Abstract
The field of cardio-oncology was born from the necessity for recognition and management of cardiovascular diseases among patients with cancer. This need for this specialty continues to grow as patients with cancer live longer as a result of lifesaving targeted and immunologic cancer therapies beyond the usual chemotherapy and/or radiation therapy. Often, potentially cardiotoxic anticancer treatment is necessary in patients with baseline cardiovascular disease. Moreover, patients may need to continue therapy in the setting of incident cancer therapy–associated cardiotoxicity. Herein, we present and discuss the concept of permissive cardiotoxicity as a novel term that represents an essential concept in the field of cardio-oncology and among practicing cardio-oncology specialists. It emphasizes a proactive rather than reactive approach to continuation of lifesaving cancer therapies in order to achieve the best oncologic outcome while mitigating associated and potentially off-target cardiotoxicities.
Key Words: cardiomyopathy, diagnosis, HER2 therapy, immunotherapy, prevention, risk factor, risk prediction, screening, treatment planning
Abbreviations and Acronyms: 5-FU, 5-fluorouracil; CAD, coronary artery disease; CV, cardiovascular; CVD, cardiovascular disease; GDMT, guideline-directed medical therapy; HER2, human epidermal growth factor receptor 2; ICI, immune checkpoint inhibitor; GLS, global longitudinal strain; HSCT, hematopoietic stem cell transplantation; LVEF, left ventricular ejection fraction; VEGF, vascular endothelial growth factor
Central Illustration
Highlights
-
•
Permissive cardiotoxicity is a terminology that represents a vital concept in cardio-oncology
-
•
It emphasizes continued cancer therapy if appropriate, while mitigating cardiotoxicities.
-
•
Its application is guided by understanding the cancer treatment, alternatives, and prognosis.
The fundamental tension in the clinical practice of cardio-oncology is the balance between effective cancer-directed therapy and the risk for developing irreversible cardiovascular (CV) complications. We propose the term permissive cardiotoxicity to represent the concept that balances the benefits of effective cancer treatment with acceptance of its associated and often off-target cardiotoxicity, while providing the best possible surveillance and attenuation of the toxicity. Cardiotoxicity in this case loosely refers to the overall toxicity to the CV system sometimes associated with cancer therapy. Permissive cardiotoxicity is a balance of clinical determinants in which optimal cancer therapy may have negative impact on the CV system, but the overall goal is to allow the best outcome from cancer treatment. Although the detection, surveillance, and management of cardiotoxicity associated with antineoplastic therapies have taken the center stage in the field of cardio-oncology, it is crucial to recognize that these therapies are providing lifesaving or sustaining benefits.
The concept of permissive cardiotoxicity emphasizes that rather than calling for cessation of the cardiotoxic treatment, the managing cardio-oncology specialist acknowledges the potential toxicity to the patient and the cancer treating team while using the most favorable CV management strategy that yields an optimal CV prognosis and enables the patient to remain on designated cancer therapy. This strategy applies principally to treatments delivered over time, with manifestations of cardiotoxicity that demand decisions regarding if and how treatment should be continued. The permissive cardiotoxicity strategy is a dynamic compromise that recognizes that not all CV complications are irreversible and that some degree of manageable cardiotoxicity can be an acceptable trade-off for highly effective cancer-directed therapies. In the subset of patients with cancer with preexisting CV conditions, permissive cardiotoxicity may have a narrower therapeutic window but still may be achievable to attain the best oncologic outcome without lethal or debilitating major adverse CV events (Figure 1).
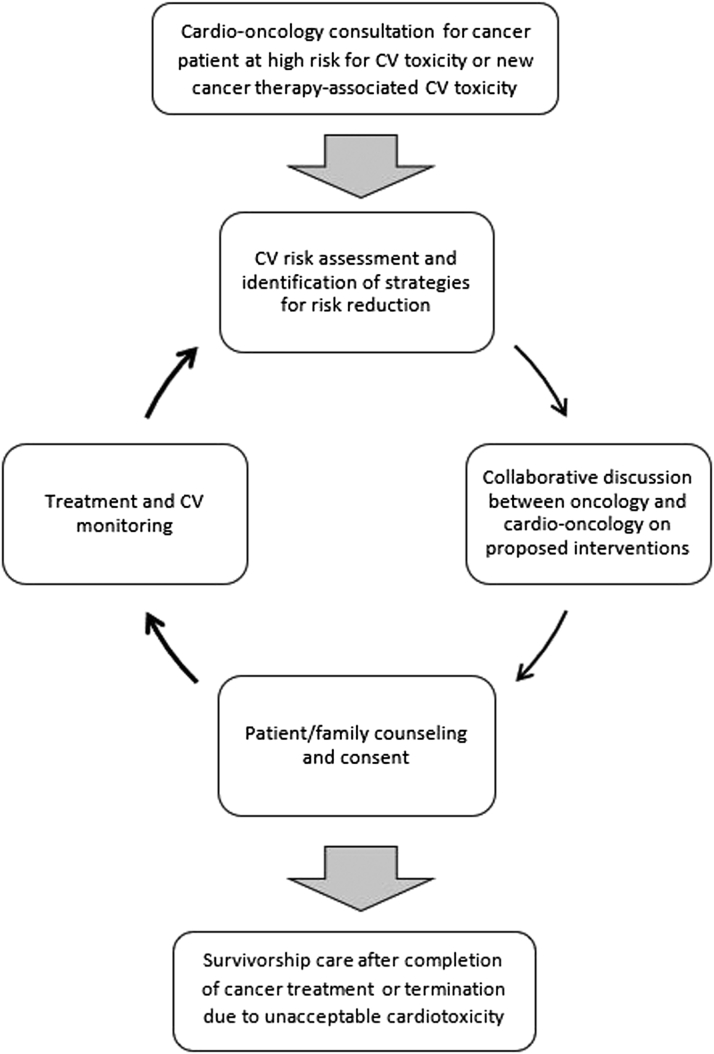
Figure 1.
The Permissive Cardiotoxicity Process
A schematic representation of the multidisciplinary approach to care of patients who develop or have the potential to develop cardiotoxicities while on cancer therapies. CV = cardiovascular.
The Necessity for Permissive Cardiotoxicity
Cancer and CV disease (CVD) are the 2 most common diseases worldwide. With an aging population and improvement in therapeutics and overall survival, greater overlap between both disease entities is observed and will continue to expand. The concept of permissive cardiotoxicity begins even prior to initiation of cancer therapies. Although newly diagnosed cancer most commonly does not present as a medical emergency, there is a sense of urgency, both from the provider and patient perspective, regarding the initiation of antineoplastic therapy to positively influence prognosis. Many of these patients may have significant underlying CV risk factors or CVD, which may increase the risk for subsequent development of cardiotoxicity. The starting point for permissive cardiotoxicity–based treatment strategies is often the recognition of this urgency to commence anticancer therapy in the setting of a new diagnosis of a hematological or solid tumor malignancy. This results in the need for urgent cardiology evaluation of CV risk status so that cancer treatment can begin without delay. Such patients may require a cardioprotective strategy implemented without the luxury of a few weeks of guideline-directed medical therapy (GDMT) for heart failure with reduced ejection fraction, or the scheduling of several diagnostic studies over a period of days or weeks, which may unnecessarily delay important cancer treatment, before the patient is deemed ready for cancer therapy. In such high-need patients, a permissive cardiotoxicity management plan may need to begin concurrently with cancer treatment, or during cancer treatment, among those who develop cardiotoxicity in the process of cancer therapy.
CV complications of cancer-directed therapies are common with such agents as anthracyclines,1 anti–human epidermal growth factor receptor 2 (HER2) targeted therapy,2,3 anti–vascular endothelial growth factor (VEGF) agents,4,5 tyrosine kinase inhibitors,6, 7, 8 proteosome inhibitors, immune checkpoint inhibitors (ICIs), and antimetabolites,9 among others. This list continues to grow with newer immune therapies (particularly ICIs)10 and adoptive cell therapies11 and other targeted therapies. In pediatrics, compared with age- and sex-matched control subjects, childhood cancer survivors are 15 times more likely to develop heart failure and 8 to 10 times more likely to die of CV complications.12 The cooccurrence of cancer and CVD is usually a reflection not only of exposure to the toxicities of the individual anticancer therapies but of individual risk factors and common underlying pathways for both CVD and cancer.13 Furthermore, interruption of anticancer treatment because of cardiotoxicity is associated with worse outcomes in patients with HER2+ breast cancer.14,15 It may be reasonable to extrapolate these findings to other anticancer agents, though data are limited.
This review is not intended to provide detailed management recommendations in the situations being discussed. Our aim is to articulate a management strategy that provides a foundation of principles and examples related to continuation of cancer therapy when the risk for recurrent cardiotoxicity may be sufficiently reduced to allow this. In such situations, cancer treatment benefits outweigh the residual CV risks during intensively monitored therapy using best available cardioprotective techniques.
Permissive Cardiotoxicity: Implications for Cancer Treatment Interruptions
Interruptions in cancer therapy can lead to suboptimal survival outcomes from dose reductions, temporary delays, or even permanent discontinuation of anticancer therapy. Most often, these interruptions are due to either symptoms associated with the cancer or side effects secondary to anticancer treatment. The detrimental impact on survival is likely more pronounced when interruptions in cancer treatment occur in patients being treated with curative intent. Several of the systemic therapy options for cancer treatment can be cardiotoxic and cause interruptions in potentially life-prolonging medications. Trastuzumab is a monoclonal antibody that targets the HER2 receptor expressed in 15% to 20% of breast cancers diagnosed in the United States. One study investigated the impact of interruptions in adjuvant trastuzumab therapy in operable HER2-positive breast cancer and revealed 29% temporary delays and 11% permanent discontinuations. Cardiotoxicity was the most common reason for the interruptions (62%). Multivariable analyses demonstrated worse disease-free (adjusted HR: 4.4; 95% CI: 1.8-10.5; P = 0.001) and overall survival (adjusted HR: 4.8; 95% CI: 2.5-9.2; P < 0.001) after adjusting for age, stage, grade, estrogen receptor status, node status, and trastuzumab-associated cardiotoxicity.14 Furthermore, chemotherapeutic agents such as anthracyclines have well-known cardiotoxicities, with a maximum lifetime cumulative dose to which a patient can be exposed. In a retrospective study, anthracycline dose reductions and delays were associated with a 3-fold increased mortality risk (HR: 3.17; 95% CI: 1.7–5.9; P < 0.001) in patients with localized breast cancer.16 Similarly, the importance of the permissive cardiotoxicity strategy when managing severe hypertension in patients receiving VEGF inhibitors cannot be overemphasized, as hypertension caused or exacerbated by these agents is associated with improved cancer outcomes in some tumors sensitive to VEGF inhibitors.17 Although uncommon, CV adverse effects associated with ICIs, such as myocarditis, pericarditis, arrhythmias, and impaired ventricular function with heart failure, have been reported.18 Current American Society of Clinical Oncology guidelines recommend permanent discontinuation of ICI therapy for any cardiac adverse effect worse than grade 1.19 Interestingly, small studies have demonstrated improved disease-free and overall survival in patients who develop any immune-related side effects. Further prospective studies are needed to investigate these reported outcomes.20, 21, 22, 23, 24 In situations in which ICI therapy has caused significant cardiotoxicity or in the case of solid organ transplant recipients, rechallenge with different types of ICIs or with modified doses may be a reasonable consideration, although long-term outcomes may not be modifiable.
The Spectrum of Permissive Cardiotoxicity
Despite the significance and frequency of cardiotoxic events, there is a growing body of evidence that continuation of potentially cardiotoxic cancer therapy in patients with mild to moderate cardiotoxicity and initiation in patients with prior cardiac disease can be safely accomplished among some patients treated with trastuzumab and anthracyclines.25,26 There are case reports and reports of experiences of successful treatment rechallenges with other agents, including 5-fluorouracil (5-FU)27 and ICIs,28 after the development of cardiotoxicity.
In essence, the question traditionally asked when a patient developed cardiotoxicity from cancer-directed therapies was “Should this therapy be discontinued?” We suggest a shift in mind-set to “How can this therapy be continued?” This may include suggesting the initiation of cardioprotective agents, interventional cardiac procedures, management of comorbid disease, or a temporary cessation in therapy (Central Illustration, Figure 1). For example, dexrazoxane has attained an expanded role as a currently off-label cardioprotective strategy for the management of patients with preexisting left ventricular dysfunction at increased risk for further cardiotoxicity with anthracycline therapy. A useful analogy is the concept of permissive hypercapnia used in critical care, in which higher blood carbon dioxide is seen as an acceptable trade-off for improved oxygenation with a lung-protective ventilation strategy.29 In contrast, in the pediatric population, it is especially difficult to find the right balance for permissive cardiotoxicity, as one must account for the child’s age and long-term consequences of cardiotoxicity. This decision is usually jointly made with the cardio-oncology specialist, the primary oncologist, and the child and parents through shared decision making. The risk and benefits must be weighed as to continuing the cancer-directed therapies and the potential for lifelong CV consequences. As such, many centers will elect to use dexrazoxane up front, for both solid tumor and hematologic malignancies, if a child’s treatment plan calls for a significant dose of anthracycline, usually >250 mg/m2 doxorubicin or equivalent.
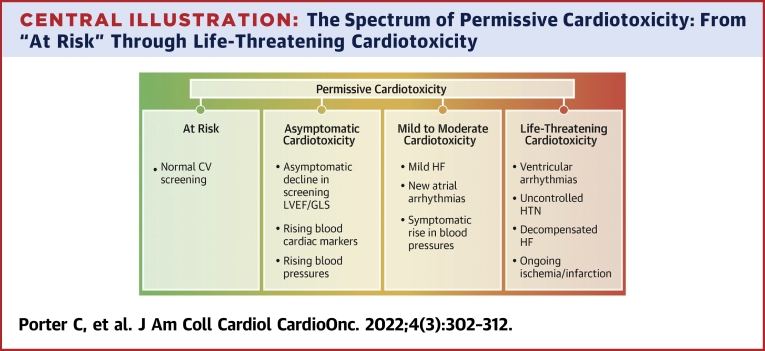
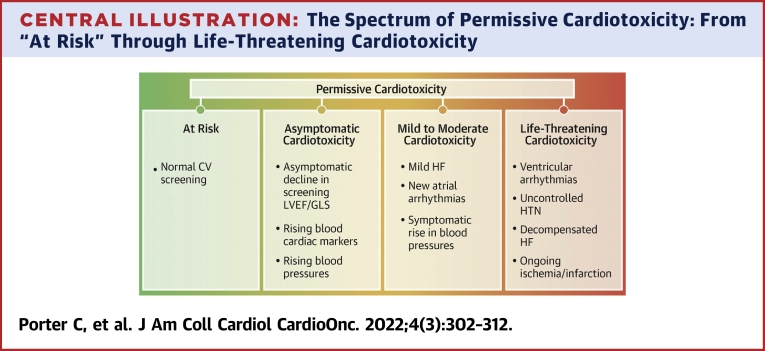
Central Illustration.
The Spectrum of Permissive Cardiotoxicity: From “At Risk” Through Life-Threatening Cardiotoxicity
A schematic representation of examples of the spectrum of cardiotoxic manifestations of cancer therapies. CV = cardiovascular; GLS = global longitudinal strain; HF = heart failure; HTN = hypertension; LVEF = left ventricular ejection fraction.
Central to the concept of permissive cardiotoxicity is screening for cardiotoxicity. If the stated goal is to intervene prior to irreversible, symptomatic effects, then screening needs to be instituted to diagnose subclinical disease. The most widely studied and accepted imaging tool for CV risk assessment among patients with cancer is echocardiography, in which left ventricular ejection fraction (LVEF) and right ventricular ejection fraction and global longitudinal strain (GLS) are the primary imaging tools used to assess cardiotoxicity risk.30, 31, 32 It is crucial to recognize and factor in the variabilities in systolic function and GLS on the basis of the loading conditions (fluid status, blood pressure, etc.), type of imaging modality, as well as interobserver and intervendor variability (particularly for GLS assessment). Performing surveillance imaging in the optimal physiological conditions with the same modality on the same or a similar machine and software may help alleviate some of the variation. This would reduce the chances of misinterpretation of differences in the findings as cardiotoxicity.33 Similarly, blood biomarkers such as brain natriuretic peptide and troponin also have prognostic value.34,35 With these available tools, several cutoff values have been suggested, but none are in widespread use. More recently, standard definitions for cardiotoxicity have been created that will hopefully allow standardization in practice and evaluation of patient outcomes.36 CV surveillance, as outlined in the 2016 American Society of Clinical Oncology practice guideline and endorsed by the American College of Cardiology and American Heart Association,37 is underused during cancer treatment and survivorship.38,39 These pitfalls are compounded in pediatric cardio-oncology, as there is a paucity of published research or guidelines that are pediatric specific.
Permissive Cardiotoxicity Cases
Herein, we present a series of hypothetical patient cases on the basis of our clinical experience, for which the concept of permissive cardiotoxicity can be used to provide optimal patient care in a multidisciplinary fashion (Figure 1). Furthermore, a list of potentially cardiotoxic anticancer agents, manifestations of cardiotoxicity, screening suggestions, and management concepts is presented in Table 1.
Table 1.
Examples of Cardiotoxic Agents, Clinical Presentations, and Management Considerations
| Therapy | Representative Cardiotoxicity Manifestations | Monitoring | When to Intervene | Intervention | Discontinuation Criteria |
|---|---|---|---|---|---|
| HER2-targeted therapya | Asymptomatic LV dysfunction and heart failure | Baseline
|
Decrease in LVEF ≥10% to absolute LVEF <50% GLS decrease >15% from baseline Rise in BNP/troponin from baseline Symptomatic heart failure |
ACE inhibitors/angiotensin receptor blockers, beta-blocker (carvedilol or nebivolol preferred) Increase frequency of echocardiography to every 4 wk until stable Decision to pause trastuzumab made jointly with patient and oncologist |
Heart failure refractory to medical management, refractory or worsening LV function despite management |
| Anthracyclines | Declines in LVEF and symptomatic heart failure, arrhythmias | Baseline
|
Symptomatic heart failure Decrease in LVEF ≥10% to absolute LVEF <50% or symptomatic heart failure Rise in BNP/troponin from baseline |
ACE inhibitors/angiotensin receptor blockers, beta-blocker, spironolactone, SGLT-2 inhibitorb ICD/CRT as indicated per standard guidelines and prognosis Frequency of imaging/biomarkers dependent on LVEF and/or symptoms Upfront dexrazoxane in patients with preexisting LV dysfunction and in those receiving high doses as in sarcoma regimens can be considered |
Heart failure refractory to medical management, refractory or worsening LV function despite management Overall prognosis and availability of alternative regimen should be considered |
| Antimetabolites (5-FU, capecitabine)35,42 | Coronary vasospasm and subsequent ventricular arrhythmias and cardiomyopathies | Baseline
|
Chest pain/acute coronary syndrome | Nitrates and calcium-channel blockade, angiography to rule out vasospasmic acute coronary syndrome Ranolazine can be considered given less impact on blood pressure and heart rate, though experience is limited In select patients with chest pain, rechallenge can be considered with prophylactic calcium-channel blockers and nitrates, after risk-benefit discussion among cardio-oncology specialist, oncologist, and patient |
Malignant ventricular arrhythmia Continued chest pain/acute coronary syndrome during rechallenge with prophylactic calcium-channel blockers and nitrates |
| VEGF inhibitors43 | Hypertension and heart failure | Baseline
|
New or worsening hypertension, asymptomatic or symptomatic decrease in LV systolic function | Aggressive management of hypertension using specific drugs (ACE inhibitors/angiotensin receptor blockers preferred upfront) Cardioprotective drugs for management of heart failure |
Refractory hypertension, refractory heart failure, other end-organ dysfunction related to VEGF inhibitors such as renal failure |
| ICIs50 | Myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure and vasculitis | Baseline
|
Any abnormal screening test result and/or symptoms warrant further workup and intervention; additional tests include as indicated BNP, echocardiography, CXR, stress test, cardiac catheterization with possible endomyocardial biopsy, cardiac MRI | Hold ICI High-dose corticosteroids (methylprednisol1 mg/kg daily for 3 d, followed by 1 mg/kg prednisone) Cardiology consult and management of cardiac symptoms according to ACC/AHA guidelines For initial steroid refractoriness, consider the addition of either mycophenolate, infliximab, or antithymocyte globulin Discuss abatacept |
Greater than CTCAE grade 1 toxicity; rare cases of rechallenge with ICI if no other options for cancer treatment |
| Ibrutinib44,45 | Atrial fibrillation and increased bleeding risk |
|
New atrial arrhythmias | DOAC for stroke risk reduction, rate/rhythm control as warranted | Refractory, symptomatic arrhythmias not controlled with conventional therapy, arrhythmias in conjunction with recurrent bleeding events, malignant ventricular arrhythmias |
This table does not include all cardiotoxic agents but rather a selection of commonly encountered agents with expected cardiotoxicities. Other agents, such as androgen deprivation therapy (risk for coronary artery disease), proteasome inhibitors (risk for cardiomyopathy), aromatase inhibitors (risk for dyslipidemia and metabolic syndrome), and many more, are not addressed here.
5-FU = 5-fluorouracil; ACC = American College of Cardiology; ACE = angiotensin-converting enzyme; AHA = American Heart Association; BNP = B-type natriuretic peptides; CRT = cardiac resynchronization therapy; CTCAE = Common Terminology Criteria for Adverse Events; CXR = chest radiography; DOAC = direct oral anticoagulant agent; ECG = electrocardiography; GLS = global longitudinal strain; HER2 = human epidermal growth factor receptor 2; ICD = implantable cardioverter-defibrillator; ICI = immune checkpoint inhibitor; LV = left ventricular; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; NT-proBNP = N-terminal pro–B-type natriuretic peptide; SGLT-2 = sodium-glucose cotransporter-2; VEGF = vascular endothelial growth factor.
Trastuzumab and anti-HER2 agents can sometimes be continued along with guideline-directed medical therapy and close monitoring in settings of LVEF lower than 50% (usually 40%-50%).26
Some data exist for benefit of SGLT-2 inhibitors in patients with cancer, though no randomized trials have been conducted.51
Case 1: trastuzumab
A 37-year-old woman with history of hypertension and early-stage HER2-positive lymph node–negative breast cancer received neoadjuvant systemic treatment with docetaxel, carboplatin, trastuzumab, and pertuzumab, followed by a mastectomy and sentinel lymph node biopsy. Her treatment plan included completion of 1-year of HER2-targeted therapy with trastuzumab. Prior to treatment initiation, echocardiography demonstrated an LVEF of 55% to 60% and GLS of −19%. After 3 months of treatment, repeat echocardiography demonstrated an LVEF of 45% to 50% with GLS of −15%. Her blood pressure had been controlled with amlodipine throughout her treatment, and she remained asymptomatic. She was referred for an expedited cardio-oncology consultation, and after discussion with the patient and primary oncologist, the decision was made to change amlodipine to carvedilol as a cardioprotective strategy and to hold trastuzumab for 1 or 2 cycles. Repeat echocardiography following these interventions demonstrated an improved LVEF to 55% to 60% and GLS to −17%. Trastuzumab was reinitiated, with repeat echocardiography the following 2 cycles and then every 3 months per the current U.S. Food and Drug Administration recommendations. Her cardiac function remained stable. She completed therapy without further complications.
Key points
-
•
Early recognition of subclinical cardiotoxicity
-
•
Rapid access to cardio-oncology specialist
-
•
Shared decision making
-
•
Treatment of hypertension known to be a significant risk factor for cancer therapy–related cardiac dysfunction
-
•
Clear monitoring plan
Note that trastuzumab and HER2-targeted therapies can sometimes be continued along with GDMT and close monitoring in settings of LVEF lower than 50% (usually 40%-50%).26,40
Case 2: anthracyclines
A 68-year-old man was diagnosed with diffuse large B-cell lymphoma. He had known coronary artery disease (CAD) and an ischemic cardiomyopathy with an LVEF of 40% to 45% on echocardiography. While planning for treatment with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy, he was evaluated by a cardio-oncology specialist at the request of his hematologist. His current heart failure regimen was already optimized, and the cardio-oncology specialist and hematologist discussed the use of dexrazoxane in this patient. The potential benefits of dexrazoxane for mitigating progressive heart failure and the potential risks of reduced treatment efficacy were presented to the patient. After joint discussions, the patient was treated with up-front dexrazoxane with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone. He successfully completed treatment and continued to follow his survivorship care plan.
Key points
-
•
Rapid access to cardio-oncology specialist
-
•
Optimization of existing therapies
-
•
Discussion related to dexrazoxane as it relates to cardioprotection vs potentially decreased cancer therapy efficacy41
-
•
Patient-oriented decision making
Case 3: 5-FU
A 53-year-old woman was diagnosed with stage IV squamous cell carcinoma of the head and neck. She was initiated on therapy with docetaxel, cisplatin, and 5-FU. She developed substernal chest pain during her first cycle and was found to have T-wave inversions on electrocardiography and an elevated troponin level. She was taken emergently for coronary angiography by cardiac catheterization, and no angiographic CAD was found. She was subsequently diagnosed with 5-FU-induced coronary vasospasm and was treated with intravenous diltiazem and nitrates, with resolution of her symptoms. She was seen prior to discharge by a cardio-oncology specialist and transitioned to oral amlodipine and long-acting nitrates per a collaboratively developed predefined protocol. Following discussion with the patient and primary oncologist, the patient was readmitted for the next cycle of treatment, and the 5-FU-based chemotherapy was reinitiated with caution; she tolerated it without complications. In a recent study, 5-FU rechallenge after pretreatment with calcium-channel blockers and nitrates, under the guidance of a cardio-oncology service, was safe and permitted continued 5-FU therapy.42
Key points
-
•
Rapid recognition of cardiotoxicity and exclusion of severe obstructive CAD
-
•
Rapid access to cardio-oncology specialist
-
•
Multiagent protocol to suppress coronary vasospasm (and associated ischemia) that can include nitrates and calcium-channel blockers
-
•
Patient-oriented decision making
-
•
Discussions leading to therapy change
Case 4: ibrutinib
A 71-year-old man was diagnosed with chronic lymphocytic leukemia. He was initiated on the Bruton’s tyrosine kinase inhibitor ibrutinib. Three months following therapy initiation, he developed symptomatic atrial fibrillation. He was subsequently initiated on metoprolol for rate control, as well as apixaban 5 mg twice daily for anticoagulation. Four months later, he developed symptomatic gastrointestinal bleeding requiring endoscopic therapies. He was seen by a cardio-oncology specialist, and a discussion was held with the referring hematologist and the patient. Given the need for anticoagulation for atrial fibrillation and concomitant concern for increased bleeding risk associated with ibrutinib, anticoagulation was reinitiated at a lower dose of apixaban 2.5 mg twice daily, and his Bruton’s tyrosine kinase inhibitor cancer therapy was switched to acalabrutinib without further complications.
Key points
-
•
Patient-oriented decision making
-
•
Longitudinal multidisciplinary care
-
•
Use of strategies to minimize the complications associated with cardiotoxicity
Case 5: bevacizumab
A 77-year-old woman was diagnosed with colorectal cancer. Given her poor performance status due to several comorbid conditions, she was initiated on therapy with capecitabine and bevacizumab. She developed new systolic hypertension, with a rise in systolic blood pressure to 170 mm Hg during regular screening after 2 weeks of therapy. She remained asymptomatic and was initiated on amlodipine 5 mg/d, which was subsequently titrated up to 10 mg/d with adequate control of her blood pressure.
Key points
-
•
Regular screening for common side effects
-
•
Prompt recognition and management of cardiotoxic effect
Of note, angiotensin-converting enzyme inhibitors are recommended as first-line agents for management of anti-VEGF-induced hypertension, mostly for their potential renoprotective effects given higher risk for proteinuria on VEGF inhibition therapy.43,44
Case 6: pediatrics
A 7-year-old girl was diagnosed with acute myeloid leukemia when she was 3 years of age. Her treatment included a daunorubicin-containing regimen, with a total dose of 150 mg/m2, and hematopoietic stem cell transplantation (HSCT). Four years later, she was diagnosed with relapsed acute myeloid leukemia. She was noted to have an LVEF of 50% to 55% at this time, with GLS of −18%, and subsequently began therapy with venetoclax with curative plans for further HSCT in the coming weeks. Routine echocardiography 1 year prior to relapse had shown an LVEF of 55% to 60% and GLS of −20%. However, pre-HSCT screening echocardiography 2 months after relapse showed an LVEF of 40% to 45%, with GLS of −14%. Her parents reported that she has seemed more tired than usual but thought it was related to the relapsed leukemia and current chemotherapy. Given the decline in her left ventricular systolic function, she was deemed not a candidate for HSCT and was quickly referred to a pediatric cardio-oncology specialist, who initiated therapy with enalapril and carvedilol. Follow-up echocardiography 1 month later demonstrated improvement in her LVEF to 50% and GLS of −17%. In discussion with her cardio-oncology specialist, primary oncologist, primary HSCT oncologist, and parents, it was decided that she was now a reasonable candidate for repeat HSCT and would continue her CV medications at least through treatment with close follow-up, including frequent echocardiography.
Key points
-
•
Prompt referral and access to a cardio-oncology specialist
-
•
Patient- and family-oriented decision making
-
•
Multidisciplinary management in limiting toxicity and continuing with cancer-directed therapies
Limitations of the Permissive Cardiotoxicity Strategy
It should be noted that permissive cardiotoxicity management strategies cannot be applied to every type of cardiotoxicity that develops during a course of cancer treatment. Permissive cardiotoxicity–based strategies can be advocated only for toxicities whose natural history during ongoing cancer treatment creates a reasonably large “safe harbor” zone in which a graduated degree of cardiotoxicity can be effectively monitored and managed in a manner that protects the patient from development of severe irreversible cardiotoxicity that will not respond to GDMT. For example, the development of severe pulmonary hypertension with dasatinib is poorly responsive to treatment and can lead to a fatal outcome if no mitigation strategy seems available. The mitigation strategy mentioned here could potentially allow a patient who had mild pulmonary hypertension to receive protective treatment that would predictably allow resumption of dasatinib. Similarly, retreatment of patients who have had ICI-related myocarditis or suggestions of other off-target effects can be selectively considered when limited options exist and could include trial of a different ICI agent. The risk for accelerated atherosclerosis is increasingly recognized as an off-target effect of ICI therapy that is not as distinctive as myocarditis. Such possibilities merit consideration in the risk-benefit assessment of resumption of ICI therapy in patients with prior cardiotoxicity. Oversight by a cardio-oncology team with advanced experience and strong collaborative relationship with oncology partners are likely prerequisites for managing such cases. The diversity of malignancies and their respective therapies mean that a “best fit” model is unlikely to be successful. Furthermore, cardiotoxicities can range from cardiomyopathies seen classically with anthracyclines,45,46 to coronary vasospasm with 5-FU,47 to dyslipidemia, metabolic syndrome, and hypertension among hormone-specific agents in breast and prostate cancer,2,48 to myocarditis with ICIs,10 and many more (Table 1, Figure 1). This results in a landscape that can be very complex given heterogeneous patient populations, therapies, and outcomes. As such, clinical care is ideally delivered in a multidisciplinary fashion involving the patient, oncologist, pharmacist, and cardio-oncology specialist in close communication. Collaborative cardio-oncology registries can provide further insight and can harness more patients and outcomes than any individual cardio-oncology specialist or institution.
Conclusions
Early detection and treatment of cancer have led to a 27% decline in death rates in the United States from 2001 to 2020.49 This progress has been driven by improved therapies but at the cost of increased CV toxicities. Both cardiologists and oncologists have a role in the detection and management of these toxicities, and there is significant benefit to regular dialogue between the cardio-oncology specialist and the oncologist. Furthermore, increasing data suggest that it may be safe to continue these potentially cardiotoxic anticancer agents with close monitoring and aggressive management, in the setting of cardiotoxicity. Overall, clinicians should be vigilant for the adverse CV effects of certain cancer therapies, such as dyslipidemia and metabolic syndrome with endocrine and androgen deprivation therapy, and hypertension with anti-VEGF and multiple receptor tyrosine kinases. Blood pressure, diabetes, and lipid screening should be a routine part of long-term surveillance in patients with cancer. Optimization of modifiable risk factors, such as poor diet, sedentary lifestyle, tobacco use, and alcohol use, often in a multidisciplinary approach, remains the foundation for minimizing cardiotoxicity during treatment and survivorship.13,37
The concept of permissive cardiotoxicity is to encourage physicians to provide optimal cancer therapy while limiting long-term, symptomatic cardiotoxicities. To do so necessarily involves assessment of the patient’s comorbidities at baseline, as well as frequent CV assessment tailored to the anticancer agents being used. It also requires the ability of health care systems to provide patients with rapid access to cardio-oncology specialists when cardiotoxicity is anticipated or noted to be present. This also necessitates greater understanding of cardio-oncology in the general cardiology and oncology practices. These challenges, although complex, are surmountable at both the institution and system levels with appropriate allocation of resources, administrative support, and education.
It is our hope that the paradigm of permissive cardiotoxicity shifts the mind-set of cancer care providers from “Should this therapy be discontinued?” to “How can we safely continue this therapy?”
Perspectives.
Competency in Systems-Based Practice Permissive cardiotoxicity is a balance of clinical determinants in which optimal cancer therapy may have negative impact on the cardiovascular system, but the overall goal is to allow the best outcome from cancer treatment. We introduce for the first time, this vital concept in cardio-oncology that emphasizes a proactive rather than reactive approach to continuation of lifesaving cancer therapies in order to achieve the best oncologic outcome; while at the same time managing the patient to mitigate associated and potentially off-target cardiotoxicities.
Funding Support and Author Disclosures
Dr Dent is a consultant to and has received honoraria from AstraZeneca and Novartis. Dr Lenihan is a consultant (modest) to AstraZeneca, Bristol Myers Squibb, Myocardial Solutions, Clementia, Intellia, Bridge Bio, OncXerna, and SecuraBio. Dr Okwuosa is a consultant to Antev. Dr Porter has received an unrestricted grant from the Sally and Cloud Cray Family Foundation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Wendy L. Schaffer, MD, PhD, served as the Guest Associate Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 2.Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Perez E.A., Romond E.H., Suman V.J., et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J.C., Haworth L., Sherry R.M., et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitland M.L., Kasza K.E., Karrison T., et al. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen A., Crandall B. Cardiovascular effects of tyrosine kinase inhibitors in patients with advanced renal cell carcinoma at the VA San Diego Healthcare System. Fed Pract. 2019;36:S18–S24. [PMC free article] [PubMed] [Google Scholar]
- 7.Manouchehri A., Kanu E., Mauro M.J., Aday A.W., Lindner J.R., Moslehi J. Tyrosine kinase inhibitors in leukemia and cardiovascular events: from mechanism to patient care. Arterioscler Thromb Vasc Biol. 2020;40:301–308. doi: 10.1161/ATVBAHA.119.313353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganatra S., Sharma A., Shah S., et al. Ibrutinib-associated atrial fibrillation. J Am Coll Cardiol EP. 2018;4:1491–1500. doi: 10.1016/j.jacep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Polk A., Vistisen K., Vaage-Nilsen M., Nielsen D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47. doi: 10.1186/2050-6511-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palaskas N., Lopez-Mattei J., Durand J.B., Iliescu C., Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganatra S., Carver J.R., Hayek S.S., et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC council perspectives. J Am Coll Cardiol. 2019;74:3153–3163. doi: 10.1016/j.jacc.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beasley G.S., Towbin J.A. Acquired and modifiable cardiovascular risk factors in patients treated for cancer. J Thromb Thrombolysis. 2021;51:846–853. doi: 10.1007/s11239-020-02273-7. [DOI] [PubMed] [Google Scholar]
- 13.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardesai S., Sukumar J., Kassem M., et al. Clinical impact of interruption in adjuvant Trastuzumab therapy in patients with operable HER-2 positive breast cancer. Cardiooncology. 2020;6:26. doi: 10.1186/s40959-020-00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong I.Y., Verma S., Yan A.T., et al. Long-term cardiovascular outcomes and overall survival of early-stage breast cancer patients with early discontinuation of trastuzumab: a population-based study. Breast Cancer Res Treat. 2016;157:535–544. doi: 10.1007/s10549-016-3823-y. [DOI] [PubMed] [Google Scholar]
- 16.Liutkauskiene S., Grizas S., Jureniene K., Suipyte J., Statnickaite A., Juozaityte E. Retrospective analysis of the impact of anthracycline dose reduction and chemotherapy delays on the outcomes of early breast cancer molecular subtypes. BMC Cancer. 2018;18:453. doi: 10.1186/s12885-018-4365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai J., Ma H., Huang F., et al. Correlation of bevacizumab-induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: a systematic review and meta-analysis. World J Surg Oncol. 2013;11:306. doi: 10.1186/1477-7819-11-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salem J.E., Manouchehri A., Moey M., et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer J.R., Lacchetti C., Schneider B.J., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S., Johnson D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi: 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman-Keller M., Kim Y., Cronin H., Richards A., Gibney G., Weber J.S. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teulings H.E., Limpens J., Jansen S.N., et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 23.Chan L., Hwang S.J.E., Byth K., et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J Am Acad Dermatol. 2020;82:311–316. doi: 10.1016/j.jaad.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Indini A., Di Guardo L., Cimminiello C., et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol. 2019;145:511–521. doi: 10.1007/s00432-018-2819-x. [DOI] [PubMed] [Google Scholar]
- 25.Nowsheen S., Aziz K., Park J.Y., et al. Trastuzumab in female breast cancer patients with reduced left ventricular ejection fraction. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leong D.P., Cosman T., Alhussein M.M., et al. Safety of continuing trastuzumab despite mild cardiotoxicity: a phase I trial. J Am Coll Cardiol CardioOnc. 2019;1:1–10. doi: 10.1016/j.jaccao.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai A., Mohammed T., Patel K.N., Almnajam M., Kim A.S. 5-Fluorouracil rechallenge after cardiotoxicity. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.924446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonaggio A., Michot J.M., Voisin A.L., et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5:1310–1317. doi: 10.1001/jamaoncol.2019.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bidani A., Tzouanakis A.E., Cardenas V.J., Jr., Zwischenberger J.B. Permissive hypercapnia in acute respiratory failure. JAMA. 1994;272:957–962. [PubMed] [Google Scholar]
- 30.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N., et al. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4:1007–1018. doi: 10.1001/jamacardio.2019.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araujo-Gutierrez R., Chitturi K.R., Xu J., et al. Baseline global longitudinal strain predictive of anthracycline-induced cardiotoxicity. Cardiooncology. 2021;7:4. doi: 10.1186/s40959-021-00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thavendiranathan P., Negishi T., Somerset E., et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77:392–401. doi: 10.1016/j.jacc.2020.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Liu J.E., Barac A., Thavendiranathan P., Scherrer-Crosbie M. Strain imaging in cardio-oncology. J Am Coll Cardiol CardioOnc. 2020;2:677–689. doi: 10.1016/j.jaccao.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skovgaard D., Hasbak P., Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0096736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenihan D.J., Stevens P.L., Massey M., et al. The utility of point-of-care biomarkers to detect cardiotoxicity during anthracycline chemotherapy: a feasibility study. J Card Fail. 2016;22:433–438. doi: 10.1016/j.cardfail.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armenian S.H., Lacchetti C., Barac A., et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 38.Koop Y., El Messaoudi S., Vermeulen H., Maas A., Atsma F. Healthcare utilization and hospital variation in cardiac surveillance during breast cancer treatment: a nationwide prospective study in 5000 Dutch breast cancer patients. Cardiooncology. 2020;6:14. doi: 10.1186/s40959-020-00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruddy K.J., Sangaralingham L.R., Van Houten H., et al. Utilization of cardiac surveillance tests in survivors of breast cancer and lymphoma after anthracycline-based chemotherapy. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.119.005984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynce F., Barac A., Geng X., et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat. 2019;175:595–603. doi: 10.1007/s10549-019-05191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganatra S., Nohria A., Shah S., et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series. Cardiooncology. 2019;5:1. doi: 10.1186/s40959-019-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zafar A., Drobni Z.D., Lei M., et al. The efficacy and safety of cardio-protective therapy in patients with 5-FU (fluorouracil)-associated coronary vasospasm. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0265767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pande A., Lombardo J., Spangenthal E., Javle M. Hypertension secondary to anti-angiogenic therapy: experience with bevacizumab. Anticancer Res. 2007;27:3465–3470. [PubMed] [Google Scholar]
- 44.Derosa L., Izzedine H., Albiges L., Escudier B. Hypertension and angiotensin system inhibitors in patients with metastatic renal cell carcinoma. Oncol Rev. 2016;10:298. doi: 10.4081/oncol.2016.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cardinale D., Colombo A., Lamantia G., et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen P.A. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. 2018;104:971–977. doi: 10.1136/heartjnl-2017-312103. [DOI] [PubMed] [Google Scholar]
- 47.Zafar A., Drobni Z.D., Mosarla R., et al. The incidence, risk factors, and outcomes with 5-fluorouracil-associated coronary vasospasm. J Am Coll Cardiol CardioOnc. 2021;3:101–109. doi: 10.1016/j.jaccao.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okwuosa T.M., Morgans A., Rhee J.W., et al. Impact of hormonal therapies for treatment of hormone-dependent cancers (breast and prostate) on the cardiovascular system: effects and modifications: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2021;14 doi: 10.1161/HCG.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention An update on cancer deaths in the United States. https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm
- 50.Alexandre J., Cautela J., Ederhy S., et al. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio-oncology guidelines. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quagliariello V., De Laurentiis M., Rea D., et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20:150. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]