Abstract
Background
Cardiomyopathy is a leading cause of late morbidity and mortality in childhood cancer survivors (CCS). Evidence-based guidelines recommend risk-stratified screening for cardiomyopathy, but the management approach for abnormalities detected when screening asymptomatic young adult CCS is poorly defined.
Objectives
The aims of this study were to build upon existing guidelines by describing the expert consensus–based cardiomyopathy screening practices, management approach, and clinical rationale for the management of young adult CCS with screening-detected abnormalities and to identify areas of controversy in practice.
Methods
A multispecialty Delphi panel of 40 physicians with expertise in cancer survivorship completed 3 iterative rounds of semi-open-ended questionnaires regarding their approaches to the management of asymptomatic young adult CCS at risk for cardiomyopathy (screening practices, referrals, cardiac testing, laboratory studies, medications). Consensus was defined as ≥90% panelist agreement with recommendation.
Results
The response rate was 100% for all 3 rounds. Panelists reached consensus on the timing and frequency of echocardiographic screening for anthracycline-associated cardiomyopathy, monitoring during pregnancy, laboratory testing for modifiable cardiac risk factors, and referral to cardiology for ejection fraction ≤50% or preserved ejection fraction with diastolic dysfunction. Controversial areas (<75% agreement) included chest radiation dose threshold to merit screening, indications for advanced cardiac imaging and cardiac serum biomarkers for follow-up of abnormal echocardiographic findings, and medical management of asymptomatic left ventricular systolic dysfunction.
Conclusions
Expert practice is largely consistent with existing risk-based screening guidelines. Some recommendations for managing abnormalities detected on screening echocardiography remain controversial. The rationale offered by experts for divergent approaches may help guide clinical decisions in the absence of guidelines specific to young adult CCS.
Key Words: cardiomyopathy, childhood cancer survivor, Delphi method, guidelines, screening
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ALVD, asymptomatic left ventricular dysfunction; AYA, adolescent and young adult; BNP, brain natriuretic peptide; CCS, childhood cancer survivor(s); cMRI, cardiac magnetic resonance imaging; COG, Children’s Oncology Group; EF, ejection fraction; GLS, global longitudinal strain; SF, shortening fraction
Central Illustration
Cardiomyopathy is a leading cause of late morbidity and mortality in long-term survivors of childhood cancer.1 It is estimated that more than one-half of all childhood cancer survivors (CCS) received treatment with known cardiotoxic agents such as anthracycline chemotherapy and/or chest radiation and are at increased risk for adverse cardiac outcomes.2 Of these, cardiomyopathy is the most prevalent, with large cohort studies reporting that 14% of exposed survivors have left ventricular ejection fractions (EFs) <50%.3 CCS have an 11% cumulative incidence of heart failure over the 40 years following treatment and are at 7 times higher risk for cardiovascular mortality compared with age- and sex-adjusted control subjects.2,4 Furthermore, CCS face lifelong cardiomyopathy risk, with no observed plateau in incidence.5 The spectrum of cardiomyopathy in CCS is broad, ranging from American College of Cardiology/American Heart Association stage B heart failure with echocardiographic findings such as diastolic dysfunction and abnormal global longitudinal strain (GLS)6 and asymptomatic left ventricular dysfunction (ALVD), defined as depressed left ventricular systolic function with EF <50% in the absence of clinical heart failure,7 to overt symptomatic (stage C) or advanced (stage D) heart failure.5
Given expected decades of time in survivorship, mitigating cardiac risk represents a key opportunity to extend life expectancy and improve quality of life for CCS. Cardiomyopathy may be clinically silent for years prior to the development of overt heart failure; thus, screening at-risk CCS with echocardiography can identify subclinical disease amenable to interventions to slow or stop disease progression.8 National and international pediatric and internal medicine oncology groups have released guidelines for risk-stratified surveillance, management of modifiable cardiac risk factors for cancer survivors, and adherence to general population guidelines for optimizing cardiovascular health.9, 10, 11 Modeling studies support the cost-effectiveness of this risk-stratified approach to screening.12, 13, 14
Routine echocardiographic surveillance of otherwise healthy adolescents and young adults (AYAs), however, may result in subclinical findings, such as borderline EF, low-grade diastolic dysfunction, or abnormal GLS, that do not have a defined management strategy. It is not known how existing general population guidelines for heart failure management apply to CCS, particularly AYA survivors. Current internal medicine guideline-directed medical therapy is focused primarily on patients with left ventricular EFs <40%.15 However, many CCS have subclinical or mild cardiomyopathy with EF >40%, for which surveillance and treatment guidelines have only recently been defined.16
Ideally, the approach to cardiomyopathy prevention and management for CCS would be guided by prospective interventional studies, but limited evidence exists. The only randomized placebo-controlled trial failed to demonstrate a benefit with the angiotensin-converting enzyme (ACE) inhibitor enalapril on the primary endpoint of maximal cardiac index at peak exercise, though it did show reduction in left ventricular wall stress.17 A nonrandomized study of ACE inhibition or angiotensin receptor blockade in CCS showed sustained improvement in GLS.18 A randomized trial of beta-blockade in CCS at risk for cardiomyopathy is ongoing.19 The practicality of interventional studies in CCS is limited by small absolute numbers of CCS, incomplete follow-up, and long latency time for the development of cardiac disease. Furthermore, randomized medication trials are challenged by a lack of clinical equipoise, as the benefits and safety of these medications in heart failure are well established. In settings in which prospective management trials are challenging, Delphi methodology is used to build clinical practice guidelines that are based on the systematic development of expert consensus.20 This Delphi study builds upon existing guidelines for cardiomyopathy screening in CCS by summarizing current practice patterns, areas of agreement and controversy, and rationale for clinical decision making on common scenarios not explicitly covered in published guidelines, among a multidisciplinary group of experts in CCS care.
Methods
Delphi panel participants
The panel of experts was a purposeful sample representing the 5 physician specialties directly involved in the management of AYA CCS at risk for cardiomyopathy: 8 pediatric oncologists, 8 primary care physicians, 9 radiation oncologists, 8 pediatric cardiologists, 1 medical oncologist, and 5 adult cardio-oncologists. Panel composition was designed to be balanced across specialty and practice location, representing all geographic regions in the United States and Canada. Selection criteria for panelists included recognition as an expert in CCS by study team members on the basis of academic and/or clinical contributions to the discipline, practice at an academic medical center affiliated with a pediatric oncology program, practice size that includes at least 5 AYA CCS at risk for cardiomyopathy a year, and commitment to participate through project completion. Eligible panelists were recruited by e-mail; 40 of 57 physicians recruited agreed to participate. Participants remained anonymous to all but the study team to avoid potential response bias due to power and prestige ranking. Individual consent was waived by the Dana-Farber Cancer Institute Institutional Review Board.
Questionnaire
The study questionnaire was developed by the study team based in part on a pilot Delphi study of regional (New England) experts in CCS.20 The open-ended questionnaire consisted of 2 parts; part 1 addressed panelists’ approaches to screening for cardiomyopathy, and part 2 consisted of clinical scenarios with common findings on screening echocardiography. To ensure that panelists had consistent background information from which to make their clinical decisions, panelists were provided with a summarized review of published research (current through January 2019) on cardiovascular outcomes in CCS; there was no confirmation that panelists reviewed the information provided. Participants’ demographic and practice information was collected with the first questionnaire. Second- and third-round questionnaires were developed on the basis of modal responses from round 1. Questionnaires were tested for content and cognitive validity by 2 nonparticipant experts in CCS care.
Data collection
Questionnaires were created in Qualtrics and completed online using individualized, secure links delivered by e-mail. Response rate was augmented by e-mail reminders. The response rate was 100% (40 of 40 participants) for all 3 rounds.
In round 1, panelists responded to open-ended questions regarding cardiomyopathy screening for a 20-year-old healthy cancer survivor (sex and race unspecified), off therapy for 15 years, considered to be at risk for cardiomyopathy. These characteristics were kept consistent, and 6 clinical scenarios were presented: the survivor had been treated with: 1) cumulative anthracycline dose <250 mg/m2; 2) anthracycline dose ≥250 mg/m2; 3) chest radiation with any anthracyclines; 4) chest radiation with no anthracyclines; 5) thoracic spine radiation 15 to 36 Gy; and 6) total body irradiation. Panelists commented on whether their screening recommendations would change on the basis of specific variations, including whether dexrazoxane was given with anthracyclines, which anthracycline was used, and the use of proton rather than photon radiation. They were asked to describe other risk factors that would change their screening approaches. Panelists also described their management of 6 asymptomatic findings on echocardiography for this 20-year-old CCS: 1) technically inadequate; 2) ALVD, represented as EF of 45% with shortening fraction (SF) of 22%; 3) EF of 50% with SF of 25%; 4) normal EF with grade 1 diastolic dysfunction; 5) normal EF with GLS >−16%; and 6) normal EF in the first trimester of pregnancy.
In round 2, the modal response for each management option was presented along with summarized clinical rationale for areas of disagreement. Participants were requested to determine whether they agreed with the modal response. If they disagreed, they were asked to suggest alternative management options and provide their rationales. In round 3, participants were presented with aggregated modal responses and alternatives from round 2. They were again asked to either agree or, if disagreeing, to explain their dissent.
Data analysis
For each round of questionnaires, mixed-methods analysis (quantitative analysis of selected screening/management options and semistructured content analysis of written responses) was completed by 2 study team members. Responses were coded, frequencies calculated, and modal responses identified. Discrepancies were resolved by a third study team member. Consensus was defined a priori as ≥90% agreement among panelists, moderate agreement defined as 75% to 89%, and disagreement as <75%. Three iterations (rounds of data gathering) were found to be sufficient either to achieve consensus or to adequately understand reasons for dissent. Categorical data are presented as counts with percentages, and continuous data are presented as median (IQR). Data analysis was performed using SPSS Statistics version 28 (IBM). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Delphi panelists
Table 1 describes the demographics of the Delphi panel participants (n = 40). All practice regions (east, central, and west, which were divided geographically on the basis of location and number of academic pediatric hematology and oncology centers) were represented. Panelists cared for a median of 72 CCS per year in their clinical practices (IQR: 24-300 patients) and had been in practice for a median of 15 years (IQR: 9-22 years).
Table 1.
Panelist Demographics
| Sex | |
| Female | 21 (52.5) |
| Male | 19 (47.5) |
| Specialty | |
| Medical oncology | 1 (2.5) |
| Cardio-oncology | 5 (12.5) |
| Pediatric cardiology | 8 (20) |
| Pediatric oncology | 9 (22.5) |
| Primary care | 8 (20) |
| Radiation oncology | 9 (22.5) |
| Practice region | |
| Central | 12 (30) |
| East | 20 (50) |
| West | 8 (20) |
| Cancer survivors treated annually by practice group | |
| 0-25 | 12 (30) |
| 26-75 | 9 (22.5) |
| 76-300 | 12 (30) |
| >300 | 6 (15) |
| Years in practice | |
| 0-10 | 13 (32.5) |
| 11-15 | 8 (20) |
| 16-20 | 5 (12.5) |
| >20 | 14 (25) |
Values are n (%).
Areas of consensus and disagreement
Screening
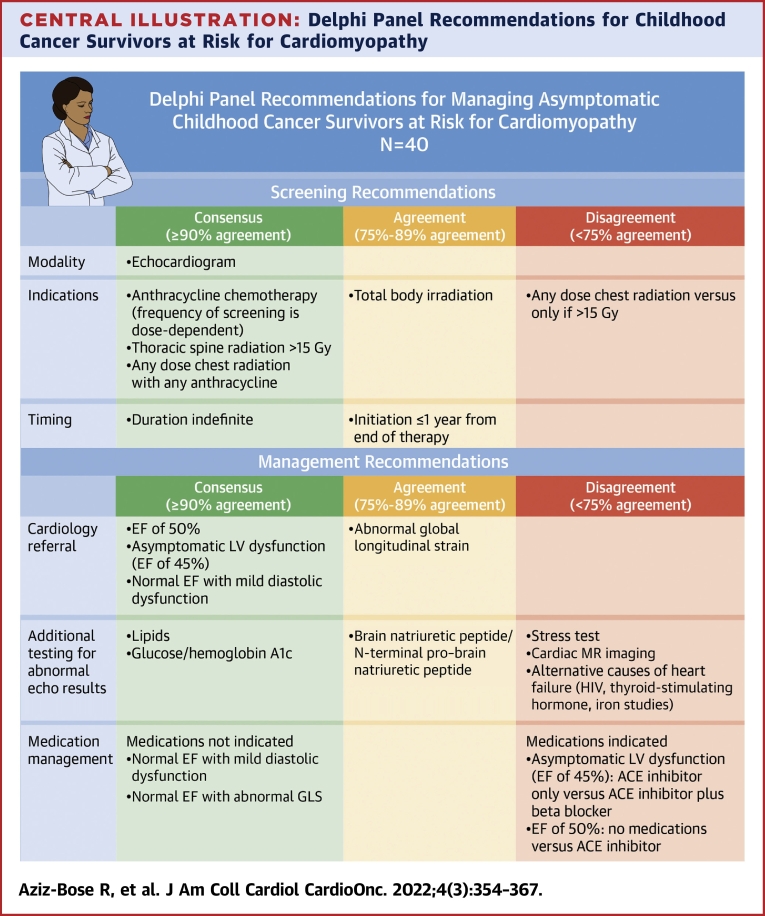
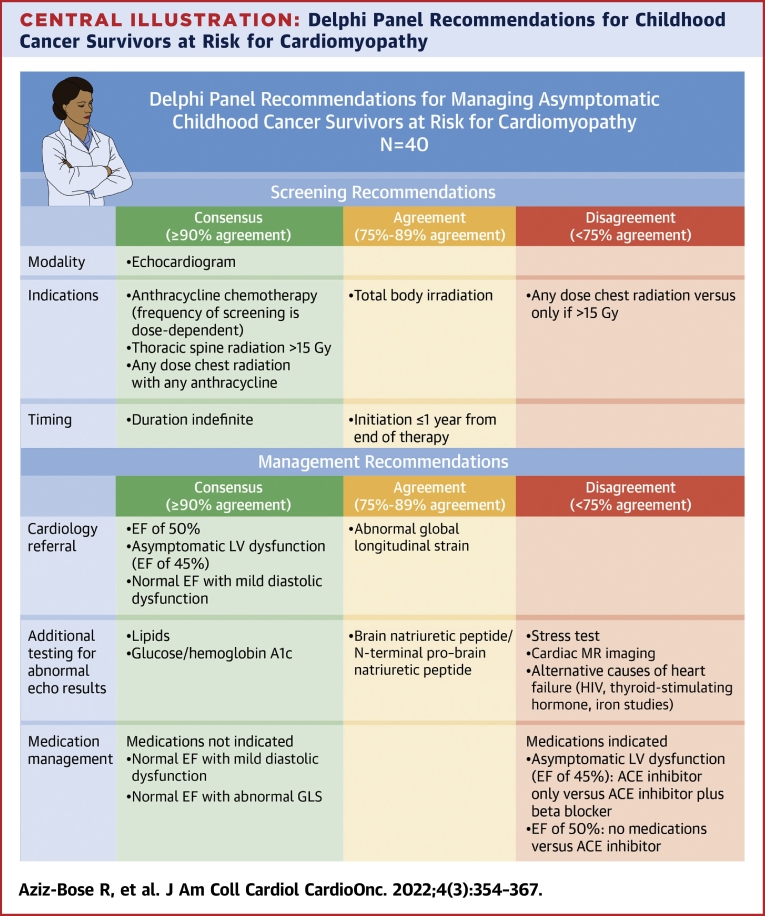
Most panelists (88%) indicated that they used the Children’s Oncology Group (COG) guidelines9 to determine patients’ screening needs, 49% reported use of the International Harmonization Group recommendations,10 and 46% the Childhood Cancer Survivorship Study cardiovascular risk calculator.21 Consensus (≥90% agreement) was reached on the use of echocardiography for screening and the need for lifelong screening, with moderate agreement (78%) to initiate screening within 1 year of completing cancer-directed therapy (Figure 1, Table 2). Consensus was reached on the need for screening echocardiography for patients in 5 of 6 clinical scenarios discussed; consensus was not reached on whether screening is required for patients who received total body irradiation without anthracyclines.
Figure 1.
Screening Recommendations
Recommendations for screening of a young adult childhood cancer survivor at risk for cardiomyopathy: areas of agreement and controversy. For areas of disagreement, alternative management options are presented within the text box. TBI = total body irradiation.
Table 2.
Representative Selection of Panelists’ Rationales for Echocardiographic Screening Childhood Cancer Survivors at Risk for Cardiomyopathy
| Scenario | Panelist Recommendation | Agree, n (%) | Comments (+) | Disagree, n (%) | Comments (−) | |
|---|---|---|---|---|---|---|
| Timing | Childhood cancer survivor at risk of cardiomyopathy | Initiation within 1 y off therapy | 31/40 (78) | Patients may develop dysfunction depending on exposure acutely, subacutely or delayed; all of these are time points of risk. Late identification of dysfunction in adult data has a significantly decreased response rate to medications. Establishing a baseline to serve as comparison is crucial, as some patients remain asymptomatic until there has been a significant decline in cardiac function. |
9/40 (22) | Screening within the first 10 y has very low yield unless acute cardiac complications occurred during treatment with anthracyclines. Delay screening until entry into survivorship care (2-5 y). |
| Duration indefinitely | 36/40 (90) | Patients may be at risk, and in fact risk may continue to increase, up to about 40 y after receipt of chemotherapy/radiation. Cardiac late effects may occur many years (decades) after exposure. Risk for CVD and heart failure increases with aging. |
4/40 (10) | For low-risk patients with no cardiac history, we stop screening if there is prolonged stability on echocardiography and only screen in presence of clinical symptoms thereafter. For high-risk patients, if start at every 1-2 y, would change to every 5 y if echocardiographic results stable over 5-10 y. Take into account what other comorbidities may affect the risk/benefit ratio of screening (ie, recurrent cancer, stroke, dementia, etc). |
||
| Treatment factors | Chest radiation, any dose (no anthracyclines) | Screening required | 22/39 (56) | Although higher doses do increase risk, risk is seen even at the lowest doses. Studies have shown even lower doses (<15 Gy) are associated with some risk (for both heart disease and cardiac mortality). There is no “safe dose” of radiation. There is no clear dose threshold for CVD risk from extension of adult breast cancer literature. Cutoffs are imperfect and can miss disease. |
17/39 (44) | Radiation is most commonly associated with coronary artery disease, which is not screen detectable with echo….RT doses <15 Gy are likely to have a very low absolute risk for screen-detectable echocardiographic abnormality. For asymptomatic patients with normal examination results, would not recommend serial screens. |
| Chest radiation threshold dose (≥15 Gy, no anthracyclines) | Screening required | 13/39 (33) | Aligns with COG long-term follow-up guidelines. >15 Gy appears to be the cutoff for moderate risk for developing cardiomyopathy. |
26/39 (67) | Highest risk is >35 Gy; in absence of other risk factors would limit screening to this higher risk group (repeat testing is not without harms). If other risk factors (eg, smoking, HTN, hyperlipidemia), would lower threshold to screen. | |
| Thoracic spinal radiation 15-36 Gy (no anthracyclines) | Screening required | 37/40 (93) | Unless using proton radiation, there is exit-dose or low-dose exposure to the heart when treating the thoracic spine. Radiation exposure to the thorax is associated with substantial risk for the subsequent development of CVD. |
3/40 (7) | If proton RT was used, the heart dose is likely negligible. | |
| Fractionated TBI (no anthracyclines) | Screening required | 30/39 (77) | TBI is associated with cardiovascular complications, including cardiomyopathy. Evidence is quite limited, but once cardiomyopathy does develop, prognosis can be quite poor, and interventions do exist to possibly improve outcomes. So until more evidence becomes available, I would err on the side of including any radiation including TBI as a risk factor. |
9/39 (33) | Insufficient data to support screening. There are harms of overmedicalizing these patients’ lives. TBI doses are <15 Gy. This recommendation would otherwise be inconsistent with the [COG LTFU] recommendation not to screen patients treated with <15 Gy and no anthracyclines. |
|
| Recommended change to frequency if… | Proton rather than photon radiation | No change to screening frequency | 32/40 (80) | I don’t feel we have enough long-term data on proton therapy to feel safe in changing the screening recommendations. Dosimetry information may not be available. |
8/40 (20) | [Screening frequency is] based on heart dose, and often protons can decrease heart dose compared with photons. |
| Patient received dexrazoxane | No change to screening frequency | 38/40 (95) | There are not enough data to determine if the short-term cardioprotective benefits of dexrazoxane translate into long-term protection. | 2/40 (5) | Dexrazoxane has been shown to be cardioprotective…based on what we do know I think 5-y screening intervals are reasonable. | |
| Age <1 y at treatment | Increase screening frequency | 22/40 (55) | In [a] registry risk model, young age at treatment (<1 y old) increased the risk of myocardial dysfunction and cardiac mortality. Younger age and developing tissues are generally at higher risk for toxicity. |
18/40 (45) | Higher risk, but no evidence that more frequent screening has an advantage. [Our group] no longer does this since publication of new [COG LTFU] guidelines. |
COG = Children’s Oncology Group; CVD = cardiovascular disease; HTN = hypertension; LTFU = long-term follow-up; RT = radiation therapy; TBI = total body irradiation.
There was disagreement regarding the dose threshold for screening CCS who had received chest radiation without anthracyclines, with 22 panelists (56%) recommending screening after any dose of chest radiation, 13 (33%) recommending screening only if chest radiation was >15 Gy, and 4 (10%) recommending screening only if >30 Gy. Panelists recommending screening after any dose of chest radiation noted the lack of a lower dose threshold for cardiotoxicity after radiation. Those recommending a higher dose threshold for screening cited the low absolute rates of echocardiography-detectable abnormalities in those who received lower dose chest radiation (Table 2).
Panelists reached moderate agreement on the frequency of echocardiographic screening on the basis of extent of prior cardiotoxic exposures, except for thoracic spine radiation, for which there was disagreement (Figure 1). Consensus was reached that panelists would increase echocardiographic screening frequency for patient preference and cardiovascular disease risk factors. Consensus was also reached that panelists would not decrease screening frequency if patients had received dexrazoxane along with anthracyclines or if treated with proton rather than photon radiation (Table 2). In keeping with the most recent version of the COG guidelines, 80% of panelists agreed not to increase the frequency of screening for patients of female sex. Consensus was not reached regarding whether to increase the frequency of screening for family history of early cardiovascular disease (55% agreed to increase frequency), smoking (50% agreed to increase frequency), and age <1 year at time of treatment (55% agreed to increase frequency).
Management
Referrals
Consensus was reached on cardiology referral in 5 of 6 clinical scenarios (technically inadequate echocardiogram because of patient anatomy; ALVD, represented as EF of 45% with SF of 22%; EF of 50% with SF of 25%; normal EF with mild diastolic dysfunction; and normal EF in the first trimester of pregnancy), with moderate agreement (88%) for cardiology referral for normal EF with abnormal GLS (Figure 2). Notably, in 4 of these scenarios (excluding technically inadequate echocardiogram and pregnancy), noncardiology panelists reached consensus to defer further management decisions to cardiologists. There was disagreement regarding the threshold for maternal-fetal medicine referral for a pregnant CCS in the first trimester, depending on cardiotoxic exposures; 50% of panelists recommended maternal-fetal medicine referral for any chest radiation or anthracycline exposure, whereas 23% recommended referral only if the anthracycline dose was >150 mg/m2 and chest radiation was >15 Gy, 7.5% only for anthracycline exposure > 250 mg/m2, and 20% for other variations (Figure 3).
Figure 2.
Management Recommendations
Recommendations for management of a young adult childhood cancer survivor at risk for cardiomyopathy. When noncardiology panelists deferred management decisions to cardiology, the following recommendations are only those of the panel’s cardiologists and those who did not defer management to cardiology. For areas of disagreement, alternative management options are presented within the text box. ACEi = angiotensin-converting enzyme inhibitor; Addl. = additional; Alt causes = additional laboratory studies to evaluate patient for alternative causes of heart failure (HIV, thyroid-stimulating hormone, iron studies); BB = beta-blocker; BNP = brain natriuretic peptide or N-terminal pro–brain natriuretic peptide; cMRI = cardiac magnetic resonance imaging; CVD = cardiovascular disease; EF = left ventricular ejection fraction; EKG = electrocardiography; GLS = global longitudinal strain; SF = shortening fraction.
Figure 3.
Management Recommendations in Pregnancy
Recommendations for management of cardiomyopathy risk during pregnancy for an asymptomatic 20-year-old childhood cancer survivor with no comorbidities. For areas of disagreement, alternative management options are presented within the text box. Anth = anthracycline; CRT = chest radiation therapy; EF = left ventricular ejection fraction; MFM = maternal-fetal medicine; Mgmt. = management.
Additional cardiac imaging
The role of follow-up cardiac testing was controversial among panelists. For the scenarios in which noncardiologists deferred management decisions to cardiology, the recommendations summarized are those of the cardiologists in the study and the noncardiologists who provided management recommendations. In particular, the use of cardiac magnetic resonance imaging (cMRI) and exercise stress testing did not reach consensus among panelists for any of the presented scenarios. There was moderate agreement on the use of cMRI as a follow-up study for technically inadequate echocardiography (82%), but in other clinical scenarios, most panelists (43%-61%) did not recommend pursuing cMRI. When recommended, panelists described the detailed tissue characterization and functional measures available with cMRI; reasons for dissent were primarily that cMRI would not change management (Table 3). Cardiac stress testing similarly was not recommended in most scenarios, though 64% agreed with pursuing cardiac stress testing for ALVD. When recommended, panelists’ rationale for stress testing was to determine functional capacity and exercise tolerance; when not recommended, panelists discussed the low likelihood of coronary artery disease and/or ischemic cardiomyopathy in a young adult CCS (Table 3).
Table 3.
Representative Selection of Panelists’ Rationales for Management of Echocardiographic Findings in a 20-Year-Old Asymptomatic Childhood Cancer Survivor
| Scenario | Panelist Recommendation | Agree, n (%) | Comments (+) | Disagree, n (%) | Comments (−) | |
|---|---|---|---|---|---|---|
| Echocardiographic findings | EF 45%, shortening fraction 22% | Stress test | 9/14 (64) | An ischemic evaluation is appropriate for the evaluation of cardiomyopathy regardless of suspected cause. May be at risk for early coronary artery disease, and [ischemic evaluation is] standard of care with low LVEF. Best assessment of functional capacity/exercise tolerance. |
5/14 (36) | I am not convinced this test would be needed in an asymptomatic individual. Too young for development of coronary disease. |
| cMRI | 8/14 (57) | Confirmation of degree of LV dysfunction and to evaluate for underlying causes of cardiomyopathy. Risk stratification including tissue characterization prior to initiation of therapy. |
6/14 (43) | Unclear that this will change management. MRI would not add value if [echocardiographic] image quality was good. |
||
| Test BNP or NT-proBNP | 12/13 (92) | These markers are prognostic. Biomarkers are useful to trend over time. Another way to assess cardiac function. Independent data on impact [of therapy]. |
1/13 (8) | Unclear that this changes management in this case. | ||
| Test for alternative etiology of heart failure (thyroid function, HIV, iron studies) | 7/14 (50) | Standard of care for new-onset cardiomyopathy. Important to be thorough in a young person with cardiomyopathy, rather than just assume. |
7/14 (50) | Unlikely that there is an alternative cause of cardiomyopathy. Only [test] as clinically indicated. These problems are unusual in this population and/or have already been assessed in oncology. |
||
| Start ACE inhibitor and beta-blocker | 9/13 (69) | Current guidelines support beta-blockers and ACE inhibitors in asymptomatic patients with LVEF <40%. In this case, would extrapolate the potential benefit to LVEF ∼45% after discussing with patient. Synergistic effect on remodeling. Standard of care for LV dysfunction, meets [definition of] AHA/ACC stage B heart failure. |
4/13 (31) | Start with ACE inhibitor first, consider beta-blocker depending on clinical response. | ||
| EF 50%, shortening fraction 25% | Repeat echocardiography in 6-12 mo | 11/14 (79%) | Close surveillance to determine if further drop in EF. Echocardiography has more interobserver variability; 10% variation [in EF may be seen] serially in absence of disease. Potential for further decline in LVEF, which would put patient in a category in which bet- blocker or ACE inhibitor would be considered. |
3/14 (21%) | Would get cMRI at this point [rather than repeat echocardiography in 6-12 mo]. An EF of 50% is not normal in this age group and meets criteria for cancer therapy–related cardiac dysfunction; [would initiate ACE inhibitor/beta-blocker now rather than repeat echocardiography in 6-12 mo]. |
|
| cMRI | 6/14 (43) | Provides incremental information [on other measures of cardiac function]; also confirms LV function as gold standard, given variability on echocardiography. cMRI is the standard for evaluation of LV mass volume, global and regional myocardial function as well as tissue characterization; in addition, cMRI is not limited by body habitus or acoustic windows. Confirmation and correlation with echocardiography. |
8/14 (57) | If the echocardiographic image quality was good, then unclear that the cMRI will change management. Does not add value unless marginal acoustic windows. Would wait for the repeat echocardiography. |
||
| Start ACE inhibitor only | 9/13 (69) | Early initiation of ACE inhibitors and beta-blockers is of extreme importance to prevent the progression of subclinical cardiac dysfunction to symptomatic heart failure in this patient population. High risk for subsequent worsening cardiomyopathy; ACE inhibitors may be protective, although data are not well established. |
4/13 (31) | Would want more data before starting therapy. Unclear benefit. Would have shared decision-making conversation with patient. |
||
| Normal EF with abnormal GLS (>−16%) | Repeat echocardiography in 6-12 mo | 14/14 (100%) | GLS predicts subsequent LV dysfunction; need to follow over time. Repeat in 6-12 mo to confirm findings. Results can be variable. |
0/14 (0%) | NA | |
| Stress test | 5/13 (38%) | Reasonable to get a baseline functional assessment. Stress test provides an assessment of exercise tolerance. |
8/13 (62%) | No clear link between abnormal strain and coronary artery disease. I would perform stress test only if [patient has] symptoms; low yield testing otherwise given age and normal LVEF. |
||
| cMRI | 4/14 (28) | Cardiac magnetic resonance is considered the gold standard for quantification of ventricular volumes, global and regional systolic function. Measures of myocardial strain by cMRI are increasingly being used to detect subclinical myocardial dysfunction. |
10/14 (72) | I do not see how this would change management at the current time. | ||
| Normal EF with grade 1 diastolic dysfunction | cMRI | 4/14 (28) | Evaluate for fibrosis and incremental information on underlying cardiomyopathy; diastolic dysfunction is not normal in this age group. | 10/14 (72) | Would not change clinical management. |
ACC = American College of Cardiology; ACE = angiotensin-converting enzyme; AHA = American Heart Association; BNP = brain natriuretic peptide; cMRI = cardiac magnetic resonance imaging; EF = ejection fraction; GLS = global longitudinal strain; LV = left ventricular; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; NA = not applicable; NT-proBNP = N-terminal pro–brain natriuretic peptide.
Laboratory testing
Consensus was reached (100%) in all scenarios on screening CCS for modifiable laboratory-based cardiac risk factors, including hemoglobin A1c and lipid panel. Although 92% of panelists agreed with obtaining cardiac biomarkers (brain natriuretic peptide [BNP] or N-terminal proBNP) in ALVD, there was disagreement regarding the role of cardiac biomarker monitoring in other scenarios, with 82% recommending BNP or N-terminal proBNP evaluation for a CCS with EF ≤50% and 57% recommending these markers for a CCS with normal EF with abnormal GLS or diastolic dysfunction. There was also disagreement (50%-57% agreed) regarding testing for alternative etiologies of cardiomyopathy such as HIV, thyroid function tests, and iron studies, with some panelists preferring to obtain this testing as standard of care for new-onset cardiomyopathy and others commenting on low likelihood of an alternative cause in a CCS with cardiotoxic exposure history (Table 3). There was consensus that laboratory assessment outside of routine obstetrical testing was not required for pregnant CCS.
Medications
For ALVD, all panelists recommended initiation of medications, though there was less consensus on specific treatment approach; most (69%) recommended combination therapy with ACE inhibition and beta-blockade, while 31% recommended ACE inhibition alone. When recommending dual therapy, panelists commented on the synergistic remodeling benefit and extrapolation of guidelines applicable to those with EFs <40%; the rationale for ACE inhibitors alone was that these panelists recommended starting medications serially rather than in parallel (Table 3). ACE inhibitor monotherapy was recommended by the majority (69%) of panelists for a CCS with an EF of 50%, though both those who agreed and those who disagreed commented that this would need to be a shared decision-making conversation with the patient. No medications were recommended for abnormal GLS or diastolic dysfunction with normal EF.
Areas of disagreement by specialty
Chest radiation threshold for screening
Proportionately more pediatric oncologists (6 of 9 [67%] agreed) and pediatric cardiologists (5 of 8 [63%] agreed) recommend echocardiographic screening after any dose of chest radiation compared with radiation oncologists (4 of 9 [44%] agreed) and cardio-oncologists (3 of 6 [50%] agreed), who were more likely to recommend screening only after a specific threshold dose was met.
Advanced cardiac imaging
Similar proportions of adult and pediatric cardiologists recommended cMRI in ALVD (4 of 6 cardio-oncologists [67%] and 4 of 8 pediatric cardiologists [50%]). In the same scenario, a higher proportion of pediatric cardiologists recommended stress testing (6 of 8 [75%]) than adult cardiologists (3 of 6 [50%]), with differing diagnostic indication (exercise tolerance and functional capacity vs ischemic evaluation; see Table 3). In the scenario of normal EF with abnormal GLS, 2 of 6 cardio-oncologists (33%) and 2 of 8 pediatric cardiologists (25%) recommended cMRI, whereas 1 of 5 oncocardiologists (20%) and 4 of 8 pediatric cardiologists (50%) would pursue a stress test.
Medication management
Cardio-oncologists were more likely than pediatric cardiologists to recommend starting both ACE inhibitors and beta-blockers for patients with ALVD (4 of 5 cardio-oncologists [80%] vs 5 of 8 pediatric cardiologists [62.5%]), as opposed to ACE inhibitors alone. For the scenario with EF of 50% and SF of 25%, 3 of 5 cardio-oncologists (60%) and 6 of 8 pediatric cardiologists (75%) recommended starting an ACE inhibitor (as opposed to not starting medications).
In this panel, other observed variations in clinical practice pattern were not clustered by practice region, years in practice, or CCS treated per year (data not shown).
Discussion
Although a growing body of research details the cardiac risks and need for screening among long-term CCS treated with a variety of cardiotoxic exposures, limited evidence exists to guide the management of subclinical cardiomyopathy, which is often found in the young adult CCS population. In this national Delphi study, we identified areas of expert agreement and controversy surrounding screening and management of AYA CCS, as well as the clinical rationale behind experts’ current practices (Central Illustration). Despite a paucity of evidence, panelists reached consensus on most decisions for cardiovascular monitoring and treatment of at-risk CCS. There was agreement on the timing and frequency of echocardiographic screening, need for close clinical and imaging monitoring during pregnancy, and identification of modifiable cardiac risk factors in CCS. Controversial areas included chest radiation threshold for screening, utility of advanced cardiac imaging, the role of cardiac biomarker measurement, and the medical management of ALVD.
Central Illustration.
Delphi Panel Recommendations for Childhood Cancer Survivors at Risk for Cardiomyopathy
Relevant areas of consensus (≥90% agreement), moderate agreement (75%-89% agreement), and disagreement (<75% agreement) among panelists are highlighted. Asymptomatic left ventricular dysfunction is defined as any ejection fraction (EF) <50% without clinical heart failure; in our study, this scenario was represented as an EF of 45% and a shortening fraction of 22% in an asymptomatic survivor.
Whom do we screen?
Our panelists reached consensus on risk-stratified screening on the basis of anthracycline exposure, in accord with existing oncology guidelines.9,10 However, there are several areas of emerging evidence for which there was absence of consensus on screening approach. For example, recent evidence shows increased risk for dosimetrically determined cardiac radiation doses exceeding 5 Gy,22,23 supporting panelists’ recommendation for echocardiographic screening after any dose of chest radiation, even in the absence of anthracyclines. This differs slightly from the current COG long-term follow-up guidelines, which suggest echocardiographic screening only for cumulative chest radiation doses >15 Gy, if the patient did not receive anthracyclines.9 There was also disagreement on whether patients should be screened more frequently if they received cardiotoxic treatment during infancy, a recommendation that was removed from the updated COG guidelines released in 2018.9
We also considered the implications of risk reduction strategies used during treatment, such as the use of dexrazoxane with anthracyclines and/or the use of proton radiation to limit effective heart dose, on future screening needs. Panelists reached consensus that they would not decrease screening frequency if patients had received dexrazoxane. Recent evidence suggests that the use of dexrazoxane can dramatically reduce the incidence of acute cardiotoxicity.24 Another analysis showed that although CCS randomized to dexrazoxane alongside anthracyclines had lower rates of a composite adverse cardiovascular outcome (cardiomyopathy, ischemic heart disease, and stroke), rates of cardiomyopathy alone did not significantly differ.25 This finding supports our panelists’ current consensus. Similarly, panelists agreed that it was inappropriate to decrease screening frequency if a patient had received thoracic proton rather than photon radiation, although rationale was often related to lack of consistent dosimetry information available regarding cardiac radiation dose. This highlights that accurate record transmission (to primary care physicians or late effects and survivorship clinics) regarding treatment exposures is necessary to ensure that patients undergo recommended screening.
How do we screen?
Two-dimensional echocardiography remains the standard screening modality selected by our panelists. Echocardiography is noninvasive, is widely available, and has established parameters for normal and abnormal findings. The echocardiographic parameter most frequently used by panelists to guide management was the left ventricular EF. Another advanced imaging modality, cMRI, has high sensitivity and provides detailed functional information, though its cost and availability limit its use for general screening of at-risk CCS.26 Given limited sensitivity of echocardiographic screening, some studies have suggested consideration of cMRI for at-risk CCS with EFs of 50% to 59% on echocardiography.3 On the basis of findings from our expert panel, cMRI is used primarily by survivorship providers for individuals in whom echocardiography is technically limited, with consideration of use in patients with inconclusive or subtly abnormal echocardiographic findings.
How do we intervene on abnormal findings?
This study highlights the importance of subspecialist cardio-oncology expertise in CCS, with panelists from all practice backgrounds planning referral to cardiology for follow-up of abnormal screening findings.
Medication management was an interesting area of nonconsensus. For ALVD with an EF of 45% detected on screening echocardiography, all the cardiologists in our study recommended initiation of a medication, though there was disagreement about whether to extrapolate guidelines for heart failure with reduced EF with initiation of ACE inhibition and beta-blockade together or whether to start these medications sequentially. Similarly, there was no consensus on the medical management of borderline EF (in this study, represented by an EF of 50%), with the majority (69%) recommending ACE inhibitors but a substantial proportion recommending no medication. Of note, recent evidence also supports our findings of discordance between adult and pediatric cardiologists in medication management of CCS.27
Panelists also considered the management of patients with abnormal GLS; GLS is an emerging echocardiographic indicator of myocardial dysfunction and has been associated with heart failure severity and cardiac mortality independent of other structural or functional indexes.6,28 Our panelists had a lower degree of agreement on cardiology referral for abnormal GLS and did not recommend any medication for the treatment of CCS with abnormal GLS or diastolic dysfunction in the setting of a preserved EF. One recent study demonstrated sustained improvement in GLS for CCS treated with ACE inhibitors or angiotensin receptor blockers18; however, another recent study published after our Delphi panel raised questions over the use of GLS as an echocardiographic parameter to guide the initiation of cardioprotective therapy.29 This highlights the evolving nature of left ventricular function assessment using echocardiography and the need for ongoing collaboration among cardiologists, oncologists, and primary care physicians to ensure that emerging evidence around imaging parameters is appropriately incorporated into CCS care.
Panelists reached consensus in every scenario on laboratory-based screening for modifiable cardiovascular disease risk factors, such as diabetes and hyperlipidemia. Management of these risk factors, along with hypertension, is a critical component of secondary prevention, with well-established and generally well-tolerated interventions. Cardiovascular risk calculators designed for the general population may significantly underestimate the risk for cardiovascular disease in CCS, who may be younger than the reference range for adult risk calculators and have additional treatment-related cardiotoxic exposures.30 CCS-specific prediction models that incorporate traditional cardiovascular risk factors as well as treatment exposures emphasize the importance of treating comorbid conditions.21,30 Of note, CCS from racial and ethnic minority groups experience an increased prevalence of cardiovascular risk factors,31 and receipt of recommended screening varies by sociodemographic factors.32 Although not addressed directly in our study, it is important for future work to consider the impact of social determinants of health on CCS’ cardiovascular risk and to design and evaluate health equity–focused interventions for CCS to improve adherence to screening and cardiovascular risk factor modification.
Strengths
We used systematic consensus-building methodology to describe national clinical practice patterns in caring for CCS at risk for cardiomyopathy. The strengths of this study are the robust panel of experts, a purposeful sample designed to represent multiple disciplines and geographic regions. There was complete participation (100% response rate) in all rounds of data collection, with anonymized, systematically coded response data to define areas of controversy and elucidate clinical rationale. Additionally, this study identified scenarios not explicitly covered in published guidelines but commonly seen in clinical practice.
Study limitations
Although purposefully designed, the nonrandom sample of panelists potentially limits the generalizability of our findings. Panel size limits the formal analysis of differences in recommendations by panelist demographics or subspecialty. Additionally, all panelists worked in large academic programs. The resources available to these practitioners (eg, cMRI) likely differ from those available to community providers, and most CCS are followed in the community. Finally, in the interest of keeping questionnaires succinct, clinical scenarios did not explore variations in patient characteristics (eg, age, time from treatment, controlled vs uncontrolled cardiac risk factors) that might influence panelists’ recommendations.
Conclusions
We describe multidisciplinary expert consensus recommendations for the care of CCS at risk for cardiomyopathy and identify clinical questions for which approach to management remains controversial. Given variability in the management approach identified in this study, important areas for future research include extension of this consensus methodology approach to a panel including cardiologists with diverse areas of expertise (pediatric, general, heart failure, cardio-oncology) focusing on areas of disagreement, particularly screening thresholds and management of subclinical cardiomyopathy. Ultimately, next steps in optimizing CCS’ cardiac health will incorporate emerging evidence around novel risk factors and interventions, consider the impact of genetic and social determinants of cardiovascular risk, and continue revision, standardization, and implementation of guidelines to ensure equitable and consistent practice.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: A Delphi panel of physicians recognized for their expertise in childhood cancer survivorship reached consensus that young adult CCS at heightened risk for cardiomyopathy receive routine risk-based echocardiographic screening, laboratory testing for modifiable cardiac risk factors, and referral to cardiology for abnormalities detected on echocardiography. Areas of disagreement included the application of advanced cardiac testing to evaluate and monitor subclinical cardiomyopathy and the medical management of ALVD. Expert recommendations developed by consensus methodology, along with rationales for divergent approaches to management, may help guide clinical decisions in the absence of guidelines specific to young adult CCS.
TRANSLATIONAL OUTLOOK: Further research is needed to examine the clinical impact of differing management approaches for subclinical cardiomyopathy in this unique but growing patient population. Implementation science approaches will be needed to ensure that patients receive recommended screening and management of abnormal findings.
Funding Support and Author Disclosures
This work was supported by National Institutes of Health T32 research training grant CA136432-12 to Dr Aziz-Bose and the Jack and Dorothy Byrne Foundation. Dr Nohria is a consultant for AstraZeneca, Bantam Pharmaceuticals, Boehringer Ingelheim, and Takeda Oncology; and has received research support from Amgen. Dr Yock has received in-kind software support from Mim Software for a multicenter Pediatric Radiation Registry (Pediatric Proton/Photon Consortium Registry). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
A preliminary version of these data was presented as an abstract at the Congress of the International Society of Paediatric Oncology in October 2021.
Carrie Lenneman, MD, served as the Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Armstrong G.T., Liu Q., Yasui Y., et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leerink J.M., de Baat E.C., Feijen E.A.M., et al. Cardiac disease in childhood cancer survivors: risk prediction, prevention, and surveillance: JACC CardioOncology state-of-the-art review. J Am Coll Cardiol CardioOnc. 2020;2(3):363–378. doi: 10.1016/j.jaccao.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong G.T., Plana J.C., Zhang N., et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30(23):2876–2884. doi: 10.1200/jco.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenian S.H., Armstrong G.T., Aune G., et al. Cardiovascular disease in survivors of childhood cancer: insights into epidemiology, pathophysiology, and prevention. J Clin Oncol. 2018;36(21):2135–2144. doi: 10.1200/jco.2017.76.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow E.J., Leger K.J., Bhatt N.S., et al. Paediatric cardio-oncology: epidemiology, screening, prevention, and treatment. Cardiovasc Res. 2019;115(5):922–934. doi: 10.1093/cvr/cvz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Yang H., Huynh Q., Nolan M., Negishi K., Marwick T.H. Diagnosis of nonischemic stage B heart failure in type 2 diabetes mellitus: optimal parameters for prediction of heart failure. J Am Coll Cardiol Img. 2018;11(10):1390–1400. doi: 10.1016/j.jcmg.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Sara J.D., Toya T., Taher R., Lerman A., Gersh B., Anavekar N.S. Asymptomatic left ventricle systolic dysfunction. Eur Cardiol. 2020;15:e13. doi: 10.15420/ecr.2019.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong G.T., Oeffinger K.C., Chen Y., et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. doi: 10.1200/jco.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Children’s Oncology Group. Children’s Oncology Group; California: 2018. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 5.0. Monrovia. [Google Scholar]
- 10.Armenian S.H., Hudson M.M., Mulder R.L., et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denlinger C.S., Sanft T., Baker K.S., et al. Survivorship, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(10):1216–1247. doi: 10.6004/jnccn.2018.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh J.M.N.A., Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160(10):661–671. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong F.L., Bhatia S., Landier W., et al. Cost-effectiveness of the Children’s Oncology Group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160(10):672–683. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrhardt M.J., Ward Z.J., Liu Q., et al. Cost-effectiveness of the International Late Effects of Childhood Cancer Guideline Harmonization Group screening guidelines to prevent heart failure in survivors of childhood cancer. J Clin Oncol. 2020;38(33):3851–3862. doi: 10.1200/jco.20.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddox T.M., Januzzi J.L., Allen L.A., et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;77(6):772–810. doi: 10.1016/j.jacc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 16.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 17.Silber J.H., Cnaan A., Clark B.J., et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22(5):820–828. doi: 10.1200/jco.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Harrington J.K., Richmond M.E., Fein A.W., Kobsa S., Satwani P., Shah A. Two-dimensional speckle tracking echocardiography-derived strain measurements in survivors of childhood cancer on angiotensin converting enzyme inhibition or receptor blockade. Pediatr Cardiol. 2018;39(7):1404–1412. doi: 10.1007/s00246-018-1910-z. [DOI] [PubMed] [Google Scholar]
- 19.Armenian S.H., Hudson M.M., Chen M.H., et al. Rationale and design of the Children’s Oncology Group (COG) study ALTE1621: a randomized, placebo-controlled trial to determine if low-dose carvedilol can prevent anthracycline-related left ventricular remodeling in childhood cancer survivors at high risk for developing heart failure. BMC Cardiovasc Disord. 2016;16(1):187. doi: 10.1186/s12872-016-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney L.B., Ames B., Margossian R., et al. Regional practice norms for the care of childhood cancer survivors at risk for cardiomyopathy: a Delphi study. Pediatr Blood Cancer. 2019;66(9) doi: 10.1002/pbc.27868. [DOI] [PubMed] [Google Scholar]
- 21.St. Jude Children’s Research Hospital CCSS Cardiovascular Risk Calculator. https://ccss.stjude.org/tools-documents/calculators-other-tools/ccss-cardiovascular-risk-calculator.html
- 22.Shrestha S., Bates J.E., Liu Q., et al. Radiation therapy related cardiac disease risk in childhood cancer survivors: updated dosimetry analysis from the Childhood Cancer Survivor Study. Radiother Oncol. 2021;163:199–208. doi: 10.1016/j.radonc.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bates J.E., Howell R.M., Liu Q., et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37(13):1090–1101. doi: 10.1200/jco.18.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liesse K., Harris J., Chan M., Schmidt M.L., Chiu B. Dexrazoxane significantly reduces anthracycline-induced cardiotoxicity in pediatric solid tumor patients: a systematic review. J Pediatr Hematol Oncol. 2018;40(6):417–425. doi: 10.1097/MPH.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow E.J., Aplenc R., Vrooman L.M., et al. Late health outcomes after dexrazoxane treatment: a report from the Children’s Oncology Group. Cancer. 2022;128(4):788–796. doi: 10.1002/cncr.33974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armenian S., Bhatia S. Predicting and preventing anthracycline-related cardiotoxicity. Am Soc Clin Oncol Educ Book. 2018;38:3–12. doi: 10.1200/edbk_100015. [DOI] [PubMed] [Google Scholar]
- 27.Bottinor W.J., Friedman D.L., Ryan T.D., et al. Cardiovascular disease and asymptomatic childhood cancer survivors: current clinical practice. Cancer Med. 2020;9(15):5500–5508. doi: 10.1002/cam4.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tröbs S.-O., Prochaska J.H., Schwuchow-Thonke S., et al. Association of global longitudinal strain with clinical status and mortality in patients with chronic heart failure. JAMA Cardiol. 2021;6(4):448–456. doi: 10.1001/jamacardio.2020.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thavendiranathan P., Negishi T., Somerset E., et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77(4):392–401. doi: 10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Chow E.J., Oeffinger K.C., et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2020;112(3):256–265. doi: 10.1093/jnci/djz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noyd D.H., Yasui Y., Li N., et al. Disparities in cardiovascular risk factors by race/ethnicity among adult survivors of childhood cancer: a report from the Childhood Cancer Survivorship Study (CCSS) J Clin Oncol. 2021;39(15_suppl) doi: 10.1200/JCO.2021.39.15_suppl.10017. 10017-10017. [DOI] [Google Scholar]
- 32.Caplin D.A., Smith K.R., Ness K.K., et al. Effect of population socioeconomic and health system factors on medical care of childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Adolesc Young Adult Oncol. 2017;6(1):74–82. doi: 10.1089/jayao.2016.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]