Abstract
Background
Monoclonal gammopathy of undetermined significance (MGUS) is associated with renal dysfunction, inflammation, and increased cardiovascular mortality, but the cardiovascular risks are not fully understood.
Objectives
The authors explored the association of MGUS with a spectrum of cardiovascular diseases using the Danish nationwide databases.
Methods
Between 1995 and 2018, patients 18 years and older with MGUS were age- and sex-matched (1:10) with control patients and followed prospectively until December 31, 2018, for the occurrence of cardiovascular diseases. Patients diagnosed with multiple myeloma, lymphoma, or amyloidosis were excluded. Multivariable adjusted hazard ratios (HRs) for cardiovascular outcomes were estimated using Cox proportional hazard regression.
Results
Patients with MGUS (n = 8,189; mean age 69.8 ± 11.7 years; 51.2% male) had higher prevalence of cardiovascular risk factors at baseline, including hypertension (48.0% vs 38.5%) and type 2 diabetes (13.0% vs 9.3%), compared with control patients. Outcomes included an increased risk of heart failure (HR: 1.55; 95% CI: 1.41-1.69), acute myocardial infarction (HR: 1.22; 95% CI: 1.06-1.40), ischemic stroke (HR: 1.16; 95% CI: 1.03-1.30), atrial fibrillation (HR: 1.32; 95% CI: 1.23-1.42), aortic aneurysm (HR: 1.55; 95% CI: 1.28-1.89), aortic stenosis (HR: 1.60; 95% CI: 1.41-1.82), aortic regurgitation (HR: 1.67; 95% CI: 1.34-2.07), heart block (HR: 1.32; 95% CI: 1.08-1.61), peripheral artery disease (HR: 1.69; 95% CI: 1.47-1.95), cor pulmonale (HR: 2.06; 95% CI: 1.55-2.73), and venous thromboembolism (HR: 1.43; 95% CI: 1.24-1.65). A sensitivity analysis including only patients without certain comorbidities (type 2 diabetes, hypertension, acute myocardial infarction, and chronic kidney disease) yielded similar results.
Conclusions
MGUS is associated with a broad spectrum of cardiovascular diseases, with greater relative risks observed for diseases previously associated with infiltrative and inflammatory disorders. Further studies are warranted to explore the underlying mechanisms.
Key Words: cardiovascular diseases, cardiovascular outcomes, epidemiology, monoclonal protein, light chain
Abbreviations and Acronyms: HR, hazard ratio; ICD, International Classification of Diseases; MGUS, monoclonal gammopathy of undetermined significance; OR, odds ratio
Central Illustration
Monoclonal gammopathy of undetermined significance (MGUS) is defined as the presence of a monoclonal immunoglobulin in the absence of lymphoproliferative disease.1 To meet the criteria for MGUS, patients must have serum M-protein <3 g/dL, <10% clonal plasma cells in the bone marrow, and the absence of a myeloma-defining event.2 Although MGUS has traditionally been viewed as a benign precursor to malignancy, more recent evidence suggests that patients with MGUS, even in the absence of a lymphoproliferative disorder, carry an increased risk of various disease states, including bone disease, recurrent infections, autoimmune disorders, peripheral neuropathy, and renal disease.3, 4, 5
There has also been an increasing interest in the risk of cardiovascular disease in patients with MGUS, with at least 2 observational studies suggesting higher cardiovascular mortality in patients with MGUS compared with age- and sex-matched control patients.6,7 An association between MGUS and deep vein thrombosis has been well described,8, 9, 10, 11 but our understanding of the cardiovascular risks and underlying pathogenesis is still limited. There are multiple mechanisms by which MGUS could lead to an increased risk of arterial and venous thrombosis. Higher levels of Factor VIII and von Willebrand factor have been demonstrated in patients with MGUS, and more recently, clonal hematopoiesis of indeterminate potential, which shares some features with MGUS, has been linked to atherosclerotic disease.12, 13, 14 Even in the absence of myeloma, MGUS is associated with several changes in the microenvironment, including increased inflammation, bone resorption, and altered angiogenesis.15,16 Paraprotein deposition is also involved in the pathogenesis of MGUS.17
In this study, we estimated the prevalence and incidence of atherosclerotic disease (acute myocardial infarction, peripheral artery disease, ischemic stroke), structural heart disease (heart failure, conduction disease), pulmonary hypertension (cor pulmonale), valvular disease (aortic stenosis, aortic regurgitation, mitral stenosis, mitral regurgitation), aortopathy (aortic dissection, aneurysm), pericarditis, and arrhythmias (atrial fibrillation) in patients with MGUS versus age- and sex-matched control patients. We also re-examined the association of venous thromboembolic disease and MGUS.
Methods
This study was exempt from ethical board approval, given its registry-based setup where individuals could not be identified. We used the Danish National Patient Registry to capture all diagnoses of MGUS, excluding multiple myeloma, lymphoma, and amyloid disease at baseline, and calculated the prevalence and incidence of a broad range of cardiovascular outcomes. In brief, the Danish National Patient Registry is a comprehensive national database that captures all inpatient and outpatient visits in Denmark (excluding primary care visits).18 Starting in 1978, every time a patient had a health care encounter, their primary and secondary diagnoses are recorded in the registry in association with the patient’s social security number. This is done using International Classification of Disease (ICD) coding (using ICD-8 from 1978 to 1993 and then using ICD-10 from 1994 onward), and data to anonymously link exposures and outcomes are accessible across institutions within Denmark. In addition, since 1995, all medications are registered using Anatomical Therapeutic Chemical classification codes that can be linked with ICD diagnoses for research purposes.19 Full diagnostic codes for outcomes and comorbidities, as well as medication use are available in Supplemental Table 1. To meet the study’s inclusion criteria, all patients needed a diagnosis of MGUS (ICD-10 code D472) between January 1, 1995, and December 31, 2018. All cases with a concomitant or prior diagnosis of multiple myeloma (ICD-10 code C90), non-Hodgkin lymphoma (ICD-10 C85), Waldenstrom’s macroglobulinemia and other B-cell lymphomas (ICD-10 C88), or amyloidosis (ICD-10 E85) were excluded. The positive predictive value of MGUS using this algorithm was estimated to be 88%.20 Endpoints included heart failure, atrial fibrillation, acute myocardial infarction, peripheral artery disease, ischemic stroke, conduction disease (including atrioventricular block or left bundle branch block), pericarditis, aortic stenosis, mitral stenosis, aortic regurgitation, mitral regurgitation, aortic dissection, aortic aneurysm, cor pulmonale, venous thromboembolism, and pacemaker/implantable cardiac defibrillator implementation. We only considered diagnoses that required hospitalization or outpatient visits, thus excluding emergency room diagnoses, which have been shown to be less valid. The majority of the studied cardiovascular endpoints have been validated with excellent positive predictive values.21 We also studied the risk of all-cause mortality and mortality from cardiovascular diseases (defined as any cardiovascular diagnosis (ICD-10 I00-I99) registered as a main or contributing cause of death in the national Causes of Death registry).22 Comorbidities were defined using ICD-8 and ICD-10 codes, as specified in Supplemental Table 1. However, because diabetes and hypertension are most often treated in a primary care setting, we used claimed prescriptions of antidiabetic drugs as a proxy for diabetes, and prescriptions of at least 2 classes of antihypertensive medications as a proxy for hypertension (this algorithm was used and validated in prior work).23
Statistical methods and analysis
Baseline characteristics are presented as mean ± SD, or as counts (percentage) (Table 1). Patients were matched (1:10) based on birth year and sex to individuals from the Danish Central Population Registry using the greedy matching principle. For the various cardiovascular diseases, individuals were followed until December 31, 2018, emigration, or death. For mortality analyses, patients were followed until December 31, 2019. Cox proportional hazard regression models were used to calculate hazard ratios (HRs), and the proportional hazards assumption was verified by visual inspection of log (-log[event-free survival probability]-log[time]) plots. Logistic regression was used to calculate odds ratios (ORs) for prevalent disease (up until the date of MGUS diagnosis). All HRs and ORs are presented with 95% CIs. An age- and sex-adjusted model was created for all regressions, as well as a multivariable model for incident disease that included hypertension, type 2 diabetes, acute myocardial infarction, atrial fibrillation, chronic kidney disease, dialysis, and all-cause cancer, as well as all medications listed in Table 1. In sensitivity analysis, we additionally adjusted for valvular disorders, heart failure, cor pulmonale, stroke, peripheral artery disease, and venous thromboembolism. Finally, cumulative incidence curves based on subdistribution hazards to account for competing risk were generated using the Fine-Gray model. As a sensitivity analysis, we also derived the subdistribution HR estimates from the Fine-Gray model for comparison with the results from the Cox model. All analyses were performed using SAS software version 9.4 (SAS Institute). A 2-sided P value <0.05 was considered statistically significant.
Table 1.
Baseline Characteristics
| MGUS (n = 8,189) | Control (n = 81,890) | |
|---|---|---|
| Age, y | 69.8 ± 11.7 | 69.8 ± 11.8 |
| Male | 4,196 (51.2) | 41,960 (51.2) |
| Hypertension | 3,933 (48.0) | 31,541 (38.5) |
| Type 2 diabetes | 1,064 (13.0) | 7,586 (9.3) |
| Heart failure | 464 (5.7) | 2,731 (3.3) |
| Atrial fibrillation | 672 (8.2) | 4,383 (5.4) |
| Acute myocardial infarction | 506 (6.2) | 4,039 (4.9) |
| Ischemic stroke | 448 (5.5) | 3,401 (4.2) |
| Aortic aneurism | 136 (1.7) | 732 (0.9) |
| Aortic dissection | 5 (0.1) | 58 (0.1) |
| Aortic stenosis | 206 (2.5) | 1145 (1.4) |
| Aortic regurgitation | 118 (1.4) | 713 (0.9) |
| Mitral stenosis | 11 (0.1) | 39 (0.1) |
| Mitral regurgitation | 105 (1.3) | 577 (0.7) |
| Heart block | 106 (1.3) | 697 (0.9) |
| Pericarditis | 74 (0.9) | 363 (0.4) |
| Peripheral artery disease | 334 (4.1) | 1,811 (2.2) |
| Cor pulmonale | 33 (0.4) | 209 (0.3) |
| Venous thromboembolism | 386 (4.7) | 2,252 (2.8) |
| Chronic kidney disease | 522 (6.4) | 1,623 (2.0) |
| Dialysis | 94 (1.2) | 309 (0.4) |
| Pacemaker/implantable cardiac defibrillator | 230 (2.8) | 1353 (1.7) |
| Cancer (all-cause) | 1,076 (13.0) | 8,969 (11.0) |
| Medications | ||
| Beta-blockers | 1,594 (19.5) | 11,727 (14.3) |
| ACE inhibitors | 1,419 (17.3) | 10,968 (13.4) |
| ARBs | 1,224 (15.0) | 9,484 (11.6) |
| Spironolactone | 296 (3.6) | 1455 (1.8) |
| Eplerenone | 9 (0.1) | 44 (0.1) |
| Aspirin | 1,524 (18.6) | 12,046 (14.7) |
| Clopidogrel | 393 (4.8) | 2,520 (3.1) |
| Statins | 2,104 (25.7) | 17,218 (21.0) |
| Direct oral anticoagulants | 84 (1.0) | 552 (0.7) |
| Warfarin | 513 (6.3) | 3,071 (3.8) |
Values are mean ± SD or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; MGUS = monoclonal gammopathy of undetermined significance.
Results
Baseline characteristics
There were 8,189 patients (51.2% male; mean age 69.8 ± 11.7 years) with MGUS included in the study between January 1, 1995, and December 31, 2018, and 81,890 individuals (51.2% male, aged 69.8 ± 11.7 years) in the matched control group. Patients with MGUS had a greater prevalence of comorbidity compared with control patients, including hypertension (48.0% vs 38.5%), type 2 diabetes (13.0% vs 9.3%), cancer (13.0% vs 11.0%), chronic kidney disease (6.4% vs 2.0%), and dialysis use (1.2% vs 0.4%). Prior cancer was present in 13% of the MGUS patients and 11% of control patients, with an equal distribution of the major cancer subtypes (eg, 18% breast cancer, 14% to 16% prostate cancer, and 15% to 16% gastrointestinal cancer) (Supplemental Table 2). Similarly, patients with MGUS had slightly higher medication use at baseline (including beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aspirin, clopidogrel, statins, direct oral anticoagulants, and warfarin). All baseline comorbidity and medication use are shown in Table 1.
Prevalence
In age- and sex-adjusted logistic regression, compared with control patients, MGUS patients had significantly greater ORs for most cardiovascular disorders, including heart failure, atrial fibrillation, acute myocardial infarction, ischemic stroke, aortic aneurysm, aortic dissection, aortic stenosis, aortic regurgitation, mitral stenosis, mitral regurgitation, conduction disease, pericarditis, peripheral artery disease, aortic aneurysm, venous thromboembolism, and cardiac device implantation (Table 2). Exceptions included similar prevalence of aortic dissection (OR: 0.86; 95% CI: 0.35-2.15) among individuals with and without MGUS.
Table 2.
Association of MGUS With Prevalent and Incident Cardiovascular Diseases
| OR (Prevalent Disease), Age- and Sex-Adjusted | HR (Incident Disease), Age- and Sex-Adjusted | HR (Incident Disease), Age-, Sex-, and Multivariable-Adjusteda | HR (Incident Disease), Age-, Sex-, and Multivariable Adjusteda, and Additional Comorbidity–Adjustedb | |
|---|---|---|---|---|
| Heart failure | 1.77 (1.59-1.96) | 1.77 (1.62-1.93) | 1.55 (1.41-1.69) | 1.52 (1.39-1.66) |
| Atrial fibrillation | 1.61 (1.48-1.75) | 1.51 (1.40-1.62) | 1.32 (1.23-1.42) | 1.32 (1.22-1.42) |
| Acute myocardial infarction | 1.28 (1.16-1.41) | 1.30 (1.14-1.49) | 1.22 (1.06-1.40) | 1.19 (1.04-1.37) |
| Ischemic stroke | 1.34 (1.21-1.49) | 1.23 (1.10-1.38) | 1.16 (1.03-1.30) | 1.15 (1.03-1.29) |
| Aortic aneurysm | 1.89 (1.57-2.27) | 1.64 (1.36-1.98) | 1.55 (1.28-1.89) | 1.51 (1.25-1.83) |
| Aortic dissection | 0.86 (0.35-2.15) | 3.78 (2.16-6.60) | 3.63 (2.06-6.39) | 3.58 (2.03-6.31) |
| Aortic stenosis | 1.84 (1.58-2.14) | 1.77 (1.56-2.00) | 1.60 (1.41-1.82) | 1.58 (1.39-1.80) |
| Aortic regurgitation | 1.67 (1.37-2.03) | 1.79 (1.44-2.22) | 1.67 (1.34-2.07) | 1.66 (1.34-2.07) |
| Mitral stenosis | 2.83 (1.45-5.52) | 2.59 (1.19-5.63) | 1.92 (0.86-4.26) | 1.87 (0.84-4.19) |
| Mitral regurgitation | 1.83 (1.49-2.26) | 1.62 (1.27-2.07) | 1.48 (1.15-1.89) | 1.48 (1.16-1.90) |
| Heart block | 1.53 (1.25-1.89) | 1.47 (1.20-1.78) | 1.32 (1.08-1.61) | 1.30 (1.07-1.59) |
| Pericarditis | 2.05 (1.59-2.63) | 1.75 (1.13-2.73) | 1.56 (0.99-2.45) | 1.52 (0.97-2.39) |
| Peripheral artery disease | 1.89 (1.68-2.13) | 1.99 (1.73-2.29) | 1.69 (1.47-1.95) | 1.67 (1.45-1.93) |
| Cor pulmonale | 1.58 (1.10-2.29) | 2.42 (1.83-3.19) | 2.06 (1.55-2.73) | 1.99 (1.50-2.64) |
| Venous thromboembolism | 1.75 (1.57-1.96) | 1.53 (1.32-1.76) | 1.43 (1.24-1.65) | 1.43 (1.24-1.65) |
| Pacemaker/implantable cardiac defibrillator | 1.74 (1.50-2.00) | 1.47 (1.20-1.78) | 1.17 (0.99-1.39) | 1.15 (0.97-1.37) |
HRs = hazard ratios; MGUS = monoclonal gammopathy of undetermined significance.
Multivariable adjustment includes diabetes, hypertension, acute myocardial infarction, atrial fibrillation, chronic kidney disease, dialysis, cancer, and medications in Table 1.
As a sensitivity analysis, the following additional comorbidities were included: prevalent valvular disorders, heart failure, cor pulmonale, stroke, peripheral artery disease, and prior venous thromboembolism.
Outcomes
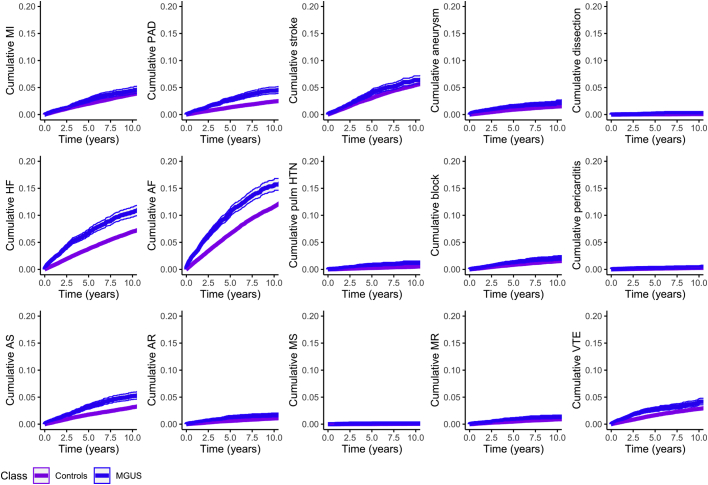
The mean follow-up times for patients with MGUS and control patients were 4.3 and 4.8 years, respectively. Median follow-up times for MGUS patients and control patients were 3.2 years (IQR: 1.5-6.0 years) and 3.6 years (IQR: 1.8-6.7 years), respectively. The cumulative incidence of each cardiovascular condition during follow-up was greater in patients with MGUS compared with control patients (Figure 1). Similarly, the HRs of all cardiovascular outcomes were elevated in patients with MGUS vs control patients in age- and sex-adjusted models (Table 2). After multivariable adjustment, estimates were partly attenuated but remained statistically significantly increased for most endpoints, including heart failure (HR: 1.55; 95% CI: 1.41-1.69), atrial fibrillation (HR: 1.32; 95% CI: 1.23-1.42), acute myocardial infarction (HR: 1.22; 95% CI: 1.06-1.40), aortic stenosis (HR: 1.60; 95% CI: 1.41-1.82), conduction disease (HR: 1.32; 95 % CI: 1.08-1.61), peripheral arterial disease (HR: 1.69; 95% CI: 1.47-1.95), cor pulmonale (HR: 2.06; 95 % CI: 1.55-2.73), and venous thromboembolic disease (HR: 1.43; 95% CI: 1.24-1.65) (Table 2). Numbers of cases and incidence rates per 100 person-years are shown in Supplemental Table 3. In addition, the overall mortality rate was higher for patients with MGUS compared with control patients: 5.86 (95% CI: 5.63-6.10) per 100 person-years for the MGUS group (total 2,432 persons) compared with 3.59 (95% CI: 3.54-3.65) per 100 person-years (total 16,619 persons) for control patients (multivariable adjusted HR: 1.55; 95% CI: 1.48-1.62).
Figure 1.
Incidence of Cardiovascular Diseases in MGUS vs Matched Controls
Cumulative incidence functions for various cardiovascular endpoints (taking the competing risk of death into account). AF = atrial fibrillation; AR = aortic regurgitation; AS = aortic stenosis; HF = heart failure; MGUS = monoclonal gammopathy of undetermined significance; MI = myocardial infarction; MR = mitral regurgitation; MS = mitral stenosis; PAD = peripheral arterial disease; pulm HTN = pulmonary hypertension; VTE = venous thromboembolism.
Sensitivity analysis
In sensitivity analysis, a cohort of patients without type 2 diabetes, hypertension, prior myocardial infarction, and chronic kidney disease was examined (n = 3,540 for MGUS cases, n = 45,534 for control patients). Hazards risks of all primary endpoints for patients with MGUS were largely similar to those in the overall analysis (Table 3), including heart failure (HR: 1.67; 95% CI: 1.46-1.91), peripheral arterial disease (HR: 2.17; 95% CI: 1.76-2.67), heart block (HR: 1.54; 95% CI: 1.15-2.05), venous thromboembolism (HR: 1.54; 95% CI: 1.15-2.05), and mortality (HR: 1.86; 95% CI: 1.72-2.01) compared with the control patients. Furthermore, we performed a sensitivity analysis that excluded the first 6 months of follow-up time after a MGUS diagnosis, and the results were similar (Supplemental Table 4). We also performed a sensitivity analysis that censored people when they developed multiple myeloma, lymphoma, or amyloidosis during follow-up. These analyses yielded similar results to the main models (Supplemental Table 5). Finally, restricting the analysis to cardiovascular mortality (7,528 events) yielded similar results to the primary analysis (multivariable adjusted HR: 1.55; 95% CI: 1.45-1.66; P < 0.0001). Subdistribution HRs obtained by the Fine-Gray model were similar to those obtained by the main Cox regression models (Supplemental Table 6).
Table 3.
HRs for Cardiovascular Outcomes in Healthy Patients With MGUS Compared to Control Patients
| HR (Incident Disease), Age-, Sex-, and Multivariable-Adjusteda | |
|---|---|
| Heart failure | 1.67 (1.46-1.91) |
| Atrial fibrillation | 1.41 (1.27-1.57) |
| Acute myocardial infarction | 1.23 (1.01-1.49) |
| Ischemic stroke | 1.14 (0.97-1.35) |
| Aortic aneurysm | 1.55 (1.18-2.05) |
| Aortic dissection | 4.48 (2.15-9.34) |
| Aortic stenosis | 1.76 (1.47-2.12) |
| Aortic regurgitation | 1.53 (1.12-2.11) |
| Mitral stenosis | 2.57 (0.72-9.11) |
| Mitral regurgitation | 1.50 (1.06-2.13) |
| Heart block | 1.54 (1.15-2.05) |
| Pericarditis | 2.33 (1.35-4.02) |
| Peripheral artery disease | 2.17 (1.76-2.67) |
| Cor pulmonale | 1.87 (1.20-2.92) |
| Venous thromboembolism | 1.54 (1.15-2.05) |
| Pacemaker/implantable cardiac defibrillator | 1.38 (1.09-1.75) |
| All-cause mortality | 1.97 (1.84-2.12) |
Healthy is defined as free of type 2 diabetes, hypertension, acute myocardial infarction, and chronic kidney disease.
Abbreviations as in Table 2.
Multivariable adjustment includes diabetes, hypertension, prior myocardial infarction, atrial fibrillation, chronic kidney disease, dialysis, cancer, and medications in Table 1.
Discussion
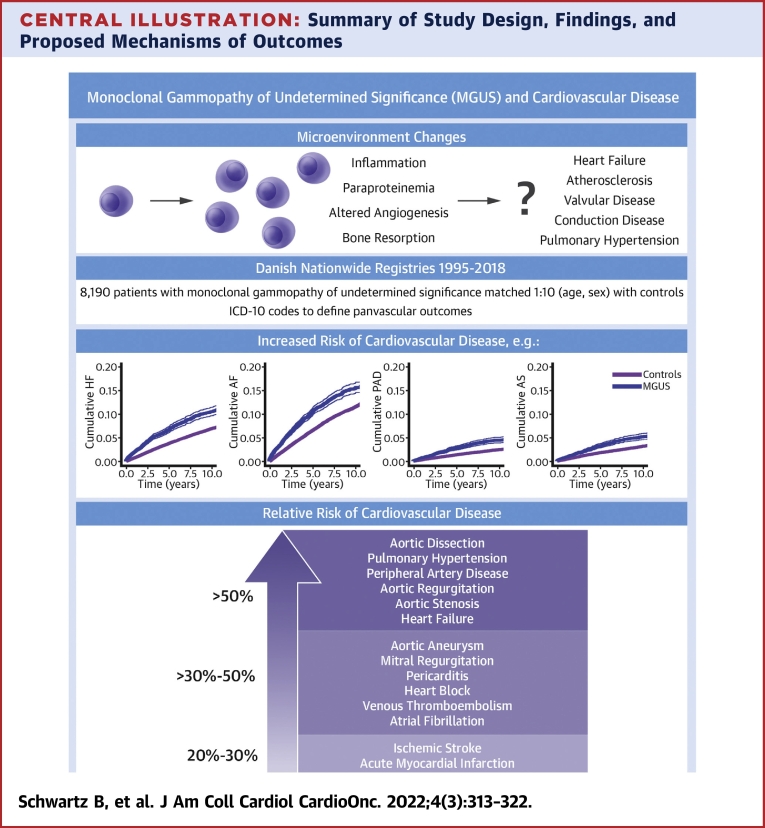
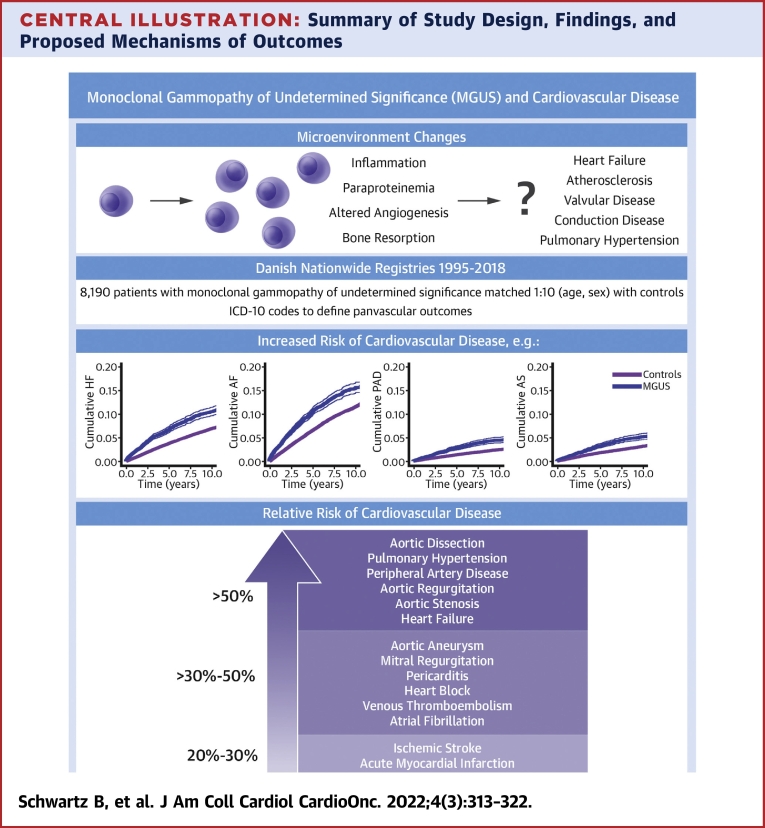
The study presented here used a large sample size of MGUS patients to measure a broad spectrum of cardiovascular outcomes associated with MGUS. We found that after adjustment for common comorbidities and medications, there was still an increased risk of most cardiovascular conditions, including ischemic stroke, acute myocardial infarction, and deep vein thrombosis. However, the magnitude of risk was somewhat greater for outcomes that have previously been associated with infiltrative and structural cardiac disease, such as heart failure, cor pulmonale, and some types of valvular disease (Central Illustration).
Central Illustration.
Summary of Study Design, Findings, and Proposed Mechanisms of Outcomes
Visual representation of proposed mechanisms of disease (top left), methodology and study design (top right), and cumulative incident curves for select outcomes and relative risk of disease in patients with MGUS vs control patients for various outcomes (bottom). AF = atrial fibrillation; AS = aortic stenosis; HF = heart failure; MGUS = monoclonal gammopathy of undetermined significance; PAD = peripheral arterial disease.
Atherosclerotic, aortic, and arterial thrombotic disease
Available studies investigating the association between atherosclerotic disease and MGUS have reported mixed results. One large retrospective cohort study from Sweden showed an increased risk of coronary artery disease (HR: 1.5; 95% CI: 1.3-1.7) and cerebrovascular disease (HR: 1.1; 95% CI: 1.0-1.3) after 5 years of follow-up, compared with control patients.24 The HRs in our study were similarly increased with MGUS, ranging from 1.22 for acute myocardial infarction to 1.69 for peripheral artery disease. Another, smaller study by Za et al25 examined similar outcomes and did not find a statistically significant increase in risk associated with MGUS vs control patients, but power was somewhat limited by a relatively smaller sample size. Our study also showed an increased incidence of aortopathies (dissection and aneurysm), whereas previous studies have not examined these outcomes.
An increased risk of atherosclerosis in MGUS may relate to the acquisition of somatic increase in certain genes absent malignancy (clonal hematopoiesis of indeterminant potential), which has recently been linked to atherosclerotic disease, and there is some evidence to suggest that a similar process may overlap with MGUS.12,13 Furthermore, biomarkers related to bone metabolism, such as osteoprotegerin and soluble receptor activator of nuclear factor-kappa B ligand (RANKL), were shown to be increased in patients with MGUS.26 These biomarkers, also reflective of vascular calcification processes, have been strongly associated with atherosclerotic disease formation and valvular disease in general populations, as well as with the burden of aortic calcification in patients with peripheral artery disease.27, 28, 29 Moreover, MGUS is often associated with a low-grade chronic inflammatory state, with elevated cytokines, such as interleukin-6.17,30 Finally, MGUS has been strongly associated with occlusive nonvasculitis vasculopathy and, more controversially, with leukocytoclastic vasculitis in case reports, possibly related to paraprotein-induced immune complex deposition and underlying inflammatory processes.31,32
Structural heart disease and arrhythmias
Our study also demonstrated a statistically significant increase in the risk of heart failure, conduction delay, pulmonary hypertension, aortic valvular disease, and atrial fibrillation among the MGUS cohort, with relative risks ranging from 1.32 to 2.06. Although there have been limited studies evaluating these outcomes, at least 1 prospective study demonstrated a higher prevalence of MGUS in heart failure patients compared with similarly aged cohorts.33 Similarly, a study that screened all emergency room patients for prevalent MGUS found that patients with heart failure were more likely to have undiagnosed MGUS compared with patients without heart failure (14% vs 5%; P = 0.012).34 Case reports have also been published on the association of myeloproliferative disorders (including MGUS) and severe pulmonary hypertension.35, 36, 37 Underlying inflammation, remodeling secondary to up-regulation of, for example, osteoprotegerin and RANK,38, 39, 40 as well as the possibility of direct infiltration of paraproteins may contribute to these associations.17,33,41 The association between MGUS and atrial fibrillation is likely related to similar pathophysiological changes, including, possibly, an atrial myopathy secondary to paraprotein infiltration.
Venous thrombotic disease
There have been multiple cohort studies on venous thromboembolic disease in patients with MGUS, and at least 5 studies have shown a significant increase in risk associated with MGUS.8, 9, 10, 11,24 One smaller study (N = 166) did not show a similar increase in risk, potentially due in part to lack of power.42 Our population-based study showed a statistically significant increase in the risk of venous thromboembolism in patients with MGUS versus control patients, with a HR of 1.43 (95% CI: 1.24-1.65). This risk was smaller than that in both studies by Kristinsson et al9,24, but was similar to the risk in Gregersen et al8 (HR: 1.4; 95% CI: 1.0-1.9).
Strengths and limitations
A few strengths of this study are worth noting. First, we examined a wide range of cardiovascular outcomes with different hypothesized mechanisms of disease within the same cohort, enabling comparisons of risk estimates across several cardiovascular disorders. We also had a large sample of MGUS patients in our database (n = 8,189), giving us the power to detect differences. Finally, we were able to add a large number of covariates to our multivariable model, decreasing the probability that statistically significant outcomes are being driven by comorbid risk or confounding. Despite the many strengths of this study, there are some limitations that need to be addressed. First, this is an observational study, and despite the many covariates added to our model, the possibility of residual confounding exists. For instance, we did not have data on smoking and obesity, which may be important, although these factors have not consistently been associated with MGUS risk.43 Second, some previous studies have shown different results based on M-protein concentration and subclass (eg, immunoglobulin G vs immunoglobulin M), but we were not able to stratify by these variables or test for interaction due to database limitations.8,24 Third, although the MGUS diagnosis was shown to have a good positive predictive value, it may be underreported.20 Fourth, some patients may have had undiagnosed amyloidosis that could influence our results. Fifth, we cannot rule out confounding by indication (ie, the reason for the test) and a component of surveillance bias as a result of the recommended monitoring of patients diagnosed with MGUS for progression to malignancy.3,44 However, the increased incidence of outcomes that are not normally subclinical and the increased risk of most endpoints, even years after diagnosis, would suggest that the results cannot entirely be explained by surveillance bias. Furthermore, HR estimates did not change significantly after excluding the first 6 months of follow-up in sensitivity analysis (Supplemental Table 3). Sixth, we could not include primary care visits in our analysis, potentially introducing a bias. Seventh, because this condition often presents asymptomatically, it is possible that some of the matched control patients also have undiagnosed MGUS as well. Finally, as the Danish National Patient Registries cover a relatively homogenous population, this study should be replicated in more diverse populations.
Conclusions
Our nationwide epidemiologic study showed that MGUS was associated with increased risk of a wide range of cardiovascular outcomes and not only the more well-known venous thromboembolic disorders. For most disorders, the risk estimates remained elevated, even after adjustment for common comorbidities and medication use, suggesting a possible causal impact of MGUS on the panvascular system. Whereas the relative risk of disease is elevated in arterial and venous thrombotic disease, the magnitude of the risk generally appears to be higher in outcomes associated with infiltrative and inflammatory disorders. Although the pathophysiologic mechanisms driving the increased cardiovascular risk in patients with MGUS are not yet fully understood, further studies are needed to test several proposed hypotheses. Moreover, population-based studies with systematic screening for MGUS (regardless of symptoms) are warranted to confirm the results from this registry-based study. In addition, future studies should assess the utility of increased screening and more aggressive management of cardiovascular risk factors in patients with MGUS.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with MGUS are at higher risk of cardiovascular disease, independent of age and sex. This risk remains even after adjustment for a wide range of cardiovascular risk factors.
TRANSLATIONAL OUTLOOK: Further studies on the hypothesized mechanisms of increased cardiovascular risk in patients with MGUS are needed. In addition, future studies should assess the utility of increased screening and more aggressive management of cardiovascular risk factors in patients with MGUS.
Funding Support and Author Disclosures
This work was supported by National Institutes of Health grant 1R38HL143584, Multi-Disciplinary Training for Promoting Research In Medical Residency (Dr Schwartz). Dr Schou has received fees from Boehringer Ingelheim, AstraZeneca, and Novo Nordisk for lectures that are unrelated to the present work. Dr Ruberg has received research funding from NIH/NHLBI (R01-HL139671), Alnylam Pharmaceuticals, Akcea Therapeutics, and Pfizer; and consulting income from Attralus and Alexion Pharmaceuticals unrelated to the present work. Dr Køber has received fees from Novartis, BMS, and AstraZeneca for lectures that are unrelated to the present work. Dr Torp-Pedersen has received study funding from Bayer and Novo Nordisk that is unrelated to the present work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Eisele L., Dürig J., Hüttmann A., et al. Heinz Nixdorf Recall Study Investigative Group. Prevalence and progression of monoclonal gammopathy of undetermined significance and light-chain MGUS in Germany. Ann Hematol. 2012;91(2):243–248. doi: 10.1007/s00277-011-1293-1. [DOI] [PubMed] [Google Scholar]
- 2.Mateos M.V., Landgren O. MGUS and smoldering multiple myeloma: diagnosis and epidemiology. Cancer Treat Res. 2016;169:3–12. doi: 10.1007/978-3-319-40320-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Kyle R.A., Larson D.R., Therneau T.M., et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241–249. doi: 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bida J.P., Kyle R.A., Therneau T.M., et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685–693. doi: 10.1016/S0025-6196(11)60518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimanovsky A., Alvarez Argote J., Murali S., Dasanu C.A. Autoimmune manifestations in patients with multiple myeloma and monoclonal gammopathy of undetermined significance. BBA Clin. 2016;6:12–18. doi: 10.1016/j.bbacli.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristinsson S.Y., Björkholm M., Andersson T.M., et al. Patterns of survival and causes of death following a diagnosis of monoclonal gammopathy of undetermined significance: a population-based study. Haematologica. 2009;94(12):1714–1720. doi: 10.3324/haematol.2009.010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregersen H., Ibsen J., Mellemkjoer L., Dahlerup J., Olsen J., Sørensen H.T. Mortality and causes of death in patients with monoclonal gammopathy of undetermined significance. Br J Haematol. 2001;112(2):353–357. doi: 10.1046/j.1365-2141.2001.02533.x. [DOI] [PubMed] [Google Scholar]
- 8.Gregersen H., Nørgaard M., Severinsen M.T., Engebjerg M.C., Jensen P., Sørensen H.T. Monoclonal gammopathy of undetermined significance and risk of venous thromboembolism. Eur J Haematol. 2011;86(2):129–134. doi: 10.1111/j.1600-0609.2010.01539.x. [DOI] [PubMed] [Google Scholar]
- 9.Kristinsson S.Y., Fears T.R., Gridley G., et al. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood. 2008;112(9):3582–3586. doi: 10.1182/blood-2008-04-151076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muslimani A.A., Spiro T.P., Chaudhry A.A., Taylor H.C., Jaiyesimi I., Daw H.A. Venous thromboembolism in patients with monoclonal gammopathy of undetermined significance. Clin Adv Hematol Oncol. 2009;7(12):827–832. [PubMed] [Google Scholar]
- 11.Srkalovic G., Cameron M.G., Rybicki L., Deitcher S.R., Kattke-Marchant K., Hussein M.A. Monoclonal gammopathy of undetermined significance and multiple myeloma are associated with an increased incidence of venothromboembolic disease. Cancer. 2004;101(3):558–566. doi: 10.1002/cncr.20405. [DOI] [PubMed] [Google Scholar]
- 12.Haefliger S., Juskevicius D., Höller S., Buser U., Dirnhofer S., Tzankov A. How to resolve a clinical and molecular puzzle: concomitant monoclonal gammopathy of undetermined significance (MGUS) with neutrophilia and clonal hematopoiesis of indeterminate potential (CHIP) Ann Hematol. 2019;98(10):2431–2432. doi: 10.1007/s00277-019-03786-9. [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal S., Natarajan P., Silver A.J., et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auwerda J.J., Sonneveld P., de Maat M.P., Leebeek F.W. Prothrombotic coagulation abnormalities in patients with paraprotein-producing B-cell disorders. Clin Lymphoma Myeloma. 2007;7(7):462–466. doi: 10.3816/clm.2007.n.027. [DOI] [PubMed] [Google Scholar]
- 15.Damasceno D., Almeida J., Teodosio C., et al. Monocyte subsets and serum inflammatory and bone-associated markers in monoclonal gammopathy of undetermined significance and multiple myeloma. Cancers (Basel) 2021;13(6):1454. doi: 10.3390/cancers13061454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann J.N., Landgren O., Landy R., et al. A prospective study of circulating chemokines and angiogenesis markers and risk of multiple myeloma and its precursor. JNCI Cancer Spectr. 2020;4(2):pkz104. doi: 10.1093/jncics/pkz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosseboeuf A., Allain-Maillet S., Mennesson N., et al. Pro-inflammatory state in monoclonal gammopathy of undetermined significance and in multiple myeloma is characterized by low sialylation of pathogen-specific and other monoclonal immunoglobulins. Front Immunol. 2017;8:1347. doi: 10.3389/fimmu.2017.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt M., Schmidt S.A., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pottegård A., Schmidt S.A.J., Wallach-Kildemoes H., Sørensen H.T., Hallas J., Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46(3) doi: 10.1093/ije/dyw213. 798-798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregersen H., Larsen C.B., Haglund A., Mortensen R., Andersen N.F., Nørgaard M. Data quality of the monoclonal gammopathy of undetermined significance diagnosis in a hospital registry. Clin Epidemiol. 2013;5:321–326. doi: 10.2147/CLEP.S50757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundboll J., Adelborg K., Munch T., et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11) doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7 suppl):26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 23.Olesen J.B., Lip G.Y., Hansen M.L., et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristinsson S.Y., Pfeiffer R.M., Björkholm M., et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115(24):4991–4998. doi: 10.1182/blood-2009-11-252072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Za T., De Stefano V., Rossi E., et al. Multiple Myeloma GIMEMA-Latium Region Working Group. Arterial and venous thrombosis in patients with monoclonal gammopathy of undetermined significance: incidence and risk factors in a cohort of 1491 patients. Br J Haematol. 2013;160(5):673–679. doi: 10.1111/bjh.12168. [DOI] [PubMed] [Google Scholar]
- 26.Politou M., Terpos E., Anagnostopoulos A., et al. Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1a) in monoclonal gammopathy of undetermined significance (MGUS) Br J Haematol. 2004;126(5):686–689. doi: 10.1111/j.1365-2141.2004.05092.x. [DOI] [PubMed] [Google Scholar]
- 27.Tschiderer L., Klingenschmid G., Nagrani R., et al. Osteoprotegerin and cardiovascular events in high-risk populations: meta-analysis of 19 prospective studies involving 27 450 participants. J Am Heart Assoc. 2018;7(16) doi: 10.1161/JAHA.118.009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abedin M., Omland T., Ueland T., et al. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study) Am J Cardiol. 2007;99(4):513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 29.Clancy P., Oliver L., Jayalath R., Buttner P., Golledge J. Assessment of a serum assay for quantification of abdominal aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26(11):2574–2576. doi: 10.1161/01.ATV.0000242799.81434.7d. [DOI] [PubMed] [Google Scholar]
- 30.DuVillard L., Guiguet M., Casasnovas R.O., et al. Diagnostic value of serum IL-6 level in monoclonal gammopathies. Br J Haematol. 1995;89(2):243–249. doi: 10.1111/j.1365-2141.1995.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 31.Llamas-Velasco M., Alegría V., Santos-Briz Á., Cerroni L., Kutzner H., Requena L. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39(9):637–662. doi: 10.1097/DAD.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 32.Drerup C., Metze D., Ehrchen J., Mitschang C., Neufeld M., Sunderkötter C. Evidence for immunoglobulin-mediated vasculitis caused by monoclonal gammopathy in monoclonal gammopathy of unclear significance prompting oncologic treatment. JAAD Case Rep. 2019;5(3):288–291. doi: 10.1016/j.jdcr.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devesa A., Rodríguez Olleros C., Kaçi X., et al. Prevalence and prognostic value of monoclonal gammopathy in heart failure patients with preserved ejection fraction: a prospective study. Cardiol J. 2020;29(2):216–227. doi: 10.5603/CJ.a2020.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkin C., Reddy-Kolanu V., Drayson M.T., Sapey E., Richter A.G. The prevalence and significance of monoclonal gammopathy of undetermined significance in acute medical admissions. Br J Haematol. 2020;189(6):1127–1135. doi: 10.1111/bjh.16487. [DOI] [PubMed] [Google Scholar]
- 35.Rajapreyar I., Joly J., Tallaj J., et al. Pulmonary vascular disease due to plasma cell dyscrasia. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):210–218. doi: 10.1016/j.mayocpiqo.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaqub S., Moder K.G., Lacy M.Q. Severe, reversible pulmonary hypertension in a patient with monoclonal gammopathy and features of dermatomyositis. Mayo Clin Proc. 2004;79(5):687–689. doi: 10.4065/79.5.687. [DOI] [PubMed] [Google Scholar]
- 37.Chinen K., Fujioka Y. Severe pulmonary hypertension caused by smoldering plasma cell myeloma: an autopsy case of POEMS syndrome. Case Rep Med. 2012;2012 doi: 10.1155/2012/836893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W., Feng W., Wang F., et al. Osteoprotegerin/RANK/RANKL axis in cardiac remodeling due to immuno-inflammatory myocardial disease. Exp Mol Pathol. 2008;84(3):213–217. doi: 10.1016/j.yexmp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Cao H., Li Q., Li M., et al. Osteoprotegerin/RANK/RANKL axis and atrial remodeling in mitral valvular patients with atrial fibrillation. Int J Cardiol. 2013;166(3):702–708. doi: 10.1016/j.ijcard.2011.11.099. [DOI] [PubMed] [Google Scholar]
- 40.Ock S., Ahn J., Lee S.H., et al. Receptor activator of nuclear factor-κB ligand is a novel inducer of myocardial inflammation. Cardiovasc Res. 2012;94(1):105–114. doi: 10.1093/cvr/cvs078. [DOI] [PubMed] [Google Scholar]
- 41.Toor A.A., Ramdane B.A., Joseph J., et al. Cardiac nonamyloidotic immunoglobulin deposition disease. Mod Pathol. 2006;19(2):233–237. doi: 10.1038/modpathol.3800524. [DOI] [PubMed] [Google Scholar]
- 42.Cohen A.L., Sarid R. The relationship between monoclonal gammopathy of undetermined significance and venous thromboembolic disease. Thromb Res. 2010;125(3):216–219. doi: 10.1016/j.thromres.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Castaneda-Avila M.A., Ulbricht C.M., Epstein M.M. Risk factors for monoclonal gammopathy of undetermined significance: a systematic review. Ann Hematol. 2021;100(4):855–863. doi: 10.1007/s00277-021-04400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyle R.A., Durie B.G., Rajkumar S.V., et al. International Myeloma Working Group Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.