute kidney injury (AKI) and multi-organ dysfunction (MODS).
Fig. 1.
Bedside Transthoracic echocardiogram with impaired mobility of the bileaflet mechanical prosthesis
A) In systole, showing the immobile valve leaflets (white arrowhead) B) In diastole.
Fig. 2.
A) Increased mitral valve gradients B) Doppler of the tricuspid regurgitation jet showing elevated pulmonary artery systolic pressures.
Table 1.
Laboratory parameters.
| Haemoglobin | 14.8 g/dl |
| Platelets | 62,000 cells/ml3 |
| WBC Count | 14800 cells/ml3 |
| Blood Urea Nitrogen (BUN) | 61 mg/dl |
| S. Creatinine | 2.12 mg/dl |
| Arterial Blood Gas | Metabolic acidosis with incomplete respiratory compensation |
| Prothrombin Time | 67.1 seconds |
| INR | 5.83 |
| aPTT | >250s |
| D-Dimer | 11 μg/ml (11000 FEU) |
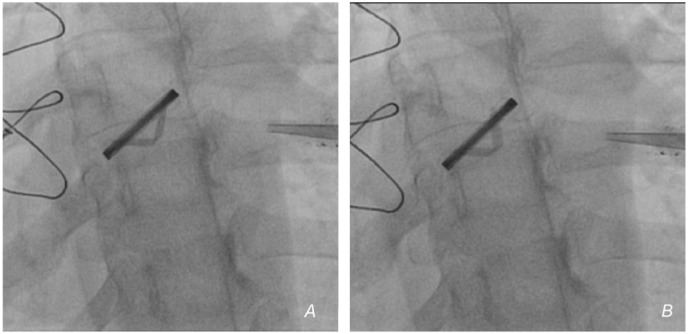
Fluoroscopy (Fig. 3 , video 2) showed poor valve mobility, confirming the diagnosis of PVT. The constellation of findings on the laboratory panel and a temporal association with vaccination led to the diagnosis of VITT (Table 2 ) and mechanical PVT. The patient was started on vasopressors and other supportive measures.
Fig. 3.
A and B) Fluoroscopy showing impaired valve leaflet mobility (video 2).
Table 2.
Case definition - Adapted from Ref. 4.
| Criteria | Symptom onset 5–30 days after ChAdOx1 nCoV-19 vaccination |
| Thrombosis | |
| Thrombocytopenia (<150000/ml3) | |
| D-Dimer > 4000 FEU | |
| PF-4 antibody positivity | |
| Definite | All five criteria present |
| Probable | Elevated D-dimer, but one of the other criteria not met |
| Possible | D-Dimer 2000–4000 FEU but one of the criteria not met |
| (Or) | |
| Two criteria not met | |
| Unlikely | Isolated Thrombocytopenia but other criteria not met |
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ihjccr.2022.09.008
The following are the supplementary data related to this article:
2D Transthoracic echocardiogram showing dilated RA, RV and the mitral mechanical valve in situ. The valve leaflets show reduced mobility.
RA - Right atrium, RV – Right ventricle.
Cinefluoroscopic loop showing the stuck mechanical valve in various projections.
Cardiothoracic surgery was consulted for possible surgery, but due to the poor overall condition, was turned down. Fresh frozen plasma (FFPs) was started and after informed consent, we instituted systemic thrombolysis with streptokinase (STK) infusion, despite coagulopathy as a last resort. But the patient continued to remain in pervasive shock, and after 6 hours of presentation, passed away.
3. Discussion
The proposed pathophysiology and mechanisms of VITT are varied5 – 1) autoantibodies to PF-4 2) adenoviral infection of megakaryocytes resulting in incorporation of the viral spike protein on platelets 3) direct platelet activation by the adenoviral vector 4) direct endothelial activation of the adenoviral vector activating the coagulation cascade or 5) increased spike protein levels post vaccination causing overwhelming immune activation. VITT is classified based on clinical and laboratory features into four types (Table 2).4
PVT occurs due to poor compliance with vitamin-k antagonists (VKA) and TTR. Acutely, it presents with a cascade of symptoms - shock, acidosis, MODS and death if not identified and treated. TTE, fluoroscopy or cardiac computed tomography (CT) can diagnose PVT rapidly. CT can differentiate thrombus from pannus. Thrombolysis with STK, low-dose alteplase or tenecteplase is often the default treatment of choice in developing countries due to their easy availability, proven efficacy and lack of timely access to emergency surgery or due to poor general condition of the patient, precluding surgery.6 At our centre, we use half-dose STK for both left and right sided PVT.
MODS, in the setting of PVT, causes deranged coagulation parameters at presentation. This necessitates both FFP infusions and thrombolysis for management. Low, half dose of STK as a slow infusion can be given for patients with PVT and coagulopathy, and in such patients who are hemodynamically stable, delayed initiation of thrombolysis can be tried.7
The proposed treatment of VITT is adapted from the management of HIT and based on expert opinion. Non heparin anticoagulation with Argatorban, fondaparinux or directly acting oral anticoagulants (DOACs) should be started immediately. Steroids, plasmapheresis,8 IVIG at a dose of 0.5–1 g/kg can be given for severe thrombocytopenia. Platelet transfusions should be avoided as it will propagate the thrombosis.
Cases of bioprosthetic PVT following VITT have been reported,9 but there are no reports of a mechanical PVT following VITT. The definition of VITT requires the identification of anti-PF-4 antibodies, but we could not get this assay done on time as the patient deteriorated very quickly. This case however fits the definition of a probable VITT (Table 2).
4. Conclusion
VITT is a catastrophic event following ChAdOx1 nCoV-19 vaccination. We highlight a case presenting with VITT associated mechanical PVT and discuss the pathogenesis and management.
Source(s) of support
None.
Presented elsewhere
No, the case report and its contents was not presented elsewhere.
Author's contributions
1. Akash Jain – Drafting the manuscript, 2. Varun Marimuthu – Drafting the manuscript, Patient care, Final approval, 3. Nagamani Alur - Patient care, Final approval.
Author's statement
The authors would like to state that the manuscript has been read and approved by all the authors, the requirements for authorship as stated earlier in this document have been met, and each author believes that the manuscript represents honest work.
Declaration of competing interest
No conflict of interest.
Acknowledgement
The authors would like to thank the patient's family for their consent.
References
- 1.Ritchie H., Mathieu E., Rodés-Guirao L., et al. https://ourworldindata.org/covid-vaccinations Coronavirus Pandemic (COVID-19). Our World Data [Internet]. 2020 Mar 5 [cited 2022 Jul 28]; Available from:
- 2.Voysey M., Costa Clemens S.A., Madhi S.A., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021 Mar 6;397(10277):881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021 Jun 3;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 Jun 3;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsilingiris D., Vallianou N.G., Karampela Ι., Dalamaga Μ. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metab Open. 2021 Sep 1;11 doi: 10.1016/j.metop.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karthikeyan G., Senguttuvan N.B., Joseph J., Devasenapathy N., Bahl V.K., Airan B. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: a systematic review and meta-analysis of observational studies. Eur Heart J. 2013 Jun 1;34(21):1557–1566. doi: 10.1093/eurheartj/ehs486. [DOI] [PubMed] [Google Scholar]
- 7.Nishanth K., Shankar M., Srinivasa K., Manjunath C., Ravindranath K. Fibrinolysis in left-sided mechanical prosthetic valve thrombosis with high INR. Eur Heart J Acute Cardiovasc Care. 2020 Oct 1;9(3_suppl):S58–S62. doi: 10.1177/2048872619846329. [DOI] [PubMed] [Google Scholar]
- 8.Patriquin C.J., Laroche V., Selby R., et al. Therapeutic plasma exchange in vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021 Aug 26;385(9):857–859. doi: 10.1056/NEJMc2109465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinnakota S., Jentzer J.C., Luis S.A. Thrombolysis for COVID-19-associated bioprosthetic mitral valve thrombosis with shock. Eur Heart J. 2021 Oct 14;42(39):4093. doi: 10.1093/eurheartj/ehab333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2D Transthoracic echocardiogram showing dilated RA, RV and the mitral mechanical valve in situ. The valve leaflets show reduced mobility.
RA - Right atrium, RV – Right ventricle.
Cinefluoroscopic loop showing the stuck mechanical valve in various projections.