Abstract
Background
Testosterone is an important hormone for the physical and mental health of men; however testosterone administration has also been suggested to adversely affect the cardiovascular system.
Aim
To investigate the effects of excessive testosterone administration on vascular endothelial and erectile function in rats.
Methods
A total of seventy-five 12-week-old rats were divided into the following groups: Sham, castrated (Cast), castrated with subcutaneous administration of 100 mg/kg/month testosterone (Cast + T1), and castrated with subcutaneous administration of 100 mg/kg/week testosterone (Cast + T4). To observe the changes in testosterone level after the administration, rats were further divided into the following groups: control; T(6.25), wherein the rats were subcutaneously injected with 6.25 mg/kg testosterone; T(25) per week, wherein the rats were subcutaneously injected with 25 mg/kg testosterone per week; and T(100), wherein the rats were subcutaneously injected with 100 mg/kg testosterone per week. The relaxation responses of aorta were measured in these rats using standardized methods, and their erectile function was also evaluated. Statistical analysis of the obtained data was performed using two-way analysis of variance (ANOVA), Tukey-Kramer's multiple comparison test, or Student's t-test.
Outcomes
At the end of the study period, endothelial function was evaluated through measurement of isometric tension, while erectile function was assessed using intracavernosal pressure (ICP), mean arterial pressure (MAP), and the expression of endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS), sirtuin 1 (Sirt1) and vascular endothelial growth factor A.
Results
The ICP/MAP ratio in the Cast group (0.42 ± 0.04) was significantly lower than that in the Sham group (0.79 ± 0.07). The ICP/MAP ratio in the Cast + T1 group (0.73 ± 0.06) was significantly higher than that in the Cast group (P < .01) and that of the Cast + T4 (0.38 ± 0.01) group was unchanged (P > .05). The T(25) and T(100) groups exhibited significantly lower responses to ACh than the control group at 4 weeks (P < .01). Meanwhile, the ICP/MAP ratios in the T(25) group (0.44 ± 0.07) and T(100) group (0.47 ± 0.03) were significantly lower than that in the control group (0.67 ± 0.05) at stimulation frequencies of 16 Hz (P < .05). The expression of androgen receptor, Sirt1, and eNOS were significantly lower while that of iNOS was higher in the T(25) group compared with the control group (P < .05).
Clinical Translation
The results based on this animal model indicate that extremely high testosterone levels may affect endothelial and erectile function.
Strengths and Limitations
We found that high-dose testosterone administration decreased endothelial function in aorta and erectile function in rats. A major limitation of this study is that the blood concentration may not be representative of that in humans, and further research is needed.
Conclusion
The findings suggest that high doses of testosterone may cause endothelial dysfunction in the aorta and erectile dysfunction in rats and that the blood concentration should be monitored after testosterone administration.
Kataoka T, Fukamoto A, Hotta Y, et al. Effect of High Testosterone Levels on Endothelial Function in Aorta and Erectile Function in Rats. Sex Med 2022;10:100550.
Key Words: Testosterone, Testosterone Replacement Therapy, Endothelial Dysfunction, Erectile Dysfunction, Risk Factor
INTRODUCTION
Testosterone is an androgenic steroid hormone synthesized from cholesterol1 that has many important physiological functions, affecting muscles, bones, sexual function, prostate gland, and central nervous system, among others. In addition, recent studies have reported that testosterone is also involved in cognitive function, metabolic syndrome, cardiovascular disease, and prognosis.2, 3, 4, 5 Testosterone is an important hormone for both the physical and mental health of men. Testosterone replacement therapy is the first choice for testosterone deficiency, and prescriptions for testosterone have increased significantly in recent years. In the United States, where testosterone therapy is becoming more widespread, the number of prescriptions for testosterone per year has increased 5-fold since 2000, reaching 53 million.6
In recent years, it has been suggested that testosterone also affects the cardiovascular system, and the relationship between blood testosterone levels and cardiovascular disease is becoming a topic of interest worldwide. In a study by Santos et al., 110 patients hospitalized for heart failure were observed, and the rate of readmission and mortality were found to be higher in the low testosterone group than in the normal group.7 In the middle-aged and elderly, testosterone replacement therapy reduced the total mortality, myocardial infarction morbidity, and stroke morbidity rates.4 In addition, in animal studies, a decrease in coronary vascular reactivity was observed in rats that were hormone-deficient due to castration, which was restored in the testosterone-administered group.8,9 Thus, many studies suggest that testosterone therapy reduces mortality and the risk of cardiovascular disease, such as myocardial infarction and erectile function.
However, it has also been suggested that administration of testosterone may adversely affect the cardiovascular system. Juul et al. reported that intramuscular injection of testosterone enanthate in patients with late onset hypogonadism (LOH) syndrome resulted in blood testosterone levels above normal for several days.10 They warned that this high testosterone level could cause a variety of adverse events, including cardiovascular disease. In addition, in a study by Basaria et al., transdermal testosterone was administered to 209 old men, and it was found that the incidence of skin, respiratory, and cardiovascular diseases was significantly higher in these men than those in the placebo group.11 Furthermore, 48 hours after testosterone enanthate was administered to healthy subjects, a significant decrease in urinary nitric oxide (NO) and antioxidant power was confirmed, and endothelial nitric oxide synthase (eNOS) expression in endothelial cells was decreased.12
On the other hand, misuse and abuse of testosterone increase the risk of adverse effects in many organ systems.13,14 Malik RD reported that many patients who were receiving testosterone did not fulfill the diagnostic criteria for testosterone deficiency, and the follow-up after testosterone administration is usually inadequate.15 Some athletes use anabolic-androgenic steroids (AAS). AAS contains testosterone and its synthetic derivatives and is prone to abuse.
Therefore, even though testosterone therapy is useful for patients with LOH syndrome, we hypothesized that “does excessive doses of testosterone increase the risk of developing cardiovascular disease?” However, the exact mechanism that adversely affects cardiovascular disease remains unclear. We also hypothesized “how high doses of testosterone administration affect erectile and cardiovascular function?” Therefore, the purpose of this study was to determine whether excessive testosterone administration has adverse effects and investigate its effects on vascular endothelial function and erectile function in rats.
MATERIAL AND METHODS
Animals
A total of seventy-five 12-week-old male Wistar/ST rats were purchased from Japan SLC, Inc. (Hamamatsu, Japan). All experimental protocols were approved by the ethics review board of Nagoya City University and conducted in accordance with our institutional standards for the care and use of animals (H25-P-09). The rats were kept in a temperature- and humidity-controlled room under a 12 hours/12 hours light/dark cycle and were given free access to normal water.
Treatment Protocols
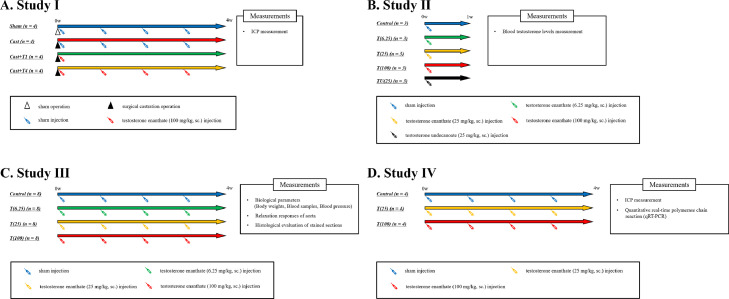
To determine the effect of excessive testosterone administration (study Ⅰ; Figure 1), the rats were divided into the following 4 groups (n = 4 in each group): castrated (Cast), castrated with subcutaneous administration of 100 mg/kg/month testosterone (Cast + T1), castrated with subcutaneous administration of 100 mg/kg/week testosterone (Cast + T4), and sham (Sham). Rats in the Cast, Cast + T1, and Cast + T4 groups were castrated as reported previously.9 The rats from each group were anesthetized briefly with isoflurane using an inhalation anesthesia apparatus (Nakazawa Seisaku-sho, Funabashi, Japan). In the Sham group, rats underwent sham laparotomy, and the incision was sutured. The rats in the Sham group were administered sesame oil as a vehicle. The rats received subcutaneous doses of testosterone enanthate (ASKA Animal Health Co., Ltd., Tokyo, Japan) or sesame oil for 4 weeks, and their erectile function was determined.
Figure 1.
Treatment protocols.
To observe changes in blood testosterone levels after the administration (study Ⅱ), the rats were divided into 5 groups (n = 3 each group): control, wherein the rats were subcutaneously injected with sesame oil; T(6.25), wherein the rats were subcutaneously injected with 6.25 mg/kg testosterone enanthate; T(25), wherein the rats were subcutaneously injected with 25 mg/kg testosterone enanthate; T(100), wherein the rats were subcutaneously injected with 100 mg/kg testosterone enanthate; and TU(25), wherein the rats were subcutaneously injected with 25 mg/kg testosterone undecanoate (Matrix Scientific, Elgin, SC, USA). The rats received subcutaneous doses of testosterone enanthate or sesame oil, and blood was collected from the tail vein at intervals before administration and 3 hours, 6 hours, 24 hours, and 1 week after administration. Testosterone undecanoate was dissolved in a vehicle composed of sesame oil (Nacalai Tesque, Kyoto, Japan) and 0.01% benzyl alcohol (Wako Pure Chemical Industries, Osaka, Japan). The rats in the control group received injections of the sesame oil as a vehicle.
The rats were divided into the following 4 groups (n = 8 each; study Ⅲ): control, wherein the rats were subcutaneously injected with sesame oil; T(6.25), wherein the rats were subcutaneously injected with 6.25 mg/kg testosterone per week; T(25), wherein the rats were subcutaneously injected with 25 mg/kg testosterone per week; and T(100), wherein the rats were subcutaneously injected with 100 mg/kg testosterone per week. In the T(6.25), T(25), and T(100) groups, the rats received subcutaneous doses of testosterone enanthate weekly for 4 weeks, while those in the control group received injections of sesame oil as vehicle. Four weeks after the administrations, the rats were euthanized with isoflurane (Mylan Pharmaceuticals, Canonsburg, PA, USA), blood was collected from the vena cava, and the aorta was harvested.
To investigate the erectile function of rats were divided into 3 groups after testosterone administration (study Ⅳ): control (n = 4), T(25) (n = 4), and T(100) (n = 4), wherein the rats were subcutaneously injected with 25 or 100 mg/kg testosterone per week. The rats received subcutaneous doses of testosterone enanthate or sesame oil for 4 weeks, and the erectile function was evaluated via intracavernous pressure (ICP) measurement, after which the corpora cavernosa were harvested.
Blood Samples and Measurement of Biological Parameters
Blood samples were obtained from all rats via the vena cava. After coagulation and centrifugal separation at 800 g for 20 minutes at 4°C, the serum samples were stored at -80°C until analysis. The biological parameters were analyzed by Fujifilm Vet Systems Co., Ltd. (Chofu, Tokyo, Japan).
Measurement of Blood Pressure
Blood pressure was measured as previously reported.16,17 Systemic arterial blood pressures of the rats were measured while the rats were awake at 0, 2, and 4 weeks, using a noninvasive automatic device (BP-98A-L; Softron, Tokyo, Japan). The rats (n = 8 each) were maintained at 37°C during the measurements by means of a warmer (THC-31; Softron). Each measurement was performed in triplicate, and the mean value was used for analysis.
Histological Evaluation of Stained Sections
Histological evaluation was performed following the method described in previous studies.9,18, 19, 20 Samples of the aorta from each group were fixed overnight in 4% formalin. Afterward, formalin was substituted sequentially with 10%, 20%, and 30% sucrose solution at intervals of at least 10 hours. Then, the samples were embedded in O.C.T. compound (Sakura Finetek Japan, Tokyo, Japan) and frozen in liquid nitrogen. The samples were stored at ˗80°C until analysis. Frozen sections (7 µm thick) from each group were adhered to charged slides and air-dried overnight. The sections were then stained with Masson's trichrome as previously described,9,18, 19, 20 microscopically examined, and photographed using an Eclipse Ti camera (Nikon, Tokyo, Japan).
Relaxation Responses of Aorta
The excised aorta (n = 8 in each each) was cleaned of adipose tissue in Krebs solution (119 mM NaCl, 4.6 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2, 15 mM NaHCO3, 11 mM d-glucose, and 1.2 mM NaH2PO4) at 4°C with 5% CO2 and 95% O2. It was cut to a length of approximately 2 mm to prepare a ring specimen. The ring strips were hung on 2 wires of the micromagnus device (Iwashiya Kishimoto Medical Instruments, Kyoto, Japan). The aorta strips were equilibrated for at least 60 minutes in an aerated organ bath containing Kreb's solution at 37°C with 5% CO2, and 95% O2. The resting force for tissue was set at 600 mg, and changes in isometric tension were recorded using a force transducer (Nihon Kohden, Tokyo, Japan) that was connected to a data acquisition board (PowerLab 4/26; ADInstruments Pty. Ltd., New South Wales, Australia). Relaxation experiments were conducted using aorta strips that were pretreated with 1 µM phenylephrine (Phe; Sigma-Aldrich, MO, USA), and the muscle relaxant effect was induced by acetylcholine (ACh; Wako Pure Chemical Industries) and sodium nitroprusside (SNP; Sigma-Aldrich). Cumulative dose (10−10−10−4 M) response curves were obtained for ACh and SNP using different tissue specimens.
Examination of Erectile Function
Erectile function was evaluated using ICP measurement as previously reported.20,21 The rats from each group were anaesthetized briefly using isoflurane using an inhalation anesthesia apparatus. The carotid artery was cannulated for continuous monitoring of the arterial pressure, and the left crus of the CC were cannulated using a 23-G needle for continuous ICP monitoring. The pressure transducer was connected through an amplifier to a data acquisition board (PowerLab 2/26, AD Instruments Pty., Ltd., New South Wales, Australia). Stainless steel bipolar wire electrodes (Unique Medical, Osaka, Japan) and a pulse generator (Nihon Kohden, Tokyo, Japan) were used for cavernous nerve stimulations with the following parameters: 1 minutes at 10 V, 20 Hz, and a square wave duration of 5 milliseconds or 1 minute at 5 V, 1–16 Hz, and a square wave duration of 5 milliseconds. As ICP is influenced by systemic arterial pressure, erectile function was evaluated using the maximum ICP/mean arterial pressure (MAP) ratio. The MAP was calculated from blood pressure measurements during electrical stimulation using LabChart ver.7 (AD Instruments Pty).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR analysis was performed as previously reported.20 Total RNA was extracted from corpus cavernosum samples using TriPure Isolation Reagent (Sigma-Aldrich) according to the manufacturer's instructions. Using a ReverTra Ace-α kit (Toyobo Co., Ltd., Osaka, Japan), 1 µg of total RNA was reverse-transcribed into complementary DNA, which served as the template for qRT-PCR performed using the KAPA SYBR Fast qPCR Kit (Roche, Pleasanton, CA, USA). The primer sequences are shown in Table 1. Amplification and detection were performed using the CFX96 Real-time System (Bio-Rad, Hercules, CA, USA), and the thermal cycler conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles each of 95°C for 15 seconds and 60°C for 1 minute, 95°C for 15 seconds, 60°C for 15 seconds, and 95°C for 15 seconds to analyze the dissociation curve. Primer specificity was verified via analysis of the dissociation curve. Target gene expression was quantified relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression using the comparative cycle threshold method.
Table 1.
Primer sequences for qRT-PCR
| mRNA | Sequence | |
|---|---|---|
| AR | Forward | 5′-ACCCTCCCATGGCACATTTT-3′ |
| Reverse | 5′-TTGGTTGGCACACAGCACAG-3′ | |
| Sirt1 | Forward | 5′-TGTTTCCTGTGGGATACCTGA-3′ |
| Reverse | 5′-TGAAGAATGGTCTTGGGTCTTT-3′ | |
| VEDF-A | Forward | 5′-GCCCTGAGTCAAGAGGACAG-3′ |
| Reverse | 5′-CAGGCTCCTGATTCTTCCAG-3′ | |
| eNOS | Forward | 5′-CCGGCGCTACGAAGAATG-3′ |
| Reverse | 5′-CAGTGCCACGGATGGAAATT-3′ | |
| iNOS | Forward | 5′-ACCTACTTCCTGGACATCAC-3′ |
| Reverse | 5′-ACCCAAACACCAAGGTCATG-3′ | |
| GAPDH | Forward | 5′-ATGATTCTACCCACGGCAAG-3′ |
| Reverse | 5′-CTGGAAGATGGTGATGGGTT-3′ |
AR = androgen receptor; eNOS = endothelial nitric oxide synthase; GAPDH = glyceraldehyde-3-phosphate dehydrogenase; iNOS = inducible nitric oxide synthase; mRNA = messenger RNA; qRT-PCR = quantitative real-time polymerase chain reaction; Sirt1 = sirtuin 1; VEGF = vascular endothelial growth factor.
Statistical Analyses
The obtained data were expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined using one-way analysis of variance (ANOVA) followed by Tukey-Kramer's multiple comparison test or Student's t-test using R statistical software program, version 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria) with EZR on R commander ver 1.41 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) on the website (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html).22 Statistical significance of the dose-responses for ACh and SNP in isometric tension study and EFS in ICP measurement was determined using two-way ANOVA using EZR on R commander ver 1.41.22 Complementary analysis was performed using Dunnett multiple comparison post hoc tests to identify differences between the control and treated groups when a significant interaction was observed. Statistical significance was set at P value < .05.
RESULTS
Testosterone Administration to Castrated Rats
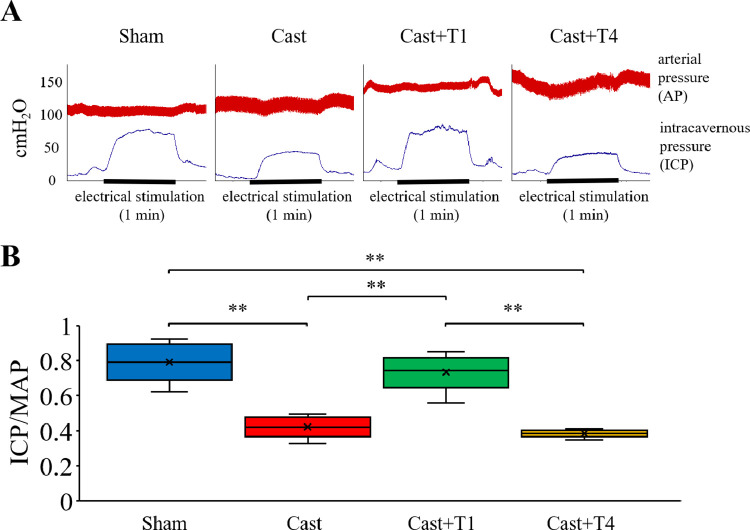
Figure 2A shows representative tracings of the ICP and atrial pressure changes during electrical stimulation of the cavernous nerve. Figure 2B shows the ICP/MAP ratios for different stimulation frequencies. The ICP/MAP ratio in the Cast group (0.42 ± 0.04) was significantly lower than that in the Sham group (0.79 ± 0.07) at a stimulation frequency of 20 Hz (P < .01). The ICP/MAP ratio in the Cast + T1 group (0.73 ± 0.06) was significantly higher than that in the Cast group (P < .01). However, the ICP/MAP ratio in the Cast + T4 (0.38 ± 0.01) group was unchanged (P > .05).
Figure 2.
(A) Representative tracings of intracavernous pressure (ICP) and arterial pressure changes during electrical stimulation at 20 Hz of the cavernous nerve in Sham, Cast, Cast + T1, and Cast + T4 rats. (B) Erectile function according to the ICP/mean arterial pressure (MAP) ratio. Data are presented as a box-and-whisker plot (n = 4). ⁎⁎P < .01 vs each group using the Tukey-Kramer's multiple comparison test.
Biological Parameters
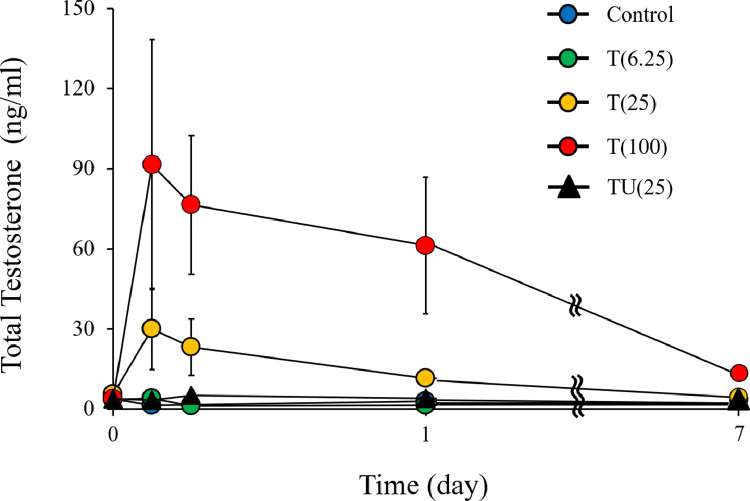
Figure 3 shows the changes in serum total testosterone levels after testosterone administration. The testosterone level was the highest 3 hours after testosterone administration (control: 3.8 ± 0.3 ng/mL, T[6.25]: 4.2 ± 0.6 ng/mL, T[25]: 30.0 ± 15.2 ng/mL, T[100]: 91.5 ± 46.8 ng/mL, and TU[25]: 5.1 ± 1.4 ng/mL), while the serum testosterone level increased in a dose-dependent manner. The testosterone level decreased with time and was reduced to original level 1 week after administration but was still high in the T(100) group (12.9 ± 2.0 ng/mL).
Figure 3.
Testosterone levels after injection. control (n = 3); T(6.25) (n = 3), wherein the rats were subcutaneously injected with 6.25 mg/kg testosterone enanthate (ASKA Animal Health Co., Ltd., Tokyo, Japan); T(25) (n = 3), wherein the rats were subcutaneously injected with 25 mg/kg testosterone enanthate; T(100) (n = 3), wherein the rats were subcutaneously injected with 100 mg/kg subcutaneous testosterone enanthate; and TU(25) (n = 3), wherein the rats were subcutaneously injected with subcutaneous testosterone undecanoate (25 mg/kg; Matrix Scientific, Elgin, SC, USA). Data are reported as the mean ± standard error of the mean.
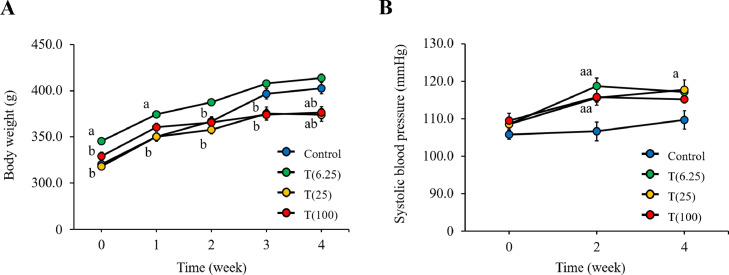
Figure 4A shows the changes in body weight during the study. There was no significant difference in the body weight of rats between the control (402.9 ± 6.1 g) and T(6.25) (414.0 ± 4.3 g) groups (P > .05). However, the body weight was significantly lower in the T (25) (373.0 ± 6.9 g) and T (100) (376.3 ± 6.7 g) groups compared with the control group at 4 weeks (both P < .05). Figure 4B shows the systolic blood pressure changes during the study. There was no significant difference in the body weight of rats between the control (109.7 ± 2.4 mm Hg), T(6.25) (117.2 ± 3.2 mm Hg), and T(100) (115.2 ± 0.8 mm Hg) groups (all P > .05). The systolic blood pressure in the T(25) group (117.8 ± 0.8 mm Hg) was significantly higher than that in the control group (P < .05).
Figure 4.
Biological parameters. (A) Body weight. (B) Systolic blood pressure. Data are reported as the mean ± standard error of the mean (n = 7-8). aP < .05 vs control, aaP < .001 vs control, bP < .05 vs T (6.25) by Tukey-Kramer's multiple comparison tests.
Table 2 shows the biological parameters of the rats. Total testosterone level was the highest in the T(100) group compared with the other groups (P < .05). There were no significant differences in electrolyte levels, liver function, renal function, and lipid levels between the groups (all P > .05).
Table 2.
Biological parameter data of the experimental rats
| Biological parameter | Unit | Control | T(6.25) | T(25) | T(100) |
|---|---|---|---|---|---|
| Testosterone | ng/mL | 3.0 ± 1.1 | 1.2 ± 0.1 | 8.4 ± 1.6 | 291.1 ± 83.5abc |
| Na | μEq/L | 147.0 ± 0.8 | 144.0 ± 0.5 | 147.0 ± 0.7 | 148.9 ± 0.7 |
| Cl | μEq/L | 99.7 ± 0.8 | 101.8 ± 0.2 | 96.9 ± 0.6 | 99.6 ± 1.2 |
| K | μEq/L | 9.6 ± 1.4 | 4.9 ± 0.1 | 11.3 ± 1.7 | 8.6 ± 1.0 |
| Ca | mg/dL | 11.0 ± 0.3 | 10.1 ± 0.1 | 10.9 ± 0.4 | 10.7 ± 0.2 |
| Total protein | g/dL | 6.0 ± 0.2 | 5.1 ± 0.1 | 6.1 ± 0.3 | 6.0 ± 0.2 |
| Albumin | g/dL | 3.4 ± 0.1 | 3.0 ± 0.0 | 3.4 ± 0.1 | 3.3 ± 0.1 |
| GPT(ALT) | IU/L | 55.6 ± 4.0 | 49.8 ± 1.6 | 48.4 ± 3.9 | 48.9 ± 3.3 |
| BUN | mg/dL | 16.4 ± 0.7 | 16.5 ± 1.0 | 14.4 ± 1.1 | 15.6 ± 0.9 |
| Creatinine | mg/dL | 0.26 ± 0.02 | 0.25 ± 0.03 | 0.26 ± 0.02 | 0.21 ± 0.01 |
| Total cholesterol | mg/dL | 74.6 ± 4.7 | 69.3 ± 2.1 | 74.7 ± 5.8 | 68.7 ± 4.6 |
| Triglyceride | mg/dL | 77.4 ± 4.7 | 56.3 ± 4.8 | 59.4 ± 10.7 | 57.5 ± 6.9 |
ALT = alanine aminotransferase; BUN = blood urea nitrogen; GPT = glutamic pyruvic transaminase. Data are reported as the mean ± standard error (n = 7-8 per group).
P < .05 vs control.
P < .05 vs T(6.25).
P < .05 vs T(25).
P values were calculated for each group using Tukey-Kramer's multiple comparison test.
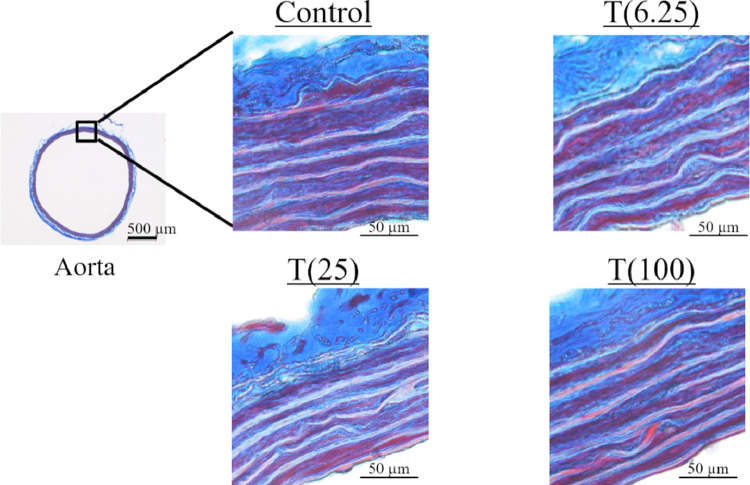
Histological Evaluation
Figure 5 shows the histological structure of the aorta. No particular changes were observed between the groups.
Figure 5.
Masson's trichrome staining of rat aorta.
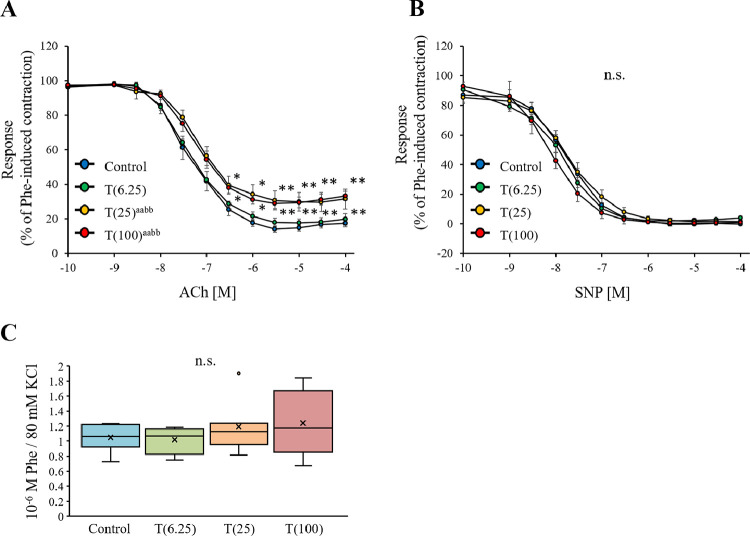
Relaxation Responses of Aorta
The relaxation responses of the Phe-pretreated rat aorta strips to increasing concentrations of ACh are shown in Figure 6A. The T(25) and T(100) groups exhibited significantly lower responses to ACh than the Control group (both P < .01). The relaxant responses of the aorta strips to increasing concentrations of SNP are shown in Figure 6B. The SNP responses at 10−10–10−4 M did not significantly differ between the groups (P > .05).
Figure 6.
Analysis of isometric tension in the aorta of rats. (A) Acetylcholine (ACh)-induced relaxation curve for rat corpus cavernosum strips. The relaxant effect of increasing concentrations of ACh (10−10-10−4 M) on aorta strips. The strips were precontracted using 10−6 M phenylephrine (Phe). (B) Sodium nitroprusside (SNP)–induced relaxation curve for rat aorta strips. The relaxant effect of increasing concentrations of SNP (10−10-10−4 M) on aorta strips. The strips were pre-exposed to 10−6 M Phe. Data are reported as the mean ± standard deviation (n = 8 per group). aaP < .001 vs control, bbP < .01 vs T (6.25) by two-way analysis of variance. *P < .05, ⁎⁎P < .01 vs control using Dunnett multiple comparison post-hoc test.
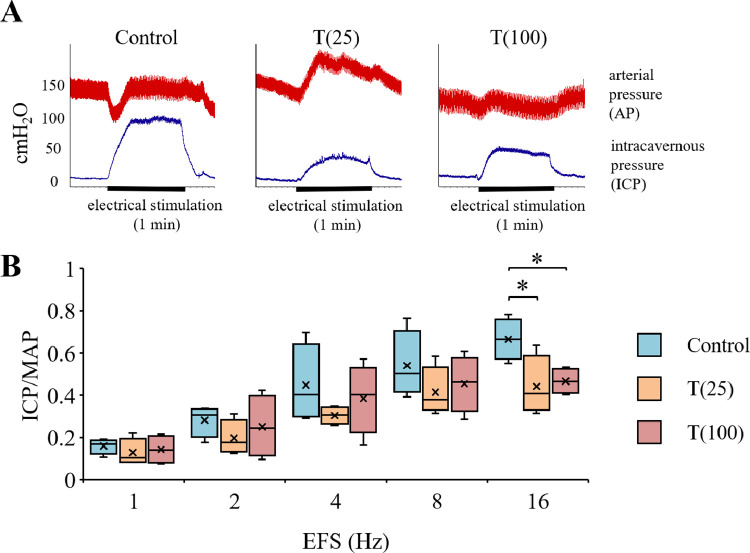
Erectile Function
Figure 7A shows representative tracings of the ICP and atrial pressure changes during electrical stimulation of the cavernous nerve. ICP values in the T(25) group at 4 weeks appeared to be significantly lower than those in the control group. Figure 7A shows the ICP/MAP ratios for different stimulation frequencies. The ICP/MAP ratio in the T(25) group (0.44 ± 0.07) and T(100) group (0.47 ± 0.03) was significantly lower than that in the control group (0.67 ± 0.05) at stimulation frequencies of 16 Hz (P < .05).
Figure 7.
(A) Representative tracings of intracavernous pressure (ICP) and arterial pressure changes during electrical stimulation at 16 Hz of the cavernous nerve in control, T(25) and T(100) rats. (B) Erectile function according to the ICP/mean arterial pressure (MAP) ratio. Data are presented as a box-and-whisker plot (n = 4). *P < .05 vs each group using Dunnett multiple comparison post-hoc test.
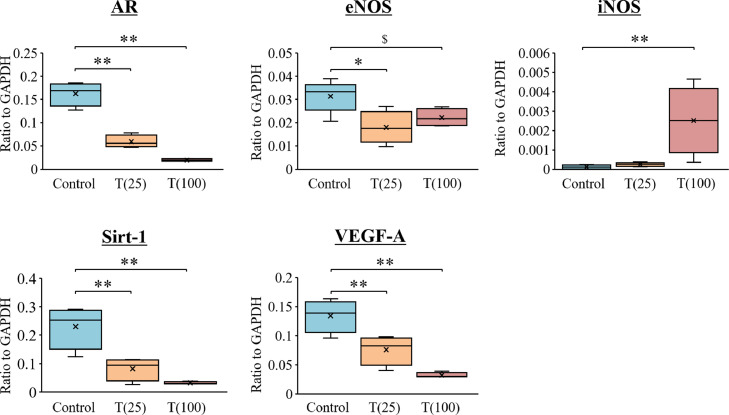
mRNA Expression Analysis
Figure 8 shows the mRNA expression levels in the corpus cavernosum. Compared with the control group, the expression of androgen receptor, sirtuin 1 (Sirt1), and VEDF-A were significantly lower in the T(25) and T(100) groups (P < .01). eNOS mRNA expression was significantly lower in the T(25) (P < .05) and tended to be lower in the T(100) groups (P = .09). iNOS mRNA expression was significantly higher in the T(100) group (P < .01).
Figure 8.
mRNA expression in the corpus cavernosum of rats. Target gene expression was quantified relative to the expression of GAPDH using the comparative CT method. Data are presented as a box-and-whisker plot (n = 4). $P < .10, *P < .05, *P < .01 vs each group using Dunnett multiple comparison post-hoc test.
DISCUSSION
This study suggests that high doses of testosterone may cause endothelial dysfunction in the aorta and erectile dysfunction in rats. It was revealed that the blood concentration became very high after the administration of testosterone enanthate. Additionally, repeated high-dose testosterone administration reduced the body weight of rats. In this study, testosterone administration did not affect renal or hepatic function, but it is considered necessary to carefully monitor the course, as excessive testosterone levels may have harmful effects rather than treatment.
In this study, 2 testosterone preparations, testosterone enanthate and testosterone undecanoate, were examined. These drugs are also widely used as long-term preparations in humans. However, in animal experiments, ART is often performed using testosterone propionate. In previous studies, testosterone propionate demonstrated a good therapeutic effect at a dose of 3 mg/kg/day.9,15,16 Performing ART with a long-term formulation can increase the dosing interval while increasing the dose per injection. As shown in this study, after administration, the concentration of testosterone enanthate in blood was high, which may adversely affect erectile function. In contrast, the concentration of testosterone undecanoate was stable after administration, suggesting a lower risk of adverse events. In addition, testosterone cypionate is also used as a long-term preparation in some countries; however, its stability in blood has not been explored, which warrants further studies.
Initially, we followed a previous study wherein 100 mg of testosterone was administered monthly to castrated rats for 1 month.23 However, because of a misunderstanding, weekly testosterone was administered weekly to castrated rats. Therefore, the erectile function did not improve, and the weight loss was more remarkable. We believed that the side effects were due to high testosterone levels. Thus, we further used rats that had not undergone castration.
After administration of testosterone, a significant increase in systolic blood pressure was observed in the T(6.25) and T(25) groups compared with the control group. Previous studies have shown that administration of an excessive amount of testosterone exceeding the physiological concentration to Wistar/ST rats, similar to what was done in this study, increases blood pressure,8,24 and in this study, testosterone administration also increased blood pressure. It has been reported that renin activity increases when testosterone is administered to rats.25 In addition, it has also been reported that the decrease in blood noradrenaline concentration due to castration was recovered via administration of testosterone and that the blood pressure was also increased.24 Rouver et al. reported that testosterone administration increased blood pressure through these mechanisms,8 and further increase in blood pressure may be observed in this study if the observation period is further extended. In addition, Alves et al. reported that high testosterone-induced vascular endothelial dysfunction was because of an enhanced nucleotide-binding oligomerization domain leucine-rich repeats pyrin domain cantaining 3 (NLRP3) inflammasome-mediated inflammatory response using knockout mice and NLRP3 receptor inhibitor.26 They showed that testosterone levels above physiological levels induced apoptosis via NLRP3, and our study results also showed an increase in iNOS in the corpus cavernosum; hence, it is considered that the ED would be caused in a similar mechanism.
Administration of excess testosterone to rats reduced the relaxation response of ACh but not of SNP, which indicates that it did not affect the relaxation response of smooth muscle but reduced that mediated by the vascular endothelium. In addition, histological examination by Masson's trichrome staining did not show any change in the structure of the aorta, suggesting that the vascular endothelial function but not the vascular smooth muscle function was impaired by the excessive dose of testosterone. Although testosterone deficiency has been widely reported to cause vascular endothelial dysfunction,27, 28, 29 high testosterone levels may also lead to vascular endothelial dysfunction.
In this study, the doses of 25 and 100 mg/kg/week of testosterone for 4 weeks reduced the body weight of rats and caused vascular endothelial dysfunction. Therefore, next, the influence of these doses on the erectile function of rats was investigated. The administration of these doses for 4 weeks significantly decreased the ICP/MAP ratio in rats, suggesting a reduced erectile function.
To clarify the mechanism of erectile dysfunction, we investigated the fluctuation in mRNA expression in the corpus cavernosum. First, the expression of androgen receptor was reduced by administration of testosterone. This is believed to be a compensatory change due to high testosterone levels but may cause a testosterone deficiency state when the expression reaches normal levels after testosterone administration. Therefore, taking a higher dose of testosterone than necessary should be avoided. In addition, high testosterone levels in the T(100) group increased iNOS mRNA expression, which can cause excessive oxidative stress to the corpus cavernosum, but reduced eNOS mRNA expression, suggesting that it may lead to a decrease in NO bioavailability. Decreased NO production due to increased oxidative stress has also been reported,30,31 which indicates that increased iNOS may be accompanied by reduced eNOS production. In addition, high testosterone levels also reduced Sirt1 and VEDF-A mRNA expression. Sirtuins have also been reported to be involved in NO production via eNOS,32,33 and it is possible that the decrease in Sirt1 decreased eNOS. It has been reported that VEDF is also involved in NO bioavailability.34,35 Taken together, the findings suggest that high-dose testosterone administration reduced NO bioavailability and erectile function in rats.
A previous study has reported that blood testosterone levels exceed the normal range even when a therapeutic dose of testosterone is administered.10 Amano et al. reported the risks of high testosterone levels in patients undergoing testosterone administration.36 The serum testosterone levels following the injection of testosterone enanthate (Enarmon depot) are much higher than the normal range. In addition, these levels decrease quickly to the original low level within 2–3 weeks.37 On the other hand, testosterone undecanoate (Nebido) maintains the testosterone levels within the normal range. However, testosterone undecanoate is not permitted in some countries. Amano et al. reported that testosterone ointment (Glowmin) was effective within the physiological range of testosterone levels in patients with LOH.38 In our study, testosterone undecanoate did not show a remarkable increase in testosterone levels, but testosterone enanthate showed a remarkable increase in blood testosterone levels. The administration method of testosterone replacement therapy should consider the transition of testosterone levels.
Previous studies have also reported that AAS abuse injures cardiovascular function by increasing oxidative stress.39,40 The production of oxidative stress by a high dose of testosterone has also been reported in the human vascular endothelial cells.41 The abuse and misuse of testosterone can cause erectile dysfunction and affect the cardiovascular system. Presently, the effect of testosterone abuse and misuse is not completely known; therefore, further detailed studies are required in the future.13,14
However, the results of this study are limited to investigation at the mRNA level, and it is necessary to directly measure the actual amount of NO produced and investigated at the protein level in the future. In addition, it is unclear whether the blood concentration in rats is representative of that in humans, and further research is needed to elucidate this. However, since side effects due to testosterone administration have been reported,10, 11, 12 it is necessary to monitor the changes in blood testosterone concentration. In addition, in this study, the blood testosterone concentration increased significantly after the administration of testosterone enanthate but not testosterone undecanoate; Therefore, the choice of drug for testosterone replacement therapy should also be considered.
CONCLUSIONS
The findings suggest that high doses of testosterone may cause endothelial dysfunction in the aorta and erectile dysfunction in rats. Although testosterone replacement therapy is a useful treatment, it is necessary to monitor the blood concentration after testosterone administration.
The thermal cycler conditions were as follows: 2 minutes at 50°C, 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C and 1 minute at 60°C, 15 seconds at 95°C, 15 seconds at 60°C, and 15 seconds at 95°C to analyze the dissociation curve. Primer specificity was verified via analysis of the dissociation curve.
STATEMENT OF AUTHORSHIP
Conceptualization, T.K. and K.K.; Data curation, T.K., A.F, Y.H., and Y.M.; Formal analysis, T.K. and A.F.; Funding acquisition, T.K., Y.H., and K.K.; Investigation, T.K., A.F.; Methodology, T.K. and Y.H.; Project administration, T.K.; Supervision, Y.H., A.S., Y.M., Y.F.H., and K.K.; Writing-original draft, T.K. and K.K.; Writing-review & editing T.K. and K.K.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI, 20K09563) of the Japan Society for the Promotion of Science (JSPS). We acknowledge the assistance of the Research Equipment Sharing Center at the Nagoya City University.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: None.
Contributor Information
Tomoya Kataoka, Email: tom.kataoka@aol.com, tkataoka@cis.ac.jp.
Kazunori Kimura, Email: kkimura@med.nagoya-cu.ac.jp.
References
- 1.Hou JW, Collins DC, Schleicher RL. Sources of cholesterol for testosterone biosynthesis in murine Leydig cells. Endocrinology. 1990;127:2047–2055. doi: 10.1210/endo-127-5-2047. [DOI] [PubMed] [Google Scholar]
- 2.Borst SE, Yarrow JF, Fernandez C, et al. Cognitive effects of testosterone and finasteride administration in older hypogonadal men. Clin Interv Aging. 2014;9:1327–1333. doi: 10.2147/CIA.S61760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Cruz E, Leibar-Tamayo A, Romero J, et al. Metabolic syndrome in men with low testosterone levels: Relationship with cardiovascular risk factors and comorbidities and with erectile dysfunction. J Sex Med. 2013;10:2529–2538. doi: 10.1111/jsm.12265. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R, Oni OA, Gupta K, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715. doi: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 5.Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 6.Vigen R, O'Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. doi: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 7.Santos MR, Sayegh AL, Groehs RV, et al. Testosterone deficiency increases hospital readmission and mortality rates in male patients with heart failure. Arq Bras Cardiol. 2015;105:256–264. doi: 10.5935/abc.20150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouver WN, Delgado NT, Menezes JB, et al. Testosterone replacement therapy prevents alterations of coronary vascular reactivity caused by hormone deficiency induced by castration. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka T, Hotta Y, Yamamoto Y, et al. Effect of late androgen replacement therapy on erectile function through structural changes in castrated rats. Sex Med. 2021;9 doi: 10.1016/j.esxm.2021.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juul A, Skakkebaek NE. Androgens and the ageing male. Hum Reprod Update. 2002;8:423–433. doi: 10.1093/humupd/8.5.423. [DOI] [PubMed] [Google Scholar]
- 11.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skogastierna C, Hotzen M, Rane A, et al. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol. 2014;21:1049–1054. doi: 10.1177/2047487313481755. [DOI] [PubMed] [Google Scholar]
- 13.Linhares BL, Miranda EP, Cintra AR, et al. Use, misuse and abuse of testosterone and other androgens. Sex Med Rev. 2021;S2050-0521:00083–00084. [Google Scholar]
- 14.Corona G, Rastrelli G, Marchiani S, et al. Consequences of anabolic-androgenic steroid abuse in males; sexual and reproductive perspective. World J Mens Health. 2022;40:165–178. doi: 10.5534/wjmh.210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik RD, Wang CE, Lapin B, et al. Characteristics of men undergoing testosterone replacement therapy and adherence to follow-up recommendations in metropolitan multicenter health care system. Urology. 2015;85:1382–1388. doi: 10.1016/j.urology.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka T, Mori T, Suzuki J, et al. Oxaliplatin, an anticancer agent, causes erectile dysfunction in rats due to endothelial dysfunction. J Sex Med. 2021;18:1337–1345. doi: 10.1016/j.jsxm.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto T, Kataoka T, Yamamoto Y, et al. High salt intake impairs erectile function in salt-sensitive rats through mineralocorticoid receptor pathway beyond its effect on blood pressure. J Sex Med. 2020;17:1280–1287. doi: 10.1016/j.jsxm.2020.04.384. [DOI] [PubMed] [Google Scholar]
- 18.Kataoka T, Hotta Y, Maeda Y, et al. Testosterone deficiency causes endothelial dysfunction via elevation of asymmetric dimethylarginine and oxidative stress in castrated rats. J Sex Med. 2017;14:1540–1548. doi: 10.1016/j.jsxm.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka T, Hotta Y, Maeda Y, et al. Assessment of androgen replacement therapy for erectile function in rats with type 2 diabetes mellitus by examining nitric oxide-related and inflammatory factors. J Sex Med. 2014;11:920–929. doi: 10.1111/jsm.12447. [DOI] [PubMed] [Google Scholar]
- 20.Kataoka T, Sanagawa A, Suzuki J, et al. Influence of anticancer agents on sexual function: An in vivo study based on the US FDA Adverse Event Reporting System. Andrology. 2022;10:166–178. doi: 10.1111/andr.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kataoka T, Hotta Y, Ohno M, et al. Limited effect of testosterone treatment for erectile dysfunction caused by high-estrogen levels in rats. Int J Impot Res. 2013;25:201–205. doi: 10.1038/ijir.2013.21. [DOI] [PubMed] [Google Scholar]
- 22.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba K, Yajima M, Carrier S, et al. Delayed testosterone replacement restores nitric oxide synthase-containing nerve fibres and the erectile response in rat penis. BJU Int. 2000;85:953–958. doi: 10.1046/j.1464-410x.2000.00598.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumai T, Tanaka M, Watanabe M, et al. Possible involvement of androgen in increased norepinephrine synthesis in blood vessels of spontaneously hypertensive rats. Jpn J Pharmacol. 1994;66:439–444. doi: 10.1254/jjp.66.439. [DOI] [PubMed] [Google Scholar]
- 25.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 26.Alves JV, da Costa RM, Pereira CA, et al. Supraphysiological levels of testosterone induce vascular dysfunction via activation of the NLRP3 inflammasome. Front Immunol. 2020;11:1647. doi: 10.3389/fimmu.2020.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buvat J, Maggi M, Guay A, et al. Testosterone deficiency in men: Systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013;10:245–284. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 28.Morgentaler A, Traish A, Hackett G, et al. Diagnosis and treatment of testosterone deficiency: Updated recommendations from the Lisbon 2018 international consultation for sexual medicine. Sex Med Rev. 2019;7:636–649. doi: 10.1016/j.sxmr.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Hotta Y, Kataoka T, Kimura K. Testosterone deficiency and endothelial dysfunction: Nitric oxide, asymmetric dimethylarginine, and endothelial progenitor cells. Sex Med Rev. 2019;7:661–668. doi: 10.1016/j.sxmr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Nunes PR, Mattioli SV, Sandrim VC. NLRP3 activation and its relationship to endothelial dysfunction and oxidative stress: Implications for preeclampsia and pharmacological interventions. Cells. 2021;10:2828. doi: 10.3390/cells10112828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerby P, Tasta O, Swiader A, et al. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021;40 doi: 10.1016/j.redox.2021.101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suades R, Cosentino F. Sirtuin 1/soluble guanylyl cyclase: A nitric oxide-independent pathway to rescue ageing-induced vascular dysfunction. Cardiovasc Res. 2019;115:485–487. doi: 10.1093/cvr/cvy297. [DOI] [PubMed] [Google Scholar]
- 33.Kilic U, Gok O, Elibol-Can B, et al. Efficacy of statins on sirtuin 1 and endothelial nitric oxide synthase expression: The role of sirtuin 1 gene variants in human coronary atherosclerosis. Clin Exp Pharmacol Physiol. 2015;42:321–330. doi: 10.1111/1440-1681.12362. [DOI] [PubMed] [Google Scholar]
- 34.Lankhorst S, Danser AH, van den Meiracker AH. Endothelin-1 and antiangiogenesis. Am J Physiol Regul Integr Comp Physiol. 2016;310:R230–R234. doi: 10.1152/ajpregu.00373.2015. [DOI] [PubMed] [Google Scholar]
- 35.Zarbin MA. Anti-VEGF agents and the risk of arteriothrombotic events. Asia Pac J Ophthalmol (Phila) 2018;7:63–67. doi: 10.22608/APO.2017495. [DOI] [PubMed] [Google Scholar]
- 36.Amano T, Imao T, Takemae K, et al. Profile of serum testosterone levels after application of testosterone ointment (glowmin) and its clinical efficacy in late-onset hypogonadism patients. J Sex Med. 2008;5(7):1727–1736. doi: 10.1111/j.1743-6109.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 37.Gyllenborg J, Rasmussen SL, Borch-Johnsen K, et al. Cardiovascular risk factors in men: The role of gonadal steroids and sex hormone-binding globulin. Metabolism. 2001;50(8):882–888. doi: 10.1053/meta.2001.24916. [DOI] [PubMed] [Google Scholar]
- 38.Amano T, Iwamoto T, Sato Y, et al. The efficacy and safety of short-acting testosterone ointment (Glowmin) for late-onset hypogonadism in accordance with testosterone circadian rhythm. Aging Male. 2018;21:170–175. doi: 10.1080/13685538.2018.1471129. [DOI] [PubMed] [Google Scholar]
- 39.Turillazzi E, Neri M, Cerretani D, et al. Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: The mutual crosstalk between ROS and NF-kB. J Cell Mol Med. 2016;20:601–612. doi: 10.1111/jcmm.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasilaki F, Tsitsimpikou C, Tsarouhas K, et al. Cardiotoxicity in rabbits after long-term nandrolone decanoate administration. Toxicol Lett. 2016;241:143–151. doi: 10.1016/j.toxlet.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 41.Skogastierna C, Hotzen M, Rane A, et al. A supraphysiological dose of testosterone induces nitric oxide production and oxidative stress. Eur J Prev Cardiol. 2014;21:1049–1054. doi: 10.1177/2047487313481755. [DOI] [PubMed] [Google Scholar]