Abstract
Successful establishment of Yersinia infections requires the type III machinery, a protein transporter that injects virulence factors (Yops) into macrophages. It is reported here that the Yersinia type III pathway responds to environmental signals by transporting proteins to distinct locations. Yersinia enterocolitica cells sense an increase in extracellular amino acids (glutamate, glutamine, aspartate, and asparagine) that results in the activation of the type III pathway. Another signal, provided by serum proteins such as albumin, triggers the secretion of YopD into the extracellular medium. The third signal, a decrease in calcium concentration, appears to be provided by host cells and causes Y. enterocolitica to transport YopE and presumably other virulence factors across the eukaryotic plasma membrane. Mutations in several genes encoding regulatory molecules (lcrG, lcrH, tyeA, yopD, yopN, yscM1, and yscM2) bypass the signal requirement of the type III pathway. Together these results suggest that yersiniae may have evolved distinct secretion reactions in response to environmental signals.
Type III secretion systems represent a common pathogenic tool of many gram-negative bacteria (20). Upon bacterial contact with host cells, type III machines deliver protein toxins across the eukaryotic plasma membrane. Once inside the cell, these proteins manipulate host signal transduction pathways, resulting in rearrangement of the cytoskeleton and in induction of an apoptotic program (45, 56). By injecting distinct sets of toxins into the host, each pathogen appears to customize the versatile type III device to suit their unique pathogenic strategy (20). Recent work suggests a temporal and/or spatial regulation of the type III secretion machinery during experimental infections caused by Salmonella, enteropathogenic Escherichia coli (EPEC), and Yersinia species. Salmonella species inject two effector proteins, SopE and SptP, which display opposing functions in the host cell (16). SopE, a GTP exchange factor for CDC42 and Rac1, first induces membrane ruffling and facilitates bacterial entry into the host cell (21). Injection of SptP, a GTPase activating factor of CDC42 and Rac1, is thought to restore the cytoskeletal rearrangements once the bacterium is inside the host cell (19). Simultaneous microinjection of both effector proteins prevents cytoskeletal rearrangements, suggesting that a temporal regulation of the type III machine is required to allow the two virulence factors to function independently of each other in the host cell cytoplasm (19).
Pathogenic Yersinia species, i.e., Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica, enter their hosts via the intestines or by flea-borne contamination of skin lesions (11). Once host barriers are breached, Yersinia colonizes lymphoid tissues or multiplies in blood to cause septicemic infections (6). The astonishing pathogenic potential of Yersinia is executed by a type III secretion machinery that transports 14 Yops (Yersinia outer proteins) (11). When bacteria establish contact with macrophages, the type III machinery injects some of these virulence factors (YopEHMOPT, effector Yops) into the cytosol of target cells (44), thereby abolishing the phagocytic process and inducing apoptosis of macrophages (35–37).
In addition to injecting toxins into host cells, the type III machine can deliver substrate proteins to other locations. One example of spatial separation of type III transport is EspA of EPEC. EspA is secreted by the type III machinery and assembled into bacterial surface appendages (27). The formation of EspA filaments is required for the delivery of EspB and Tir into host cells, indicating that the type III machinery must distinguish secretion substrates to allow for differential delivery during infection (26, 27). Yersinia species also deliver type III secretion substrates to distinct locations. During infection of tissue culture cells, yersiniae secrete YopB, YopD, and YopR into the extracellular milieu (30), whereas effector Yops (YopE, YopH, YopM, YopO, YopP, and YopT) are injected into the cytosol of host cells (5, 45). Thus, as reported for EPEC, yersiniae may also distinguish between different sets of secretion substrates.
What are the environmental signals that activate secretion via the Yersinia type III pathway? Early work established a requirement for calcium to allow growth of pathogenic yersiniae at 37°C on artificial medium (28). Later studies showed that the chelation of calcium together with a temperature shift to 37°C triggers the type III secretion of Yops (18, 33, 49). The calcium concentration of extracellular host fluids (1.2 mM) is well above the threshold required for induction of type III machines (12). Nevertheless, Yersinia type III machines are activated during infection, a phenomenon that is referred to as the calcium paradox (12). It has been proposed that phagocytosis exposes bacteria to the low-calcium environment of the endocytic pathway, activating type III gene expression and providing a mechanism for Yersinia target cell selection (40). This possibility now seems somewhat remote, as the type III injection of effector Yops is believed to be catalyzed by extracellular Yersinia (45).
The observation that bacterial contact with host cells causes an increase in virulence gene expression provides a clue to understanding calcium signaling during type III secretion (38). It is thought that receptors on the bacterial surface may interact with specific ligands on the surface of host cells (11). This interaction may activate the type III machinery and remove the YopN-mediated block of the type III pathway (11, 17). However, Yersinia surface receptors that are necessary for type III injection or the corresponding ligand on the surface of eukaryotic cells have thus far not been identified. It is reported here that host cells may generate the calcium signal that leads to activation of the type III pathway. A mechanism is proposed whereby yersiniae measure the intracellular calcium of target cells. Further, activation of the Yersinia type III pathway is shown to require two other signals, i.e., serum amino acids (glutamate, glutamine, aspartate, or asparagine) and proteins such as albumin. Yersiniae respond to temperature shift and glutamate with the expression and assembly of the type III machinery. Albumin triggers Yersinia type III secretion of YopD into the extracellular milieu. Host cell contact transforms the type III machinery into an injection device that transports YopE across the plasma membrane into the eukaryotic cytosol. Mutations in Yersinia regulatory genes (lcrG, lcrH, tyeA, yopD, yopN, yscM1, and yscM2) disrupt this type III secretion program and bypass the requirement for specific signals.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Y. enterocolitica O:9 strain W22703 (13) and isogenic variants with frameshift mutations in Δ(yopB) (30), Δ(yopD) (32), Δ(yopN) (29), Δ(yopQ) (2), and Δ(yopR) (30) or deletions in Δ(lcrG) (15), Δ(lcrV) (31), Δ(lcrH) (D. M. Anderson, K. Ramamurthi, C. Tam, and O. Schneewind, submitted for publication), Δ(sycH) (7), Δ(tyeA) (9), and Δ(yscM1/yscM2) (7) have been previously described. Plasmids pDA35 and pDA37 have also been previously described (1).
Type III secretion in DMEM.
Yersinia strains were grown overnight at 26°C with shaking. Cultures were diluted 1:20 into 30 ml of fresh Luria broth and incubated at 26°C for 2 h. Bacteria were collected by centrifugation and were washed, and 2.5 × 108 CFU was added to 10 ml of Dulbecco's modified Eagle medium (DMEM) in 75-cm2 tissue culture flasks. Cultures were incubated for 3 h at 37°C and 5% CO2. Bacteria were scraped off the flasks, and the culture was transferred to a 15-ml conical tube. Yersinia strains were sedimented by centrifugation at 12,000 × g. Five milliliters of the culture supernatant was removed and precipitated with methanol-chloroform. The remaining supernatant was discarded. The Yersinia sediment was suspended in 1 ml of phosphate-buffered saline (PBS), and a 0.5-ml aliquot was precipitated with methanol-chloroform. Proteins were collected at the interphase after centrifugation at 12,000 × g. After removal of the aqueous phase, proteins were washed with methanol and sedimented by centrifugation at 12,000 × g. The precipitate was dried, suspended in sample buffer, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting. Immunoreactive species were quantified as chemiluminescent signals on X-ray film using laser densitometry scanning.
Digitonin fractionation.
Overnight cultures of Yersinia strains were diluted 1:20 into 30 ml of fresh Luria broth and grown for 2 h at 26°C with shaking. Bacteria were sedimented at 8,000 × g for 10 min and suspended in PBS. HeLa cells were grown to 80% confluency in 75-cm2 tissue culture flasks with DMEM and 10% fetal bovine serum (FBS). Prior to infection, cells were washed twice with PBS, covered with 10 ml of DMEM, and warmed to 37°C for 30 min. Aliquots of HeLa cells were counted, and each flask was infected with yersiniae at a multiplicity of infection of 10 and incubated for 3 h at 37°C with 5% CO2. Culture media were removed and centrifuged at 32,000 × g for 15 min to separate soluble proteins from nonadherent bacteria in the sediment. HeLa cells as well as adherent bacteria were scraped off the flasks into 10 ml of 1% digitonin in PBS and placed on a rotary shaker for 20 min. Samples were centrifuged at 32,500 × g for 15 min. A 7-ml aliquot was withdrawn and precipitated with methanol-chloroform, while the remaining supernatant was discarded. The sediment was suspended in 10 ml of PBS, and a 7-ml aliquot was precipitated with methanol-chloroform. Protein precipitates were solubilized in sample buffer, separated on SDS-PAGE, and analyzed by immunoblotting with specific antiserum. Immunoreactive species were quantified as chemiluminescent signals on X-ray film using laser densitometry scanning.
Preparation of environmental signals.
Unless otherwise indicated, glutamate was prepared as a stock of 68 mM monosodium glutamate (Fisher BP378) in water, sterile filtered, and diluted 1:5,000 to yield a final concentration of 135 μM in DMEM. Unless otherwise indicated, purified bovine albumin (Sigma A-7638) was prepared as a stock of 1.5 mM albumin in PBS (10% wt/vol), sterile filtered, and diluted 1:1,000 to yield a final concentration of 1.5 μM in DMEM. DMEM without (GIBCO-BRL 21068-028) or with (GIBCO-BRL 11960-051) 1.8 mM calcium was used in these experiments. Heat-inactivated FBS (Gemini Bioproducts) was stored in frozen aliquots, melted, and diluted to a final concentration of 0.2% (vol/vol). For protease digestion, proteinase K (100 μl of a 100-mg/ml solution in PBS [Roche]) was incubated with 10 ml of FBS for 6 days at 4°C. Protease digestion was quenched by the addition of phenylmethylsulfonyl fluoride to 0.1 mM. Ten milliliters of FBS was dialyzed once against 100 ml of PBS for 12 h at 4°C using a membrane with a permeability barrier of 8 kDa (Spectrum Laboratories 132650); the dialysate was collected and used at a 2% (vol/vol) dilution in DMEM. After two additional dialysis steps, i.e., dialysis against 500 ml of PBS each for 12 h at 4°C using the same membrane, dialyzed FBS was recovered and tested for induction of type III secretion at a dilution of 0.2% in DMEM. Cohn's serum fractions were purchased from Sigma (Cohn I [fibrinogen], F-4753; Cohn II/III [γ-globulin], G-5009; Cohn IV-1, G-8512; Cohn IV-4, G-8637; Cohn V [albumin]; and A-8022). Cohn fractions were prepared as a 10% stock (wt/vol) in PBS, sterile filtered, and diluted 1:1,000 to yield a final concentration of 0.01% in DMEM.
RESULTS
Yersinia infection of HeLa cells in the presence and absence of calcium.
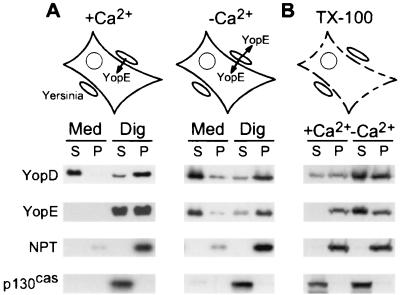
We wondered whether the specificity of effector Yop injection is controlled by Yersinia recognition of a host signal, presumably a change in calcium concentration. Activation of the Yersinia type III machinery was measured by infecting and fractionating tissue cultures by the digitonin technique (29). Briefly, HeLa cell cultures were infected with Y. enterocolitica W22703 for 3 h. The growth medium was removed and centrifuged to separate nonadherent bacteria from the extracellular medium. HeLa cells and yersiniae adherent to the culture flasks were treated with digitonin, a detergent that disrupts the eukaryotic plasma membrane but not the bacterial envelope (29). Samples were centrifuged to separate the HeLa cell cytosol from the bacterial sediment. During HeLa cell infection in DMEM tissue culture medium with 1.8 mM calcium, Y. enterocolitica W22703 secreted YopD into the extracellular medium and injected YopE into the cytosol (Fig. 1A). Small amounts of YopD were also observed in the supernatant of digitonin-extracted HeLa cells. As a control for proper fractionation, p130cas, a protein in the HeLa cell cytosol, was solubilized by digitonin extraction of tissue culture cells. In contrast, Yersinia Npt (neomycin phosphotransferase, encoded by npt carried by pDA37) was not solubilized by digitonin treatment and sedimented with the bacteria after centrifugation of tissue culture extracts. When HeLa cells were infected in DMEM lacking calcium, Yersinia strains secreted both YopD and YopE into the extracellular medium (Fig. 1A). These results suggest that the specific injection of YopE via the Yersinia type III pathway requires extracellular calcium.
FIG. 1.
A calcium signal induces Yersinia type III targeting of YopE but not type III secretion of YopD. (A) HeLa cells in DMEM with (+Ca2+) or without (−Ca2+) 1.8 mM calcium were infected with Y. enterocolitica W22703. The culture medium (Med) was decanted and centrifuged, separating the extracellular medium (S, supernatant) from the bacterial sediment (P, pellet). HeLa cells were extracted with digitonin (Dig). After centrifugation, proteins in the cytosol of HeLa cells (S, supernatant) were separated from the bacterial sediment (P, pellet). Samples were analyzed by immunoblotting. p130cas is located in the cytosol of HeLa cells, whereas Npt is located in the bacterial cytoplasm. (B) HeLa cells in DMEM with or without 1.8 mM calcium were lysed with Triton X-100 (TX-100). Cell lysates were infected with Yersinia and centrifuged to separate the extracellular medium (S) from the bacterial sediment (P).
Can the cytosol of HeLa cells generate the calcium signal for the injection of YopE? To address this possibility, HeLa cells were lysed with 0.1% Triton X-100 in DMEM with 1.8 mM calcium, an extraction that also disrupts the calcium gradient across the plasma membrane. As a control, detergent treatment solubilized p130cas, indicating that the plasma membrane, and therefore the calcium gradient across the plasma membrane, had been disrupted (Fig. 1B). Infection of the HeLa cell lysate with yersiniae in the presence of 1.8 mM calcium failed to activate YopE secretion. In contrast, secretion of YopD still occurred, albeit at a greatly reduced rate. To examine whether the removal of extracellular calcium can substitute for the calcium signal that is generated by intact HeLa cells, tissue cultures were lysed with 0.1% Triton X-100 in DMEM without calcium. Infection of lysate without calcium resulted in Yersinia type III transport of YopD and YopE (Fig. 1B). Further, the removal of calcium ions from HeLa cell extracts led to an increase in the concentration of YopD and YopE, suggesting that yop gene expression may also be stimulated under these conditions (see Table 1 and Table 2 for the regulatory effects of low-calcium signaling). Together these results suggest that intact HeLa cells may generate the calcium signal that activates the transport of YopE. Results similar to those described above were obtained when HeLa cells were lysed by sonication, shearing, or digitonin treatment (data not shown), suggesting that the method of HeLa cell lysis does not affect the calcium signaling in this experiment.
TABLE 1.
Expression of YopE by Y. enterocolitica W22703(pDA35) grown in DMEM at 37°C
| Signal(s) added | Amt. of YopE in culture with (+) or without (−) Ca2+a
|
|||
|---|---|---|---|---|
| −Ca2+b | −Ca2+c | +Ca2+b | +Ca2+c | |
| 79 (20) | 55 (7) | 74 (26) | 65 (18) | |
| Glutamate | 140 (105) | 99 (12) | 98 (54) | 78 (16) |
| Albumin | 62 (28) | 80 (32) | 64 (8) | 75 (15) |
| Glutamate and albumin | 150 (14) | 237 (72) | 94 (17) | 123 (3) |
| FBS | 150 (14) | 267 (151) | 100d | 100d |
Numbers indicate the percent amount of YopE present in Y. enterocolitica cultures. Numbers in parentheses represent the standard deviation of the measurement.
Data were generated by trichloroacetic acid precipitating cultures, separating proteins on SDS-PAGE, and immunoblotting with YopE-specific antiserum. Numbers represent averages of three independent experiments. Data were multiplied with a correction factor for the bacterial density, which was determined by measuring the optical density at 660 nm of Yersinia cultures.
Data were generated as described above and multiplied with a correction factor for an immunoreactive signal obtained with chloramphenicol acetyltransferase-specific antibodies. cat is carried by pDA35 and is not part of the yop regulon.
The average amount of YopE in Y. enterocolitica W22703 cultures grown in DMEM supplemented with FBS was arbitrarily assigned a value of 100%.
TABLE 2.
Expression of YopD by Y. enterocolitica W22703(pDA35) grown in DMEM at 37°C
| Signal(s) added | Amt. of YopD in culture with (+) or without (−) Ca2+a
|
|||
|---|---|---|---|---|
| −Ca2+b | −Ca2+c | +Ca2+b | +Ca2+c | |
| 77 (4) | 55 (12) | 73 (4) | 68 (32) | |
| Glutamate | 174 (127) | 124 (23) | 118 (59) | 99 (27) |
| Albumin | 65 (31) | 85 (39) | 54 (14) | 63 (22) |
| Glutamate and albumin | 181 (34) | 289 (111) | 97 (16) | 128 (5) |
| FBS | 113 (29) | 241 (122) | 100d | 100d |
Numbers indicate the percent amount of YopD present in Y. enterocolitica cultures. Numbers in parentheses represent the standard deviation of the measurement.
Data were generated by trichloroacetic acid precipitating cultures, separating proteins on SDS-PAGE, and immunoblotting with YopD-specific antiserum. Numbers represent averages of three independent experiments. Data were multiplied with a correction factor for the bacterial density, which was determined by measuring the optical density at 660 nm of Yersinia cultures.
Data were generated as described above and multiplied with a correction factor for an immunoreactive signal obtained with chloramphenicol acetyltransferase-specific antibodies.
The average amount of YopD in Y. enterocolitica W22703 cultures grown in DMEM supplemented with FBS was arbitrarily assigned a value of 100%.
Calcium concentration required for Yersinia type III secretion.
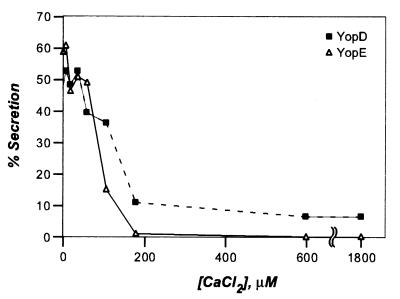
To determine the critical concentration of calcium required to induce type III secretion, Y. enterocolitica W22703(pDA37) was grown in DMEM supplemented with 0.2% FBS (final concentration) and various amounts of calcium chloride (CaCl2) at 37°C for 3 h. Variations in the concentration of calcium were achieved by mixing DMEM without calcium and DMEM with calcium. The concentration of calcium was verified by measuring these ions directly with a calcium electrode. Yersinia cultures were centrifuged, and the extracellular medium was separated with the supernatant from the sedimented bacteria in the pellet). Proteins in both fractions were precipitated with chloroform-methanol and analyzed by SDS-PAGE and immunoblotting with specific antisera. In Fig. 2 the proportion of secreted Yop (percent amount of the total) is plotted against the extracellular calcium chloride concentration. We observed a reduction in the amount of secreted YopE when the extracellular calcium chloride concentration reached 100 μM. At 200 μM calcium chloride, all Y. enterocolitica secretion of YopE was blocked. These observations corroborate the previous finding that Y. pestis activates the expression of a yopK (yopQ)-lacZ transcriptional fusion when the calcium chloride concentration drops below 200 μM (40). It should be noted, however, that these studies measured reporter protein expression but not type III secretion. These findings are consistent with the observed expression of YopD and YopE shown in Fig. 1B. It therefore appears that the low-calcium signal may regulate yop gene expression as well as the activity of the type III machinery. In contrast to the block in secretion of YopE, a low-level secretion of YopD (about 10%) was observed even at a calcium chloride concentration of 1,800 μM. Nevertheless, reducing the calcium chloride concentration below 100 μM caused Y. enterocolitica to increase the secretion of YopD (40 to 50%). During Yersinia infection of HeLa tissue cultures or HeLa lysates, comparatively more YopD was secreted in the presence of 1.2 mM calcium. The reason for this discrepancy is unknown. In summary, the critical concentration of calcium required to activate Yersinia type III transport of YopE is ≤80 μM (Fig. 2), a level that is well above the intracellular calcium concentration of HeLa cells (100 nM) (3). We propose that Yersinia strains may have evolved a mechanism to measure calcium ions across the eukaryotic plasma membrane. Presumably, this mechanism requires contact between Yersinia cells and eukaryotic cells. Hoiczyk and Blobel reported that the YscF-containing needle complexes of Y. enterocolitica are responsible for the formation of a type III targeting conduit between bacteria and eukaryotic cells (23). These results may have identified the device with which yersiniae sense calcium. As Yersinia needle complexes are assembled prior to host cell contact (23), the insertion of this structure into eukaryotic cells may allow bacteria to measure the change in environmental calcium concentration and thereby activate the type III pathway.
FIG. 2.
Yersinia type III secretion of YopE and YopD is regulated by extracellular calcium ions. Y. enterocolitica W22703(pDA37) was grown in DMEM supplemented with 0.2% fetal bovine serum for 3 h. Cultures were centrifuged, and the extracellular medium was separated with the supernatant from the bacterial sediment. Protein in both fractions was precipitated with methanol-chloroform, separated on SDS-PAGE, and analyzed by immunoblotting with antiserum raised against purified YopD or YopE.
Glutamate activates the Yersinia type III machinery.
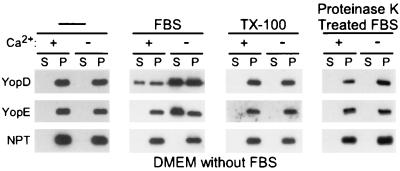
When bacteria are grown in brain heart infusion or other complex media and incubated at 37°C in the absence of calcium ions, Yersinia type III secretion is induced (34). Growth of Y. enterocolitica in DMEM tissue culture medium at 37°C without HeLa cells failed to activate the type III transport of YopD and YopE, even in the absence of calcium ions (Fig. 3). This was a surprising result, as the removal of calcium and the incubation at 37°C were hitherto thought to be sufficient as signals for the activation of the Yersinia type III pathway (14). Secreted extracellular Yops are known to aggregate and to precipitate on the surface of glass and plastic containers (34). We wondered whether the lack of YopD and YopE secretion observed in Fig. 3 is caused by the aggregation of Yops. Yersinia cultures were grown in the presence of 0.1% Triton X-100, a condition that is known to prevent the aggregation of some Yops (1). However, growth of Yersinia in the presence of 0.1% Triton X-100 did not result in the appearance of soluble, extracellular YopD or YopE. It seems that YopD and YopE sediment with the bacteria when yersiniae are grown in DMEM without FBS, because the type III secretion machinery is not activated under these conditions.
FIG. 3.
FBS activates the Yersinia type III pathway. Y. enterocolitica W22703(pDA37) was grown in DMEM without FBS and with or without calcium. The medium was supplemented with 0.2% FBS or 0.1% Triton X-100 (TX-100) as indicated. Type III secretion was measured as described in the legend to Fig. 2. S, supernatant; P, pellet.
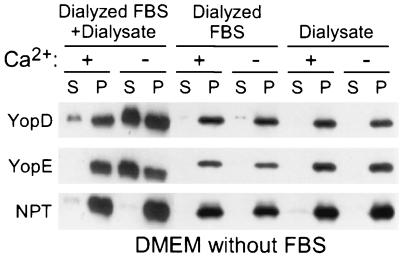
Supplementation of DMEM with 0.2% FBS restored the ability of Y. enterocolitica W22703 to secrete YopD in the presence of calcium as well as YopD and YopE in the absence of calcium (Fig. 3). These data suggest that Yersinia requires serum signals to activate the type III pathway. Similar observations have been made for Salmonella and Shigella species, suggesting that many gram-negative pathogens activate the type III pathway in response to serum components (32, 55). FBS was dialyzed to remove small molecules (≤8 kDa). This treatment removed the signal(s) required to induce Yersinia type III secretion (Fig. 4). However, when equal parts of dialyzed FBS and dialysate were mixed, the inducing signal(s) could be reconstituted, as yersiniae were once again activated for YopD and YopE secretion in the absence of calcium as well as for YopD secretion in the presence of calcium (Fig. 4). DMEM contains most of the small molecules (amino acids, carbohydrates, ions, and salts) that are present in serum or extracellular fluids. However, 6 amino acids are absent (glutamine, glutamate, asparagine, aspartate, proline, and alanine). The addition of glutamate, glutamine, aspartate, or asparagine to DMEM without calcium each activated Yersinia type III secretion of YopD and YopE, whereas addition of proline or alanine had no effect (Fig. 5A) (data not shown). The critical concentration of amino acids required for activation of type III secretion is ≥70 μM (data not shown), a level that is below that of the amino acid concentration in serum (300 μM) (48). Glutamate proved to be the most potent inducer of the type III pathway and was used at a final concentration of 135 μM for all subsequent experiments. Glutamate and aspartate chelate calcium ions; the concentration at which these amino acids are added to DMEM does not lead to significant changes in the concentration of extracellular calcium ions that could explain the observed activation of the type III pathway (data not shown).
FIG. 4.
Dialysis of FBS abolishes the Yersinia type III inducing activity. Y. enterocolitica W22703(pDA37) was grown in DMEM with or without calcium supplemented with 0.2% dialyzed FBS, the dialysate of this reaction mixture, or a mixture of both. Type III secretion was measured as described in the legend to Fig. 2. S, supernatant; P, pellet.
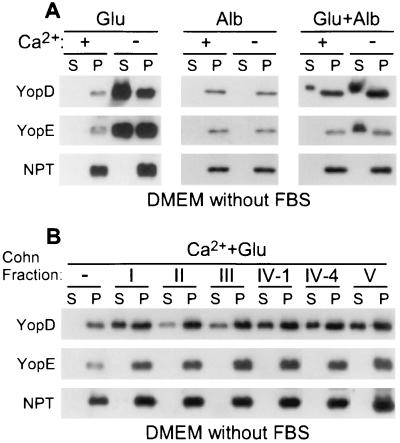
FIG. 5.
Glutamate and albumin activate the Yersinia type III pathway. (A) Y. enterocolitica W22703(pDA37) was grown in DMEM with or without calcium supplemented with glutamate (Glu) and albumin (Alb), alone or in combination, as indicated. Type III secretion was measured as described in the legend to Fig. 2. (B) Y. enterocolitica W22703(pDA37) was grown in DMEM with calcium supplemented with glutamate and a Cohn serum fraction (I, II, III, IV-1, IV-4, or V), as indicated. S, supernatant; P, pellet.
Albumin activates the type III secretion of YopD.
Digestion of FBS with proteinase K abolished the signal(s) that activates type III secretion (Fig. 3). Addition of glutamate alone to DMEM with calcium also did not activate Yersinia type III secretion of YopD (Fig. 5). It therefore appears that glutamate, as well as glutamine, aspartate, and asparagine, induces the low-calcium transport of Yops; however, additional serum components seem required to activate type III secretion of YopD in the presence of calcium. When combined with glutamate, dialyzed FBS stimulated the secretion of YopD in DMEM with calcium (data not shown). To test whether serum proteins (>8 kDa) activate type III secretion, we analyzed various Cohn fractions (I through V; Sigma Pharmaceuticals), generated by ethanol precipitation of bovine serum (10). When added to DMEM with glutamate and calcium, each of the five Cohn fractions (100 μg of protein/ml) activated type III secretion of YopD (Fig. 5B). This result suggests that many different serum proteins are capable of activating the type III secretion pathway. To test this prediction, albumin, a serum protein that is abundantly present in the blood of many mammals (42), was examined (42). When added to DMEM with glutamate and calcium, bovine albumin induced Yersinia type III secretion of YopD (Fig. 5A). The concentration of albumin required for induction of type III secretion (≥0.3 μM; data not shown) was below that present in human serum (600 μM) (42). Extracellular YopD and YopE, secreted by yersiniae growing in the presence of albumin, migrated more slowly on SDS-PAGE than intrabacterial YopD and YopE. This was explained as an overloading of the SDS-PAGE with albumin, a condition that may artifactually alter the mobility of faster-migrating proteins such as YopD and YopE. The molecular properties of serum proteins that activate type III secretion are not known. It is conceivable that peptide sequences, folded structure, or posttranslational modifications function as a signal.
The addition of glutamine, albumin, FBS, or calcium to DMEM appears to affect the expression of yopE or yopD. If so, the regulation of YopD and YopE secretion that is reported here could, in fact, be caused by a regulatory effect of gene expression. To address this possibility, Y. enterocolitica W22703(pDA35) cultures were grown in DMEM with or without various inducers. The cell density was measured, and proteins in the culture were precipitated with methanol-chloroform. After separation of proteins on SDS-PAGE and immunoblotting, YopE and YopD were quantified by chemiluminescent measurement of immunoreactive signals and their expression levels were calculated (Table 1 and Table 2). The addition of glutamate and albumin and the addition of FBS as well as the omission of calcium resulted in a weak stimulation of yopD and yopE expression. The addition of glutamine and albumin alone had little or no effect. These data suggest that glutamine, albumin, and calcium cause weak regulatory effects on yopD and yopE expression in addition to regulating the secretion of YopD and YopE polypeptides by the Yersinia type III machinery.
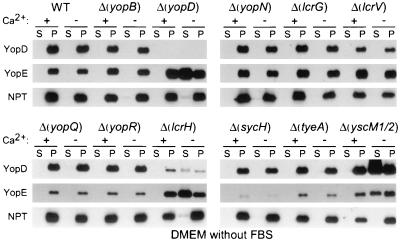
Mutations in yopD, lcrH, or yscM1/M2 bypass the glutamate signal.
The type III pathway is modulated by glutamate, albumin, and calcium signals. Presumably, each of these signals modulates the activity of the secretion machinery by a distinct mechanism, requiring the function of regulatory genes. If so, mutations in regulatory genes should abolish the signal requirement of the type III machinery. This prediction was first tested for glutamate. Previous work identified genes that regulate the Yersinia type III pathway (4, 8, 14, 25, 41, 43, 47, 54). We wondered whether mutations that result in a loss-of-function phenotype for yopBDNQR, lcrGVH, sycH, tyeA, or yscM1/yscM2 abolish the glutamate requirement of the Yersinia type III pathway. Yersiniae were grown in DMEM without calcium, glutamate, albumin, or FBS. Mutations in yopD, lcrH (sycD), or yscM1/yscM2 allowed yersiniae to secrete YopE, whereas all other mutations had no effect (Fig. 6). lcrH and yscM1/yscM2 mutants failed to secrete YopD and YopE in DMEM with calcium, suggesting that these mutations bypass the requirement for glutamate but not for albumin or calcium (Fig. 6; data for albumin not shown). Furthermore, yopD mutants do not secrete YopB in DMEM with calcium (data not shown). YopD, LcrH, and YscM1/YscM2 (LcrQ) seem to control Yop secretion at the same step and appear to function as a switch that allows secretion of type III substrates in response to glutamate. The lcrH mutant strain contained reduced amounts of YopD; this can be explained by the lacking chaperone function of LcrH (SycD), a small cytoplasmic protein that binds to YopD (50). The sycH mutant Yersinia strain synthesized very little, if any, YopD and YopE (Fig. 6). SycH acts as a secretion chaperone and functional inhibitor for YscM1/YscM2, the negative regulators of the Y. enterocolitica type III pathway (7, 47). Thus, the sycH mutant phenotype can be explained as YscM1/YscM2-mediated inhibition of yopE expression (7).
FIG. 6.
Knockout mutations in yopD, lcrH, and yscM1/M2 bypass the glutamate requirement for Yersinia type III secretion. Y. enterocolitica strains were grown in DMEM without FBS and without glutamate. Type III secretion was measured as described in the legend to Fig. 2. S, supernatant; P, pellet.
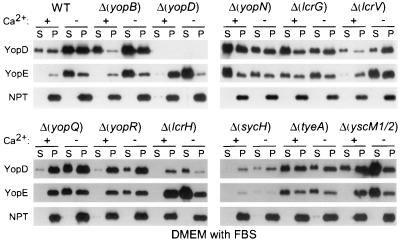
Mutations in yopN, lcrG, and tyeA bypass the calcium signal.
When bacteria are grown in rich medium, mutations in yopN (lcrE) abrogate the calcium signal requirement of Yersinia and activate the type III machinery in the presence of 1.8 mM calcium (54). YopN has been proposed to act as both a calcium sensor on the surface of Yersinia and as a stop valve that occludes the type III machinery (11, 17). Type III export of YopN itself is regulated in a complex manner. The secretion chaperones YscB and SycN bind to the N-terminal portion of YopN (14). TyeA, a small polypeptide that interacts with the C-terminal end of YopN (24), seems to act as a negative regulator of YopN secretion (9 and L. W. Cheng, O. Kay, and O. Schneewind, submitted for publication). Yersinia carrying deletion mutations in sycN, tyeA, and yscB display a calcium-blind phenotype similar to that of yopN mutants (14, 25). We asked whether mutations in yopBDNQR, lcrGVH, sycH, tyeA, or yscM1/yscM2 abolish the calcium regulation of the Yersinia type III pathway. Yersinia strains were grown in DMEM with calcium and 0.2% FBS. Mutations in yopN, lcrG, or tyeA allowed yersiniae to secrete YopE in the presence of calcium, whereas all other mutations had no effect (Fig. 7). These data suggest that DMEM supplemented with 0.2% FBS resembles other rich laboratory media and allows induction of type III secretion upon removal of calcium ions. Mutations in lcrG are known to cause a calcium-blind phenotype in mutant yersiniae which is corroborated by the data shown in Fig. 7 (15, 46).
FIG. 7.
Knockout mutations in yopN, lcrG, and tyeA bypass the calcium requirement for Yersinia type III secretion. Y. enterocolitica strains were grown in DMEM with FBS. Type III secretion was measured as described in the legend to Fig. 2. S, supernatant; P, pellet.
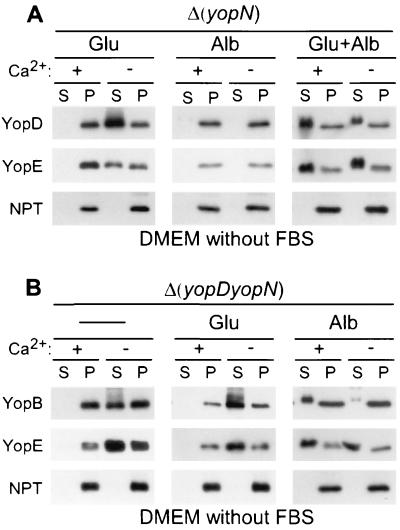
yopN mutant yersiniae failed to secrete Yops when grown in DMEM without glutamate and calcium (Fig. 8A). Apparently, these mutants are still capable of regulating type III secretion and may require glutamate for induction. Although glutamate allowed secretion in the absence of calcium, yopN mutants failed to activate the type III pathway in DMEM with glutamate and calcium (Fig. 8A). Only the addition of glutamate and albumin triggered yopN mutants to secrete Yops in the presence of calcium (Fig. 8A). Several conclusions can be drawn from these experiments. (i) Full induction of the type III pathway requires all three signals: glutamate, albumin, and calcium. (ii) The three signals activate Yersinia in a sequential manner: glutamate and then albumin and calcium. (iii) When yersiniae are grown in DMEM with glutamate, calcium signaling of yopN mutants occurs in a manner similar to that of wild-type strains. This result suggests that YopN cannot function as a calcium sensor for the type III pathway. (iv) As suggested previously, mutations in yopN, tyeA, sycN, and yscB bypass the calcium signal requirement of the Yersinia type III pathway (17, 24), suggesting that these genes provide a stop valve function for the type III machinery (12). (v) The stop valve is not implemented unless yersiniae receive glutamate and albumin signals.
FIG. 8.
A program of Yersinia type III secretion events is triggered by specific host signals. Y. enterocolitica strains were grown in DMEM without FBS and supplemented with glutamate (Glu) and/or albumin (Alb) as indicated. Type III secretion was measured as described in the legend to Fig. 2. (A) Knockout mutations in yopN; (B) knockout mutations in yopD and yopN. S, supernatant; P, pellet.
Sequential activation of the type III machinery.
The type III pathway appears to be activated by a sequence of signals: glutamate and then albumin and calcium. If so, albumin alone should activate the type III pathway of Δ(yopDN) yersiniae, as these mutants are predicted to bypass the Yersinia requirement for glutamate and calcium. This was tested, and Δ(yopDN) yersiniae secreted YopB and YopE in DMEM with albumin and calcium (Fig. 8B). The Δ(yopDN) mutant strain appears to be capable of sensing calcium, as YopB and YopE secretion in DMEM without albumin occurred only in the absence but not in the presence of calcium (Fig. 8B). Further, the Δ(yopDN) mutant secretes YopE in DMEM alone (Fig. 8), similar to yopD but unlike yopN mutant yersiniae (Fig. 6). These data suggest that the regulatory function of yopD is epistatic over that of yopN.
Type III secretion occurs prior to target cell contact.
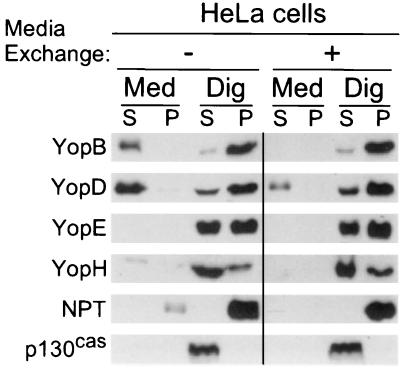
Glutamate and albumin induce Yersinia type III secretion of YopD. Bacterial sensing of these signals should occur immediately upon host entry and before Yersinia encounters immune cells. In contrast, type III injection of YopE requires bacterial contact with the target cell and occurs later during infection. We wondered whether the secretion machinery is modified by target cell contact. In this scenario one would expect nonadherent yersiniae to catalyze type III secretion of YopB, YopD, and YopR. Target cell contact should transform the machinery into an injection device that promotes type III targeting but not secretion. To test this, HeLa cells were infected with Y. enterocolitica W22703. After 1 h of infection, more than 90% of the yersiniae adhered to tissue culture cells (data not shown). The medium and nonadherent bacteria were removed, and cells were washed and replenished with fresh DMEM. After further incubation for 2 h, the cultures were fractionated by the digitonin technique and analyzed by immunoblotting. Removal of nonadherent yersiniae seemed to reduce the secretion of YopB and YopD, whereas targeting of YopE and YopH was not affected (Fig. 9). These results support the hypothesis that Y. enterocolitica transport of Yops may occur in a sequential manner, beginning with type III secretion of YopBD and followed by type III targeting of YopEH into host cells.
FIG. 9.
Type III secretion occurs prior to target cell contact. HeLa cell cultures were infected with Y. enterocolitica W22703(pDA37). After 1 h of infection, the medium of HeLa cells was removed to separate nonadherent bacteria from Yersinia that had established target cell contact. The samples were washed twice with PBS, and HeLa cells with adherent yersiniae were covered with fresh DMEM and incubated for 2 h. Samples were fractionated and analyzed by immunoblotting. S, supernatant; P, pellet; Med, culture medium; Dig, digitonin.
DISCUSSION
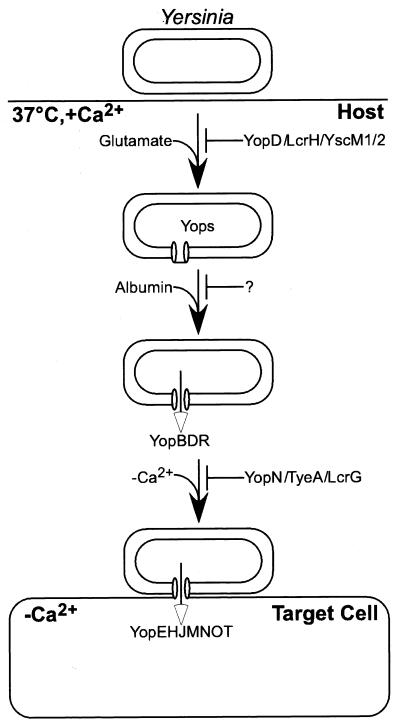
A model is proposed whereby yersiniae enter the host and respond to changes in temperature as well as to glutamate, glutamine, asparagine, or aspartate with the assembly of the type III machinery (Fig. 10). Albumin and other serum proteins activate Yersinia to secrete YopB, YopD, and YopR into the extracellular medium. Contact with immune cells transforms the type III machinery into an injection device. The molecular nature of calcium sensing is unknown. Electron microscopy experiments revealed the insertion of type III needles into the plasma membrane of tissue culture cells (23). Such needle insertion may not only serve Yop transport across membranes but could also provide for the measurement of calcium ions. Once stimulated by a low-calcium signal, Yersinia transports effector proteins (YopE, YopH, YopM, YopO, YopP, and YopT) across the plasma membrane. Several regulatory genes control this cascade of type III transport reactions. lcrF (virF) encodes a transcription factor that activates the expression of type III genes when the temperature is shifted to 37°C (22, 51, 53). YopD, LcrH, and YscM1/YscM2 (LcrQ) prevent activation of the type III pathway in the absence of glutamate. Recent work suggests that YopD, LcrH, and YscM1/YscM2 (LcrQ in Y. pestis and Y. pseudotuberculosis) function in posttranscriptional regulation of yop gene expression (D. M. Anderson et al., submitted). As albumin is required to activate type III secretion, other (hitherto unidentified) genes may prevent transport of YopB, YopD, and YopR. YopN, TyeA, SycN, YscB, and LcrG block the type III injection step until Yersinia receives the calcium signal.
FIG. 10.
Host signals trigger a Yersinia type III secretion program. The drawing depicts a model for events that occur during Yersinia infection. Entry of pathogenic yersiniae into their host causes changes in temperature and extracellular glutamate that induce expression of the type III machinery and yop genes. Serum albumin triggers secretion of YopBDR into the extracellular milieu. Contact with host cells halts Yersinia secretion and transforms the type III machinery into an injection device. The cytosol of host cells generates the calcium signal that activates injection of effector Yops. Repressor molecules control the activity of type III secretion machines. YopD, LcrH, and YscM are thought to regulate the translation of secretion substrates, whereas YopN, TyeA, SycN, and YscB function as stop valves for the type III secretion machinery. Mutations in repressor genes bypass the requirement of the type III machinery for activation by specific signals. Mutations in regulatory genes that bypass the albumin signal are still unknown (?).
This model predicts that mutations in regulatory genes may disrupt the Yersinia type III pathway at discrete steps. yopN, lcrG, sycN, yscB, and tyeA mutations cause premature secretion of effector Yops into the extracellular medium (Los phenotype, for loss of type III targeting specificity) (15, 29, 45), consistent with their presumed repressor function for the type III pathway. yscM1/yscM2 mutant yersiniae transport massive amounts of effector Yops into target cells, whereas sycH mutants (YscM1/YscM2 chaperone) inject only small amounts of YopEMOPT (7). These data suggest that the gene products of yscM1/yscM2 and sycH alter the amplitude of Yop expression without affecting the transport reactions of effector Yops. In contrast, knockout mutations in yopD abolish type III targeting without affecting secretion (Not phenotype, for no type III targeting) (30, 45). The Not phenotype cannot be explained by the repression of the type III pathway alone but must be accounted for by the specific function of secreted proteins (45). Thus, in order to advance through the type III program, yersiniae not only receive glutamate, albumin, and calcium signals but also catalyze specific transport reactions, such as the secretion of YopD or the injection of YopN and YscM1/YscM2.
What is the role of serum signals during Yersinia infection of a mammalian host? It is presumed here that during host infection, glutamate and albumin provide signals that lead to the activation of the Yersinia type III pathway. Further, it is presumed that Yersinia receives the serum signals during the infection of tissue culture cells. Experimental verification of these assumptions are hindered by the fact that both animal hosts of Yersinia infection as well as cultured human cells or tissues release glutamate, glutamine, and proteins into the extracellular medium (serum) (39, 42). Thus, simple omission of glutamate and serum proteins from the media of tissue culture cells cannot be achieved in an experimental protocol that measures type III secretion and targeting during a prolonged time interval (3 h). It should be emphasized again that many serum proteins in addition to albumin can activate the secretion of YopBDR in the presence of calcium. It appears, therefore, that yersiniae recognize a common property of serum proteins and respond to this signal with type III secretion.
ACKNOWLEDGMENTS
V.T.L. acknowledges support by a fellowship from the National Science Foundation and the Warsaw Family Fellowship. S.K.M. acknowledges support by the Predoctoral Training Program in Genetic Mechanisms at the University of California—Los Angeles (GM07104). This work was supported by U.S. Public Health Service grant AI42797 from the National Institutes of Health-National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch.
REFERENCES
- 1.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Schneewind O. Yersinia enterocolitica type III secretion: an mRNA signal that couples translation and secretion of YopQ. Mol Microbiol. 1999;31:1139–1148. doi: 10.1046/j.1365-2958.1999.01254.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrero M J, Montero M, Alvarez J. Dynamics of [Ca2+] in the endoplasmic reticulum and cytoplasm of intact HeLa cells. J Biol Chem. 1997;272:27694–27699. doi: 10.1074/jbc.272.44.27694. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann T, Hakansson S, Forsberg A, Norlander L, Macellaro A, Backman A, Bolin I, Wolf-Watz H. Analysis of V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of lcrH and lcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Burrows T W, Bacon G A. The basis of virulence in Pasteurella pestis: antigen determining virulence. Br J Exp Pathol. 1956;37:481–493. [PMC free article] [PubMed] [Google Scholar]
- 7.Cambronne E D, Cheng L W, Schneewind O. LcrQ/YscM1, regulators of the Yersinia yop virulon, are injected into host cells by a chaperone dependent mechanism. Mol Microbiol. 2000;37:263–273. doi: 10.1046/j.1365-2958.2000.01974.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L W, Schneewind O. Type III machines of pathogenic Yersinia deliver the goods. Trends Microbiol. 2000;8:214–220. doi: 10.1016/s0966-842x(99)01665-0. [DOI] [PubMed] [Google Scholar]
- 9.Cheng L W, Schneewind O. Yersinia enterocolitica TyeA, an intracellular regulator of the type III machinery, is required for the specific targeting of YopE, YopH, YopM, and YopN into the cytosol of eukaryotic cells. J Bacteriol. 2000;182:3183–3190. doi: 10.1128/jb.182.11.3183-3190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn E J, Strong L E, Hughes W L, Jr, Mulford D J, Ashworth J N, Melin M, Taylor H L. Preparation and properties of serum and plasma proteins. IV. A system for the separation into fractions of the protein and lipoprotein components of biological tissues and fluids. J Am Chem Soc. 1946;68:459–475. doi: 10.1021/ja01207a034. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis G R. The Yersinia deadly kiss. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis G R, Boland A, Boyd A P, Geuijen C, Iriarte M, Neyt C, Sory M-P, Stainier I. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis G R, Colson C. Restriction of DNA in Yersinia enterocolitica detected by the recipient ability for a derepressed R factor from Escherichia coli. J Gen Microbiol. 1975;87:285–291. doi: 10.1099/00221287-87-2-285. [DOI] [PubMed] [Google Scholar]
- 14.Day J B, Plano G V. A complex composed of SycN and YscB functions as a specific chaperone for YopN in Yersinia pestis. Mol Microbiol. 1998;30:777–789. doi: 10.1046/j.1365-2958.1998.01110.x. [DOI] [PubMed] [Google Scholar]
- 15.DeBord, K., V. T. Lee, and O. Schneewind. On the role of LcrG and LcrV during the type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 16.Donnenberg M S. Salmonella strikes a balance. Nature. 1999;401:218–219. doi: 10.1038/45690. [DOI] [PubMed] [Google Scholar]
- 17.Forsberg A, Viitanen A-M, Skunik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg A, Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-pIB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988;2:121–133. doi: 10.1111/j.1365-2958.1988.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 19.Fu Y, Galan J E. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- 20.Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1333. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 21.Hardt W D, Chen L M, Schuebel K E, Bustelo X R, Galan J E. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell. 1998;93:815–826. doi: 10.1016/s0092-8674(00)81442-7. [DOI] [PubMed] [Google Scholar]
- 22.Hoe N P, Minion F C, Goguen J D. Temperature sensing in Yersinia pestis: regulation of yopE transcription by lcrF. J Bacteriol. 1992;174:4275–4286. doi: 10.1128/jb.174.13.4275-4286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoiczyk E, Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc Natl Acad Sci USA. 2001;98:4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson M W, Day J B, Plano G V. YscB of Yersinia pestis functions as a specific chaperone for YopN. J Bacteriol. 1998;180:4912–4921. doi: 10.1128/jb.180.18.4912-4921.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 27.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kupferberg L L, Higuchi K. Role of calcium ions in the stimulation of growth of virulent strains of Pasteurella pestis. J Bacteriol. 1958;76:120–121. doi: 10.1128/jb.76.1.120-121.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee V T, Anderson D M, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee V T, Tam C, Schneewind O. Yersinia enterocolitica type III secretion. LcrV, a substrate for type III secretion, is required for toxin-targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- 32.Menard R, Sansonetti P, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michiels T, Cornelis G. Nucleotides sequence and transcription analysis of yop51 from Yersinia enterocolitica W22703. Microb Pathog. 1988;5:449–459. doi: 10.1016/0882-4010(88)90006-x. [DOI] [PubMed] [Google Scholar]
- 34.Michiels T, Wattiau P, Brasseur R, Ruysschaert J-M, Cornelis G. Secretion of Yop proteins by yersiniae. Infect Immun. 1990;58:2840–2849. doi: 10.1128/iai.58.9.2840-2849.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbouch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persson C, Carballeira N, Wolf-Watz H, Fallman M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesion. EMBO J. 1997;16:2307–2318. doi: 10.1093/emboj/16.9.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petterson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 39.Piva T J, McEvoy-Bowe E. Oxidation of glutamine in HeLa cells: role and control of truncated TCA cycles in tumour mitochondria. J Cell Biochem. 1998;68:213–225. doi: 10.1002/(sici)1097-4644(19980201)68:2<213::aid-jcb8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 40.Pollack C, Straley S C, Klempner M S. Probing the phagolysosomal environment of human macrophages with a Ca2+-responsive operon fusion in Yersinia pestis. Nature. 1986;322:834–836. doi: 10.1038/322834a0. [DOI] [PubMed] [Google Scholar]
- 41.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Putnam F W, editor. The plasma proteins. 2nd ed. New York, N.Y: Academic Press; 1975. [Google Scholar]
- 43.Rimpilainen M, Forsberg A, Wolf-Watz H. A novel protein, LcrQ, involved in the low-calcium response of Yersinia pseudotuberculosis shows extensive homology to YopH. J Bacteriol. 1992;174:3355–3363. doi: 10.1128/jb.174.10.3355-3363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosqvist R, Forsberg A, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stainier I, Iriarte M, Cornelis G R. YscM1 and YscM2, two Yersinia enterocolitica proteins causing downregulation of yop transcription. Mol Microbiol. 1997;26:833–843. doi: 10.1046/j.1365-2958.1997.6281995.x. [DOI] [PubMed] [Google Scholar]
- 48.Stein W H, Moore S. The free amino acids of human blood plasma. J Biol Chem. 1954;211:915–926. [PubMed] [Google Scholar]
- 49.Straley S C, Plano G V, Skrzypek E, Haddix P L, Fields K A. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol Microbiol. 1993;8:1005–1010. doi: 10.1111/j.1365-2958.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 50.Wattiau P, Bernier B, Deslee P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wattiau P, Cornelis G R. Identification of DNA sequences recognized by VirF, the transcriptional activator of the Yersinia yop regulon. J Bacteriol. 1994;176:3878–3884. doi: 10.1128/jb.176.13.3878-3884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams A W, Straley S C. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J Bacteriol. 1998;180:350–358. doi: 10.1128/jb.180.2.350-358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yother J, Chamness T W, Goguen J D. Temperature controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986;165:443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yother J, Goguen J D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985;164:704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zierler M K, Galan J E. Contact with cultured epithelial cells stimulates secretion of Salmonella typhimurium invasion protein InvJ. Infect Immun. 1995;63:4024–4028. doi: 10.1128/iai.63.10.4024-4028.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]