Central Illustration
Key Words: cardiac amyloidosis, heart failure, Middle East
Cardiac amyloidosis has garnered increasing attention as an underdiagnosed cause of heart failure (HF). The ability to confirm cardiac involvement noninvasively and the advent of effective treatment options for common forms of cardiac amyloidosis have likely contributed to this growing interest.1 The premise that earlier identification of affected patients will help maximize benefits from available therapies has driven large-scale efforts to raise awareness about disease “red flags” and to emphasize the importance of multidisciplinary evaluation of patients with suspected amyloid heart disease.2,3 As a result, an increase in the prevalence of cardiac amyloidosis among hospitalized patients with HF in North America and Europe has recently been reported.4, 5, 6
However, little is known about the current level of awareness about cardiac amyloidosis as an important cause of HF in the Middle East and Arabian Gulf (MEG) region. Interestingly, geographic disparities in cardiac amyloidosis recognition have also been reported even within the United States, such that the disease is more frequently diagnosed in proximity to amyloidosis centers but remains widely underdiagnosed across the rest of the country.7 Furthermore, areas where health care providers had decreased awareness about the disease also had less public interest, manifesting as lower online search volumes for cardiac amyloidosis and related terms.8 Determining whether such disparities exist in other parts of the world, including the MEG region, is critical, as lack of awareness about cardiac amyloidosis as a cause of HF can potentially delay initiation of disease-modifying therapies and adversely affect patient outcomes.
Accordingly, a group of cardiologists practicing in the MEG region sought to identify gaps in knowledge about cardiac amyloidosis in the region. Results of the cardiac amyloidosis awareness survey and recommendations based on these findings are summarized.
Cardiac Amyloidosis Awareness Survey
A group of cardiologists who shared interest in cardiac amyloidosis and practiced in 5 MEG countries (the United Arab Emirates, Kuwait, Bahrain, Qatar, and Oman) convened in 2020. In addition, a senior member from Italy (S.P.) with extensive experience in cardiac amyloidosis joined the working group as a consultant and to foster collaboration between interested physicians in the region and leading amyloidosis programs in Italy. Working group members had various training backgrounds, including cardiac imaging, HF, cardiovascular outcomes research, and electrophysiology.
The mission of the working group was defined by the following objectives: 1) to assess physicians’ knowledge about cardiac amyloidosis and their familiarity with contemporary recommendations for evaluation of patients with suspected amyloid heart disease; 2) to develop a strategic plan that addresses gaps in knowledge and aims to raise awareness about cardiac amyloidosis among health care providers in the region; and 3) to provide local health care providers with necessary resources to optimize care for patients with confirmed or suspected cardiac amyloidosis.
Working group members embarked first on identifying existing gaps in knowledge about cardiac amyloidosis by conducting the cardiac amyloidosis awareness survey (CAAS). This was a voluntary and anonymous survey of physicians practicing in the 5 MEG countries represented in the working group. Eligible physicians received invitations to participate in the survey via e-mails containing a unique link to a web-based electronic survey. Participants were not compensated for completing the survey. The CAAS was created using a free, online survey development software (SurveyMonkey) and included 23 closed-ended questions that covered demographics of survey participants, awareness about manifestations and diagnosis of cardiac amyloidosis, perceived barriers to cardiac amyloidosis diagnosis in the region, and needs assessment and participants’ preferences for future educational activities. Responses were recorded on a 4- or 5-point Likert-type scale and are presented as percentages or as mean ± SD. The survey was open between May 1, 2020, and June 30, 2020, with periodic e-mail reminders. Institutional Review Board deemed the survey to be exempt from full review.
Survey Participants
The survey was completed by 320 physicians (81.3% men). Of all participating physicians, 43.4% were between 41 and 50 years of age and 28.4% between 25 and 40 years of age, while the rest were older than 50 years. General cardiology was the most common specialty among responding physicians (56.0%), and 13.4% were cardiology subspecialists (cardiac imagers or advanced HF specialists), while the remaining were internists or internal medicine subspecialists or of other specialties. Most respondents (95.9%) practiced in hospital-based settings, most commonly at government-run hospitals (84.8%) and with teaching affiliations (63.9%). Survey participants were relatively experienced, with 39.2% practicing in their respective current specialties for >10 years and 28.2% for >20 years. Last, most participating physicians (88.6%) provided care for patients with HF in their practices.
Knowledge About Diagnosis of Cardiac Amyloidosis
Overall, the survey indicated a high level of perceived awareness about cardiac amyloidosis, with 82.7% of participants considering themselves to be “somewhat or extremely familiar” with signs and symptoms of cardiac amyloidosis. However, survey respondents did not suspect the diagnosis of cardiac amyloidosis frequently enough, with 61.4% having suspected this diagnosis less than once a month on average and another 25.0% who had never suspected cardiac amyloidosis in patients they evaluated. Furthermore, when asked about disease processes considered during the evaluation of patients with HF and preserved ejection fraction, amyloidosis, with a mean response of 3.6 ± 1.5, was less likely to be considered by survey participants compared with hypertension (4.4 ± 0.9), diabetes (4.1 ± 1.0), and obesity (3.8 ± 1.2).
Importantly, the survey identified gaps in physicians’ knowledge about the role of noninvasive modalities in diagnosing amyloid heart disease. Whereas echocardiography and cardiac magnetic resonance imaging were believed to be useful for the diagnosis of cardiac amyloidosis by 82.0% and 91.9% of survey participants, respectively, only 60.5% of physicians considered cardiac scintigraphy with bone-avid radiotracers to be useful, while 33.3% were unsure of the role of this modality in confirming cardiac amyloidosis diagnosis. Moreover, the survey uncovered significant gaps in the perceived importance of laboratory studies to exclude monoclonal protein abnormalities, with 52.9% of participants reporting serum and urine electrophoresis to be extremely useful, whereas serum free light-chain assays and immunofixation studies (serum and urine) were considered useful by 49.4% and 41.9% of survey participants, respectively. Urine free light-chain assay, not routinely obtained for evaluation of plasma cell dyscrasias, was believed to be useful by 42.1% of physicians completing the survey.
Perceived Barriers to Cardiac Amyloidosis Diagnosis
Overall, 89.2% of survey respondents were aware that the diagnosis of cardiac amyloidosis is usually delayed, and 88.8% agreed that delayed initiation of treatment for cardiac amyloidosis may lead to adverse health outcomes for patients. Additionally, survey participants were cognizant of the importance of timely diagnosis of cardiac amyloidosis, with 63.2% considering timely diagnosis to be “extremely critical” and 30.3% considering it “somewhat critical.”
With regard to perceived barriers to prompt diagnosis of cardiac amyloidosis, lack of knowledge about the disease “red flags” was the most important barrier, with a mean response of 3.4 ± 0.7, followed by limited access to imaging and laboratory tools necessary to diagnose cardiac amyloidosis (3.3 ± 0.7) and heterogeneity in disease manifestations and presentation (3.1 ± 0.6). Conversely, lack of available treatment options once cardiac amyloidosis is diagnosed and the perception that cardiac amyloidosis can be diagnosed only on endomyocardial biopsy were less important barriers to cardiac amyloidosis diagnosis according to CAAS participants, with mean responses of 2.9 ± 0.8 and 2.8 ± 1.0, respectively.
Discussion
This survey identified major gaps in physicians’ knowledge about amyloid heart disease in the MEG region and showed that the disease remains underrecognized as a cause of HF, despite a high level of perceived awareness. In addition, one-third of participating physicians were not sure of the role of bone-avid tracers in diagnosing cardiac involvement in amyloidosis. This observation mirrors findings from prior surveys and indicates the need to update practicing cardiologists on best practices for evaluation of patients with suspected amyloid heart disease, particularly transthyretin cardiomyopathy.9 Moreover, the survey uncovered a significant deficiency in relation to the utility of urine studies in detecting coexisting gammopathies, with almost one-half of survey participants considering urine free light-chain assays “extremely useful” in diagnosing plasma cell abnormalities, which is not consistent with the prevailing evidence.10
The unique region-specific insight provided by this survey sheds additional light on potential obstacles to correct and timely diagnosis of cardiac amyloidosis. Together, input from CAAS offers a strong foundation for a strategic plan aiming to raise awareness about cardiac amyloidosis through future educational programs. Whereas formal assessment of survey validity was not performed as part of this project, it is unlikely that this limitation affected the robustness of the present findings given the nature of the topic addressed with this survey. Additionally, the response rate for this survey could not be accurately determined given the strategy used to identify survey recipients, and the results of the survey must be interpreted in the context of this limitation.
A call to action
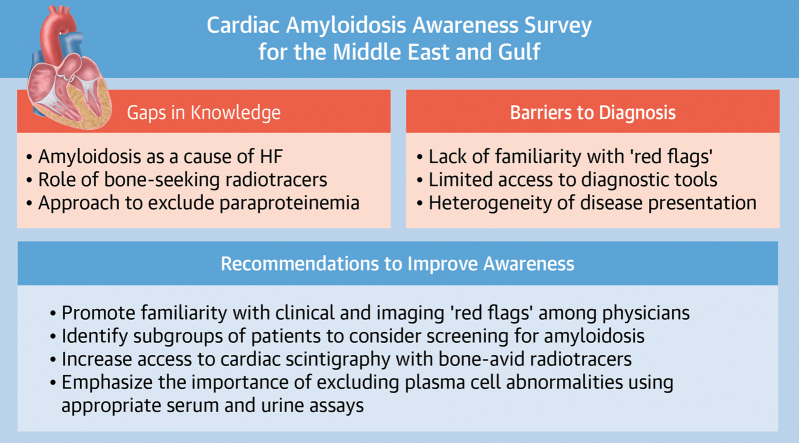
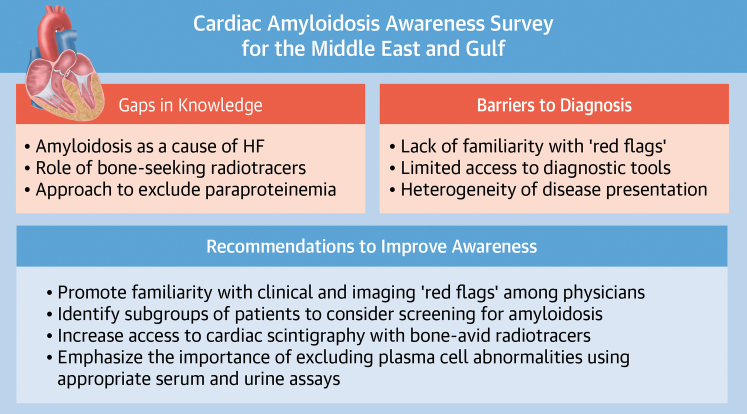
Accordingly, several focus areas were identified, inspired by the findings of the survey (Figure 1). First, educational campaigns will emphasize the importance of recognizing clinical and imaging “red flags” for cardiac amyloidosis and also will highlight “high-yield” patient subgroups in which the likelihood of cardiac amyloidosis is high, including HF with unexplained ventricular hypertrophy, severe low-flow, low-gradient aortic stenosis, and HF with concomitant neuropathy. Furthermore, the central role of radionuclide imaging using bone-seeking radiotracers in confirming the diagnosis of transthyretin amyloid cardiomyopathy will also be emphasized during educational programs, as will be the optimal approach to evaluate patients with suspected plasma cell disorders, along with clear referral pathways for a dedicated hematologic evaluation, as necessary. The working group will also prioritize increasing access to cardiac scintigraphy with bone-seeking radiotracers, through close collaboration with interested international nuclear medicine and nuclear cardiology societies to encourage adherence with standardized image acquisition and processing protocols and international quality standards.
Figure 1.
Cardiac Amyloidosis Working Group Call to Action
Working group recommendations to raise awareness about cardiac amyloidosis in the Middle East and Persian Gulf region. HF = heart failure.
Conclusions
In the first formal assessment of physicians’ knowledge about cardiac amyloidosis in the MEG region, several important deficiencies were identified, especially in physicians’ familiarity with contemporary recommendations to diagnose cardiac amyloidosis. Efforts to bridge these gaps can potentially circumvent existing delays in diagnosing patients with cardiac amyloidosis, which may in turn improve patients’ health outcomes.
Funding Support and Author Disclosures
Pfizer Gulf supported creation of the electronic version of the survey through collaboration with Innovacom. Pfizer Gulf did not influence data collection, analysis, or interpretation, nor did it contribute to the writing or editorial review of the manuscript. No honoraria were provided for authorship. Dr Al Badarin has served on an advisory board for Pfizer Gulf. Dr Sabbour has received speaker fees from Pfizer Gulf, AstraZeneca, and Novartis (all honoraria are donated to local charities). Dr Perlini has received honoraria and travel fees from Pfizer, Novartis, and Alnylam; and has received a research grant from Pfizer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors acknowledge Drs Haluk Alibazoglu (Cleveland Clinic Abu Dhabi, Abu Dhabi, United Arab Emirates), Juwairia Al Ali (Rashid Hospital, Dubai, United Arab Emirates), and Amr Badr (Hamad Medical Center, Doha, Qatar) for their contributions to the working group activities.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Kittleson M.M., Maurer M.S., Ambardekar A.V., et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142:e7–e22. doi: 10.1161/CIR.0000000000000792. [DOI] [PubMed] [Google Scholar]
- 2.Maurer M.S., Bokhari S., Damy T., et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorbala S., Ando Y., Bokhari S., et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 2 of 2—diagnostic criteria and appropriate utilization. J Card Fail. 2019;25:854–865. doi: 10.1016/j.cardfail.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Gilstrap L.G., Dominici F., Wang Y., et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service Medicare beneficiaries in the United States. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane T., Fontana M., Martinez-Naharro A., et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140:16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 6.Sperry B.W., Saeed I.M., Raza S., Kennedy K.F., Hanna M., Spertus J.A. Increasing rate of hospital admissions in patients with amyloidosis (from the National Inpatient Sample) Am J Cardiol. 2019;124:1765–1769. doi: 10.1016/j.amjcard.2019.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Alexander K.M., Orav J., Singh A., et al. Geographic disparities in reported US amyloidosis mortality from 1979 to 2015: potential underdetection of cardiac amyloidosis. JAMA Cardiol. 2018;3:865–870. doi: 10.1001/jamacardio.2018.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuong J., Singh A., Falk R.H., Merchant R., Dorbala S. Geographic variation in public interest about amyloidosis in the United States and English speaking countries. Amyloid. 2020;27:210–212. doi: 10.1080/13506129.2020.1724941. [DOI] [PubMed] [Google Scholar]
- 9.Mircsof D. Diagnosis of amyloidosis: a survey of current awareness and clinical challenges among cardiologists in Switzerland. Cardiol Ther. 2020;9:127–138. doi: 10.1007/s40119-019-00160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejoie T., Corre J., Caillon H., et al. Serum free light chains, not urine specimens, should be used to evaluate response in light-chain multiple myeloma. Blood. 2016;128:2941–2948. doi: 10.1182/blood-2016-07-726778. [DOI] [PMC free article] [PubMed] [Google Scholar]