Abstract
To investigate the function of ribosomal proteins and translational factors in Bacillus subtilis, we developed an in vivo assay system to measure the level of nonsense readthrough by utilizing the LacZ-LacI system. Using the in vivo nonsense readthrough assay system which we developed, together with an in vitro poly(U)-directed cell-free translation assay system, we compared the processibility and translational accuracy of mutant ribosomes with those of the wild-type ribosome. Like Escherichia coli mutants, most S12 mutants exhibited lower frequencies of both UGA readthrough and missense error; the only exception was a mutant (in which Lys-56 was changed to Arg) which exhibited a threefold-higher frequency of readthrough than the wild-type strain. We also isolated several ribosomal ambiguity (ram) mutants from an S12 mutant. These ram mutants and the S12 mutant mentioned above (in which Lys-56 was changed to Arg) exhibited higher UGA readthrough levels. Thus, the mutation which altered Lys-56 to Arg resulted in a ram phenotype in B. subtilis. The efficacy of our in vivo nonsense readthrough assay system was demonstrated in our investigation of the function of ribosomal proteins and translational factors.
Aminoglycoside antibiotics, such as streptomycin, inhibit protein synthesis in ribosomes. Streptomycin-resistant mutants have been isolated since the time when streptomycin was first used clinically. Many such ribosomal mutants have been isolated and characterized; they are mutants of various organisms, but they are mainly mutants of Escherichia coli. These mutants have made great contributions to research on ribosomal proteins and to elucidating the functions of these proteins. Of the ribosomal proteins that have been studied, proteins S12, S4, and S5 are the best characterized. Analyses of mutants over the past three decades have revealed that these proteins play an essential role in maintaining the accuracy of protein synthesis (for a review see reference 19). Most mutations able to confer a high level of resistance to streptomycin are mutations in the rpsL gene, which encodes ribosomal protein S12 (2, 9, 11, 30, 32), and the effects of these mutations have been discussed with respect to growth rate, nonsense codon readthrough, missense error frequency, and peptide elongation rate (19, 29). It is evident that most rpsL mutations result in hyperaccurate translation and that the mutations are frequently associated with phenotypes such as a streptomycin requirement for growth or severely impaired growth. Additional second-site mutations that phenotypically reverse streptomycin dependence or impaired growth have been found in the rpsD, rpsE, and rplL genes, which encode proteins S4, S5, and L7/L12, respectively (6, 14, 17). Since these mutations result in enhancement of the translational error, they are called ribosomal ambiguity (ram) mutations.
Numerous microorganisms, including members of the genus Bacillus, produce a variety of antibiotics and extracellular enzymes. Working with members of the genera Streptomyces and Bacillus, we have demonstrated that antibiotic production by Streptomyces lividans or Streptomyces coelicolor is activated when streptomycin, tetracycline, or hygromycin is added to the growth medium at sublethal concentrations (25, 30). Moreover, introduction of rpsL mutations, which produced mutant strains with streptomycin or paromomycin resistance, induced antibiotic production (12, 30). The efficacy of rpsL mutations for activating antibiotic production has been demonstrated in several other microorganisms, including Bacillus subtilis (11, 13). Although several ribosomal mutants of B. subtilis have been isolated and characterized with respect to their ability to grow and sporulate (9, 10), the functions of the ribosomal proteins involved have been studied less than the functions of the E. coli proteins. Our objective is to fully understand the functions of ribosomes in initiating sporulation and secondary metabolism in microorganisms. In the present study, we developed a system for quantifying the frequency of nonsense readthrough in vivo. Using this in vivo nonsense readthrough assay system and an in vitro poly(U) translation assay system, we characterized various ribosomal mutants with respect to the accuracy of protein synthesis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. To construct a vector with a selectable marker to detect the rpsD mutation, the chloramphenicol acetyltransferase gene (cat) was amplified with the following primers: Cm Sse 5′-2 (5′-GTTACCCTTATTA TCACCTGCAGGAAGAAAG-3′) and Cm Sse 3′-2 (5′-TACAGTCGGCATTCCTGCAGGTTATAAAAG-3′), both of which include an Sse8387I site (underlined nucleotides). Plasmid pAG58 (15), which carries the cat gene, was used as a template for PCR. The Sse8387I-digested PCR product containing the cat gene was cloned into plasmid pCR2.1, resulting in pCR2.1-cat. The rpsD-tyrS region was also amplified from genomic DNA with the following primers: rpsD-F (5′-ATGGCTCGCTATACAGGTC-3′) and tyrS-R (5′-TATGGAAGTTGACAGCACCC-3′). The fragment generated was cloned into pCR2.1, resulting in pCR2.1-DS. An HindIII-EcoRI fragment containing the noncoding region between the 3′ end of rpsD and the 3′ end of tyrS was ligated to the corresponding restriction enzyme sites in pUC18, resulting in pUC18-DS. Then, the Sse8387I fragment containing the cat gene from pCR2.1-cat was inserted into the PstI site in the noncoding region of pUC18-DS, resulting in pUC18-DCS. The rpsE1 mutation, which results in alteration of Gly-28 to Val in ribosomal protein S5, was isolated from a spontaneously generated mutant which was able to resist selection with spectinomycin (50 μg/ml). For site-directed mutagensis of the rpsL gene, the complete coding region of rpsL was amplified with the following primers: rpsL-F (5′-ATGCCTACAATTAATCAGCTAATT-3′) and rpsL-R (5′-TTATTTTGCTTTAGGTTTTTTCG-3′). The PCR product was cloned into pCR2.1, and the resulting plasmid was designated pCR2.1-rpsL. The HindIII-PstI fragment containing the rpsL gene from pCR2.1-rpsL was ligated into pKF19k, which is a vector designed for site-directed mutagenesis (Mutan-Super Express Km; Takara). Oligonucleotide K56D (5′-AGTTCGGTTTGTCCGGTGTCATTG-3′), which includes the mutation sites (underlined nucleotides), was used to change Lys-56 to Asp in order to generate rpsL7 (8). The resulting plasmid, pKF19k-rpsL7, was linearized with HindIII and introduced into the chromosomal DNA of strain 168, and transformants were selected for resistance to streptomycin (1,000 μg/ml). For random mutagenesis, strain 168 was transformed with PCR products of the rpsL gene and mutants resistant to 50 μg of streptomycin per ml were selected. Spontaneous suppressor mutants which were able to grow as well as the wild-type strain were isolated from the rpsL7 mutant, which exhibited severely impaired growth. The rpsD mutations (rpsD1 and rpsD2) and the rpsE mutation (rpsE7) were isolated by congression by using TI53 or TI32 as the recipient strain. E. coli JM109 was used as the host strain for gene cloning, and MV1184 was used for oligonucleotide-directed mutagenesis. When necessary, the nucleotide sequences of the ribosomal genes were confirmed by sequencing (ABI310; PE Biosystems).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or phenotype | Construction, source, or reference |
|---|---|---|
| B. subtilis strains | ||
| 168 | trpC2 | Laboratory stock |
| WE1 | trpC2 rpsE1 Spcr | Spontaneous rpsE mutant → 168a |
| UOT1803 | hisH101 lys-1 aprEΔ3 nprE18 nprR2 | F. Kawamura |
| YO-005 | hisH101 | UOT1803 → 168 |
| TI32 | hisH101 rpsE1 Spcr | WE1 → YO-005 |
| TI53 | hisH101 rpsD(wt)-cat-tyrS Cmr | pUC18-DCS → YO-005 |
| WL1 | trpC2 rpsL1 Smr | Spontaneous rpsL mutant → 168 |
| WL2 | trpC2 rpsL2 Smr | Spontaneous rpsL mutant → 168 |
| WL3 | trpC2 rpsL3 Smr | Spontaneous rpsL mutant → 168 |
| WL4 | trpC2 rpsL4 Smr | Spontaneous rpsL mutant → 168 |
| WL7 | trpC2 rpsL7 Smr | pKF19k-rpsL7 → 168 |
| WL9 | trpC2 rpsL9 Smr | PCR product → YO-005 |
| sup7–9 | trpC2 rpsL7 rpsD1 Smr | Suppressor mutant of WL7 |
| sup7–13 | trpC2 rpsL7 rpsD2 Smr | Suppressor mutant of WL7 |
| sup7–10 | trpC2 rpsL7 rpsE7 Smr | Suppressor mutant of WL7 |
| WD1 | trpC2 rpsD1 | sup7–9 → TI53 |
| WD2 | trpC2 rpsD2 | sup7–13 → TI53 |
| WE7 | trpC2 rpsE7 | sup7–10 → TI32 |
| SG81 | trpC2 Ω(lacA::neo) | J. Erringtonb |
| TI8 | trpC2 Ω(lacA::neo) | SG81 → 168 |
| TI10-W | trpC2 Ω(lacA::neo) amyE::pTMI-W (lacI wt) | pTMI-W → TI8 |
| TI10-TGA | trpC2 Ω(lacA::neo) amyE::pTMI-TGA | pTMI-TGA → TI8 |
| TI10-TAG | trpC2 Ω(lacA::neo) amyE::pTMI-TAG | pTMI-TAG → TI8 |
| TI10-TAA | trpC2 Ω(lacA::neo) amyE::pTMI-TAA | pTMI-TAA → TI8 |
| E. coli strains | ||
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′[traD36 proAB+ lacIqlacZΔM15] | Takara |
| MV1184 | ara Δ(lac-proAB) rpsL thi (φ80 lacZΔM15) Δ(srl-recA)306::Tn10(Tetr)/F′ [traD36 proAB+lacIqlacZΔM15] | Takara |
| Plasmids | ||
| pCR2.1 | Cloning vector for PCR product, Apr | Invitrogen |
| pUC18 and pUC19 | Cloning vector, Apr | Takara |
| pKF18k and pKF19k | Vector for site-directed mutagenesis, Kmr | Takara |
| pMutinT3 | Vector, Apr Emr | 22 |
| pCR2.1-cat | pCR2.1 containing a cat casette | This study |
| pUC18-DCS | pUC18 containing 3′-rpsD(wt)-cat-3′-tyrS | This study |
| pKF19k-rpsL7 | pKF19k containing rpsL7 | This study |
| pTMI-W | pMUTIN3 containing 5′amyE at BglII site | This study |
| pTMI-TGA | pTMI-W containing E105UGA in lacI gene | This study |
| pTMI-TAG | pTMI-W containing E105UAG in lacI gene | This study |
| pTMI-TAA | pTMI-W containing E105UAA in lacI gene | This study |
The strain to the right of the arrow was transformed with the chromosomal DNA, PCR product, or plasmid to the left of the arrow.
See reference 5.
Growth conditions.
B. subtilis strains were grown aerobically at 37°C in L medium (10 g of tryptone per liter, 5 g of yeast extract per liter, 5 g of NaCl per liter). Spizizen's salts medium [14 g of K2 HPO4 per liter, 6 g of KH2PO4 per liter, 2 g of (NH4)2SO4 per liter, 1 g of sodium citrate per liter, 0.2 g of MgSO4 · 7H2O per liter, 5 g of glucose per liter] supplemented with 1 μM MnCl2 and the required amino acid (50 μg/ml) was used as the minimum medium and also as the transformation medium for B. subtilis. If necessary, 0.05% yeast extract was added. The following antibiotics were added to the media: for selection of B. subtilis transformants, streptomycin (50 to 1,000 μg/ml), spectinomycin (50 μg/ml), chloramphenicol (5 μg/ml), neomycin (7.5 μg/ml), and erythromycin (0.5 μg/ml); and for selection of E. coli transformants, ampicillin (50 μg/ml) and kanamycin (20 or 100 μg/ml).
Measurement of nonsense readthrough in vivo.
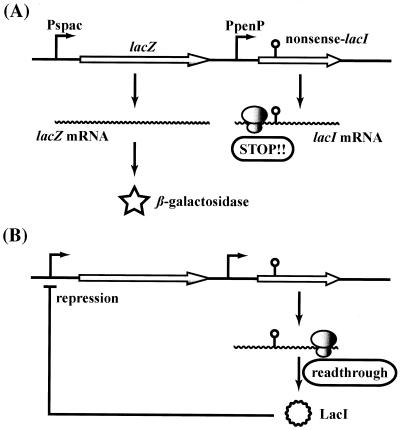
The level of nonsense readthrough for each mutant was estimated by measuring the degree of repression of lacZ expression. Plasmid pMutinT3 (24) carries the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible lacZ gene and the lacI gene encoding a lacZ repressor. A 1-kb fragment containing the amyE 5′ region was amplified with the following primers: amyE-F (5′-TCATTTGGATCCGGCAGGAC-3′) and amyE-R (5′-CTCCCTCAGATCTGGAAAAG-3′), which include sites for BamHI and BglII, respectively (underlined nucleotides). A BamHI-BglII fragment of the PCR product was cloned into the BgIll site of pMutinT3 in the direction opposite that of the lacZ gene, resulting in pTMI-W. Site-directed mutagenesis of lacI at Glu-105 was carried out with oligonucleotides E105UGA (5′-AAGCGGCGTCTGAGCCTGTAAAG-3′), E105UAG (5′-AAGCGGCGTCTAGGCCTGTAAAG-3′), and E105UAA (5′-AAGCGGCGTCTAAGCCTGTAAAG-3′), which include mutation sites (underlined nucleotides). Each plasmid was introduced into the amyE locus of B. subtilis TI8, which lacks endogenous β-galactosidase (β-Gal), and the resulting mutants were designated TI10-W, TI10-TGA, TI10-TAG, and TI10-TAA. Chromosomal DNA from strains TI10-W, TI10-TGA, TI10-TAG, and TI10-TAA were introduced into the chromosomes of various ribosomal mutants by congression with Nmr and Emr. To measure nonsense readthrough, cells were grown to an optical density at 650 nm of 0.3 to 0.5 in Spizizen's salts medium containing a required compound in the presence of 10 mM IPTG or in the absence of IPTG. When strain YO-005 (Trp+ His−) was used, the cells were grown in the presence of 50 μg of tryptophan per ml or in the absence of tryptophan with 50 μg of histidine per ml. β-Gal activities were measured by the method described by Miller (23). Repressor (LacI) activity was represented by β-Gal activity (Fig. 1), and the β-Gal activities as induced by IPTG differed to some extent from strain to strain. Therefore, the nonsense readthrough levels were expressed as induction ratios (the β-Gal activity of the culture supplemented with IPTG divided by the activity of the culture without IPTG).
FIG. 1.
Scheme of the system used to measure in vivo readthrough in B. subtilis. Plasmid pMutinT3 (see reference 24 for details concerning this plasmid), which carries the IPTG-inducible lacZ gene and the lacI gene encoding its repressor protein, was introduced into the amyE locus of the chromosome. Pspac and PpenP are the promoters of the lacZ gene and the lacI gene, respectively. Three different in-frame nonsense codons are introduced into the lacI gene as described in Materials and Methods. In hyperaccurate mutants, production of LacI protein is incomplete, resulting in greater induction of lacZ expression (A). On the other hand, in ram mutants, expression of lacZ can be repressed due to suppression of nonsense codons, resulting in a decrease in β-Gal activity when cells are grown in the medium without IPTG (B).
Poly(U)-directed cell-free translation.
In order to study peptide elongation ability and the missense error frequency of ribosomes, we used the poly(U)-directed cell-free translation system of Legault-Demare and Chambliss (20), with a slight modification. Cells were grown in L medium at 37°C to an optical density at 650 nm of 0.7, harvested by centrifugation, and ground with aluminum oxide for 5 min. Extracts were centrifuged twice at 15,000 × g for 10 min. The supernatants were centrifuged at 30,000 × g for 30 min, dialyzed for 3 h, and then centrifuged at 30,000 × g for 20 min. These procedures were all carried out at 4°C. The resulting supernatants (designated S30) contained about 20 mg of ribosomes per ml and were used in the poly(U)-dependent in vitro translation system, as follows. Ribosome mixtures contained 0.1 mg of S30 ribosomes, 55 mM HEPES–KOH (pH 7.5), 1.7 mM dithiothreitol, 210 mM potassium acetate, 27.5 mM ammonium acetate, 10.7 mM magnesium acetate, and 68 mM folinic acid. These mixtures were preincubated at 30°C for 10 min to remove the endogenous mRNA. Then, 1.2 mM ATP, 0.8 mM GTP, 0.64 mM 3′,5′-cyclic AMP, 80 mM creatine phosphate, 0.25 mg of creatine kinase per ml, 0.5 U of RNase inhibitor per ml, 0.3 mg of B. subtilis tRNA per ml, 0.75 mg of poly(U) per ml, 5 mM spermine, and 0.13 μM l-[2,3,4,5,6-3H]phenylalanine (0.1 MBq; Amersham Pharmacia Biotech) or 0.13 μM l-[4,5-3H]leucine (0.13 MBq; Amersham Phamacia Biotech) were added. The reaction mixtures (usually 0.1 ml) were incubated at 30°C for the appropriate time. A reaction mixture lacking poly(U) was also incubated in parallel to confirm that the endogenous mRNA was removed. After incubation, 10-μl aliquots were applied to filter disks (GF/F glass filter; Whatman) which had previously been permeated with 10% trichloroacetic acid (TCA). The disks were boiled in 10% TCA for 10 min and washed for 3 min twice with 10% TCA, once with ethanol, once with ethanol-diethyl ether (1:1), and finally once with diethyl ether. After air drying, the radioactivities of the filter disks were measured with a liquid scintillation counter. The missense error frequency was expressed as the ratio of incorporation of [3H]leucine to incorporation of [3H]phenylalanine.
RESULTS
Construction and isolation of ribosomal mutants.
In E. coli, mutational analysis of the rpsL gene, which codes for ribosomal protein S12, demonstrated that the mutations result in amino acid substitutions in the two short domains, namely, at amino acid positions 40 to 43 and 87 to 93 (32). Working with B. subtilis, we found previously that mutations conferring a high level of streptomycin resistance result in a change at Lys-56, which corresponds to Lys-43 in E. coli protein S12 (11). To investigate the function of ribosomal protein S12, we constructed two additional rpsL mutants in the present study. In one mutant Lys-56 was changed to the acidic amino acid Asp by site-directed mutagenesis. In the other mutant Pro-104 (corresponding to Pro-91 in E. coli) was changed to Ser by PCR random mutagenesis (see above). These rpsL mutations are summarized in Table 2. The mutants harboring these rpsL mutations grew as well as the wild-type strain, except for the rpsL7 mutant (in which Lys-56 was changed to Asp), which exhibited strikingly restrictive growth in nutrient medium and was unable to grow in minimum medium. The rpsL7 mutant gave rise to spontaneous suppressor mutants at a high frequency, and these mutants formed large colonies. Since these suppressor mutants could have been ram mutants, we sequenced their genes, focusing on rpsD, rpsE, and rplL on the basis of the results obtained previously with E. coli. We found that the majority (16 of 24) of the mutants had a mutation in one of three genes; several representatives are shown in Table 3. These mutants still had the original rpsL mutation. In the rpsD mutants, an amino acid substitution at Glu-46 or a short duplication or deletion in this region was detected, as previously shown by Henkin et al. (10). In the rpsE mutants, there was an amino acid substitution at Arg-112 or Gly-104, positions which correspond to the positions of rpsE ram mutations in E. coli (14, 27). Three-factor transformation analysis in which rpsL7 (Smr), ybaC::cat (Cmr), and rpsE1 (Spcr) were used as selectable markers suggested that two of the mutations, rpsE8 and rpsE9, have a lethal effect in the absence of the rpsL7 mutation (data not shown). As in the E. coli ram mutant (17), the mutation in rplL encoding L7/L12 was found to result in deletion of five amino acids at residues 42 to 46, which is the flexible hinge region of this protein. In the present study, we used three mutations (rpsD1, rpsD2, and rpsE7) as possible ram mutations for further analysis.
TABLE 2.
rpsL alleles used in this study
| rpsL allele | Nucleotide sequence of:a
|
Amino acid at:
|
||
|---|---|---|---|---|
| Codon 56 | Codon 104 | Position 56 | Position 104 | |
| Wild type | AAA | CCA | Lys | Pro |
| rpsL1 | AGA | Arg | ||
| rpsL2 | AAT | Asn | ||
| rpsL3 | ACA | Thr | ||
| rpsL4 | ATA | Ile | ||
| rpsL7 | GAC | Asp | ||
| rpsL9 | TCA | Ser | ||
The numbers start at the start codon (ATG) of the open reading frame.
TABLE 3.
Mutations found in suppressor mutants obtained from rpsL7 strain
| Strain | Altered gene | Nucleotide change | Amino acid position(s)a | Amino acid change |
|---|---|---|---|---|
| sup7–23 | rpsL13 | GAG → AAG | 75 | Glu → Lys |
| sup7–9 | rpsD1 | GCT GGC AAA CTA → duplicate | 75–78 | Ala Gly Lys Leu → duplicate |
| sup7–13 | rpsD2 | GCT GGC AAA CTA → Δb | 75–78 | Ala Gly Lys Leu → Δ |
| sup7–16 | rpsD3 | GAA → AAA | 46 | Glu → Lys |
| sup7–10 | rpsE7 | CGT → CAT | 112 | Arg → His |
| sup7–18 | rpsE8 | CGT → TGT | 112 | Arg → Cys |
| sup7–19 | rpsE9 | GGA → AGA | 104 | Gly → Arg |
| sup7–8 | rplL1 | GCT GGC GGA GCT GCT → Δ | 42–46 | Ala Gly Gly Ala Ala → Δ |
The numbers start at the start codon (ATG) of the open reading frame.
Δ, the nucleotides or amino acids are deleted.
The levels of resistance to streptomycin determined in this study were >1,000 μg/ml for rpsL1, rpsL2, rpsL3, rpsL4, and rpsL7 mutants, 50 μg/ml for rpsL9 mutants, and 5 μg/ml for rpsD1, rpsD2, rpsE1, and rpsE7 mutants and the wild-type strain, as determined on L agar plates.
Construction of an accuracy assay system.
We next attempted to construct a system for measuring translational accuracy in vivo. To do this, we used plasmid pMutinT3, which carries the IPTG-inducible lacZ gene and the lacI gene which encodes the repressor protein. The scheme of this assay system is shown in Fig. 1. Previously, Kleina and Miller (18) reported that Glu-105 of the LacI protein was tolerant to substitutions with 12 different amino acids, and none of these substitutions resulted in any change in repressor activity. Therefore, we introduced three different nonsense codons (UGA, UAG, and UAA) at the Glu-105 site by site-directed mutagenesis (see above). Each resulting plasmid was integrated into the amyE locus of the TI8 strain, which lacks endogenous β-Gal activity. Active LacI protein can be generated when introduced nonsense codons are read through, which eventually leads to repression of lacZ expression. Therefore, the level of readthrough of each nonsense codon can be detected as a decrease in β-Gal activity when cells are grown in medium without IPTG.
Translational properties of ribosomal mutants.
Using the system described above, we studied the effect of each ribosomal mutation on the readthrough of three different nonsense codons. It is notable that even in the wild-type strain, the UGA readthrough frequency was significantly higher (about sixfold higher) than the readthrough frequencies of the UAG and UAA codons (data not shown). Although the UGA codon functions as one of the termination codons in B. subtilis, this codon is also known to be decoded by tRNATrp (22). Since an excess of tryptophan (50 μg/ml) was added to the medium as a requirement, the effect of tryptophan on the readthrough level was examined with strain YO-005 (Trp+ His−). As a result, addition of tryptophan or histidine had no effect on readthrough at the UGA codon (data not shown). These results raise the possibility that the UGA codon is a leaky termination codon in B. subtilis. Most of the rpsL mutations (all except rpsL1) significantly reduced the readthrough frequency at the UGA codon compared to that in the wild-type strain (Table 4). In contrast, lacZ expression in rpsD1, rpsD2, and rpsE7 mutants was almost completely repressed by the readthrough product of the lacI gene harboring the in-frame UGA codon. These three mutations can, therefore, be considered ram mutations. The rpsE1 mutation, which changes Gly-28 to Val in ribosomal protein S5, was isolated in a spectinomycin-resistant mutant and has been known to result in a non-ram phenotype. As expected, the UGA readthrough frequency was not affected by the rpsE1 mutation when the rpsE1 mutant was compared with the wild-type strain (Table 4). The rpsL7 mutant could not be used with this assay system because of its genetic instability. Thus, most rpsL mutations restrict UGA readthrough, and ram mutations act as strong suppressors of the UGA codon. Of interest is the fact that the rpsL1 mutation clearly resulted in a threefold increase in UGA readthrough (Table 4). As in liquid cultures, the readthrough of each ribosomal mutant at the UGA codon could be detected when the mutant was grown on an L agar plate containing 0.008% X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (data not shown). Peptide release factor 2 (RF-2) plays a role in recognizing UGA and UAA as stop codons (26). Western blot analysis performed with an anti-RF-2 antibody showed that the intensities of the RF-2 protein from the rpsL1 and rpsL2 mutants were similar to the intensity of the RF-2 protein from the wild-type strain, indicating that the observed difference in readthrough levels at the UGA codon in rpsL mutants was not due to the different amounts of RF-2 (data not shown). We also estimated the missense error rates of mutant ribosomes in vitro by using the poly(U)-dependent translation assay system. Consistent with the UGA readthrough frequencies in vivo, we detected significant decreases in misincorporation rates for leucine in most rpsL mutants except rpsL1 (Table 4). However, there was no substantial difference between the missense error frequencies of the rpsL1 and ram mutant (rpsD1, rpsD2, rpsE7) ribosomes and the wild-type ribosomes, although the cells harboring these mutations exhibited high levels of UGA readthrough in vivo. Unlike UGA readthrough, readthrough of UAG and UAA was below the detectable level even in the rpsL1 and ram mutants (data not shown), although we have no explanation for these findings. Eventually, we concluded that the in vivo UGA readthrough system is sufficiently useful to investigate the ribosomal mutations.
TABLE 4.
Characterization of translation in mutant ribosomes in vivo and in vitro
| Mutation | In vitro missense error rate (10−2)a | In vivo readthrough induction ratios for Glu-105 codon of LacIb
|
|
|---|---|---|---|
| GAA (native) | UGA | ||
| Wild type | 4.4 | 102 | 4.9 |
| rpsL1 | 5.8 | 101 | 16 |
| rpsL2 | 0.92 | 100 | 0.75 |
| rpsL3 | 0.77 | 67 | 0.83 |
| rpsL4 | 0.81 | 83 | 0.95 |
| rpsL9 | 0.86 | 125 | 1.2 |
| rpsD1 | 3.6 | 50 | 37 |
| rpsD2 | 4.6 | 44 | 45 |
| rpsE1 | NDc | 105 | 4.2 |
| rpsE7 | 3.7 | 59 | 44 |
The missense error rates of ribosomes were measured by using the poly(U)-dependent cell-free translation system described in Materials and Methods.
Overnight cultures were diluted 50-fold in Spizizen's salts medium with or without 10 mM IPTG and were cultivated until the optical density at 650 nm was 0.3 to 0.5. Then, cells were harvested, and β-Gal activities were measured. At least three independent experiments were performed, and the average values of the induction ratios (see text) are presented.
ND, not determined.
DISCUSSION
The subject of the present study, translational fidelity and ribosomal protein mutations that affect this process, is of general interest, particularly in light of recent structural information on 30S and 50S ribosomal subunits. The goal of the work reported here was to obtain greater understanding of features of B. subtilis translation. Ribosomal proteins S12, S4, and S5 play an important role in the translational accuracy of the ribosomes. Previous studies indicated that rpsL mutations result in increased accuracy by affecting the proofreading step (28) and that a conformation change in 16S rRNA during translation is facilitated by S5 and S12 (21). A more recent study (3) based on 30S crystal structure analysis suggested that error-prone or restrictive mutations cause the ribosomes to stabilize at a higher or lower tRNA affinity state. Probably, these ribosomal mutations affect codon-anticodon arrangement and tRNA selection at the ribosomal A site by changing the conformation of rRNA.
In the present study, we developed an in vivo system for measuring the readthrough frequencies of three different nonsense codons and characterized the translational apparatus of various ribosomal mutants. Most S12 alterations, which confer streptomycin resistance, reduce both UGA readthrough and missense error frequencies (Table 4). In E. coli, the mutants with hyperaccuracy are known to have low tRNA affinity, which results in an increased chance of proper selection of cognate tRNA at the ribosomal A site (3). Importantly, unlike the previously described cases, we demonstrated that alteration of Lys-56 to Arg can apparently result in UGA readthrough frequencies higher than those in the wild-type strain. In E. coli (19), a mutant carrying the equivalent mutation (Lys-43 changed to Arg) was shown to be unable to exhibit hyperaccuracy. Recently, Bjökman et al. (2) found that the streptomycin-dependent phenotype of a certain Salmonella enterica serovar Typhimurium S12 mutant (in which Pro-90 was changed to Leu) can become streptomycin independent because of a mutation in the S12 area, as we demonstrated above. Moreover, alteration of Lys-62 to Arg in the yeast S28 protein (corresponding to the bacterial S12 protein) is known to act as an omnipotent suppressor (1, 31). We therefore concluded that this alteration of S12 results in a weak ram phenotype in a wide variety of organisms. The UGA readthrough frequency appears to be affected by a wider range of ribosomal mutations, at least in B. subtilis. Therefore, UGA readthrough can be used as a good marker for monitoring the accuracy of ribosomes in B. subtilis.
Lovett et al. (22) reported previously that in B. subtilis the UGA codon introduced into the cat86 mRNA is translated as tryptophan at a relative efficiency as high as 6% compared with the wild-type product. The UGA readthrough frequency detected with our assay system (approximately 5%) is close to this value. Since these readthrough frequencies are considered significantly high, we speculate that UGA readthrough has an important biological function in B. subtilis. In fact, a deleterious effect on the ability to grow and sporulate was observed in certain rpsL (rpsL7) mutants rather than in the ram mutants. An unusual decoding mechanism, such as reprogrammed genetic decoding (recoding), is believed to be widely distributed in various organisms (7). RF-2 has a UGA codon in its gene. Several reports have shown that during translational regulation of RF-2, the UGA codon is used as a cis element for recoding (4, 16). Although the amounts of RF-2 produced by the rpsL mutants and the wild-type strain were not substantially different under our experimental conditions (see above), the different levels of readthrough at the UGA codon might influence the synthesis of such recoding products, at least under certain physiological conditions. Further clarification of the function of the leaky termination codon should help in understanding the mechanism of activation of the secondary metabolism caused by S12 mutations at the molecular level (11–13, 25, 30).
ACKNOWLEDGMENTS
This work was supported by a grant from the Organized Research Combination System (ORCS) of the Science and Technology Agency of Japan.
We are grateful to J. Errington and F. Kawamura for providing strains SG81 and UOT1802.
REFERENCES
- 1.Alksne L E, Anthony R A, Liebman S W, Warner J R. An accuracy center in the ribosome conserved over 2 billion years. Proc Natl Acad Sci USA. 1993;90:9538–9541. doi: 10.1073/pnas.90.20.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Björkman J, Samuelsson P, Andersson D I, Hughes D. Novel ribosomal mutations affecting translational accuracy, antibiotic resistance and virulence of Salmonella typhimurium. Mol Microbiol. 1999;31:53–58. doi: 10.1046/j.1365-2958.1999.01142.x. [DOI] [PubMed] [Google Scholar]
- 3.Carter A P, Clemons W M, Brodersen D E, Morgan-Warren R J, Wimberly B T, Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 4.Craigen W J, Caskey C T. Expression of peptide chain release factor 2 requires high-efficiency frameshift. Nature. 1986;322:273–275. doi: 10.1038/322273a0. [DOI] [PubMed] [Google Scholar]
- 5.Daniel R A, Haiech J, Denizot F, Errington J. Isolation and characterization of the lacA gene encoding beta-galactosidase in Bacillus subtilis and a regulator gene, lacR. J Bacteriol. 1997;179:5636–5638. doi: 10.1128/jb.179.17.5636-5638.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deuser E, Stöffler G, Wittmann-Liebold H G. Altered S4 proteins in Escherichia coli mutants from streptomycin dependence to independence. Mol Gen Genet. 1970;109:298–302. doi: 10.1007/BF00267699. [DOI] [PubMed] [Google Scholar]
- 7.Gesteland R F, Weiss R B, Atkins J F. Recoding: reprogrammed genetic decoding. Science. 1992;257:1640–1641. doi: 10.1126/science.1529352. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto-Gotoh T, Mizuno T, Ogasahara Y, Nakagawa M. An oligodeoxyribonucleotide-directed dual amber method for site-directed mutagenesis. Gene. 1995;152:271–275. doi: 10.1016/0378-1119(94)00750-m. [DOI] [PubMed] [Google Scholar]
- 9.Henkin T M, Chambliss G H. Genetic analysis of a streptomycin-resistant oligosporogenous Bacillus subtilis mutant. J Bacteriol. 1984;157:202–210. doi: 10.1128/jb.157.1.202-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henkin T M, Chambliss G H, Grundy F J. Bacillus subtilis mutants with alterations in ribosomal protein S4. J Bacteriol. 1990;172:6380–6385. doi: 10.1128/jb.172.11.6380-6385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosoya Y, Okamoto S, Muramatsu H, Ochi K. Acquisition of certain streptomycin-resistant (str) mutations enhances antibiotic production in bacteria. Antimicrob Agents Chemother. 1998;42:2041–2047. doi: 10.1128/aac.42.8.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosoya Y O, Sato T, Ochi K. Resistance to paromomycin is conferred by rpsL mutations, accompanied by an enhanced antibiotic production in Streptomyces coelicolor A3(2) J Antibiot. 2000;53:1424–1427. doi: 10.7164/antibiotics.53.1424. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Ochi K. Novel approach for improving the productivity of antibiotic-producing strains by inducing combined resistant mutations. Appl Environ Micobiol. 2001;67:1885–1892. doi: 10.1128/AEM.67.4.1885-1892.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh T, Wittmann H G. Amino acid replacements in proteins S5 and S12 of two Escherichia coli revertants from streptomycin dependence to independence. Mol Gen Genet. 1973;127:19–32. doi: 10.1007/BF00267779. [DOI] [PubMed] [Google Scholar]
- 15.Jaacks K J, Healy J, Losick R, Grossman A D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami K, Nakamura Y. Autogenous suppression of an opal mutation in the gene encoding peptide release factor 2. Proc Natl Acad Sci USA. 1990;87:8432–8436. doi: 10.1073/pnas.87.21.8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsebom L A, Amons R, Isaksson L A. Involvement of ribosomal protein L7/L12 in control of translation accuracy. Proc Natl Acad Sci USA. 1985;82:717–721. doi: 10.1073/pnas.82.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleina L G, Miller J H. Genetic studies of the lacI repressor. XIII. Extensive amino acid replacements generated by the use of natural and synthetic nonsense suppressors. J Mol Biol. 1990;212:295–318. doi: 10.1016/0022-2836(90)90126-7. [DOI] [PubMed] [Google Scholar]
- 19.Kurland C G, Hughes D, Ehrenberg M. Limitations of translational accuracy. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology Press; 1996. pp. 979–1004. [Google Scholar]
- 20.Legault-Demare L, Chambliss G H. Natural messenger ribonucleic acid directed cell-free protein-synthesizing system of Bacillus subtilis. J Bacteriol. 1974;120:1300–1307. doi: 10.1128/jb.120.3.1300-1307.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lodmell J S, Dahlberg A E. A conformational switch in Escherichia coli 16S ribosomal RNA during decoding of messenger RNA. Science. 1997;277:1262–1267. doi: 10.1126/science.277.5330.1262. [DOI] [PubMed] [Google Scholar]
- 22.Lovett P S, Ambulos N P, Jr, Mulbry W, Noguchi N, Robers E J. UGA can be decoded as tryptophan at low efficiency in Bacillus subtilis. J Bacteriol. 1991;173:1810–1812. doi: 10.1128/jb.173.5.1810-1812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N. Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24.Moriya S, Tsujikawa E, Hassan A K, Asai K, Kodama T, Ogasawara N. A Bacillus subtilis gene-encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol Microbiol. 1998;29:179–187. doi: 10.1046/j.1365-2958.1998.00919.x. [DOI] [PubMed] [Google Scholar]
- 25.Ochi K, Zhang D, Kawamoto S, Hesketh A. Molecular and functional analysis of the ribosomal L11 and S12 protein genes (rplK and rpsL) of Streptomyces coelicolor A3(2) Mol Gen Genet. 1997;256:488–498. doi: 10.1007/pl00008614. [DOI] [PubMed] [Google Scholar]
- 26.Pel H J, Rep M, Grivell L A. Sequence comparison of new prokaryotic and mitochondrial members of the polypeptide chain release factor family predicts a five-domain model for release factor structure. Nucleic Acids Res. 1992;20:4423–4428. doi: 10.1093/nar/20.17.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishnan V, White S W. The structure of ribosomal protein S5 reveals sites of interaction with 16S rRNA. Nature. 1992;358:768–771. doi: 10.1038/358768a0. [DOI] [PubMed] [Google Scholar]
- 28.Ruusala T, Kurland C G. Streptomycin preferentially perturbs ribosomal proofreading. Mol Gen Genet. 1984;198:100–104. doi: 10.1007/BF00328707. [DOI] [PubMed] [Google Scholar]
- 29.Ruusala T, Andersson D, Ehrenberg M, Kurland C G. Hyper- accurate ribosomes inhibit growth. EMBO J. 1984;3:2575–2580. doi: 10.1002/j.1460-2075.1984.tb02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shima J, Hesketh A, Okamoto S, Kawamoto S, Ochi K. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2) J Bacteriol. 1996;178:7276–7284. doi: 10.1128/jb.178.24.7276-7284.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Synetos D, Frantziou C P, Alksne L E. Mutations in yeast ribosomal proteins S28 and S4 affect the accuracy of translation and alter the sensitivity of the ribosomes to paromomycin. Biochim Biophys Acta. 1996;1309:156–166. doi: 10.1016/s0167-4781(96)00128-5. [DOI] [PubMed] [Google Scholar]
- 32.Timms A R, Steingrimsdottir H, Lehmann A R, Bridges B A. Mutant sequences in the rpsL gene of Escherichia coli B/r: mechanistic implications for spontaneous and ultraviolet light mutagenesis. Mol Gen Genet. 1992;232:89–96. doi: 10.1007/BF00299141. [DOI] [PubMed] [Google Scholar]