Abstract
Background
Wearing a face mask is a primary public health method to reduce SARS-CoV-2 transmission.
Methods
We performed a nested case-control analysis within the North Carolina COVID-19 Community Research Partnership (NC-CCRP) of adults who completed daily surveillance surveys, April 2020 - February 2022. We assessed the association between self-reported mask wearing behavior during nonhousehold interactions and COVID-19 infection during 3 pandemic periods using conditional logistic regression models of risk of infection that were adjusted for demographics, vaccination status, and recent known exposure to COVID-19.
Results
Among 3,901 cases and 27,813 date-matched controls, there was a significant interaction between mask use and time period (P < .001). Prior to July 2021, the odds of a reported infection were 66% higher (aOR = 1.66, 95% CI = 1.43-1.91) among participants reporting ≥1 day not wearing a mask compared to those who reported no days (1,592 cases, 11,717 controls). During the Delta-predominant period, the results were similar (aOR = 1.53, 95% CI = 1.23-1.89; 659 cases, 4,649 controls). This association was attenuated during the Omicron-predominant period, where odds of an infection was 16% higher (aOR = 1.16, 95% CI = 1.03-1.32; 1,563 cases, 10,960 controls).
Conclusions
While the effect of not wearing a mask remains significant, during the Omicron-predominant period we observed a decrease in the association between self-reported mask wearing and risk of SARS-CoV-2 infection.
Key words: Mask use, Epidemiology, Infection prevention and control
Wearing a facemask has been a primary public health method to reduce SARS-CoV-2 transmission throughout the pandemic.1, 2, 3 Facemasks, along with other non-pharmaceutical interventions, may remain important even with increasing vaccination coverage,4 and as protection from vaccination and prior infection wane over time. There is evidence to suggest that wearing masks protects the wearer by reducing inhalation of droplets, protects others around a potentially infectious individual by reducing emission of droplets by the wearer, and lastly provides community protection with widespread mask wearing.5 As SARS-CoV-2 becomes endemic, vaccinated individuals may consider layers of protection. Information about the effectiveness of different methods to minimize risk for the wearer is particularly important in light of the highly transmissible Omicron variant and its subvariants.
Most prior studies, however, have focused on community spread rather than protection for the individual mask-wearer or are limited to a specific setting or set of conditions. There remains limited information on the benefit of real-world mask use to the individual mask-wearer and specifically in the era after the introduction of vaccines. How well typical mask-wearing behavior conveyed protection during each variant wave, including the most recent Omicron wave, has yet to be evaluated. Using prospectively collected data from the North Carolina COVID-19 Community Research Partnership (CCRP), we assessed the association between self-reported mask use when interacting with others outside the household and risk of COVID-19 infection during three periods of the pandemic.
Methods
Study participants
The NC-CCRP6 is a prospective, multi-site cohort COVID- 19 syndromic surveillance study of a convenience sample of adults enrolled at 6 NC healthcare systems from April 2020 through June 2021 (http://www.covid19communitystudy.org/). Participants were recruited through patient portals, public websites, and community outreach. Data were collected via a secure, Health Insurance Portability and Accountability Act of 1996 (HIPAA)-compliant, online platform through March 2022. All participants provided informed consent, and Institutional Review Board (IRB) approval was provided by the Wake Forest School of Medicine IRB. Study sites included: Atrium Health (Charlotte, NC, USA), Campbell University School of Osteopathic Medicine (Lillington, NC, USA), New Hanover Regional Medical Center (Wilmington, NC, USA), Vidant Health (Greenville, NC, USA), Wake Forest Baptist Health (Winston-Salem, NC, USA), and WakeMed Health and Hospitals (Raleigh, NC, USA). The study is registered with ClinicalTrials.gov, NCT04342884.
Data collection
For this report, eligible participants were ≥18 years, completed daily surveys, did not participate in a vaccine trial, and did not self-report prior COVID-19 infection at enrollment (Fig 1 ). Demographic data including age, sex, race/ethnicity, county of residence and healthcare worker status were self-reported at the time of enrollment. We classified counties of residence as urban, rural, or suburban based on population density estimates. Education level, household size and comorbidities were collected on a subset of participants using a supplemental survey. For a subset of participants, EHR data were available. Daily online surveys asked about COVID-19-like symptoms (fever, chills, cough, shortness of breath, fatigue, muscle pain, headache, loss of taste/smell, sore throat, congestion/runny nose, nausea/vomiting), test results, receipt of COVID-19 vaccination, and risk behavior including mask wearing and contact with a person with COVID-19. Not wearing a mask was defined as responding “no” at least once in the ten days preceding the match date to the daily survey question, “In the last 24 hours, have you worn a face mask or face covering every time you interacted with others (not in your household) within a distance of less than 6 feet?” Known exposure to COVID-19 was reported daily as “Yes” or “No” to the question: “Did you have close contact with someone who has tested positive for COVID-19 infection?” Vaccination status was ascertained using a combination of data from the daily survey and an updated set of daily survey questions that began in September 2021, and included date, dose, and product of any COVID-19 vaccine received, along with EHR data when available.
Fig 1.
Inclusion flow.
Analysis design
To summarize mask use over time, we categorized mask use as “yes” or “no” based on whether there were any days not wearing a mask “when interacting with others outside the household” in the 10 days preceding the match date. Similarly, we categorized recent exposure as “yes” or “no” based on whether a participant had one or more close contacts with a person with COVID-19 during the ten days before the match date.
We performed a nested case-control analysis to compare self-reported cases to controls who never self-reported a positive test for SARS-CoV-2 infection. To account for differences in the risk of infection over calendar time and to allow for stratification by variant-predominant period, we matched up to 10 controls to each case on calendar time of first self-reported positive test. Case participants self-reported at least positive viral test during study follow-up. We used an optimal matching algorithm maximizing the number of case-participants with at least one self-reported positive test included in the analysis and number of matching controls per case.7 The number of controls per case ranged from 1 to 10 (median = 7). Conditional logistic regression models of COVID-19 infection were adjusted for enrollment site, age group, race/ethnicity, county population density (urban/suburban/rural), healthcare worker occupation, vaccination status, and recent known exposure to COVID-19 (within 10 days preceding match date). After assessing whether there was an interaction between variant predominant period and mask use, we evaluated 3 periods during the pandemic: Pre-Delta (July 1 2020-June 30, 2021), Delta (July 1, 2021-November 30, 2021), and Omicron (December 1, 2021-February 28, 2022) predominance. Analyses were performed using R (V.4.0.3, R Foundation for Statistical Computing).
Results
Of 3,901 cases and 27,813 date-matched controls, participants were majority female (71.5% of cases; 68.6% of controls) and non-Hispanic white (89.1% of cases; 87.5% of controls). Healthcare worker occupation was common (35.4% of cases; 26.8% of controls) and most participants were ≥14 days after a second mRNA vaccine dose (53.7% of cases; 55.7% of controls) (Table 1 ). The pre-Delta predominant period accounted for 42.5% of cases, compared to 17.1% during the Delta-predominant period and 40.5% during the Omicron-predominant period.
Table 1.
Characteristics of participants
| Case participants (Self-reported positive test) | Control participants (Never self-reported positive test) | P-value* | |
|---|---|---|---|
| N | 3,901 | 27,813 | |
| Site of enrollment | <.001 | ||
| Atrium | 1,106 (28.4%) | 6,101 (21.9%) | |
| Campbell | 44 (1.1%) | 553 (2.0%) | |
| New Hanover | 38 (1.0%) | 617 (2.2%) | |
| Vidant | 48 (1.2%) | 1,034 (3.7%) | |
| Wake Forest | 2,356 (60.4%) | 17,089 (61.4%) | |
| Wake Med | 309 (7.9%) | 2,419 (8.7%) | |
| Period of Match | .424 | ||
| Pre-Delta (July2020-June2021) | 1,641 (42.5%) | 12,062 (43.6%) | |
| Delta (July-Nov 2021) | 659 (17.1%) | 4,649 (16.8%) | |
| Omicron (Dec 2021-March 2022) | 1,563 (40.5%) | 10,960 (39.6%) | |
| Age in years | <.001 | ||
| 18-39 y | 1,067 (27.4%) | 8,025 (28.9%) | |
| 40-54 y | 1,463 (37.5%) | 8,072 (29.0%) | |
| 55-64 y | 844 (21.6%) | 5,735 (20.6%) | |
| 65 y† | 527 (13.5%) | 5,981 (21.5%) | |
| Sex | <.001 | ||
| Female | 2,788 (71.5%) | 19,074 (68.6%) | |
| Male | 1,113 (28.5%) | 8,739 (31.4%) | |
| Race and ethnicity | <.001 | ||
| Hispanic | 114 (2.9%) | 661 (2.4%) | |
| NH Black | 169 (4.3%) | 1,505 (5.4%) | |
| NH White | 3,477 (89.1%) | 24,336 (87.5%) | |
| Other | 141 (3.6%) | 1,311 (4.7%) | |
| County classification | <.001 | ||
| Rural | 907 (23.3%) | 5,758 (20.7%) | |
| Suburban | 884 (22.7%) | 5,363 (19.3%) | |
| Urban | 2,110 (54.1%) | 16,692 (60.0%) | |
| Healthcare worker occupation | <.001 | ||
| No | 2,521 (64.6%) | 20,365 (73.2%) | |
| Yes | 1,380 (35.4%) | 7,448 (26.8%) | |
| Vaccination status§ | <.001 | ||
| mRNA ≥14 days after 2nd dose | 2,096 (53.7%) | 15,490 (55.7%) | |
| mRNA ≥1 dose | 158 (4.1%) | 1,358 (4.9%) | |
| Unvaccinated | 1,647 (42.2%) | 10,965 (39.4%) | |
| Education level‡ | <.001 | ||
| College degree | 2,469 (71.7%) | 14,371 (75.3%) | |
| No College degree | 974 (28.3%) | 4,713 (24.7%) | |
| Household size‡ | <.001 | ||
| >2 people | 1,576 (60.0%) | 6,358 (46.2%) | |
| 1-2 people | 1,050 (40.0%) | 7,401 (53.8%) | |
| Any comorbidity§ | .009 | ||
| Yes | 979 (25.8%) | 7,009 (27.9%) | |
| No | 2,811 (74.2%) | 18,132 (72.1%) |
P-values for Pearson's Chi-squared test comparing cases to controls.
Vaccination status at the time of index/match date. We defined vaccination using self-report and categorized participants into 3 categories: ≥14 days after receiving second dose of either the Pfizer BioNTech BNT162b2 or Moderna mRNA-1273 vaccine, receiving at least one dose of either mRNA vaccine or unvaccinated. Participants who reported receiving a non-mRNA vaccine or had an undetermined vaccine status were excluded.
Available on a subset of participants who completed a supplemental survey (N = 3,443 case-participants and N=19,084 control-participants).
Defined as any comorbidity self-reported or in EHR (autoimmune disease, cancer, CVD, diabetes, immunocompromised, liver disease, renal disease, obesity, pulmonary disease, other disease, neurologic disease, substance use disorder, mental health condition). Available on a subset of participants who completed a supplemental survey or had linked EHR data available (N = 3,790 case-participants and N = 25,141 control-participants).
The survey response rate in the ten days preceding the match date was 73.1% for cases and 65.3% for controls. Reporting not wearing a facemask when interacting with others outside the household was more prominent among cases prior to the index date (date self-reported positive test) and less prominent afterwards. Approximately 42.5% of cases and 36.3% of controls responded at least once that they did not wear a mask in the 10 days preceding the match date (Table 2 ). Over half of the cases (54.1%) reported a known exposure to someone who recently tested positive for COVID-19 in the ten days preceding the index date compared to only 9.3% of controls.
Table 2.
Self-reported behavioral characteristics, 10 days preceding match date
| Case participants (self-reported positive test) | Control participants (never self-reported positive test) | P-value* | |
|---|---|---|---|
| N | 3,901 | 27,813 | |
| Daily survey response rate | 0.731±0.297 | 0.653±0.327 | <.001 |
| Any days interaction without mask† | <.001 | ||
| No (no days) | 2,244 (57.5%) | 17,706 (63.7%) | |
| Yes (at least 1 day) | 1,657 (42.5%) | 10,107 (36.3%) | |
| Proportion days interaction without mask† | 0.18±0.28 | 0.16±0.28 | <.001 |
| Any known exposure | <.001 | ||
| No | 1,789 (45.9%) | 25,227 (90.7%) | |
| Yes | 2,112 (54.1%) | 2,586 (9.3%) | |
| Any days reporting at least one symptoms | <.001 | ||
| No | 703 (18.0%) | 20,174 (72.7%) | |
| Yes | 3,192 (82.0%) | 7,593 (27.3%) | |
| Any days reporting 3+ symptoms | <.001 | ||
| No | 1,642 (42.1%) | 25,747 (92.6%) | |
| Yes | 2,259 (57.9%) | 2,066 (7.4%) | |
| Sought treatment | <.001 | ||
| No | 3,086 (79.1%) | 26,780 (96.3%) | |
| Yes | 815 (20.9%) | 1,033 (3.7%) |
P-values for Pearson's Chi-squared test for categorical variables and Welch's 2-sample t-test for continuous variables.
Participants who responded “No” to the question: “In the last 24 hours, have you worn a face mask or face covering every time you interacted with others (not in your household) within a distance of less than 6 feet?.”
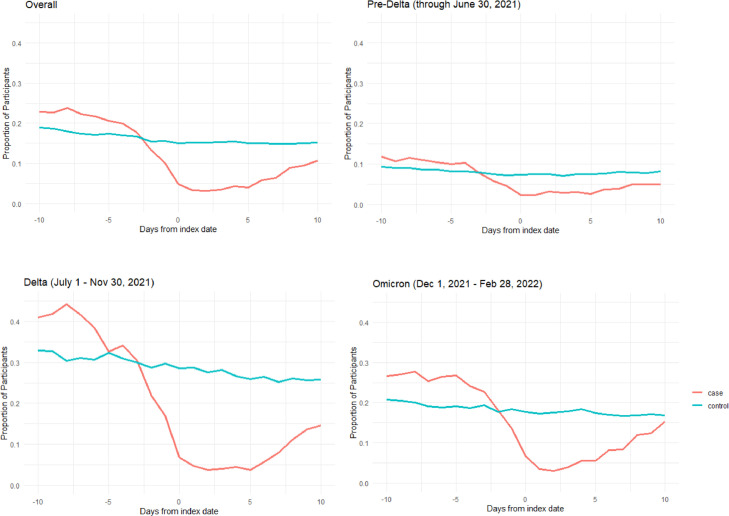
Mask wearing behavior varied by period in the pandemic (Fig 2 ). During the Pre-Delta period, reports of not wearing a mask was the lowest of the 3 periods for both cases and controls; whereas, during the Delta-predominant period, reports of not wearing a mask was comparatively highest among both cases and controls compared to behavior reported in the other 2 periods. The relative relationship between cases and controls was consistent over time with a greater proportion of cases reporting days not wearing a mask prior to the match date and fewer cases reporting days not wearing a mask immediately following their self-reported infection.
Fig 2.
Proportion of participants responding to daily survey that they did not wear a face mask or face covering every time they interacted with others preceding and following match date (Day 0), by variant-predominant period.
The association between mask use and SARS-CoV-2 infection varied by period (P-interaction <.001). During the pre-Delta predominant period, the odds of SARS-CoV-2 was 66% higher (adjusted odds ratio [aOR] = 1.66, 95% CI = 1.43-1.91) among participants reporting at least one day not wearing a mask in the 10 days preceding the index date compared to those who reported no days (Table 3 ). During the Delta-predominant period, the results were similar (aOR = 1.53, 95% CI = 1.23-1.89). This association was attenuated but remained significant during the Omicron-predominant period, where the odds of SARS-CoV-2 was 16% higher (aOR = 1.16, 95% CI = 1.03-1.32) for those reporting at least one day not wearing a mask compared with those who reported consistent mask wearing.
Table 3.
Effect of self-reporting any days not wearing a mask when interacting with others outside household on self-reported SARS-CoV-2 infection by period
| Time | N cases | N controls | aOR | CI lower | CI upper |
|---|---|---|---|---|---|
| Pre-Delta | 1,592 | 11,717 | 1.655 | 1.433 | 1.911 |
| Delta | 659 | 4,649 | 1.527 | 1.232 | 1.892 |
| Omicron | 1,563 | 10,960 | 1.162 | 1.025 | 1.317 |
Conditional logistic regression models (date-matched) adjusted for site, age group, race/ethnicity, urban/rural, vaccination status, healthcare worker occupation, known exposure in the 10 days preceding the match date.
Discussion
In this community-based observational study, we found not wearing a mask was associated with increased odds of SARS-CoV-2 infection after adjusting for demographics and recent known exposure. While the effect of not wearing a mask on disease transmission during non-household interactions remained significant during the entire study period, we observed a decrease in this association during the Omicron-predominant period. This variation was seen against decreasing overall mask use across the 3 periods.
Our findings during the pre-Delta and Delta predominant periods are consistent with previous studies suggesting that wearing a mask consistently is related to lowered odds of infection.2 Population-based studies,8, 9, 10, 11, 12, 13, 14, 15 specific settings16, 17, 18 and studies outside the U.S. have all demonstrated the benefits of mask-wearing.19, 20, 21, 22 These studies focused largely on the impact of masking mandates and of universal masking on community rates of COVID-19 and were unable to draw conclusions about benefit to the individual mask wearer.8, 9, 10, 11, 12, 13, 14, 15 Additionally, all of these studies took place in 2020 prior to the Delta or Omicron predominant periods and prior to availability of vaccines in the U.S. Only a few studies focused on individual benefit of mask-wearing include data collected after the widespread availability of vaccines and only extend through December 2021.2 , 23 To the best of our knowledge, this is the first study to extend into the Omicron predominant period and compare the protectiveness of masks.
This study includes prospectively collected data over 2 years on a large number of participants, reducing recall bias following infection, with data available throughout much of the pandemic (through February 28, 2022). However, our findings may be limited by selection and reporting bias and may not be generalizable to other geographic regions. Other limitations include the use of self-report to determine mask use and a lack of nuance in the masking question to allow for improper use, type of mask, duration of use, and frequency and duration of interactions.
Our results suggest decreased protection for the wearer from masks during the Omicron-predominant wave. Findings may also be explained by more frequent exposures outside of one's household, increased transmissibility of the Omicron variant, high rates of vaccination and increasing population immunity, and a decrease in mask wearing as guidance for vaccinated individuals evolved over time.24 , 25 Within the NC-CCRP study population, while masking continued to be one of the valuable tools to decrease risk of COVID-19 infection, the association between consistent mask-wearing behavior and COVID-19 infection decreased during the Omicron phase of the pandemic.
Ethics approval and consent to participate
Activity was determined to meet the definition of research [45 CFR 46.102(l)] involving human subjects [45 CFR 46.102 (e)(1)] and Institutional Review Board (IRB) approval was provided by Wake Forest University.
Consent to participate
All participants in the COVID-19 Community Research Partnership provided written consent for participation.
Consent for publication
Not applicable. No identifying data from any individual person is contained in the manuscript.
Availability of data and materials
Results of the COVID-19 CRP are being disseminated on the study website (https://www.covid19communitystudy.org/) as well as in publications and presentations in medical journals and at scientific meetings. At end of the study, the databases will be made publicly available in a de-identified manner according to CDC and applicable U.S. Federal policies.
Acknowledgments
The COVID-19 Community Research Partnership gratefully acknowledges the commitment and dedication of the study participants. Programmatic, laboratory, and technical support was provided by Vysnova Partners, Inc., Javara, Inc., Oracle Corporation, LabCorp, Scanwell Health, and Neoteryx. This publication was supported by the Centers for Disease Control and Prevention (CDC) [contract #75D30120C08405] and the CARES Act of the U.S. Department of Health and Human Services (HHS) [Contract # NC DHHS GTS #49927]. Fifty percent of the current project was funded by the CDC/HHS award and fifty percent by the CARES Act/HHS award. The Partnership is listed in clinicaltrials.gov (NCT04342884). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, HHS, or the U.S. Government. A complete list of Study Sites, investigators, and staff can be found in the Appendix.
Footnotes
Funding/support: This publication was supported by the Centers for Disease Control and Prevention (CDC) [Contract #75D30120C08405] and the CARES (Coronavirus Aid, Relief, and Economic Security) Act of the U.S. Department of Health and Human Services (HHS) [Contract # NC DHHS GTS #49927].
Conflicts of interest: The authors declare that they have no relevant competing interests.
Author contributions: A.H.T. and S.L.E. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: A.H.T., S.L.E. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: A.H.T.
Authorship appendix: The COVID-19 Research Group (*site principal investigator)
Wake Forest School of Medicine: Thomas F Wierzba PhD, MPH, MS*, John Walton Sanders, MD, MPH, David Herrington, MD, MHS, Mark A. Espeland, PhD, MA, John Williamson, PharmD, Morgana Mongraw-Chaffin, PhD, MPH, Alain Bertoni, MD, MPH, Martha A. Alexander-Miller, PhD, Paola Castri, MD, PhD, Allison Mathews, PhD, MA, Iqra Munawar, MS, Austin Lyles Seals, MS, Brian Ostasiewski, Christine Ann Pittman Ballard, MPH, Metin Gurcan, PhD, MS, Alexander Ivanov, MD, Giselle Melendez Zapata, MD, Marlena Westcott, PhD, Karen Blinson, Laura Blinson, Mark Mistysyn, Donna Davis, Lynda Doomy, Perrin Henderson, MS, Alicia Jessup, Kimberly Lane, Beverly Levine, PhD, Jessica McCanless, MS, Sharon McDaniel, Kathryn Melius, MS, Christine O'Neill, Angelina Pack, RN, Ritu Rathee, RN, Scott Rushing, Jennifer Sheets, Sandra Soots, RN, Michele Wall, Samantha Wheeler, John White, Lisa Wilkerson, Rebekah Wilson, Kenneth Wilson, Deb Burcombe, Georgia Saylor, Megan Lunn, Karina Ordonez, Ashley O'Steen, MS, Leigh Wagner.
Atrium Health: Michael S. Runyon MD, MPH*, Lewis H. McCurdy MD*, Michael A. Gibbs, MD, Yhenneko J. Taylor, PhD, Lydia Calamari, MD, Hazel Tapp, PhD, Amina Ahmed, MD, Michael Brennan, DDS, Lindsay Munn, PhD RN, Keerti L. Dantuluri, MD, Timothy Hetherington, MS, Lauren C. Lu, Connell Dunn, Melanie Hogg, MS, CCRA, Andrea Price, Marina Leonidas, Melinda Manning, Whitney Rossman, MS, Frank X. Gohs, MS, Anna Harris, MPH, Jennifer S. Priem, PhD, MA, Pilar Tochiki, Nicole Wellinsky, Crystal Silva, Tom Ludden PhD, Jackeline Hernandez, MD, Kennisha Spencer, Laura McAlister.
MedStar Health Research Institute: William Weintraub MD*, Kristen Miller, DrPH, CPPS*, Chris Washington, Allison Moses, Sarahfaye Dolman, Julissa Zelaya-Portillo, John Erkus, Joseph Blumenthal, Ronald E. Romero Barrientos, Sonita Bennett, Shrenik Shah, Shrey Mathur, Christian Boxley, Paul Kolm, PhD, Ella Franklin, Naheed Ahmed, Moira Larsen.
Tulane: Richard Oberhelman MD*, Joseph Keating PhD*, Patricia Kissinger, PhD, John Schieffelin, MD, Joshua Yukich, PhD, Andrew Beron, MPH, Johanna Teigen, MPH.
University of Maryland School of Medicine: Karen Kotloff MD*, Wilbur H. Chen MD, MS*, DeAnna Friedman-Klabanoff, MD, Andrea A. Berry, MD, Helen Powell, PhD, Lynnee Roane, MS, RN, Reva Datar, MPH, Colleen Reilly.
University of Mississippi: Adolfo Correa MD, PhD*, Bhagyashri Navalkele, MD, Yuan-I Min, PhD, Alexandra Castillo, MPH, Lori Ward, PhD, MS, Robert P. Santos, MD, MSCS, Pramod Anugu, Yan Gao, MPH, Jason Green, Ramona Sandlin, RHIA, Donald Moore, MS, Lemichal Drake, Dorothy Horton, RN, Kendra L. Johnson, MPH, Michael Stover.
Wake Med Health and Hospitals: William H. Lagarde MD*, LaMonica Daniel, BSCR.
New Hanover: Patrick D. Maguire MD*, Charin L. Hanlon, MD, Lynette McFayden, MSN, CCRP, Isaura Rigo, MD, Kelli Hines, BS, Lindsay Smith, BA, Monique Harris, CCRP, Belinda Lissor, AAS, CCRP, Vivian Cook, MA, MPH, Maddy Eversole, BS, Terry Herrin, BS, Dennis Murphy, RN, Lauren Kinney, BS, Polly Diehl, MS, RHIA, Nicholas Abromitis, BS, Tina St. Pierre, BS, Bill Heckman, Denise Evans, Julian March, BA, Ben Whitlock, CPA, MSA, Wendy Moore, BS, AAS, Sarah Arthur, MSW, LCSW, Joseph Conway.
Vidant Health: Thomas R. Gallaher MD*, Mathew Johanson, MHA, CHFP, Sawyer Brown, MHA, Tina Dixon, MPA, Martha Reavis, Shakira Henderson, PhD, DNP, MS, MPH, Michael Zimmer, PhD, Danielle Oliver, Kasheta Jackson, DNP, RN, Monica Menon, MHA, Brandon Bishop, MHA, Rachel Roeth, MHA.
Campbell University School of Osteopathic Medicine: Robin King-Thiele DO*, Terri S. Hamrick PhD*, Abdalla Ihmeidan, MHA, Amy Hinkelman, PhD, Chika Okafor, MD (Cape Fear Valley Medical Center), Regina B. Bray Brown, MD, Amber Brewster, MD, Danius Bouyi, DO, Katrina Lamont, MD, Kazumi Yoshinaga, DO, (Harnett Health System), Poornima Vinod, MD, A. Suman Peela, MD, Giera Denbel, MD, Jason Lo, MD, Mariam Mayet-Khan, DO, Akash Mittal, DO, Reena Motwani, MD, Mohamed Raafat, MD (Southeastern Health System), Evan Schultz, DO, Aderson Joseph, MD, Aalok Parkeh, DO, Dhara Patel, MD, Babar Afridi, DO (Cumberland County Hospital System, Cape Fear Valley).
George Washington University Data Coordinating Center: Diane Uschner PhD*, Sharon L. Edelstein, ScM, Michele Santacatterina, PhD, Greg Strylewicz, PhD, Brian Burke, MS, Mihili Gunaratne, MPH, Meghan Turney, MA, Shirley Qin Zhou, MS, Ashley H Tjaden, MPH, Lida Fette, MS, Asare Buahin, Matthew Bott, Sophia Graziani, Ashvi Soni, MS.
George Washington University Mores Lab: Christopher Mores, PhD, Abigail Porzucek, MS.
Oracle Corporation: Rebecca Laborde, Pranav Acharya.
Sneez LLC: Lucy Guill, MBA, Danielle Lamphier, MBA, Anna Schaefer, MSM, William M. Satterwhite, JD, MD.
Vysnova Partners: Anne McKeague, PhD, Johnathan Ward, MS, Diana P. Naranjo, MA, Nana Darko, MPH, Kimberly Castellon, BS, Ryan Brink, MSCM, Haris Shehzad, MS, Derek Kuprianov, Douglas McGlasson, MBA, Devin Hayes, BS, Sierra Edwards, MS, Stephane Daphnis, MBA, Britnee Todd, BS.
Javara Inc: Atira Goodwin.
External Advisory Council: Ruth Berkelman, MD, Emory, Kimberly Hanson, MD, U of Utah, Scott Zeger, PhD, Johns Hopkins, Cavan Reilly, PhD, U. of Minnesota, Kathy Edwards, MD, Vanderbilt, Helene Gayle, MD MPH, Chicago Community Trust, Stephen Redd.
References
- 1.Brooks JT, Butler JC. Effectiveness of mask wearing to control community spread of SARS-CoV-2. JAMA. 2021;325:998–999. doi: 10.1001/jama.2021.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrejko KL, Pry JM, Myers JF, et al. Effectiveness of face mask or respirator use in indoor public settings for prevention of SARS-CoV-2 infection - california, february-december 2021. MMWR Morb Mortal Wkly Rep. 2022;71:212–216. doi: 10.15585/mmwr.mm7106e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talic S, Shah S, Wild H, et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. BMJ. 2021;375 doi: 10.1136/bmj-2021-068302. e068302-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartsch SM, O'Shea KJ, Chin KL, et al. Maintaining face mask use before and after achieving different COVID-19 vaccination coverage levels: a modelling study. Lancet Public Health. 2022;7:e356–e365. doi: 10.1016/S2468-2667(22)00040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Science brief: community use of masks to control the spread of SARS-CoV-2. Updated 2021. Accessed May 24, 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/masking-science-sars-cov2.html [PubMed]
- 6.Sanders J., The COVID-19 Community Research Partnership The COVID-19 community research partnership: objectives, study design, baseline recruitment, and retention. medRxiv. 2022 http://medrxiv.org/content/early/2022/02/10/2022.02.09.22270272.abstract 2022.02.09.22270272. Accessed May 24, 2022. [Google Scholar]
- 7.Mamouris P, Nassiri V, Molenberghs G, van den Akker M, van der Meer J, Vaes B. Fast and optimal algorithm for case-control matching using registry data: application on the antibiotics use of colorectal cancer patients. BMC Med Res Method. 2021;21:62. doi: 10.1186/s12874-021-01256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitze T, Kosfeld R, Rode J, Wälde K. Face masks considerably reduce COVID-19 cases in germany. Proc Natl Acad Sci U S A. 2020;117:32293–32301. doi: 10.1073/pnas.2015954117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dyke ME, Rogers TM, Pevzner E, et al. Trends in county-level COVID-19 incidence in counties with and without a mask mandate - kansas, june 1-august 23, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1777–1781. doi: 10.15585/mmwr.mm6947e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyu W, Wehby GL. Community use of face masks and COVID-19: evidence from A natural experiment of state mandates in the US. Health Aff (Millwood) 2020;39:1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- 11.Karaivanov A, Lu SE, Shigeoka H, Chen C, Pamplona S. Face masks, public policies and slowing the spread of COVID-19: evidence from canada. J Health Econ. 2021;78 doi: 10.1016/j.jhealeco.2021.102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324:703–704. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallaway MS, Rigler J, Robinson S, et al. Trends in COVID-19 incidence after implementation of mitigation measures - arizona, january 22-august 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1460–1463. doi: 10.15585/mmwr.mm6940e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader B, White LF, Burns MR, et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: a cross-sectional study. Lancet Digit Health. 2021;3:e148–e157. doi: 10.1016/S2589-7500(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamachari B, Morris A, Zastrow D, Dsida A, Harper B, Santella AJ. The role of mask mandates, stay at home orders and school closure in curbing the COVID-19 pandemic prior to vaccination. Am J Infect Control. 2021;49:1036–1042. doi: 10.1016/j.ajic.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in beijing, china. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrix MJ, Walde C, Findley K, Trotman R. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy - springfield, missouri, may 2020. MMWR Morb Mortal Wkly Rep. 2020;69:930–932. doi: 10.15585/mmwr.mm6928e2. [DOI] [PubMed] [Google Scholar]
- 18.Payne DC, Smith-Jeffcoat SE, Nowak G, et al. SARS-CoV-2 infections and serologic responses from a sample of U.S. navy service members - USS theodore roosevelt, april 2020. MMWR Morb Mortal Wkly Rep. 2020;69:714–721. doi: 10.15585/mmwr.mm6923e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doung-Ngern P, Suphanchaimat R, Panjangampatthana A, et al. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, thailand. Emerg Infect Dis. 2020;26:2607–2616. doi: 10.3201/eid2611.203003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in danish mask wearers : a randomized controlled trial. Ann Intern Med. 2021;174:335–343. doi: 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lio CF, Cheong HH, Lei CI, et al. Effectiveness of personal protective health behaviour against COVID-19. BMC Public Health. 2021;21:825–827. doi: 10.1186/s12889-021-10680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Gan Y, Zheng D, et al. Relationship between COVID-19 infection and risk perception, knowledge, attitude, and four nonpharmaceutical interventions during the late period of the COVID-19 epidemic in china: Online cross-sectional survey of 8158 adults. J Med Internet Res. 2020;22:e21372. doi: 10.2196/21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrejko KL, Pry J, Myers JF, et al. Predictors of SARS-CoV-2 infection following high-risk exposure. Clin Infect Dis. 2022;75:e276–e288. doi: 10.1093/cid/ciab1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calamari LE, Tjaden AH, Edelstein SL, et al. Self-reported mask use among persons with or without SARS CoV-2 vaccination —United states, December 2020–August 2021. Preventive Med Rep. 2022;28 doi: 10.1016/j.pmedr.2022.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crane MA, Shermock KM, Omer SB, Romley JA. Change in reported adherence to nonpharmaceutical interventions during the COVID-19 pandemic, april-november 2020. JAMA. 2021;325:883–885. doi: 10.1001/jama.2021.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 1.Tjaden Ashley, Gibbs Michael, William Weintraub, et al. Association between Self-reported Masking Behavior and SARS-CoV-2 Infection Wanes from Pre-Delta to Omicron-Predominant Periods — North Carolina COVID-19 Community Research Partnership. medRxiv. 2022 doi: 10.1101/2022.05.27.22275689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Results of the COVID-19 CRP are being disseminated on the study website (https://www.covid19communitystudy.org/) as well as in publications and presentations in medical journals and at scientific meetings. At end of the study, the databases will be made publicly available in a de-identified manner according to CDC and applicable U.S. Federal policies.