Abstract
Background
To assess the existence of association between neutrophil to lymphocyte ratio (NLR) and the risk of sarcopenia in COVID-19 patients.
Methods
A retrospective cross-sectional study was conducted in a university hospital with patients with an active COVID-19 infection admitted to the nursing ward or intensive care unit (ICU) between September to December 2020. Sarcopenia risk was assessed using the Strength, Assistance for walking, Rise from a chair, Climb stairs and Falls (SARC-F). Biochemical analyses were assessed by circulating of C-reactive protein, D-dimer, neutrophils, lymphocytes count and NLR. Sixty-eight patients were evaluated and divided into tertiles of NLR values and the association between NLR and sarcopenia risk were tested using the linear regression analyses and p < 0.05 were considered as significant.
Results
Sixty-eight patients were evaluated and divided in NLR tertiles being the 1st (men = 52.2%; 71.1 ± 9.0 y; NLR: 1.1–3.85), 2nd (women = 78.3%; 73.2 ± 9.1 y; NLR: 3.9–6.0) and 3rd (men = 72.7%; 71.7 ± 10.4 y; NLR: 6.5–20.0). There was a difference between the tertiles in relation to the first to the biochemical parameters of total neutrophils count (p = 0.001), C-reactive protein (p = 0.012), and D-dimer (p = 0.012). However, no difference was found in linear regression analysis between tertiles of NLR and SARC-F, if in total sample (p = 0.054) or divided by sex, if men (p = 0.369) or women (p = 0.064).
Conclusion
In elderly patients hospitalized with COVID-19, we do not find an association between the risk of sarcopenia and NLR.
Keywords: Inflammation, COVID-19, Sarcopenia, SARC-F
Abstract
Objetivo
Evaluar la existencia de asociación entre la relación neutrófilos a linfocitos (RNL) y el riesgo de sarcopenia en pacientes con COVID-19.
Métodos
Se realizó un estudio transversal retrospectivo en un hospital universitario con pacientes con infección activa por COVID-19 ingresados en sala de enfermería o unidad de cuidados intensivos (UCI) entre septiembre a diciembre de 2020. El riesgo de sarcopenia se evaluó utilizando: fuerza, ayuda para caminar, levantarse de una silla, subir escaleras y caídas (SARC-F). Los análisis bioquímicos se evaluaron mediante circulación de proteína C reactiva, dímero D, neutrófilos, recuento de linfocitos y RNL. Sesenta y ocho pacientes fueron evaluados y divididos en terciles de valores de RNL y la asociación entre RNL y el riesgo de sarcopenia se evaluó mediante análisis de regresión lineal; p < 0,05 se consideró significativo.
Resultados
Sesenta y ocho pacientes fueron evaluados y divididos en terciles de RNL, siendo el primero (hombres = 52,2%; 71,1 ± 9,0 años; RNL: 1,1-3,85), el segundo (mujeres = 78,3%; 73,2 ± 9,1 años; RNL: 3,9-6,0) y el tercero (hombres = 72,7%; 71,7 ± 10,4 años; RNL: 6,5-20,0). Hubo diferencia entre los terciles con relación al primero en los parámetros bioquímicos de recuento de neutrófilos totales (p = 0,001), proteína C reactiva (p = 0,012) y dímero D (p = 0,012). Sin embargo, no se encontró diferencia en el análisis de regresión lineal entre terciles de RNL y SARC-F, si en muestra total (p = 0,054) o dividida por sexo, si hombres (p = 0,369) o mujeres (p = 0,064).
Conclusiones
En pacientes ancianos hospitalizados con COVID-19 no encontramos asociación entre el riesgo de sarcopenia y RNL.
Palabras clave: Inflamación, COVID-19, Sarcopenia, SARC-F
Introduction
COVID-19 triggered by the SARS-Cov-2 virus emerged to the world in mid-2020 as a serious and major disease.1 Symptoms such as coughing, weakness and muscle pain, fever and shortness of breath are commonly observed in SARS-Cov-2 infection, and may progress to death.2 The sarcopenia and cachexia can be observed in patients with COVID-19 contributing to bed restriction and demand for respiratory support.3 Added to these conditions, the loss of muscle strength leads to a condition known as sarcopenia that is associated with prolonged hospitalization of critical patients at Intensive Care Unit (ICU).4 Sarcopenia was initially described as a natural process of muscle loss.5 Currently, the diagnosis of sarcopenia requires the presence of muscle strength deficit associated with low quality and muscle quantity.6 In the elderly, sarcopenia is related to low quality of life, besides being an important factor for morbidity and mortality.7, 8 In this sense, the Strength Assistance for Walking, Rise from a chair, Climb stairs and Falls (SARC-F) is a simple tool for screening the risk of sarcopenia that can be applied during the clinical practice.4, 9, 10, 11
Sarcopenia and respiratory failure are correlated in ICU patients with COVID-19 hospitalized, due to the high degree of inflammation and muscle degradation.11 Moreover it is known that the new SARS-Cov-2 coronavirus causes an imbalance in the immune response in infected patients, providing a storm of inflammatory cytokines that impact the clinical outcome of the disease.12 For this reason, early detection of COVID-19 infection through immunoinflammatory parameters may result in better treatment strategies, could increase cure rate and reduce risk of death and length of hospitalization.12 Neutrophil and lymphocyte defense cells constitute the main anti-inflammatory apparatus of the human body.13 In this sense, the presence of inflammatory diseases promotes unbalance in the count of circulating neutrophils (neutrophilia) and lymphocytes (lymphopenia), impacting the neutrophil–lymphocyte ratio (NLR).14 NLR is an important indicator of the inflammatory process in SARS-Cov-2 infection, as it is low cost and a good marker of outcome's patient with COVID-19.12, 15 Recent studies have concluded that NLR guides the development of accurate strategies in the treatment of elderly patients with COVID-19.16, 17 However, although the NLR is an important tool in the prognosis of prediction of cell damage due to viral infections,15, 16 there were no studies investigating the association of NLR and SARC-F tool in hospitalized COVID-19 patients. Therefore, we aimed to investigate the association of NLR and the risk of sarcopenia in hospitalized patients with COVID-19.
Methods
This retrospective cross-sectional study was conducted at the UFG Clinics Hospital. The information was obtained from electronic medical records of patients with an active COVID-19 infection presenting symptoms of dyspnea, intense fatigue, tachypnea, desaturation and high fever, admitted to the nursing ward or ICU between September to December 2020. Inclusion criteria were patients of both sexes, over 60 years, who answered the questionnaires during the nutritional assessment and were not intubated. We elected 74 patients; however six patients were excluded for not having answered the SARC-F tool. The study was approved by the Ethical Committee of the HC-UFG/EBSERH (4.592.437). SARC-F questionnaire was applied to evaluate the risk of sarcopenia, as previously described.18 Biochemical analyses were evaluated by circulating C-reactive protein and D-dimer using a turbidimetric method using immunoglobulin (immunoturbidimetry) in a Roche Cobas© 6000 C501, (Roche Diagnostics, Mannheim, Germany), the absolute value of neutrophils was divided by the absolute value of lymphocytes, both obtained by blood count through Automated Electronic Counts (Sysmex Corporation, Kobe, Japan), to calculate the neutrophil–lymphocyte ratio (NLR). The sample was divided into tertiles of NLR values and described as mean ± standard deviation or percentage and confidence interval. Associations between categorical variables were assesses using p of trend or Fisher's exact test and for continuous variables by the trend p. Differences between the tertiles were identified by the Pairwise test. The association between NLR and risk of sarcopenia (SARC-F) was tested using the linear regression analyses. Stata 12.0 software (StataCorp LP, TX, USA) was used for analyses and p < 0.05 were considered as significant.
Results
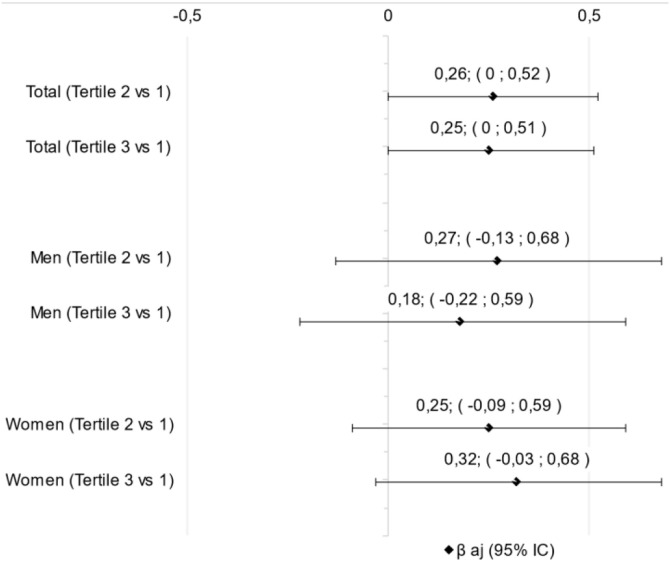
Sixty-eight patients were evaluated and divided in NLR tertiles being the 1st (men = 52.2%; 71.1 ± 9.0 y; NLR: 1.1–3.85), 2nd (men = 21.7%; 73.2 ± 9.1 y; NLR: 3.9–6.0) and 3rd (men = 72.7%; 71.7 ± 10.4 y; NLR: 6.5–20.0) (Table 1 ). The biochemical parameters were higher in the upper tertiles than the first tertil for total neutrophils count, C-reactive protein, and D-dimer (Table 1). However, there was no difference in linear regression analysis between NLR tertiles and SARC-F in any classification, if total sample or divided by sex (Fig. 1 ).
Table 1.
Characterization of Covid-19 patients by tertile of neutrophil-to-lymphocyte ratio.
| Variables | Tertile 1 | Tertile 2 | Tertile 3 | p-Valuea |
|---|---|---|---|---|
| Demographic | N = 23 | N = 23 | N = 22 | |
| Age, y | 71.1 ± 9.0 | 73.2 ± 9.1 | 71.7 ± 10.4 | 0.814 |
| Sex, % | 0.184 | |||
| Male | 52.2 (30.9; 73.4) | 21.7 (4.2; 39.3) | 72.7 (53.3; 92.1) | |
| Female | 47.8 (26.6; 69.1) | 78.3 (60.7; 95.8) | 27.3 (7.9; 46.7) | |
| Health conditionsd | ||||
| Neurological diseases, % | 0.979 | |||
| Yes | – | 8.7 (−3.3; 20.7) | – | |
| No | – | 91.3 (79.3; 103) | – | |
| Cardiovascular diseases, % | 0.149 | |||
| Yes | 78.3 (60.7; 95.8) | 87.0 (72.6; 101) | 59.1 (37.7; 80.5) | |
| No | 21.7 (4.2; 39.3) | 13.0 (−1.3; 27.4) | 40.9 (19.5; 62.3) | |
| Disorders of the digestive tract, % | 0.392 | |||
| Yes | – | 4.4 (−4.3; 13.0) | 4.6 (−4.5; 13.6) | |
| No | – | 95.6 (86.9; 104) | 95.4 (86.4; 104) | |
| Diseases of the Genitourinary system, % | 0.555 | |||
| Yes | 8.7 (−3.3; 20.7) | 4.4 (−4.3; 13.0) | 4.6 (−4.5; 13.6) | |
| No | 91.3 (79.3; 103) | 95.6 (86.9; 104) | 95.4 (86.4; 104) | |
| Respiratory system diseases, % | 0.076 | |||
| Yes | 17.4 (1.3; 33.5) | 13.0 (−1.3; 27.4) | – | |
| No | 82.6 (66.5; 98.7) | 86.9 (72.6; 101) | – | |
| Endocrine diseases, % | 0.661 | |||
| Yes | 56.5 (35.4; 77.6) | 52.2 (30.9; 73.4) | 50 (28.2; 71.8) | |
| No | 43.5 (22.4; 64.6) | 47.8 (26.6; 69.1) | 50 (28.2; 71.8) | |
| Infections, % | 0.392 | |||
| Yes | – | 4.4 (−4.3; 13.0) | 4.5 (−4.5; 13.6) | |
| No | – | 95.6 (86.9; 104) | 95.4 (86.4; 104) | |
| Neoplasms, % | 0.661 | |||
| Yes | 13.0 (−1.3; 27.4) | 8.7 (−3.3; 20.7) | 9.1 (−3.4; 21.6) | |
| No | 87.0 (72.6; 101) | 91.3 (79.3; 103) | 90.9 (78.4; 103) | |
| Mental ilnesess, % | 0.311 | |||
| Yes | 4.4 (−4.3; 13.0) | 13.0 (−1.3; 27.4) | 13.6 (−1.3; 28.6) | |
| No | 95.6 (89.9; 104) | 87.0 (72.6; 101) | 86.4 (71.4; 101) | |
| Anthropometric data | ||||
| Weight, kg | 73.1 ± 14.2 | 68 ± 15.5 | 73.9 ± 24.7 | 0.901 |
| Height, m | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 0.189 |
| Body mass index, kg/m2 | 27.7 ± 5.4 | 26.5 ± 6.2 | 26.7 ± 8.0 | 0.595 |
| Sarcopenia risk | ||||
| Sarc-f (score) | 4.4 ± 2.8 | 5.9 ± 2.1 | 5.6 ± 2.6 | 0.099 |
| Risk of sarcopenia, % | 56.5 (35.4; 77.6) | 82.6 (66.5; 98.7) | 81.8 (65.0; 98.6) | 0.058 |
| No risk of sarcopenia, % | 43.5 (22.4; 64.6) | 17.4 (1.3; 33.5) | 18.2 (1.4; 34.9) | |
| Hospitalization | 0.381b | |||
| ICU | 21.7 (4.2; 39.3) | 34.8 (14.5; 55.1) | 40.9 (19.5; 62.3) | |
| Ward | 78.3 (60.7; 95.8) | 65.2 (44.9; 85.5) | 59.1 (37.7; 80.5) | |
| Biochemical parameters | ||||
| Neutrophil-to-lymphocyte ratio | 2.4 ± 0.8c | 5.0 ± 0.6c | 8.8 ± 3.0c | <0.001* |
| Neutrophils count | 4261.7 ± 2284.9c | 7449.0 ± 2689.6c | 11,821.3 ± 12,546.0c | 0.001* |
| Lymphocytes count | 1790.2 ± 814.1 | 1519.0 ± 616.5 | 1324.9 ± 1155.5 | 0.080 |
| C-reactive protein, mg/dL | 68.9 ± 117.8c | 88.5 ± 109.0c | 157.1 ± 116.0c | 0.012* |
| D-dimer, ng/mL | 1119.2 ± 1133.1c | 2214.6 ± 2014.5c | 3490.4 ± 4621.8c | 0.012* |
Data are described as mean ± standard deviation or percentage (confidence interval).
p of trend or
Fisher's exact.
Pairwise test.
Health events prior to the diagnosis of covid-19. Tertile of neutrophil-to-lymphocyte ratio: tertile 1: 1.1–3.85; tertile 2: 3.9–6 and tertile 3: 6.5–20.
p-value is statistically significant.
Fig. 1.
Linear regression between tertiles of neutrophil-to-lymphocyte ratio and SARC-F. Tertile of neutrophil-to-lymphocyte ratio: tertile 1: 1.1–3.85; tertile 2: 3.9–6 and tertile 3: 6.5–20.
Discussion
We did not find association between NLR and the risk of sarcopenia in elderly hospitalized patients with COVID-19. Although COVID-19 is an acute disease, we observed that patients presented high SARC-F values independent of tertile.
It is known that aging naturally promotes inflammation and age is a determinant of COVID-19 severity.19 In this sense, when considering COVID-19, Belice, Demir and Yüksel (2020), observed higher NLR values in patients older than 65 years of age, higher mortality risk and even higher NLR in men (9.1 ± 0.5) compared to women (4.4 ± 0.8).20 However, in the present study we also did not observe any difference between the NLR in relation to sex, since there is a great oscillation of the NLR values. In the first tertile, the age and presence of COVID-19, the mean ranged from 1.1 to 3.8, corresponding to normal values at the least for a healthy adult population.21 However, Long et al. found that an NLR ≥ 2.973 in patients with COVID-19 may already indicate severity in Sars-CoV-2 infection.22 Similarly, Liu et al. observed high criticality of COVID-19 in patients aged ≥ 50 years and with NLR ≥ 3.13.23 In the second and third tertiles, we found increased values of the mean NLR, varying in the second tertile of 3.9–6.0 and in the third of 6.5–20.0, corresponding to values associated with a higher severity of COVID-19.17 A cross-sectional study with 123 cancer patients found that an NLR ≥ 6.5 was associated with a higher risk of sarcopenia.24 The positive association between NLR and sarcopenia has been described in several types of cancer.25, 26 A study of 508 patients with a mean age of 67 years, diagnosed with COVID-19, revealed that an NLR > 6.5 is associated with severity, increasing by 5 times the risk of death.27 Increased levels of NLR above 11 ≥ 17, and may exceed 30, may express the criticality of the disease, hyperinflammation and reach of pathological stress.28 Therefore, considering the proportion of patients with high NLR values in the second and third tertiles, the high C-reactive protein and D-dimer values and the high SARC-F values, we confirmed that they are severe and inflamed patients, and that possibly the small sample size of our study justifies the absence of associations.
Despite the existence of studies evaluating the NLR with COVID-1915, 19 and the risk of sarcopenia in patients with COVID-19,4 our study was the first to evaluate the association between these two variables. On the other hand, this study presents some limitations such as (i) the small sample size and absence of sample size calculus, because it study was conducted during pandemic disease; and (ii) the lack of differentiation between patients in ICU or medical ward; thus, large cross-sectional studies are crucial to confirm the data.
In conclusion, a high NLR was not associated with the risk of sarcopenia in our sample of hospitalised elderly patients with COVID-19.
Authors’ contributions
JS, BMG, PCBL, VAA and GDP contributed to the design of the research; JS, BMG and VAA participated to the acquisition of the data; JS, BMG and PCBL done the statistical analysis; JS, BMG, PCBL, VAA and GDP partaken to the interpretation of the data; All authors drafted the article. All authors revised the manuscript, read and approved the final version of manuscript.
Funding
No funding was received for this work.
Conflict of interest
None declared.
Acknowledgements
GDP would like to thank The Brazilian National Council for Scientific and Technological Development (CNPq, Brazil, 312252/2019-6).
References
- 1.Adil M.T., Rahman R., Whitelaw D., Jain V., Al-Taan O., Rashid F., et al. SARS-CoV-2 and the pandemic of COVID-19. Postgrad Med J. 2021;97:110–116. doi: 10.1136/postgradmedj-2020-138386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esakandari H., Nabi-Afjadi M., Fakkari-Afjadi J., Farahmandian N., Miresmaeili S.M., Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced Online. 2020;22:1–10. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley J.E., Kalantar-Zadeh K., Anker S.D. COVID-19: a major cause of cachexia and sarcopenia? J Cachexia Sarcopenia Muscle. 2020;11:863–865. doi: 10.1002/jcsm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierdsma N.J., Kruizenga H.M., Konings L.A., Krebbers D., Jorissen J.R., Joosten M.H.I., et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin Nutr ESPEN. 2021 doi: 10.1016/j.clnesp.2021.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg I.H. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Bauer J., Morley J.E., Schols A.M.W.J., Ferrucci L., Cruz-Jentoft A.J., Dent E., et al. Sarcopenia: a time for action an SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10:956–961. doi: 10.1002/jcsm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kizilarslanoglu M.C., Kuyumcu M.E., Yesil Y., Halil M. Sarcopenia in critically ill patients. J Anesth. 2016;30:884–890. doi: 10.1007/s00540-016-2211-4. [DOI] [PubMed] [Google Scholar]
- 8.Papadopoulou S.K. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. 2020:12. doi: 10.3390/nu12051293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krznarić Ž., Bender D.V., Laviano A., Cuerda C., Landi F., Monteiro R., et al. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr. 2020;39:1983–1987. doi: 10.1016/j.clnu.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riesgo H., Castro A., del Amo S., San Ceferino M.J., Izaola O., Primo D., et al. Prevalence of risk of malnutrition and risk of sarcopenia in a reference hospital for COVID-19: relationship with mortality. Ann Nutr Metab. 2021:1–6. doi: 10.1159/000519485. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y., He M., Hou L.S., Xu S., Huang Z.X., Zhao N., et al. The role of SARC-F scale in predicting progression risk of COVID-19 in elderly patients: a prospective cohort study in Wuhan. BMC Geriatr. 2021;21:4–11. doi: 10.1186/s12877-021-02310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pimentel G.D., Dela Vega M.C.M., Laviano A. High neutrophil to lymphocyte ratio as a prognostic marker in COVID-19 patients. Clin Nutr ESPEN. 2020;40:101–102. doi: 10.1016/j.clnesp.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang J., Liu R., Yu X., Yang R., Xu H., Mao Z., et al. The neutrophil–lymphocyte count ratio as a diagnostic marker for bacteraemia: a systematic review and meta-analysis. Am J Emerg Med. 2019;37:1482–1489. doi: 10.1016/j.ajem.2018.10.057. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z., Fu Z., Huang W., Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38:641–647. doi: 10.1016/j.ajem.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92:1733–1734. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eid M.M., Al-Kaisy M., Regeia W.A.L., Khan H.J. The prognostic accuracy of neutrophil–lymphocyte ratio in covid-19 patients. Front Emerg Med. 2021;5:1–5. doi: 10.22114/ajem.v0i0.472. [DOI] [Google Scholar]
- 17.Yang A.P., Ping L.J., Qiang T.W., Ming L.H. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Rocha A.Q., Lobo P.C.B., Pimentel G.D. Muscle function loss and gain of body weight during the COVID-19 pandemic in elderly women: effects of one year of lockdown. J Nutr Heal Aging. 2021;25:1028–1029. doi: 10.1007/s12603-021-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciccullo A., Borghetti A., Zileri Dal Verme L., Tosoni A., Lombardi F., Garcovich M., et al. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. 2020:56. doi: 10.1016/j.ijantimicag.2020.106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belice T., Demir I., Yüksel A. Role of neutrophil–lymphocyte-ratio in the mortality of males diagnosed with covid-19. Iran J Microbiol. 2020;12:194–197. doi: 10.18502/ijm.v12i3.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forget P., Khalifa C., Defour J.P., Latinne D., Van Pel M.C., De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10:1–4. doi: 10.1186/s13104-016-2335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long L., Zeng X., Zhang X., Xiao W., Guo E., Zhan W., et al. Short-term outcomes of COVID-19 and risk factors for progression. Eur Respir J. 2020;318:50–52. doi: 10.1183/13993003.00990-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:1–12. doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges T.C., Gomes T.L., Pichard C., Laviano A., Pimentel G.D. High neutrophil to lymphocytes ratio is associated with sarcopenia risk in hospitalized cancer patients. Clin Nutr. 2021;40:202–206. doi: 10.1016/j.clnu.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Petrova M.P., Donev I.S., Radanova M.A., Eneva M.I., Dimitrova E.G., Valchev G.N., et al. Sarcopenia and high NLR are associated with the development of hyperprogressive disease after second-line pembrolizumab in patients with non-small-cell lung cancer. Clin Exp Immunol. 2020;202:353–362. doi: 10.1111/cei.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Q., Mao W., Wu T., Xu Z., Yu J., Wang C., et al. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are associated with sarcopenia risk in hospitalized renal cell carcinoma patients. Front Oncol. 2021;11:1–8. doi: 10.3389/fonc.2021.736640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haghjooy Javanmard S., Vaseghi G., Manteghinejad A., Nasirian M. Neutrophil-to-Lymphocyte ratio as a potential biomarker for disease severity in COVID-19 patients. J Glob Antimicrob Resist. 2020;22:862–863. doi: 10.1016/j.jgar.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahorec R. Neutrophil-to-lymphocyte ratio past, present and future perspectives. Bratislava Med J. 2021;122:474–488. doi: 10.4149/BLL. [DOI] [PubMed] [Google Scholar]