Abstract
Cervical cancer is the fourth most common malignancy in women worldwide. Although infection from human papillomavirus (HPV) has been the leading cause of cervical cancer, HPV-negative cervical cancer accounts for approximately 3-8% of all cases. Previous research studies on cervical cancer have focused on HPV-positive cervical cancer due to its prevalence, resulting in HPV-negative cervical cancer receiving considerably less attention. As a result, HPV-negative cervical cancer is poorly understood. Its etiology remains elusive mainly due to limitations in research methodology such as lack of defined markers and model systems. Moreover, false HPV negativity can arise from inaccurate diagnostic methods, which also hinders the progress of research on HPV-negative cervical cancer. Since HPV-negative cervical cancer is associated with worse clinical features, greater attention is required to understand HPV-negative carcinoma. In this review, we provide a summary of knowledge gaps and current limitations of HPV-negative cervical cancer research based on current clinical statistics. We also discuss future directions for understanding the pathogenesis of HPV-independent cervical cancer.
Keywords: Cervical cancer subtypes, HPV negative cervical cancers, HPV test, Human cervical cancer models, Mutations in cervical cancer
INTRODUCTION
In 2020, more than 606,000 women were diagnosed with cervical cancer and around 341,831 women died due to cervical cancer worldwide (1). This figure represents that cervical cancer is the fourth most common cancer type in women (6.5% of all cancer incidence in women) (1). It has the fourth highest mortality of all cancer cases (7.5% of all cancer related deaths in women) (1). Since the discovery of human papillomavirus (HPV) in early 1980s (2), the majority of cervical cancer cases are associated with HPV infection. Most of cervical cancer research studies have focused on HPV-positive cervical cancer, aiming to develop diagnostic methods, HPV vaccines, and targeted therapies.
The reported incidence rate of HPV-positive cervical cancer has increased from 85.9% in 1990 to 92.9% in 2010 (3). The upward trend in HPV-positive cases is due to improvements in HPV detection methods and histological diagnosis (4). This means that the number of HPV-negative cases has been declined in the same time period. Nonetheless, it is worth to note that about 3-8% of cervical cancer cases are truly HPV-negative. Cervical cancer cases that are HPV-independent are being reported steadily in clinical practice (5-7).
HPV negative cervical cancer shows the following clinical features. Cervical adenocarcinoma is frequently (15-38%) HPV-negative (8), although cervical squamous cell carcinoma is mostly HPV-positive. HPV-negative cervical cancer patients show significantly worse prognosis due to advanced International Federation of Gynecology and Obstetrics (FIGO) stage with lymphatic invasion at diagnosis than HPV-positive cervical cancer patients. Although the molecular etiology of HPV-negative cervical adenocarcinoma is unknown, several studies have suggested that mutations in TP53, PIK3CA, and CDKN2A are involved (9-12).
To mitigate the poor prognosis of HPV-negative cervical cancer such as poor overall survival (OS) and disease-free survival (DFS), greater attention is needed for the treatment plan of these cases. Since there is no targeted therapy for HPV-negative cervical cancer, more research studies are needed to uncover the etiology of HPV-negative cervical cancer and to provide better therapeutic options for patients with HPV-negative cervical cancer.
In this review, we will provide an overview of the current status of HPV-negative cervical cancer in the perspective of clinic and research and discuss new studies to overcome current limitations of HPV-negative cervical cancer research with the aim to encourage more basic and translational research on HPV-negative cervical cancer.
INCIDENCE OF HPV-NEGATIVE CERVICAL CANCER
The association of HPV positivity with cervical cancer was identified in the early 1980s (2). Since then, most research studies on cervical cancer have focused on HPV-positive cervical cancer. However, several studies have also reported the incidence of HPV-negative cervical cancer. We will discuss the true portion of HPV independent cervical cancer cases in the clinic and its impact on treatment plans.
The number of reported cases of HPV-negative cervical cancer has been decreased over time. In 2001, Baay et al. reported observational data from Belgian patients with cervical cancer and HPV infection and found that HPV-negative cases accounted for 13% of women with cervical cancer before 2000 (8). However, a subsequent study reported that HPV-negative cases were decreased, accounting for only 7.1% of women with cervical cancer between 2001 and 2008 (13).
Decreased numbers of HPV-negative cervical cancer in more recent studies could be largely explained by improved HPV test and histological classification of cervical cancer. Sensitivity and specificity of HPV test are critical to determine HPV infection status. Castle and colleagues showed cervical cancer screening results with HPV test and Papanicolaou (Pap) test as two common test methods for US women (6). Out of 526 patients with cervical cancer, 98 (18.6%) women showed HPV-negative results from the HPV DNA test using Hybrid Capture 2 (HC2). However, the HC2 test can only detect 13 sub-types of high-risk HPVs, leading to a false negativity in 18.6% of HPV negative cases. Improved pathological diagnosis also contributes to more accurate identification of truly HPV-negative cervical cancer. A recent study with 371 biopsy-proven primary cervical cancers showed that 68% of HPV-negative cervical cancer cases were in fact non-cervical cancers (5). These results suggest that the number of HPV-negative cervical cancer cases can be overestimated due to false negative HPV tests and wrong histological diagnosis.
To obtain accurate statistics of HPV-negative cervical cancer, improved test methods and clinical categorization are needed. The Cancer Genome Atlas Research Network (TCGA) analysed primary tumors and blood samples from women with cervical cancer and showed that 5% of core-set tumors were genotypically HPV-negative (7). Since whole genome sequencing is not feasible for routine HPV test, multiplex polymerase chain reaction (PCR) based test could be an alternative (14). Comparing HPV test methods between HC2 and highly sensitive PCR techniques showed that 14 (10.2%) out of 136 women with cervical cancer in Spain were HPV negative based on HC2 test, whereas only 8 (5.8%) cases were confirmed to be truly HPV-negative based on PCR test (15). Another study showed consistent results (16). A PCR based virus testing has been widely used. Its accuracy and applicability in population level have been proven during the SARS-CoV-2 pandemic as well (17).
In the clinic, the diagnosis of HPV negative cervical cancer can be wrong due to limitation of HPV testing methods (i.e., HC2 and Pap test). Establishing a standard and more accurate HPV test with specific diagnosis criteria will lead us to collect consistent data from clinical practice. It can also expedite the progress of future research on HPV negative cervical cancer. Lastly, it is worth to note that most cervical cancer studies have used data from Caucasian populations. Considering the incidence of HPV negative cervical cancer may vary from different ethnic groups, studies on multi-ethnic groups are needed to provide more information.
KNOWLEDGE GAP IN HPV-NEGATIVE CERVICAL CANCER
Lack of interest in HPV-negative cervical cancer
After zur Hausen and colleagues detected HPV 16 DNA in cervical cancer cells in 1983 (2), following studies have revealed that not only HPV 16, but also other high-risk types of HPV including HPV 18 are strongly associated with adenocarcinoma of the cervix (18-20). The majority of studies about cervical cancer have aimed to discover the viral oncogenic mechanism of HPV and develop diagnostic methods for early detection, HPV vaccines, and targeted drugs for patients with HPV-positive cancer (21). This research trend might have skewed the research community to consider HPV infection as the sole cause of cervical cancer. This might have also resulted in the very limited number of studies covering HPV-negative cervical cancer.
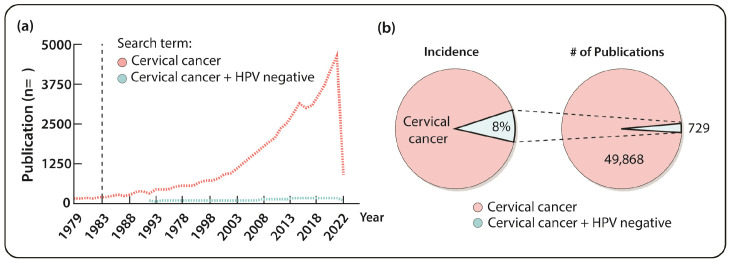
The number of studies focusing on HPV-negative cancer is extremely low compared to that on HPV-negative cancer. As of February 2022, PubMed search with keyword of “cervical cancer” has reached 49,868 entries since 1983 (Fig. 1A). As discussed earlier, about 3-8% of human cervical cancers are HPV independent based on a conservative estimate. However, the number of publications including two search terms, “HPV negative” and “cervical cancer”, is only 729, accounting for 1.46% of publications on cervical cancer in PubMed. This percentage is less than a third of the conservatively estimated incidence rate of HPV-negative cervical cancer (Fig. 1B). Although the number of publications on HPV-negative cervical cancer has slightly increased since 2014, the lack of interest in HPV-negative cervical cancer compared to the general interest in cervical cancer needs to be improved.
Fig. 1.
Understudied HPV-negative cervical cancer. (A) PubMed search results indicate that the number of published works on HPV-negative cervical cancer (blue) is significantly lower than that on overall cervical cancer research (red). (B) The percentage of published work on HPV-negative cervical cancer (1.46%, right) is lower than the actual incidence rate of HPV-negative cervical cancers in the clinic (3-8%, left).
Underestimated clinical significance
Due to less attention to HPV-negative cervical cancer, the clinical significance of HPV-negative cervical cancer might have been underestimated. Several research studies have suggested that HPV-negative cancer might represent worse clinical features with distinct biological characteristics compared to HPV-positive cervical cancer (22-24). The first study that evaluated the difference in clinical features between patients with HPV-positive cervical cancer and HPV-negative cervical cancer had a cohort of 136 women with cervical cancer (8). Through PCR-based tests, the cohort was divided into two groups, a HPV-positive group and a HPV-negative group. Clinical data showed that patients with HPV-negative cervical cancer had significantly lower DFS than those with HPV-positive cervical cancer. In addition, patients with HPV-negative cervical cancer tended to have poorer OS than those with HPV-positive cervical cancer, although the difference was not statistically significant (8). Another noticeable result is that HPV-negative cervical cancer is associated with a higher risk of progression and mortality than HPV-positive cervical cancer. HPV-negative cervical cancer shows advanced FIGO stage at diagnosis, which might explain its increased progression and mortality. The previous study (8) has also indicated that patients with HPV negative cervical cancer have a higher rate of lymph node metastasis than patients with HPV positive cervical cancer. The limitation of that study was a small number of samples. Thus, the clinical significance of HPV-negative cervical cancer warranties further research with a larger population.
Another research analysed clinical and pathological data from 214 women with cervical cancer in Spain from 2012 to 2015 (13). It also showed the clinical significance of HPV-negative cervical cancer (13). Through a highly sensitive PCR test and p16 immunostaining for 214 cervical cancer specimens, 21 (10%) tumors were found to be HPV-negative. These HPV-negative cervical cancers were associated with advanced FIGO stage and lymph node metastases. In terms of clinical characteristics, patients with HPV-negative cervical cancer had significantly worse DFS and OS as well than those with HPV-positive cervical cancer. Consistently, a meta-analysis study (14) using data from 2,838 cervical cancer cases with HPV DNA status in 17 published studies showed that patients with HPV-positive cervical cancer had a better prognosis including OS and DFS than patients with HPV-negative cervical cancer. These studies showed the clinical significance of HPV-negative cervical cancer, suggesting that HPV-negative cervical carcinoma might arise via unknown biologically distinct pathways from relatively well-characterized HPV-dependent pathways (9).
Distinct biological characteristics of HPV-negative cervical cancer
Several studies have suggested that HPV-negative cervical cancer might have distinct biological features from HPV-positive carcinomas (2, 9, 21, 22, 25). Cervical cancers can be divided into two types: squamous cell carcinomas and adenocarcinomas. HPV-negative cervical cancer tumors are significantly associated with adenocarcinomas (2, 9, 21, 22). HPV-negative cervical carcinomas also show different histological types such as gastric, clear cell, mesonephric, and endometrioid types from HPV-positive ones (25).
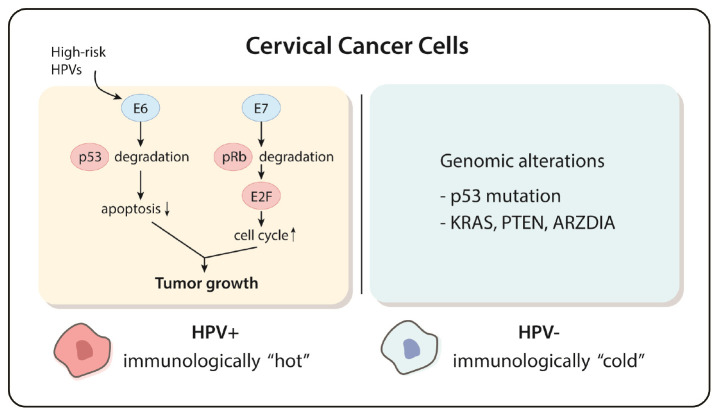
HPV-negative cervical cancer may arise due to distinctive pathological pathway compared to HPV-positive cervical cancer. Nicolás and colleagues showed higher prevalence of p53 mutation in HPV-negative cervical cancers than in HPV-positive ones, with 71% of HPV-negative cervical cancer cases displaying aberrant p53 immunostaining pattern with p16 overexpression (9). Since the strong link between p53 mutation and poor prognosis was shown by a previous research (26), the phenotypical characteristic might be the reason why HPV-negative cervical cancer showed more aggressive features including advanced FIGO stage, higher rate of metastasis, and poorer prognosis than HPV-positive one (11). Loss of function mutation of TP53, a tumor suppressor gene encoding tumor suppressor p53, is easily found in many other types of cancer (26). Interestingly, viral oncoprotein E6 encoded by HPVs can also block apoptosis induced by p53, resulting in outgrowth of infected cells (12). Further molecular studies that investigate p53 mutations in HPV-negative cervical cancer are required to delineate distinct molecular etiologies of HPV-positive and HPV-negative cervical cancers.
Genetic mutations in cervical cancer can provide therapeutic targets. Most HPV-positive cervical cancers have either one or both mutations of PI3K-MAPK and TGFb signaling pathways. These genetic pathways are considered as therapeutic targets for HPV-positive cervical cancers (7). According to the TCGA study, HPV-negative cervical tumors were significantly associated with additional genomic mutations in KRAS, ARID1A, and PTEN compared to HPV-positive cervical tumors (7). This study highlights that unraveling mechanisms of PTEN and ARID1A mutations in HPV-negative cervical carcinoma can be a milestone to discover novel therapeutic targets. The discovery of distinct molecular pathways that lead to HPV negative cervical carcinomas can highlight the biological diversity of cervical cancer development.
No targeted drugs
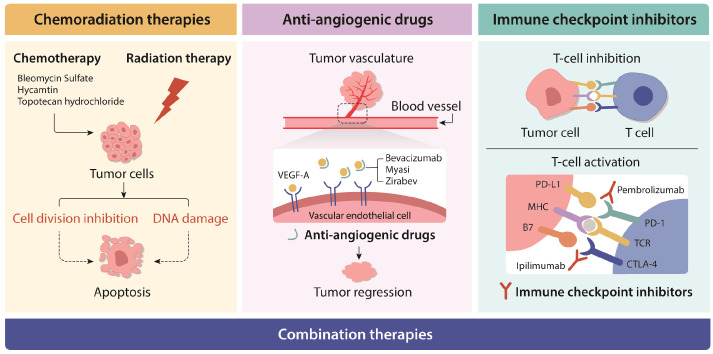
Of nine FDA-approved drugs and two combination therapies for cervical cancer treatment, there are no targeted drugs that are specific for HPV-positive or HPV-negative cervical cancers (27). Primary treatment option of both HPV-positive and HPV-negative cervical cancers is determined by results of clinical staging and diagnostic imaging (28). Approved cervical cancer therapies include chemoradiation therapies, anti-angiogenic drugs, immune checkpoint inhibitors, and combination therapies (Fig. 2). Bleomycin Sulfate, hycamtin, and topotecan hydrochloride are conventional chemotherapy drugs. A recent clinical research has shown that adjuvant radiation therapy (RT) or concurrent chemoradiation therapy (CCRT) after radical hysterectomy in early cervical cancer can lead to improved therapeutic benefits (29). Anti-angiogenic drugs in cervical cancer treatment that can inhibit the secretion of vascular endothelial growth factor A (VEGF-A) include inhibitors such as bevacizumab, myasi, and zirabev (30). The combination of bevacizumab and paclitaxel with cisplatin or topotecan has been used for patients who are not suitable for platinum therapy in the EU (31).
Fig. 2.
Approved cervical cancer therapies. Standard treatment options include surgery, chemo-radiation therapies, targeted therapy (or anti-angiogenic therapy), immunotherapy, and a combination of these. New treatment options are also being tested in clinical trials. Further research studies will lead to the development of more effective novel therapeutic options.
Another notable therapeutic option for treating cervical cancer is an immunotherapy using immune checkpoint inhibitors. In 2018, the first immune checkpoint inhibitor, Pembrolizumab, targeting PD-1 in cervical cancer was approved by FDA (32). Previous clinical studies have demonstrated that blocking PD-1 pathway is an effective therapeutic option to suppress tumor growth in PD-L1 expressing cervical cancers (32-34). Immunotherapies are considered attractive options for HPV-positive cervical cancer which is referred to as an immunologically “hot” tumor (Fig. 3) (34). In particular, a recent in vitro study has indicated that HPV infection can increase the expression level of PD-L1 in infected cells. Elevated PD-L1 expression level has been frequently reported in HPV-positive cervical cancers (30). Several successful clinical trials that tested the efficacy of pembrolizumab in combination with other chemotherapeutic drugs in cervical cancer (34, 35) have resulted in FDA approval of pembrolizumab as a neoadjuvant therapy with bevacizumab (35). Another immune checkpoint inhibitor, ipilimumab, a CTLA4 inhibitor, is also being tested in a clinical trial (36).
Fig. 3.
Distinct molecular etiology of HPV-positive and HPV-negative cervical cancers. HPV-positive cervical cancers are known to be immunologically “hot” with viral proteins driving the oncogenesis. Although some mutations of oncogenes or tumor suppressor genes have been reported in HPV-negative cervical cancers, more research studies on the molecular etiology of this type of cancer are needed to elucidate its unique mechanisms of oncogenesis.
The limitation of immunotherapy is that drugs are selectively effective only in patients with PD-L1 or CTLA4 positive cervical carcinomas. In addition, HPV-negative cervical cancers are considered as immunologically “cold” as they are not associated with viral infection (36). Not surprisingly, immunologically “cold” tumors are known to be not responsive to immunotherapies (37).
To overcome limited therapeutic options in HPV-negative cervical cancers, more fundamental research studies on the molecular etiology and identifying novel therapeutic targets of HPV-negative cervical cancer are needed (Fig. 3). Current treatment guidelines do not demonstrate specialized treatment options based on the histological type of genomic signature of cervical carcinomas (38). Optimization of subtype-specific therapeutic strategies based on pathobiological features of cervical carcinoma can improve therapeutic outcomes (39). Novel therapeutic options may be suggested by discovering distinctive underlying molecular mechanisms of tumorigenesis in HPV-negative cervical carcinomas.
CURRENT LIMITATIONS OF HPV-NEGATIVE CERVICAL CANCER RESEARCH
Lack of defined markers and classification
One of the main causes that hinders the progress of HPV negative cervical cancer research is the lack of specific definition to classify HPV-negative cervical cancers. Currently, HPV negative cervical cancer cases are distinguished from HPV-positive cancer cases by HPV detection tests. As discussed earlier, HPV infection diagnostic tests are not sufficient to classify HPV negative cervical cancer because the test method varies in diverse clinical practice environment. The two most clinically validated molecular diagnostic methods of HPV infection are the HC2 test and an in-house PCR test (40). HC2 test is based on a nucleic acid hybridization assay. By using antibodies bound to a microtiter plate, specific HPV DNA-RNA hybrids are detected and the intensity of emitted light from a luminescent product generated by the reaction is measured as relative light unit (RLU). The value of RLU represents the relative amount of specific DNA in the specimen. Conventional HC2 test is only able to detect 13 high-risk HPV subtypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68). A more recently developed HC2 test from several providers can detect five low-risk HPV subtypes (HPV 6, 11, 42, 43, and 44) in addition to the 13 high-risk HPV subtypes (41).
Compared to the HC2 test, a PCR-based test shows a higher sensitivity to detect HPV infection from specimens since an amplification process of PCR can enhance the sensitivity of detecting low virus copy numbers in samples. By using multiplex PCR-based test, the number of HPV subtypes that can be detected simultaneously can be significantly increased up to 19 HPV subtypes (42). While the PCR test can confirm the existence of HPV in a specimen, it cannot determine whether the HPV infection is transient or promoting tumorigenesis (43).
Another diagnostic marker for detecting a high-risk HPV infection is p16 immunohistochemistry (IHC). Overexpression of p16 has been observed in specific types of cervical carcinoma such as cervical preneoplasia and invasive cervical cancer (44). In contrast, decreased p16 expression has been observed from normal cervix or other cervical cancer subtypes such as metaplastic cervical carcinomas (45). The overexpression of p16 seems to be independent of HPV infection in cervical carcinomas because up to 57% of HPV negative cervical cancers display p16 protein expression (46, 47).
For better classification of HPV-negative cervical cancers, specific histopathologic markers are required. For example, gastric-type adenocarcinoma of the cervix (GAS), a novel type of cervical carcinoma, is a HPV-negative cervical adenocarcinoma (48). GAS shows worse prognosis than HPV-positive adenocarcinoma (48), consistent with other studies showing poor clinical outcomes of HPV-negative cervical carcinomas (8, 10, 15, 26). To improve the accuracy of GAS diagnosis, IHC staining has been suggested to measure intra-cytoplasmic gastric type mucin, a GAS specific marker. Likewise, classifying subtypes of HPV-negative cervical carcinomas based on their histopathological characteristics will be essential to uncover tumorigenesis mechanisms of HPV-negative cervical carcinomas and identify novel biomarkers.
Limitation of cervical cancer model systems
One of the general challenges in cervical cancer research and drug discovery is generating cancer models. The most common in vitro cervical cancer model is using human immortalized cancer cell lines. HeLa cell line named after Henrietta Lacks who died of an aggressive cervical cancer in 1951 is the oldest and most widely used cells in cancer research. Additionally, eight more immortalized cervical cancer cell lines have been established for cervical cancer research (Table 1). Two cervical cancer cell lines, C33A and OMC-4, represent HPV-negative cervical cancer. Other common cell lines, CaSki and SiHa, are HPV16-positive cervical cancer cell lines. Immortalized cervical cancer cell lines with HPV18 include HeLa, KB-V1/Vbj, and TMCC-1. ME180 is a cell line containing another high-risk HPV type, HPV68. The noticeable difference between HPV-negative and HPV-positive cervical cell lines is that the p53 tumor suppressor gene is mutated only in two HPV-negative cervical cell lines (49). It is plausible to speculate that p53 mutation is one of the critical steps in tumorigenesis of HPV-negative cervical carcinomas.
Table 1.
List of human cervical cancer cell lines
| Cell line | HPV status | Cancer type | Description | Reference |
|---|---|---|---|---|
| C33A | Negative | Squamous cell carcinoma | Mutation of the p53 tumor suppressor gene Point mutation at codon 273 |
Lee et al., 2005; Hirchaud et al., 2013; Kim MS et al., 2013 |
| OMC-4 | Negative | Adenocarcinoma | Mutation of the p53 tumor suppressor gene well differentiated | Noguchi et al., 2006 |
| CaSki | HPV16 | Squamous cell carcinoma | 200-400 copies of HPV-16 | Ahn et al., 2003a; Lee et al., 2005; Shin et al., 2008; Lee et al., 2011; Cherry et al., 2013; Hirchaud et al., 2013; Kim et al., 2013 |
| SiHa | HPV16 | Squamous cell carcinoma | One to two integrated copies of HPV16 | Yokoyama et al., 2004; Lee et al., 2005; Singh et al., 2010; Lee et al., 2011; Di Domenico et al., 2012; Kim et al., 2013 |
| HeLa | HPV18 | Adenocarcinoma | 10-50 copies of HPV18 | Wang et al., 2001; Samama et al., 2002; Guo et al., 2004; Totta et al., 2004; Virgili et al., 2004; Zhang et al., 2006; Hirchaud et al., 2013 |
| KB-V1/Vbl | HPV18 | Adenocarcinoma | Multidrug resistant | Pluchino et al., 2012 |
| TMCC-1 | HPV18 | Adenocarcinoma | Poorly differentiated | Yokoyama et al., 2004; Noguchi et al., 2006; Yokoyama et al., 2008 |
| ME180 | HPV68 | Squamous cell carcinoma | Absence of estrogen receptor isoforms | Yokoyama et al., 2004; Wang et al., 2001 |
Recent studies have highlighted that conventional cell lines are limited to recapitulate biological characteristics of primary cervical cancer cells (50). Using immortalized cervical cancer cell lines is particularly discouraged for cancer drug development research as it is impossible to permit squamous differentiation of the ectocervix with these cell lines. Alternatively, organotypic epithelial raft cultures have been suggested to overcome limitations of immortalized cervical cancer cell lines in vitro (50).
For in vivo cervical cancer research, syngeneic and xenograft mouse models have been established. The most common mouse models of cervical cancer are syngeneic mouse models with TC-1 Luc cell line and C3 cell line (51). The TC-1 Luc cell line is generated by immortalizing primary lung cells of C57BL/6 mouse with E6 and E7 oncogenes from HPV16 (52). The C3 cell line is also a murine cell line from C57BL/6 embryonic cells. It contains both E and L oncogenes from HPV16 (53). Thus, syngeneic mouse models with C3 cell line can be used for studies targeting HPV16 L protein. The TC-1 Luc cell line is more frequently used for research studies focusing on HPV E proteins. Both cell lines are used for not only HPV-positive cervical cancer research, but also for HPV-positive head and neck cancer and HPV vaccine studies (41). Although both TC-1 Luc and C3 cell lines are widely used in studies of HPV infection associated diseases, mouse models from those cell lines have failed to mimic the specific pathogenesis of cervical carcinoma because the origin of these cells is not from cervix (52, 53). In addition, those syngeneic models can only represent HPV-positive cervical cancers as they are transduced to express viral proteins. Alternatively, U14 cell line, a murine uterine cervical cancer cell line, is another cell line used for generating cervical cancer mouse models. It was established from a murine primary cervical carcinoma not associated with HPV infection (54). However, whether murine cervical carcinoma and human cervical carcinoma share common pathobiological characteristics is currently unknown. Such knowledge gap may limit the use of syngeneic mouse model with U14 cell line in human cervical cancer research.
For generating cervical cancer xenograft mouse models, SiHa and HeLa cell lines are used. SiHa cell line is a squamous carcinoma cell line that contains HPV 16 oncogenes. HeLa cell line is an epithelial adenocarcinoma cell line that contains HPV18 oncogene (55). Human cell line-derived xenograft (CDX) models also have limitations as they can only be used for HPV-positive cervical cancer studies. For HPV-positive cervical cancer research, patient-derived xenograft (PDX) models have been established using two major subtypes of human cervical cancer: adenocarcinomas and squamous cell carcinoma (56). However, the PDX mouse model for HPV-negative cervical carcinoma has not been developed yet. The lack of HPV-negative cervical cancer in vivo models may be partially explained by the little demand of experimental models in research community or by our limited understanding on the tumorigenesis mechanisms of HPV-negative cervical carcinomas.
FUTURE RESEARCH DIRECTIONS
Distinct tumorigenesis mechanisms of HPV-negative cervical cancer
Several studies have suggested that HPV-negative cervical cancer might have distinct tumorigenic mechanisms as it has different pathobiological characteristics from HPV-positive cervical cancer (7, 9, 15, 48). As discussed earlier, our current understanding of HPV-negative cervical cancer etiology and progression is very poor compared to that for HPV infection associated carcinogenic pathways. Recently, a comprehensive genomic study was conducted to discover immunogenic alterations in HPV-positive and HPV-negative cervical cancers using RNA-seq, copy number variation, and genetic mutation analysis (57). This study identified that nine immune signature genes (BIR2, IL1RAP, MMP1, MMP3, MMP13, PIK3CA, PLD1, TFRC, and TNFSF10) were up-regulated with frequent genomic amplifications in HPV-positive cervical cancer, leading us to speculate that immune alterations in HPV-negative cervical cancer might be different from those in HPV-positive cervical cancer. Such distinct immune response can be a novel therapeutic target to improve current treatment and develop personalized treatment options. More research studies are required to obtain a comprehensive understanding on distinct tumorigenesis mechanisms as well as differential immune responses of HPV-negative and HPV-positive cervical cancers.
Mutations of tumor suppressor genes and oncogenes in other cancer types may provide some clues for deciphering the molecular etiology of HPV-negative cervical cancers. The KRAS oncogene is mutated in most HPV-negative cervical adenocarcinomas (7). Since KRAS mutation is also frequently observed in lung adenocarcinomas (58), these two distinct tumors may share a common tumorigenesis mechanism. Similarly, as mentioned earlier, HPV-negative cervical carcinomas are strongly associated with adenocarcinomas, including several rare subtypes such as clear cell and gastric types (25). Cervical clear cell adenocarcinomas are more likely to be observed in older cervical cancer patients accompanied by the loss of PTEN expression and increases of EGFR and HER2 expression (59). Gastric type endocervical adenocarcinoma shows frequent genetic alterations of STK11/LKB1 and TP53 genes (59, 60). Identifying molecular characteristics of HPV-negative carcinoma subtypes with integrated genomics approaches will shed light on new research avenues to discover distinct oncogenic pathways of HPV-negative cervical cancer and new treatment options.
Improvement of cervical cancer model system
To overcome limitations of current in vivo and in vitro cervical cancer models, new ideas to generate more human cancer-relevant models have been proposed. Human cervical organoid is one of the most attractive tools because it can address critical limitation of current 2D immortalized cell line models that fail to represent female 3D reproductive tract and intra-tumor heterogeneity (61, 62). Several studies have shown that the establishment of human-derived normal ecto- and endo-cervical organoids as well as tumoroid can successfully recapitulate the tissue of origins (61-63). It is clear that cervical organoids can be used as an experimental platform for both HPV-positive and HPV-negative cervical cancer research.
Patient-derived organoids can play an important role in discovering molecular mechanisms of oncogenesis while maintaining tumor heterogeneity as well as in drug discovery research. Patient-derived organoids represent the pathogenic diversity of cervical cancer subtypes including HPV-negative cervical adenocarcinomas (62). Recently, a patient-derived organoid model from a HPV-negative cervical clear cell carcinoma (cCCC), an extremely rare cervical cancer subtype, has been established (63). This study revealed that HPV negative cCCC organoid had different histological and genetic features compared to squamous cell carcinomas and that the oncogene MET was overexpressed in cCCC due to copy number gain of MET. Interestingly, MET downstream signaling pathways such as MEK/ERK and PI3K/AKT were not activated in MET overexpressing cCCC, suggesting noncanonical pro-oncogenic effects of MET (63). Taken together, those progresses support the idea that establishment of human-derived normal and tumorous cervical organoids provides a valuable tool for cervical cancer research, especially for studies on HPV-negative cervical carcinomas.
Exosomes
Exosomes are extracellular vesicles secreted by cells that play a critical role in intercellular communication. Exosomes carry cell-specific cargos of proteins, nucleic acids, and lipids (64). Contents of exosomes vary by cell origin and by pathological and physiological conditions of exosome-secreting cells (65). Tumor cells can secrete tumor-specific exosomes such as dysfunctional miRNAs involved in cancer pathogenesis (66). As exosomes can exist in body fluids, exosomal miRNAs can be used as non-invasive diagnostic biomarkers (67) and novel therapeutic targets to treat cervical cancer.
A recent study has revealed the role of miR-221-3p, an exosomal miRNA, in promoting angiogenesis in cervical cancer (68). For both HPV-positive and HPV-negative cell lines (SiHa and CC3A), miR-221-3p can be transported via exosomes from cancer cells to vessel endothelial cells. miR-221-3p is correlated with the density of microvascular in vitro. It can improve tumor growth in vivo (68). With these results, Wu et al. have concluded that exosomal miRNA-221-3p can be a potential therapeutic target and a diagnostic biomarker to study the progression of cancer (69). Zhang and colleagues have shown that other exosomal genetic materials such as lncRNAs are differentially expressed between patients with and without cervical cancer (70). Hence, the role of exosomes in cervical cancer requires more investigation to understand clinical implications for both improved diagnosis and treatment.
Recently, Bhat et al. have suggested that there are quantitative and qualitative differences in exosomal cargos between HPV-positive and HPV-negative cervical cancer cells (67). Using HPV-positive and HPV-negative cell lines, they showed that HPV-positive exosomes induced relatively more migration and angiogenesis, highlighting the role of exosomes as potential pharmacological targets for metastasis of cervical cancer. In a follow-up study, they screened exosomes from HPV-positive and HPV-negative cervical cancer cells and identified 3,099 differentially expressed transcripts of lncRNAs (71). These studies show that exosomal lncRNAs can be used to differentiate HPV-positive and HPV-negative cervical cancer cases. Differences between exosomes secreted by HPV-positive and HPV-negative cervical cancer cells need further investigation as they may serve as a more accurate diagnostic tool for HPV-negative cervical cancer.
CONCLUSIONS
In this review, we explored current clinical statistics of HPV independent cervical cancers. Although HPV-negative cervical cancer accounts for only less than 10% of total cervical cancer cases, its clinical impacts are not neglectable because HPV-negative cervical cancer patients show advanced FIGO stage at diagnosis and poorer prognosis than HPV-positive cases. Moreover, HPV-negative cervical cancers seem to have distinctive molecular features compared to HPV-positive cervical cancers, highlighting the possible discovery of novel targeted therapies for this type of cancer. However, our understanding on molecular etiology and mechanisms of cancer progression of HPV-negative cervical cancer is very limited largely due to very few research studies focusing on this cancer type. Considering limitations of currently available cervical cancer models, development of novel cancer models is urgently needed for basic and translational research studies. In that regard, recent achievements in developing more physiologically relevant cancer models especially for HPV-negative cervical cancers such as patient-derived organoid model are prominent for future research studies.
ACKNOWLEDGEMENTS
This work was supported by grants from NIH grants P20GM 121176 from NIGMS (mentored PI in AIM CoBRE) and P30 CA118100 from NCI (UNM Comprehensive Cancer Center Support Grant), METAvivor, and Department of Pathology, University of New Mexico (start-up funds) to T-H.K.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Durst M, Gissmann L, Ikenberg H, zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983;80:3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–935. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 4.Xing B, Guo J, Sheng Y, Wu G, Zhao Y. Human papillomavirus-negative cervical cancer: a comprehensive review. Front Oncol. 2020;10:606335. doi: 10.3389/fonc.2020.606335.4c9c2921f61b4a0aa3696e74aa718f7a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petry KU, Liebrich C, Luyten A, Zander M, Iftner T. Surgical staging identified false HPV-negative cases in a large series of invasive cervical cancers. Papillomavirus Res. 2017;4:85–89. doi: 10.1016/j.pvr.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle PE. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:566. doi: 10.1002/cncy.21572. [DOI] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research N, author; Albert Einstein College of M, author; Analytical Biological S, author et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baay MF, Tjalma WA, Weyler J, et al. Prevalence of human papillomavirus in elderly women with cervical cancer. Gynecol Obstet Invest. 2001;52:248–251. doi: 10.1159/000052984. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas I, Marimon L, Barnadas E, et al. HPV-negative tumors of the uterine cervix. Mod Pathol. 2019;32:1189–1196. doi: 10.1038/s41379-019-0249-1. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Tan Y, Zhu LX, et al. Prognostic value of HPV DNA status in cervical cancer before treatment: a systematic review and meta-analysis. Oncotarget. 2017;8:66352–66359. doi: 10.18632/oncotarget.18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zampronha Rde A, Freitas-Junior R, Murta EF, et al. Human papillomavirus types 16 and 18 and the prognosis of patients with stage I cervical cancer. Clinics (Sao Paulo) 2013;68:809–814. doi: 10.6061/clinics/2013(06)14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tommasino M, Accardi R, Caldeira S, et al. The role of TP53 in cervical carcinogenesis. Hum Mutat. 2003;21:307–312. doi: 10.1002/humu.10178. [DOI] [PubMed] [Google Scholar]
- 13.Tjalma WA, Trinh XB, Rosenlund M, et al. A cross-sectional, multicentre, epidemiological study on human papillomavirus (HPV) type distribution in adult women diagnosed with invasive cervical cancer in Belgium. Facts Views Vis Obgyn. 2015;7:101–108. [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Pastrana F. Detection and typing of human papilloma virus by multiplex PCR with type-specific primers. ISRN Microbiol. 2012;2012:186915. doi: 10.5402/2012/186915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Carunchio L, Soveral I, Steenbergen RD, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG. 2015;122:119–127. doi: 10.1111/1471-0528.13071. [DOI] [PubMed] [Google Scholar]
- 16.Iftner T, Germ L, Swoyer R, et al. Study comparing human papillomavirus (HPV) real-time multiplex PCR and Hybrid Capture II INNO-LiPA v2 HPV genotyping PCR assays. J Clin Microbiol. 2009;47:2106–2113. doi: 10.1128/JCM.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarrom D, Elston L, Washington J, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid Based Med. 2022;27:33–45. doi: 10.1136/bmjebm-2020-111511. [DOI] [PubMed] [Google Scholar]
- 18.Syrjanen K, Mantyjarvi R, Vayrynen M, et al. Human papillomavirus (HPV) infections involved in the neoplastic process of the uterine cervix as established by prospective follow-up of 513 women for two years. Eur J Gynaecol Oncol. 1987;8:5–16. [PubMed] [Google Scholar]
- 19.Pratili MA, Le Doussal V, Harvey P, et al. [Human papillomaviruses in the epithelial cells of the cervix uteri: frequency of types 16 and 18. Preliminary results of a clinical, cytologic and viral study]. J Gynecol Obstet Biol Reprod (Paris) 1986;15:45–50. [PubMed] [Google Scholar]
- 20.Weisbrod CR, Chavez JD, Eng JK, Yang L, Zheng C, Bruce JE. In vivo protein interaction network identified with a novel real-time cross-linked peptide identification strategy. J Proteome Res. 2013;12:1569–1579. doi: 10.1021/pr3011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 22.Barreto CL, Martins DB, de Lima Filho JL, Magalhaes V. Detection of human papillomavirus in biopsies of patients with cervical cancer, and its association with prognosis. Arch Gynecol Obstet. 2013;288:643–648. doi: 10.1007/s00404-013-2803-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins GD, Davy M, Roder D, Uzelin DM, Phillips GE, Burrell CJ. Increased age and mortality associated with cervical carcinomas negative for human papillomavirus RNA. Lancet. 1991;338:910–913. doi: 10.1016/0140-6736(91)91773-N. [DOI] [PubMed] [Google Scholar]
- 24.Riou G, Favre M, Jeannel D, Bourhis J, Le Doussal V, Orth G. Association between poor prognosis in early-stage invasive cervical carcinomas and non-detection of HPV DNA. Lancet. 1990;335:1171–1174. doi: 10.1016/0140-6736(90)92693-C. [DOI] [PubMed] [Google Scholar]
- 25.Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. 2018;42:214–226. doi: 10.1097/PAS.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 27.Reed N, Balega J, Barwick T, et al. British Gynaecological Cancer Society (BGCS) cervical cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2021;256:433–465. doi: 10.1016/j.ejogrb.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 29.Yeung AR, Pugh SL, Klopp AH, et al. Improvement in patient-reported outcomes with intensity-modulated radiotherapy (RT) compared with standard RT: a report from the NRG Oncology RTOG 1203 Study. J Clin Oncol. 2020;38:1685–1692. doi: 10.1200/JCO.19.02381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yetkin-Arik B, Kastelein AW, Klaassen I, et al. Angiogenesis in gynecological cancers and the options for anti-angiogenesis therapy. Biochim Biophys Acta Rev Cancer. 2021;1875:188446. doi: 10.1016/j.bbcan.2020.188446. [DOI] [PubMed] [Google Scholar]
- 31.Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25:1044–1052. doi: 10.1093/annonc/mdu098. [DOI] [PubMed] [Google Scholar]
- 32.Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 33.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594–1602. doi: 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 34.Wendel Naumann R, Leath CA., 3rd Advances in immunotherapy for cervical cancer. Curr Opin Oncol. 2020;32:481–487. doi: 10.1097/CCO.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 36.Da Silva DM, Enserro DM, Mayadev JS, et al. Immune activation in patients with locally advanced cervical cancer treated with ipilimumab following definitive chemoradiation (GOG-9929) Clin Cancer Res. 2020;26:5621–5630. doi: 10.1158/1078-0432.CCR-20-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada M, Tokunaga H, Kigawa J, Yaegashi N. Impact of histopathological risk factors on the treatment of stage IB-IIB uterine cervical cancer. Tohoku J Exp Med. 2020;252:339–351. doi: 10.1620/tjem.252.339. [DOI] [PubMed] [Google Scholar]
- 39.Arezzo F, Cormio G, Loizzi V, et al. HPV-negative cervical cancer: a narrative review. Diagnostics (Basel) 2021;11:952. doi: 10.3390/diagnostics11060952.98d0a4d3aa334914b91dda1a56be5afa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meijer CJ, Berkhof J, Castle PE, et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int J Cancer. 2009;124:516–520. doi: 10.1002/ijc.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poljak M, Cuzick J, Kocjan BJ, Iftner T, Dillner J, Arbyn M. Nucleic acid tests for the detection of alpha human papillomaviruses. Vaccine. 2012;30 Suppl 5:F100–106. doi: 10.1016/j.vaccine.2012.04.105. [DOI] [PubMed] [Google Scholar]
- 42.Gheit T, Landi S, Gemignani F, et al. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human papillomavirus types. J Clin Microbiol. 2006;44:2025–2031. doi: 10.1128/JCM.02305-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prigge ES, Arbyn M, von Knebel Doeberitz M, Reuschenbach M. Diagnostic accuracy of p16(INK4a) immunohistochemistry in oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. Int J Cancer. 2017;140:1186–1198. doi: 10.1002/ijc.30516. [DOI] [PubMed] [Google Scholar]
- 44.Klaes R, Friedrich T, Spitkovsky D, et al. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 45.Jedpiyawongse A, Homcha-em P, Karalak A, ivatanakul P., Sr Immunohistochemical overexpression of p16 protein associated with cervical cancer in Thailand. Asian Pac J Cancer Prev. 2008;9:625–630. [PubMed] [Google Scholar]
- 46.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 47.Larque AB, Hakim S, Ordi J, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27:343–351. doi: 10.1038/modpathol.2013.155. [DOI] [PubMed] [Google Scholar]
- 48.Pirog EC. Diagnosis of HPV-negative, gastric-type adenocarcinoma of the endocervix. Methods Mol Biol. 2015;1249:213–219. doi: 10.1007/978-1-4939-2013-6_16. [DOI] [PubMed] [Google Scholar]
- 49.Sak K. Characteristic features of cytotoxic activity of flavonoids on human cervical cancer cells. Asian Pac J Cancer Prev. 2014;15:8007–8019. doi: 10.7314/APJCP.2014.15.19.8007. [DOI] [PubMed] [Google Scholar]
- 50.Lohmussaar K, Boretto M, Clevers H. Human-derived model systems in gynecological cancer research. Trends Cancer. 2020;6:1031–1043. doi: 10.1016/j.trecan.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Arbeit JM. Mouse models of cervical cancer. Comp Med. 2003;53:256–258. [PubMed] [Google Scholar]
- 52.Sharma RK, ivastava AK, Sr, Yolcu ES, et al. SA-4-1BBL as the immunomodulatory component of a HPV-16 E7 protein based vaccine shows robust therapeutic efficacy in a mouse cervical cancer model. Vaccine. 2010;28:5794–5802. doi: 10.1016/j.vaccine.2010.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Immel TA, Grutzke M, Spate AK, Groth U, Ohlschlager P, Huhn T. Synthesis and X-ray structure analysis of a heptacoordinate titanium(IV)-bis-chelate with enhanced in vivo antitumor efficacy. Chem Commun (Camb) 2012;48:5790–5792. doi: 10.1039/c2cc31624b. [DOI] [PubMed] [Google Scholar]
- 54.Zhao X, Pang L, Qian Y, et al. An animal model of buccal mucosa cancer and cervical lymph node metastasis induced by U14 squamous cell carcinoma cells. Exp Ther Med. 2013;5:1083–1088. doi: 10.3892/etm.2013.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pappa KI, Kontostathi G, Makridakis M, et al. High resolution proteomic analysis of the cervical cancer cell lines secretome documents deregulation of multiple proteases. Cancer Genomics Proteomics. 2017;14:507–521. doi: 10.21873/cgp.20060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudary N, Jaluba K, Pintilie M, Hill RP. Establishment of orthotopic primary cervix cancer xenografts. Methods Mol Biol. 2015;1249:381–391. doi: 10.1007/978-1-4939-2013-6_28. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, Luan S, Xia B, et al. Integrated analysis of HPV-mediated immune alterations in cervical cancer. Gynecol Oncol. 2018;149:248–255. doi: 10.1016/j.ygyno.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 58.Roman M, Baraibar I, Lopez I, et al. KRAS oncogene in non-small cell lung cancer: clinical perspectives on the treatment of an old target. Mol Cancer. 2018;17:33. doi: 10.1186/s12943-018-0789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pirog EC. Cervical adenocarcinoma: diagnosis of human papillomavirus-positive and human papillomavirus- negative tumors. Arch Pathol Lab Med. 2017;141:1653–1667. doi: 10.5858/arpa.2016-0356-RA. [DOI] [PubMed] [Google Scholar]
- 60.Lin M, Kim KR, Ro J. Gastric-type endocervical adenocarcinoma: review of clinicopathologic characteristics and recent advances. J Gynecol Res Obstet. 2020;6:72–75. doi: 10.17352/jgro.000091. [DOI] [Google Scholar]
- 61.Chumduri C, Gurumurthy RK, Berger H, et al. Opposing Wnt signals regulate cervical squamocolumnar homeostasis and emergence of metaplasia. Nat Cell Biol. 2021;23:184–197. doi: 10.1038/s41556-020-00619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lohmussaar K, Oka R, Espejo Valle-Inclan J, et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell. 2021;28:1380–1396. doi: 10.1016/j.stem.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 63.Maru Y, Tanaka N, Ebisawa K, et al. Establishment and characterization of patient-derived organoids from a young patient with cervical clear cell carcinoma. Cancer Sci. 2019;110:2992–3005. doi: 10.1111/cas.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li C, Zhou T, Chen J, et al. The role of Exosomal miRNAs in cancer. J Transl Med. 2022;20:6. doi: 10.1186/s12967-021-03215-4.1490fd5fb14849e089b225e05f4ce1ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhat A, Yadav J, Thakur K, et al. Transcriptome analysis of cervical cancer exosomes and detection of HPVE6*I transcripts in exosomal RNA. BMC Cancer. 2022;22:164. doi: 10.1186/s12885-022-09262-4.3376027e21ae49108e720963858d72f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jimenez-Avalos JA, Fernandez-Macias JC, Gonzalez-Palomo AK. Circulating exosomal MicroRNAs: New non-invasive biomarkers of non-communicable disease. Mol Biol Rep. 2021;48:961–967. doi: 10.1007/s11033-020-06050-w. [DOI] [PubMed] [Google Scholar]
- 67.Bhat A, Yadav J, Thakur K, et al. Exosomes from cervical cancer cells facilitate pro-angiogenic endothelial reconditioning through transfer of Hedgehog-GLI signaling components. Cancer Cell Int. 2021;21:319. doi: 10.1186/s12935-021-02026-3.1850bc356971434baa6f93fe8fd8804c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. doi: 10.1186/s13578-019-0282-2.da51882df7154674bfb25b5c231e5d0f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu XG, Zhou CF, Zhang YM, et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis. 2019;22:397–410. doi: 10.1007/s10456-019-09665-1. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, Liu SC, Luo XH, et al. Exosomal long noncoding RNAs are differentially expressed in the cervicovaginal lavage samples of cervical cancer patients. J Clin Lab Anal. 2016;30:1116–1121. doi: 10.1002/jcla.21990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]