Abstract
Cardio-oncology research studies often require consideration of potential competing risks, as the occurrence of other events (eg, cancer-related death) may preclude the primary event of interest (eg, cardiovascular outcome). However, the decision to conduct competing risks analysis is not always straightforward, and even when deemed necessary, misconceptions exist about the appropriate choice of analytical methods to address the competing risks. R researchers are encouraged to consider competing risks at the study design stage and are provided provide an assessment tool to guide decisions on analytical approach on the basis of study objectives. The existing statistical methods for competing risks analysis, including cumulative incidence estimations and regression modeling are also reviewed. Cardio-oncology-specific examples are used to illustrate these concepts and highlight potential pitfalls and misinterpretations. R code is also provided for these analyses.
Key Words: censoring, competing risks, cumulative incidence, epidemiology, survival analysis, statistics
Abbreviations and Acronyms: CIF, cumulative incidence function; csH, cause-specific hazard; csHR, cause-specific HR; CV, cardiovascular; KM, Kaplan-Meier; sdH, subdistribution hazard; sdHR, subdistribution HR; VAD, ventricular assist device
Central Illustration
Highlights
-
•
Cardio-oncology research studies often require consideration of competing risks.
-
•
Need for competing risks analysis should be examined at the study design stage.
-
•
CIF curves should be interpreted with simultaneous evaluation of both event types.
-
•
Both sdH and csH regression models are useful depending on the question of interest.
Survival analyses are commonly used in cancer research to examine clinical questions, including treatment efficacy. Overall survival has historically been the primary focus, in which the outcome of interest is time to death of all causes. However, with improving treatments and prognosis across a wide range of malignancies, considerations of outcomes other than death are becoming increasingly important. The field of cardio-oncology research focuses on cardiovascular (CV) outcomes among patients with cancer, in the setting of a growing array of cancer treatments with potential adverse cardiac effects as well as improved cancer-specific survival. When an outcome other than overall survival (such as a CV outcome) is being evaluated, competing risks become unavoidable as patients can have other events that preclude the primary event of interest. For example, when a patient dies of cancer, they cannot subsequently experience a CV outcome.
The issue of competing risks has been extensively discussed in statistical research1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and more recently in medical research.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Most of these papers describe statistical methods assuming that a competing risks analysis is needed, but this decision is not always straightforward, as we illustrate in this review. We therefore provide an assessment tool to align analytical approaches with the study objectives, when competing risks are a potential concern. Moreover, once competing risks analysis is deemed necessary, misconceptions surrounding the appropriate choice of statistical methods still exist, as some familiar analytical properties in standard survival analysis are not valid in the competing risks setting. Therefore, we review the statistical methods for competing risks analysis by comparing and contrasting concepts to standard survival analysis approaches. Throughout the paper, we illustrate the statistical quantities and techniques in the context of cardio-oncology research.
The review is structured accordingly. In the next section we outline considerations to determine whether competing risks are relevant for a given study objective. In the third section, we review various statistical methods for competing risks analysis, such as cumulative incidence estimation and regression modeling. We then illustrate these methods using examples of clinical studies from cardio-oncology research in the fourth section. We close with concluding remarks.
Competing Risks Overview
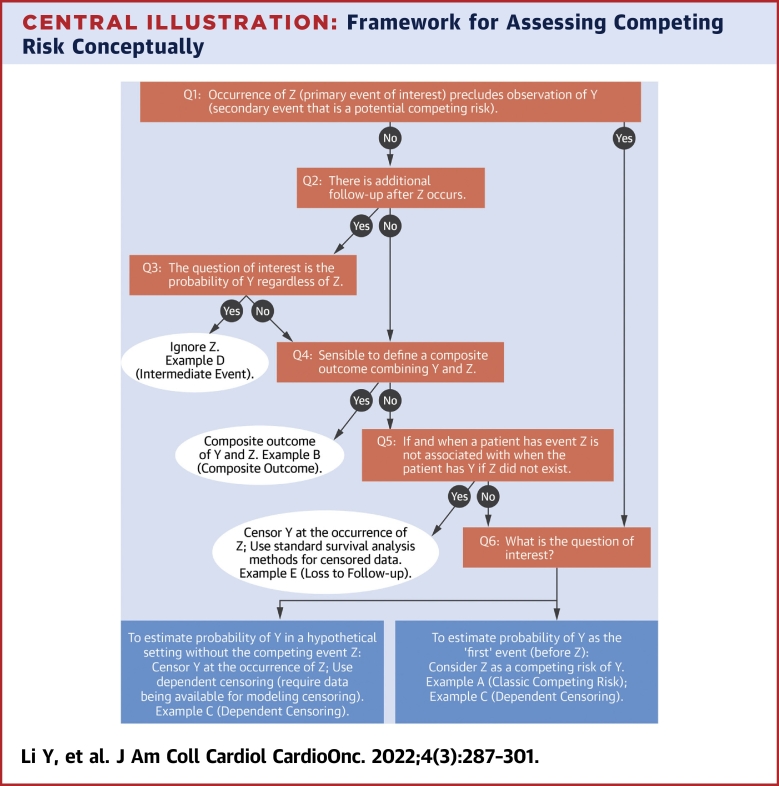
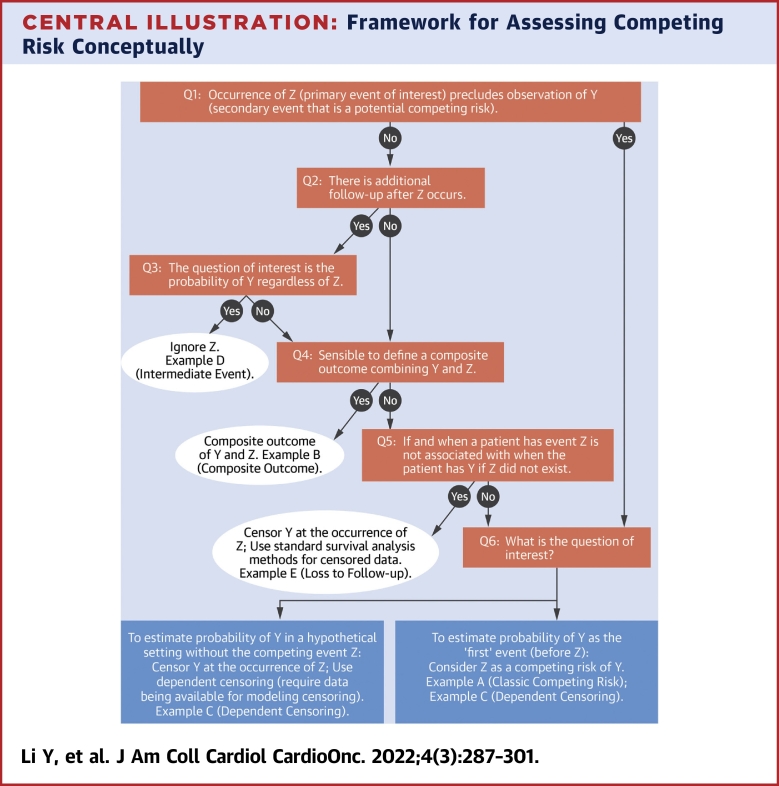
In survival analysis, a competing risk is defined as an event whose occurrence precludes observation of the primary event of interest. The concept of competing risks should be considered at study design to ensure that the capture of primary and competing events over the duration of follow-up aligns with the research objective. The presence of competing risks is obvious in some settings but is more ambiguous in others, as illustrated in the following examples. Appropriate treatment of potential competing risks depends on the study objectives; the Central Illustration outlines an assessment to determine whether competing risks are relevant and the best approach for subsequent analysis in each scenario. Importantly, the research question of interest is the primary driver of the type of analytical approach. Once the research question is established, the next step is to determine the capability of the data to answer the question. In the figure, Y represents the primary event of interest, and Z represents a secondary event that is potentially a competing risk for Y. Boxes represent various analytical options with corresponding examples in hypothetical studies.
Central Illustration.
Framework for Assessing Competing Risk Conceptually
Here Y represents the primary event of interest, and Z represents the secondary event that is potentially a competing risk for Y. Examples A (classic competing risk), B (composite outcome), and C (dependent censoring) are further explained in the text (in the section “Competing Risks Overview”), and examples D (intermediate event) and E (loss to follow-up) are explained in the Supplemental Appendix. Q1 = Question 1; Q2 = Question 2; Q3 = Question 3; Q4 = Question 4; Q5 = Question 5; Q6 = Question 6.
In example A (classic competing risk), a study aims to estimate the probability of cardiac-related death (Y), in the presence of deaths of all other causes (Z), including cancer-related deaths. In this example, it is obvious that the occurrence of non-cardiac-related death precludes the observation of cardiac-related death (the response to question 1 is yes). Additionally, it is difficult to imagine a setting without the competing event of non-cardiac-related death (ie, the response to question 6 unlikely to follow the left arrow). Therefore, Z is a competing risk of Y. A similar example would be a study that aims to estimate the probability of cancer relapse (Y), in the presence of all-cause death (Z), in which death is a clear competing risk of relapse.
In example B (composite outcome), a study aims to estimate the probability of cardiac-related event(s), which could be heart attack, stroke, or heart failure. In this case, the occurrence of 1 event (eg, stroke) does not preclude the occurrence of the other events (eg, heart attack), but study follow-up typically stops at the documented occurrence of the first event (ie, the answers to both questions 1 and 2 in the Central Illustration are no). In addition, as all of these events are unfavorable outcomes, a reasonable approach would be to combine them into a composite outcome. The operationalization of the commonly used endpoint of major adverse cardiac events, typically defined as the earliest occurrence of heart failure, stroke, myocardial infarction, or cardiac-related death, is an example of this composite outcome approach. A similar example would be evaluations of relapse-free survival, a composite endpoint that includes both death and relapse.
In example C (dependent censoring), a trial aims to estimate the probability of death (Y) among patients implanted with ventricular assist devices (VADs) for treatment of advanced heart failure, but follow-up for study endpoints ends at the receipt of a heart transplant (Z). In this scenario, the receipt of a heart transplant does not preclude observation of death (no to question 1), but the study does not have additional follow-up for endpoints after the receipt of a heart transplant (no to question 2). Furthermore, depending on the research question, it may not always be appropriate to combine death and heart transplantation into a composite outcome (no to question 4). For example, if the VAD is intended to be a bridge to heart transplantation, then heart transplantation becomes the goal for improving survival (ie, a favorable outcome), while death is an unfavorable outcome.
A naive approach commonly used in such scenarios is to censor patient follow-up at the occurrence of Z (receipt of heart transplantation) and use standard survival analysis for censored data (such as Kaplan-Meier [KM] analysis). However, as question 5 indicates, this approach is appropriate only if censoring is independent; that is, time to Z is not related to the time to Y. When this assumption is violated, naive censoring could introduce a selection bias. In our specific example C, patients with VADs who have clinical deterioration related to VAD complications may be prioritized to undergo heart transplantation to prolong survival, and thus censoring caused by transplantation is not independent of hazard of death.
Instead, one could consider 2 alternative approaches depending on the study objective. If the objective is to estimate the probability of death with a VAD before undergoing heart transplantation or the probability of death as the first event before heart transplantation (ie, follow the right arrow from question 6), then receipt of a heart transplant should be treated as a competing risk. Otherwise, if the probability of death (Y) in a hypothetical setting without heart transplantation (Z) (ie, the marginal survival) is of interest (ie, follow the left arrow from question 6), then censoring at the occurrence of Z is appropriate only if more advanced methods to handle dependent censoring are applied. These methods require modeling of the censoring process as a function of the observed covariates followed by inverse probability weighting or multiple imputations to adjust for the bias induced by the dependent censoring. We refer readers to several publications for detailed discussions on the implementation of these methods.25, 26, 27, 28, 29 Note that this approach is valid only when data on the covariates that determine the censoring process are collected and available for analyses. This highlights the importance of a well-defined study question and careful consideration of analytical approach at the design stage, to ensure that adequate information is collected to conduct valid analyses that match the research objective. If the study objective is to estimate marginal survival but a competing risks analysis is performed instead, then the results are not directly interpretable in addressing the study question of interest.
Additional examples for other analytical options to answer various study questions are presented in the Supplemental Appendix. In summary, how to handle the secondary event Z in the analysis of the primary event Y requires careful consideration. Treating Z as censoring allows estimation of marginal quantities, such as the probability of Y in a hypothetical setting in which Z does not exist; however, the standard survival analysis for censored data assumes independent censoring and is biased when this assumption is violated. Treating Z as a competing risk could provide estimation of the probability of Y as the “first” event (before Z) but does not formally account for nonindependent censoring. To estimate marginal survival while accounting for nonindependent censoring, advanced methods such as inverse probability weighting (and corresponding data elements) must be used. Similar discussions on choice of analytical strategies for different study questions were also deliberated in van Geloven et al22 and Stegherr et al24 which we recommend for further reading. For the remainder of the present review, we focus on the scenario in which a competing risks analysis is determined to be necessary (ie, consistent with the box at the bottom right of the Central Illustration).
Key Concepts in Competing Risks Analysis in Contrast to Standard Survival Analysis
In this section we review key concepts and statistical quantities in competing risks analysis, with a focus on how they differ from those in standard survival analysis (Table 1). In standard survival analysis, there is only 1 type of event (eg, all-cause death), and time to this event is the endpoint of interest. As not all subjects will experience the event of interest (death) by the end of follow-up, subjects are censored at the last observed time if the event has not yet occurred. Thus, the outcome categorization D in standard survival analysis involves 2 values, indicating occurrence of event (D = 1) or censoring (D = 0), and the time variable T denotes the earliest time of event or censoring. Note that in this paper we focus on right censoring, as it is the most common type of censoring, and we refer readers to other publications for methods to handle other types of censoring and truncations in survival times.5,30,31
Table 1.
Key Concepts in Competing Risks Analysis in Contrast to Standard Survival Analysis
| Standard Survival Analysis | Competing Risk Analysis | |
|---|---|---|
| Data structure | Outcome categorization D: two values, event (D = 1) vs censoring (D = 0) Time variable T: the earliest time of event or censoring |
Outcome categorization D: three values, primary event (D = 1) vs competing event (D = 2) vs censor (D = 0) Time variable T: the earliest time of primary event, competing event, or censoring |
| Hazard function | Hazard of the event, denoted as h(t) | Cause-specific hazard for the primary event, denoted as Cause-specific hazard for the competing event, denoted as |
| Failure or survival functiona | Failure function for the event, denoted as F(t) Survival function for the event, denoted as S(t) S(t) = 1 − F(t) |
Failure function for the primary event, denoted as F1(t) Failure function for the competing event, denoted as F2(t) Overall survival function: probability of surviving both events, denoted as Soverall(t) Soverall(t) = 1 − F1(t) − F2(t) |
| Relationship between hazard and failure probability | Simple 1-to-1 relationship between F(t) and h(t) |
No simple 1-to-1 relationship between F1(t) and ; calculation of F1(t) involves both and |
| Estimandb of interest in the study | Hazard and cumulative incidence are compatible | Requires precise description; must specify whether the estimand is the (cause-specific) hazard of the event or the cumulative incidence of the event |
Failure function is equivalent to cumulative incidence function.
Estimand is the target quantity of interest to be estimated.
In contrast, in competing risks analysis, subjects can experience more than 1 type of event, the primary event of interest and the competing event. Thus, the outcome can be categorized by 3 values, indicating occurrence of the event of primary interest (D = 1), the competing event (D = 2), or censoring (D = 0), and the time variable T denotes the earliest time of any type of event or censoring. Although in this review we consider only a single competing event for simplicity, these concepts and methods are generally applicable to settings with multiple competing events.
Cause-specific hazard
One important function in the standard survival analysis is the hazard function, which quantifies the instantaneous rate of the event at some time t as the probability of failing at time t, conditional on surviving up until t. That is,
h(t)= Prob(event occurs in the next instant, given that have not experienced the event by t)/Δt.
In this definition, hazard is a ratio of a “conditional” probability and a time interval Δt (where the time interval is very small), so hazard is an instantaneous rate. Conditioning on survival up until time t means that the hazard is calculated for the risk set of subjects who have not yet experienced the event by t.
In the competing risk setting, in contrast to standard survival analysis, the hazard function is modified to accommodate the possibility of 2 types of events. The cause-specific hazard (csH) function is used to quantify the instantaneous rate for the specific type of event at time t, conditional on surviving all event types until t. That is,
= Prob(the type j event occurs in the next instant, given that the patient has not experienced any types of event
by t)/Δt, j = 1, 2
Therefore, denotes the csH for the primary event, and denotes the csH for the competing event. This csH definition is very similar to the standard hazard function, except that the probability of the event is type specific, and the risk set reflects the occurrence of all event types. Specifically, the risk set here includes subjects who have not yet experienced any type of event and have not been censored, so all patients with any event (primary event or the competing event) before time t are removed from the risk set at that time point. Suppose the observed ordered event times (regardless of event type) are t1, t2,…, tL; then at each event time tl, can be estimated simply as the number of individuals who experience the type j event at time tl divided by the number of subjects at risk at time tl.
Cumulative incidence function
Another important function in the standard survival analysis is the survival function, S(t), which quantifies the probability of surviving (or having not experienced) the event by time t. A related function is the failure function, F(t), which quantifies the probability of having the event by time t. By definition these 2 functions are the complement of each other: S(t) = 1 − F(t). In research studies, survival probabilities are more commonly reported than failure probabilities, but one can easily calculate the failure probability from a reported survival probability.
In the competing risk setting, because the outcome has more than 2 categories, 1 survival function and 1 failure function are no longer sufficient to describe the situation. Failure functions now need to be specific for the event type, so that Fj(t) represents the probability of having the type j event by time t. That is, F1(t) is the probability of having the primary event by time t, and F2(t) is the probability of having the competing event by time t. Recall that the 2 types of events are mutually exclusive of each other, and any subject can only experience 1 type of event, so that in the foregoing definition, having the type j event also implies having this type j event before having the other type of event. In competing risks analysis, the term cumulative incidence function (CIF) is preferred over the term failure function, but these 2 functions are the same. We use the term CIF hereafter. Given that 2 types of events could occur, the standard survival function is no longer a relevant quantity. Instead, the overall survival function is defined as the probability of surviving both events, with a relationship to the CIFs as
Soverall(t) = 1 − F1(t) − F2(t)
Because the CIF is specific to the event type whereas overall survival function is not, it is more useful to report the CIF for each event type in the setting of competing risks. This is an important difference from standard survival analysis, in which the survival function is more commonly reported than the failure function.
In the absence of censoring, CIF for event type j at each event time tl can be easily estimated as the proportion of subjects experiencing the type j event by time tl among all the study subjects. In the presence of censoring, one can estimate CIF for event type j at each event time tl as follows3,30:
Here the estimate is a sum of a series of quantities at ti, with the summation taken over all the times up to tl. Each quantity at ti is the product of the overall survival probability at the previous time point ti−1 and the type j csH at time ti. The overall survival probability could be estimated using the KM method, and the csH can be estimated as described in the section “Cause-Specific Hazard.” Two alternative estimators have also been proposed and proved to be equivalent to this one.30
After the CIF at each event time is estimated, the CIF curve for each event type can be plotted over time. The estimated CIF curve is a step-up function, with the steps occurring at the times when the type j event occurs. From the CIF curves for the primary event and the competing event, one could easily evaluate the cumulative probabilities of having had the primary event, a competing event, and no events in the presence of each other, and these 3 probabilities sum to 1. One common mistake related to CIF estimation is the approach that naively censors subjects at the occurrence of the competing event and uses the complement of the KM survival estimate for CIF estimation. As noted by multiple investigators,3,11,23 this approach overestimates the cumulative incidence of the primary event.
Last, if comparison between treatment groups is of interest, the CIF curves can be plotted by groups. Gray’s test1 can be used to compare CIF curves, in contrast to the log-rank test for comparing KM curves in standard survival analysis.
Relationship between the csH and the CIF
Both hazard function and survival function (or CIF) are often the target quantity of interest to be estimated in a study (namely, the estimand), and they are related and translatable to each other mathematically. In standard survival analysis, the hazard function and the CIF have a simple 1-to-1 relationship such that one function can be easily derived from the other:
This also implies that any treatment (or exposure or covariate) effect on one function is consistent with that on the other function. For example, if a treatment is associated with a higher hazard of the event, then it is also associated with a higher cumulative incidence of the event.
However, competing risks complicate the relationship between the csH and the CIF. For example, for the primary event, and F1(t) no longer have a simple 1-to-1 relationship, and instead the calculation of F1(t) involves both and :
This illustrates that the CIF for the primary event is influenced by both the hazard of the primary event and the hazard of the competing event. Therefore, compared with the KM survival curves in the standard survival analysis, CIF curves can be more complex to interpret. The CIF for the primary event may appear to be low if the hazard for the competing event is high. In other words, populations may have a low cumulative incidence of the primary event, because they often experience the competing event first, which leads to a lack of opportunity to experience the primary event. Therefore, it is recommended to always examine together the CIF curves for each type of event and the overall survival to fully understand and interpret the incidence of various events in a given study.17,23
Moreover, it is possible to obtain inconsistent treatment effects between one analysis that focuses on the csH and another that focuses on the CIF. For example, the results may suggest no association between the treatment and the csH, but suggest a higher CIF associated with the treatment, because of the association between the treatment and the competing event. Because of this complexity, it is essential for investigators to be precise in stating the target estimand (csH or CIF) and delineating study hypotheses and avoid defaulting to a broad term such as risk, which is often used and leads to confusion in interpretation of results in the setting of competing risks. The 2 hypotheses “treatment is associated with lower (cause-specific) hazard of the event of interest” and “treatment is associated with lower cumulative incidence of the event of interest” are distinct and may result in analytical contrasts with different, even opposing, conclusions depending on the nature and extent of the competing risks.
Regression Models in Competing Risks Analysis
To this point, we have framed competing risks in the context of the measure of outcome occurrence and the estimation of a probability of the event. However, it is common in clinical studies to compare outcome occurrence between groups, where group is defined by treatment assignment, exposure status, or covariate values. The same considerations of competing risks extend to such studies, and the measure of association is simply the contrast (difference or ratio) of the treatment (exposure) group-specific event probabilities.
Although statistical tests (such as the log-rank test and Gray’s test) can be performed to compare survival or CIF curves after they are estimated using the methods described in the section “Key Concepts in Competing Risks Analysis in Contrast to Standard Survival Analysis,” such comparisons do not account for multiple risk factors or address potential confounding. Regression models are important tools to address these issues and quantify the association between the treatment (or exposure or covariate) and the outcome while adjusting for other risk factors. In the standard survival analysis, the most popular regression model is the Cox proportional hazards model, in which the treatment effect is summarized as an HR. In the setting of competing risks, 2 popular regression models are the csH model2 and the Fine and Gray subdistribution hazard (sdH) model.4 Key elements and differences of these 2 models are summarized in Table 2 and reviewed in this section.
Table 2.
Comparison of Cause-Specific Hazard Model and Subdistribution Hazard Model for Competing Risks Analysis
| csH Model | sdH Model | |
|---|---|---|
| Risk set for the hazard of the primary event | Subjects who are still at risk for the primary event (ie, those not yet censored or not experiencing any type of event) | Subjects who are still at risk for the primary event and those who already experienced the competing event (ie, those not yet censored or not experiencing the primary event) |
| Interpretation of the hazard | csH is a hazard function; interpretable | sdH is not a true hazard function; direct interpretation is difficult |
| Relationship between hazard and CIF | No simple 1-to-1 relationship, but CIF can be derived from the csH of the primary and competing events Covariate’s effect measured by csHR is not always consistent with its effect on CIF |
sdH is designed to have simple 1-to-1 relationship with CIF Covariate’s effect measured by sdHR is consistent with its effect on CIF, with respect to direction and significance, although the magnitude of the effect is not directly translatable |
| Estimated hazard ratio of the primary event for a covariate | csHR Although estimated in the same way as in the standard Cox model, interpretation is different from a standard HR because it is cause specific Measures the direct association between the covariate and the csH of the primary event among the subjects who are still at risk for the primary event |
sdHR Better to interpret as the covariate effects on the cumulative incidence (rather than the hazard) of the primary event Measures the association due to both the association of the covariate with the primary event and the possibly differential impact of the competing event on the risk set for the patients with different covariate values |
| When to use | If interested in estimating etiologic or biological association between the exposure/treatment/covariate and the hazard of the primary event | If interested in estimating the prognostic effect of the exposure/treatment/covariate on the cumulative incidence of the primary event |
CIF = cumulative incidence function; csH = cause-specific hazard; csHR = cause-specific HR; sdH = subdistribution hazard; sdHR = subdistribution HR.
csH model
One commonly used csH regression model takes the same form as the Cox proportional hazards model for the standard survival analysis, except that it models the csH instead.2 The model assumes that the csH function is proportional for the groups defined by the covariates in the model. With X representing the covariate and bj representing the regression coefficient, the model specifies
Here is the arbitrary baseline csH (similar to the baseline hazard in Cox proportional hazards model), where “baseline” means for a subject with covariate X = 0. One model is constructed for each type of event, and a covariate effect is estimated for each type of event. So b1 corresponds to the covariate effect on the primary event, b2 corresponds to the covariate effect on the competing event, and these effects do not have to be the same. The covariate effect is then summarized as the cause-specific HR (csHR), which equals exp(bj) and can be interpreted as the relative change in the csH for the event type j corresponding to a 1-unit increase in the covariate X. When X is binary (eg, exposed vs unexposed), the csHR is simply the ratio of the csH for one group vs the other.
Recall that in the definition of csH, the risk set at time t includes subjects who are still at risk for the primary event (ie, those who are not yet censored or have not yet experienced any type of event), so the subjects who experienced the competing event before t are removed. Therefore, csHR measures the association of the covariate and the csH among the subjects who are still at risk for the primary event of interest. When estimating csHR, censoring and competing risks are treated the same, and therefore csHR is estimated in the same way as the simple HR from a standard Cox model. However, the interpretation of csHR is different from the standard Cox HR because it is cause specific. In contrast to the standard survival analysis in which the direction (and statistical significance) of the HR implies the direction of covariate’s effect on the cumulative incidence (eg, HR <1 suggests that the treatment reduces the hazard of event, and equivalently the treatment reduces the cumulative incidence of the event), in the presence of competing risks, the direction (and statistical significance) of the csHR does not correspond directly to the covariate effect on the CIF.5,13 Nevertheless, as noted in the section “Relationship between the csH and the CIF,” the CIF could be derived based the csH for both the primary event and the competing event. Therefore, after csH models are fitted for both event types, model-based CIF curves could be estimated (see the Supplemental Appendix for R code to implement this).
sdH model
The sdH function was proposed by Fine and Gray,4 for the purpose of having a direct connection with CIF. The definition of sdH for the type j event is the instantaneous rate of occurrence of the type j event at time t in subjects who have not yet experienced the type j event. That is,
= Prob(the type j event occurs in the next instant,
given that the patient has not experienced type j
event by t)/Δt, j = 1, 2
So the rate of the event is assessed in the subjects who are either event free or have experienced the competing event. The key difference between the sdH and the csH lies in the construction of risk set; the subjects who had the competing event before time t are removed from the risk set for csH but are included in the risk set for the sdH. Because the subjects who had the competing event previously cannot possibly have the primary event but are included in the risk set, the sdH is not a true hazard function, and its direct interpretation is difficult.
Nevertheless, one can construct a proportional hazards model from the sdH (called the sdH model or Fine and Gray model) in a similar fashion to the csH model:
Here is the arbitrary baseline sdH, and βj is the regression coefficient. Again, 1 model is constructed for each type of event, so β1 corresponds to the covariate effect on the primary event, and β2 corresponds to the covariate effect on the competing event. The subdistribution HR (sdHR), which equals exp(βj), summarizes the relative change in the sdH for the event type j corresponding to a 1-unit increase in the covariate X. However, when reporting the sdHR, it is important to bear in mind the definition of the sdH with the unique risk set construction. For example, the sdHR for the primary event measures the association of the covariate and the sdH for the primary event, among the subjects who have not experienced any type of event and who have experienced the competing event.
In contrast, the sdHR is designed to have a direct correspondence with the CIF, so the direction (and statistical significance) of the sdHR implies the direction of covariates effect on the CIF. For example, an sdHR for the primary event that is significantly >1 for the exposed vs unexposed group suggests that the exposed subjects have significantly higher cumulative incidence of the primary event. Given the difficulty of direct interpretation of the sdH and its 1-to-1 correspondence with the CIF, it has been recommended to interpret the results from sdH models as the covariate effect on the cumulative incidence (rather than the hazard) of the event.9,23 One caveat is that the magnitude of the relative effect of the covariate on the sdH is different from the magnitude of the covariate’s relative effect on the CIF, so the numeric value of the sdHR cannot be directly used. For example, when sdHR = 2, one cannot infer that the cumulative incidence of the event for the exposed group is 2 times of that for the unexposed group.
Comparison and choice of the 2 models
Although both the csHR and the sdHR may be viewed as measures of association, the associations they measure are different. csHRj=1 (the csHR for the primary event) measures the direct association between the covariate and the csH of the primary event among the subjects who are still at risk for the primary event, and the competing event contributes only by passively removing subjects from the risk set. In contrast, sdHRj=1 (the sdHR for the primary event) measures the association due to both the association of the covariate with the primary event and the possibly differential impact of the competing event on the risk set for the patients with different covariate values, and the competing event actively contributes to the risk set.
Numeric values of csHRj=1 and sdHRj=1 are generally different. The magnitude and direction of the difference between csHRj=1 and sdHRj=1 depend on how the covariate is associated with the competing event, and we refer readers to Latouche et al6 and Lau et al13 for more details. It is possible, for example, that csHRj=1 = 1, suggesting no direct association of the exposure and the csH of the primary event. However, csHRj=2 (the csHR for the competing event) <1 suggests that the exposure decreases the csH of the competing event, and as a result sdHRj=1 >1, because the exposed subjects are less likely to experience the competing event and thus have more opportunity to experience the primary event. In this example, although there is no direct exposure association with the primary event, the exposed subjects are more likely to have the primary event because of the exposure’s impact on the competing event and its differential alteration of the risk set.
Therefore, as noted by others, the choice of model depends on the study objective.6, 7, 8, 9,13,19,20,23 If the objective is to assess the etiologic or biological association between the exposure (or treatment or covariate) and the outcome, then the csH model is more appropriate. However, if the objective is to assess the prognostic effect of the exposure (or treatment or covariate) on the cumulative incidence of outcome, then the sdH model is more appropriate. Last, if the objective is to derive a prediction model for the occurrence of outcomes over time, both models could be considered. Although the sdH model has the advantage of the 1-to-1 relation with the CIF, making prediction on the basis of the sdH model easier, a csH model may fit the data better with respect to the proportional hazards assumption and thus yield better prediction. Moreover, the predicted CIFs from Fine and Gray sdH models may lead to a cumulative total probability of event that exceeds 1 for some covariate patterns.10 This again highlights the importance of clear and precise specification of the study objective and hypothesis in the setting of competing risks analysis.
Analysis of the Cardio-Oncology Example
We consider a cardio-oncology example of a randomized trial comparing an investigational antiandrogen agent against standard first-line therapy for patients with metastatic prostate cancer. One study outcome of interest is time to the occurrence of a CV event (ie, major adverse cardiac events as defined previously). Clearly, cancer-related death is a competing risk for the outcome of CV events because the occurrence of cancer-related death precludes any subsequent CV events. The trial is designed to have 5 years of follow-up, and patients are censored at the last contact if they are lost to follow-up before 5 years (most likely for random reasons). Importantly, on the basis of previous research showing increased cardiac risk associated with antiandrogen therapy,32, 33, 34, 35 it is expected that the investigational agent may improve cancer survival (csHR <1) but also increase the risk for CV event (csHR >1).
We simulate a hypothetical dataset using statistical software to mimic a randomized clinical trial. The simulation settings were informed by previously published clinical trials32, 33, 34, 35 to reflect the example described previously. We assume a total of 1,000 patients with 1:1 equal randomization to the 2 treatment arms. Patients are followed for 5 years with the possibility of random censoring. Five-year cancer-related death is set to be approximately 60% in the standard arm,32,34 and the 5-year CV event rate is set to be approximately 8% in the standard arm.33, 34, 35 For the purpose of illustration, we simulate data under various true associations between the investigational agent and the potential outcomes: scenario 1, a strong positive association with CV event (csHR = 2) and a strong inverse association with cancer-related death (csHR = 0.5); scenario 2, a weak positive association with CV event (csHR = 1.25) and a strong inverse association with cancer-related death (csHR = 0.5); and scenario 3, a weak positive association with CV event (csHR = 1.25) and a weak inverse association with cancer-related death (csHR = 0.8).
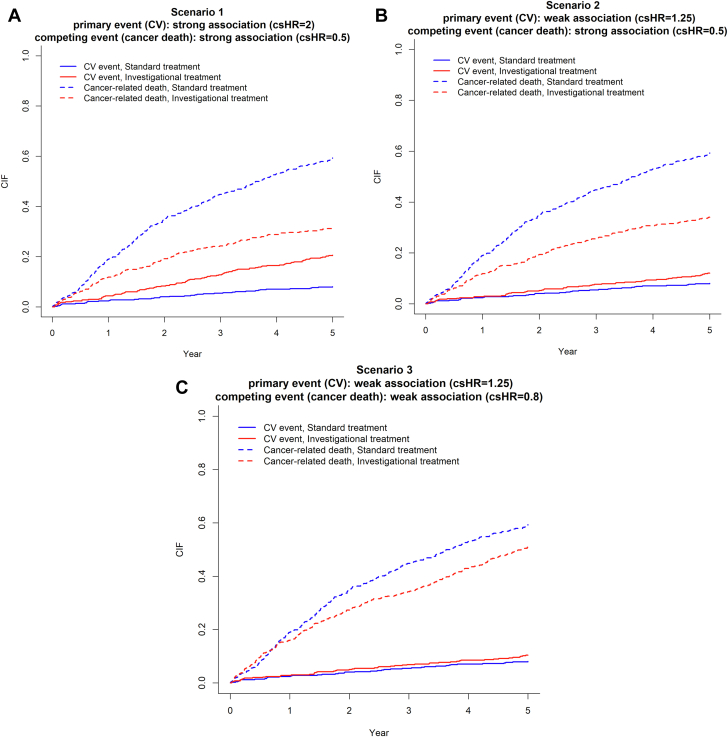
CIF and 1 − KM estimates for the primary event
For each scenario, we constructed CIF curves for both the primary event (CV event) and the competing event (cancer-related death) by treatment groups, using the methods described in the section “Cumulative Incidence Function” (Figure 1). We then focused on the CIF estimate for the primary event at 5 years and summarized the estimates and their 95% CIs under the 3 scenarios (Table 3). For the standard treatment group, the CIF estimate is the same across scenarios (8.1%; 95% CI: 5.9%-15.2%) because the true hazards for both primary and competing events are unchanged in the simulation settings. Because the positive association between the investigational agent and the hazard of the primary event (CV event) is strong (csHR = 2) in scenario 1 and weak (csHR = 1.25) in scenario 2, the corresponding CV event CIF estimate for the investigational treatment group is higher in scenario 1 (20.7%; 95% CI: 17.25%-24.4%) than in scenario 2 (12.1%; 95% CI: 9.4%-15.2%).
Figure 1.
CIF Curves of Primary and Competing Events by Treatment Groups
The primary event is a cardiovascular (CV) event, and the competing event is cancer-related death. (A) Scenario 1: the investigational treatment has a strong positive association with the primary event (cause-specific HR [csHR] = 2) and a strong inverse association with the competing event (csHR = 0.5). (B) Scenario 2: the investigational treatment has a weak positive association with the primary event (csHR = 1.25) and a strong inverse association with the competing event (csHR = 0.5). (C) Scenario 3: the investigational treatment has a weak positive association with the primary event (csHR = 1.25) and a weak inverse association with the competing event (csHR = 0.8).
Table 3.
Estimated 5-Year Cumulative Incidence of Primary Event (CV Event) From CIF and KM Methods
| Scenario | Treatment Group | CIF (95% CI) | 1 − KM (95% CI) | Illustrated Concept |
|---|---|---|---|---|
| Scenario 1: primary event (CV event), strong positive association (csHR = 2); competing event (cancer death), strong inverse association (csHR = 0.5) | Standard treatment | 8.1% (5.9%-10.7%) | 13.2% (9.0%-17.1%) | KM method overestimates cumulative incidence. |
| Investigational treatment | 20.7% (17.2%-24.4%) | 26.5% (21.9%-30.0%) | ||
| Scenario 2: primary event (CV event), weak positive association (csHR = 1.25); competing event (cancer death), strong inverse association (csHR = 0.5) | Standard treatment | 8.1% (5.9%-10.7%) | 13.2% (9.0%-17.1%) | Compared with scenario 1, CIF estimate of the primary event (CV event) for the investigational treatment group is lower, reflecting weaker positive treatment association with primary event. |
| Investigational treatment | 12.1% (9.4%-15.2%) | 15.6% (11.8%-19.2%) | ||
| Scenario 3: primary event (CV event), weak positive association (csHR = 1.25); competing event (cancer death), weak inverse association (csHR = 0.8) | Standard treatment | 8.1% (5.9%-10.7%) | 13.2% (9.0%-17.1%) | Compared with scenario 2, CIF estimate of the primary event (CV event) for the investigational treatment group is even lower, despite no change in the treatment association with the primary CV event, because the inverse treatment association with the competing event (cancer-related death) changes from strong to weak. This highlights that the CIF estimate of the primary event is also influenced by the hazard of the competing event because an increasing hazard of the competing event results in less opportunity to experience the primary event. |
| Investigational treatment | 10.4% (7.9%-13.3%) | 15.3% (11.2%-19.3%) |
The CIF method treats cancer-related death as a competing risk and uses the method described in the section “Cumulative Incidence Function.” The naive KM method treats cancer-related death as censoring and uses 1 − KM to estimate cumulative incidence.
CV = cardiovascular; KM = Kaplan-Meier; other abbreviations as in Table 2.
Note that the CIF estimate of the primary event (CV event) for the investigational treatment group is lower in scenario 3 (10.4%; 95% CI: 7.9%-13.3%) than in scenario 2 (12.1%; 95% CI: 9.4%-15.2%), even though the hazard of the primary event associated with the investigational agent is the same in the 2 scenarios (csHR = 1.25). This occurs because the inverse association between the investigational agent and the hazard of the competing event (cancer-related death) is weak (csHR = 0.8) in scenario 3 but strong (csHR = 0.5) in scenario 2. It highlights that the CIF estimate of the primary event is affected by the hazard of the competing event, in addition to the hazard of the primary event itself, because an increasing hazard of the competing event results in less opportunity to experience the primary event.
Table 3 also presents the estimated incidence of the primary event at 5 years obtained from the naive KM method by censoring patients at occurrence of the competing event (the “1 − KM” column). In all 3 scenarios and for both treatment groups, the naive KM method overestimates the incidence compared with the CIF method. For example, in the standard treatment group, the estimated incidence is 13.2% (95% CI: 9.0%-17.1%) for the naive KM method and 8.1% (95% CI: 5.9%-10.7%) for the CIF method, so the magnitude of overestimation is substantial (relatively 63%). This illustrates a common drawback of KM survival analysis when applied inappropriately in the setting of competing risks.
Treatment group comparison and regression models
Next, we estimated the association of the investigational agent with the primary event (CV event) and the competing event (cancer-related death) using 2 competing risks regression models, the csH model and the sdH model, as described in the section “Regression Models in Competing Risks Analysis” (Table 4). In scenario 1, for the primary event (CV event), the estimated sdHR (2.7; 95%: 1.9-3.9) is higher than the csHR (2.1; 95% CI: 1.5-3.1) because the investigational treatment group has a lower hazard of the competing event (cancer-related death) and thus more opportunity to experience the primary CV event. In contrast, focusing on the event of cancer-related death and considering CV event as a competing risk, the estimated csHR (0.46; 95% CI: 0.38-0.56) and sdHR (0.44; 95% CI: 0.36-0.53) for cancer-related death are similar. This is because although the investigational treatment is associated with a higher hazard of CV event and also differentially alters the risk set for cancer-related death, the incidence of CV event (about 8%) is much lower compared with the cancer-related death (about 60%), so the differential alteration of the risk set caused by the occurrence of CV event is nearly negligible.
Table 4.
Estimated Treatment Associations With Primary CV Event and Competing Event of Cancer-Related Death
| Scenario | Event Type | True Direct Investigational Treatment Association | csH Model: csHR (95% CI), P Value | sdH Model: sdHR (95% CI), P Value | Illustrated Concept |
|---|---|---|---|---|---|
| Scenario 1 | CV event | Strong positive (csHR = 2) | 2.1 (1.5-3.1), 0.001 | 2.7 (1.9-3.9), 0.001 | Because the investigational treatment group has the lower hazard of the competing event, sdHR is higher than csHR, suggesting a stronger positive association between the investigational treatment and the primary event. |
| Cancer-related death | Strong inverse (csHR = 0.5) | 0.46 (0.38-0.56), 0.001 | 0.44 (0.36-0.53), 0.001 | ||
| Scenario 2 | CV event | Weak positive (csHR = 1.25) | 1.19 (0.80-1.79), 0.387 | 1.51 (1.01-2.25), 0.045 | When the association of the investigational treatment is weak (positive) for the primary event and strong (inverse) for the competing event, sdHR is higher than csHR, and this difference could lead to a different conclusion on the basis of the statistical significance. |
| Cancer-related death | Strong inverse (csHR = 0.5) | 0.48 (0.39-0.58), 0.001 | 0.48 (0.39-0.58), 0.001 | ||
| Scenario 3 | CV event | Weak positive (csHR = 1.25) | 1.17 (0.77-1.77), 0.456 | 1.29 (0.86-1.96), 0.220 | When the association of the investigational treatment is weak for both the primary and competing events, sdHR is similar to csHR. |
| Cancer-related death | Weak inverse (csHR = 0.8) | 0.79 (0.67-0.94), 0.006 | 0.79 (0.66-0.93), 0.005 |
In scenario 2, for the primary event (CV event), again the sdHR (1.51; 95% CI: 1.01-2.25; P = 0.045) is higher than the csHR (1.19; 95% CI: 0.80-1.79; P = 0.387). Because the true association between the investigational agent and the primary event (CV event) is weak in this scenario, such an increase in the sdHR (compared with the csHR) could lead to a different conclusion if interpretation focuses on strictly on statistical significance (ie, P value associated with the sdHR interpreted as a significantly positive association).
Last, in scenario 3, when the association of the investigational treatment is weak for both the primary event and competing event, the csHR and sdHR are generally similar. For example, for the CV event, the csHR is 1.17 (95% CI: 0.77-1.77) and the sdHR is 1.29 (95% CI: 0.86-1.96).
R markdown code for data simulations and analyses is provided in the Supplemental Appendix.
Conclusions
Cardio-oncology research studies often require careful consideration of potential competing events. Appropriate handling of these events is important, and the choice of analytical method should be guided by the study objective. As we illustrate in the assessment tool in the Central Illustration, competing risks analysis is a commonly used approach, but not the only one. Because different methods are appropriate to address different study objectives, the need for a competing risks analysis should be evaluated at the time of study design, rather than at the time of analysis.
Once a competing risks analysis is deemed necessary, multiple statistical methods exist and can be easily implemented in the standard software, as we have reviewed in this paper. For estimating cumulative incidence of the outcome, the naive KM method leads to inflated estimates; instead, the CIF method should be used. When reporting and interpreting CIF curves, it is essential to evaluate the CIF for both the primary event of interest and the competing event, because the CIF estimate of the primary event is influenced by both the hazard of the primary event and the competing event. This also implies that results across studies are not directly comparable if different events are treated as competing risks.
When reporting regression models for competing risks, the sdHR from the Fine and Gray sdH model is often interpreted as if it is equivalent to an HR from a standard Cox model. However, as we note in this paper, sdH is not a true hazard function, and thus the sdHR should not be directly interpreted as an HR without noting the unique risk set for sdH. Instead, it is better to interpret the sdH model results from the perspective of the treatment (or exposure or covariate) effect on the cumulative incidence (rather than hazard). Moreover, there is a misconception that the Fine and Gray sdH model should always be used when competing risks are present. However, both sdH and csH models are useful tools and provide valid estimates of different types of associations. The sdH model estimates the prognostic association of the treatment or exposure with the cumulative incidence of the event, whereas the csH model estimates the etiologic or biological association of treatment or exposure with the csH of the event. Therefore, in practice, the model that is aligned with the study objective should be chosen as the primary analysis, and the other model may be presented as a secondary analysis, which enables a more complete understanding and interpretation of the relationships between the treatment or exposure and the outcome.
We defined a competing risk as an event that precludes the observation of the primary event of interest; one could also consider it as time to the first event and the type of the event (either the primary event of interest or the competing event), as described by Schmoor et al.18 With this, competing risks can be viewed as a special case of the multistate framework that models events as transitions between states.5,18 The csH is then equivalent to the transition intensity (the rate of transitioning from one state to another) in the multistate model. Although competing risks analyses focus on the first event, general multistate models are able to model outcomes after the first nonfatal event, such as death after transplantation (or relapse).
In this paper we focus on describing methods that are applicable to the most common scenarios encountered in studies with potential competing risks concerns, but other scenarios occur that require distinct advanced analytical methods, which we have only briefly mentioned here. One example is accounting for dependent censoring when the study objective is to estimate marginal survival, as in example C described in the section “Competing Risks Overview.” Another example is handling of competing risks under types of censoring and truncation other than the right censoring, as noted in the section “Key Concepts in Competing Risks Analysis in Contrast to Standard Survival Analysis.” The application of appropriate advanced methods in these settings is necessary to obtain unbiased estimated and make valid inferences. Last, causal interpretation of the results of a competing risks analysis is very challenging,36 because the csH may suffer from the same selection mechanism as the standard hazard by generating a selective risk set over time (ie, removing those who experienced the event differentially between the comparison groups).37, 38, 39 Furthermore, the CIF also does not reflect the causal effect, as it is influenced by the effect of the treatment or exposure on the competing event. Research in this area is ongoing,40 and a detailed discussion is beyond the scope of this paper.
Funding Support and Author Disclosures
Dr Getz has received support for research effort from a National Heart, Lung, and Blood Institute career development grant (5K01HL143153). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Joerg Herrmann, MD, served as the Guest Associate Editor for this paper. Kathryn J. Ruddy, MD, MPH, served as the Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For additional examples for analytical options in the Central Illustration and R code for the simulations and analyses, please see the online version of this paper.
Appendix
References
- 1.Gray R.J. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 2.Lunn M., McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 3.Gooley T.A., Leisenring W., Crowley J., Storer B.E. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 5.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 6.Latouche A., Boisson V., Chevret S., Porcher R. Misspecified regression model for the subdistribution hazard of a competing risk. Stat Med. 2007;26(5):965–974. doi: 10.1002/sim.2600. [DOI] [PubMed] [Google Scholar]
- 7.Beyersmann J., Schumacher M. Misspecified regression model for the subdistribution hazard of a competing risk. Stat Med. 2007;26(7):1649–1651. doi: 10.1002/sim.2727. [DOI] [PubMed] [Google Scholar]
- 8.Koller M.T., Raatz H., Steyerberg E.W., Wolbers M. Competing risks and the clinical community: irrelevance or ignorance? Stat Med. 2012;31(11-12):1089–1097. doi: 10.1002/sim.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin P.C., Fine J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin P.C., Steyerberg E.W., Putter H. Fine-Gray subdistribution hazard models to simultaneously estimate the absolute risk of different event types: cumulative total failure probability may exceed 1. Stat Med. 2021;40(19):4200–4212. doi: 10.1002/sim.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H.T. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559–565. doi: 10.1158/1078-0432.ccr-06-1210. [DOI] [PubMed] [Google Scholar]
- 12.Dignam J.J., Kocherginsky M.N. Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol. 2008;26(24):4027–4034. doi: 10.1200/jco.2007.12.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dignam J.J., Zhang Q., Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.ccr-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen P.K., Geskus R.B., de Witte T., Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41(3):861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noordzij M., Leffondré K., van Stralen K.J., Zoccali C., Dekker F.W., Jager K.J. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–2677. doi: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 17.Latouche A., Allignol A., Beyersmann J., Labopin M., Fine J.P. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66(6):648–653. doi: 10.1016/j.jclinepi.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Schmoor C., Schumacher M., Finke J., Beyersmann J. Competing risks and multistate models. Clin Cancer Res. 2013;19(1):12–21. doi: 10.1158/1078-0432.ccr-12-1619. [DOI] [PubMed] [Google Scholar]
- 19.Wolkewitz M., Cooper B.S., Bonten M.J., Barnett A.G., Schumacher M. Interpreting and comparing risks in the presence of competing events. BMJ. 2014;349:g5060. doi: 10.1136/bmj.g5060. [DOI] [PubMed] [Google Scholar]
- 20.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/circulationaha.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warnock D.G. Competing risks: you only die once. Nephrol Dial Transplant. 2016;31(7):1033–1035. doi: 10.1093/ndt/gfv455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Geloven N., le Cessie S., Dekker F.W., Putter H. Transplant as a competing risk in the analysis of dialysis patients. Nephrol Dial Transplant. 2017;32(suppl_2):ii53–ii59. doi: 10.1093/ndt/gfx012. [DOI] [PubMed] [Google Scholar]
- 23.Oyama M.A., Shaw P.A., Ellenberg S.S. Considerations for analysis of time-to-event outcomes subject to competing risks in veterinary clinical studies. J Vet Cardiol. 2018;20(3):143–153. doi: 10.1016/j.jvc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Stegherr R., Beyersmann J., Jehl V., et al. Survival Analysis for adverse Events With Varying Follow-Up Times (SAVVY): rationale and statistical concept of a meta-analytic study. Biometr J. 2021;63(3):650–670. doi: 10.1002/bimj.201900347. [DOI] [PubMed] [Google Scholar]
- 25.Robins J.M., Finkelstein D.M. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 26.Willems S., Schat A., van Noorden M.S., Fiocco M. Correcting for dependent censoring in routine outcome monitoring data by applying the inverse probability censoring weighted estimator. Stat Methods Med Res. 2018;27(2):323–335. doi: 10.1177/0962280216628900. [DOI] [PubMed] [Google Scholar]
- 27.Grafféo N., Latouche A., Le Tourneau C., Chevret S. ipcwswitch: An R package for inverse probability of censoring weighting with an application to switches in clinical trials. Comput Biol Med. 2019;111 doi: 10.1016/j.compbiomed.2019.103339. [DOI] [PubMed] [Google Scholar]
- 28.Hsu C.H., Taylor J.M. Nonparametric comparison of two survival functions with dependent censoring via nonparametric multiple imputation. Stat Med. 2009;28(3):462–475. doi: 10.1002/sim.3480. [DOI] [PubMed] [Google Scholar]
- 29.Jackson D., White I.R., Seaman S., Evans H., Baisley K., Carpenter J. Relaxing the independent censoring assumption in the Cox proportional hazards model using multiple imputation. Stat Med. 2014;33(27):4681–4694. doi: 10.1002/sim.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geskus R.B. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67(1):39–49. doi: 10.1111/j.1541-0420.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 31.Hudgens M.G., Li C., Fine J.P. Parametric likelihood inference for interval censored competing risks data. Biometrics. 2014;70(1):1–9. doi: 10.1111/biom.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James N.D., de Bono J.S., Spears M.R., et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iacovelli R., Ciccarese C., Bria E., et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645–e653. doi: 10.1016/j.clgc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Fizazi K., Tran N., Fein L., et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/s1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 35.Shore N.D., Saad F., Cookson M.S., et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382(23):2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 36.Geskus R.B. In: Handbook of Statistics. Srinivasa Rao A.S.R., Rao C.R., editors. Elsevier; New York: 2020. Chapter 5: competing risks: aims and methods; pp. 249–287. [Google Scholar]
- 37.Hernán M.A. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aalen O.O., Cook R.J., Røysland K. Does Cox analysis of a randomized survival study yield a causal treatment effect? Lifetime Data Anal. 2015;21(4):579–593. doi: 10.1007/s10985-015-9335-y. [DOI] [PubMed] [Google Scholar]
- 39.Stensrud M.J., Aalen J.M., Aalen O.O., Valberg M. Limitations of hazard ratios in clinical trials. Eur Heart J. 2019;40(17):1378–1383. doi: 10.1093/eurheartj/ehy770. [DOI] [PubMed] [Google Scholar]
- 40.Stensrud M.J., Young J.G., Didelez V., Robins J.M., Hernán M.A. Separable effects for causal inference in the presence of competing events. J Am Stat Assoc. 2022;117(537):175–183. doi: 10.1080/01621459.2020.1765783. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.