Abstract
The performance of a solar photocatalysis reactor as pretreatment for the removal of total organic carbon (TOC) and turbidity from municipal wastewater was achieved by implementing an integrated system as tertiary treatment. The process consisted of ultraviolet (UV) sunlight, UV sunlight/H2O2, and UV sunlight/TiO2 nanocatalysts as pretreatment steps to prevent ultrafiltration (UF) membrane fouling. The characterization of TiO2 was conducted with X-ray diffraction spectroscopy, Fourier-transform infrared spectroscopy, scanning electron microscopy , and Brunauer–Emmett–Teller surface area analysis. This study investigated the effect of time and solar radiation using UV, UV/H2O2, and UV/TiO2 to remove TOC and turbidity. The transmembrane pressure improvement was studied using a UF membrane system to pretreat wastewater with different UV doses of sunlight for 5 h and UV/H2O2 and UV/TiO2. The results showed that the highest removal efficiency of the turbidity and TOC reached 95% and 31%, respectively. The highest removal efficiency of the turbidity reached 40, 75, and 95% using UV, UV/H2O2, and UV/TiO2, respectively, while the optimal removal efficiency of TOC reached 20%, 30%, and 50%, respectively.
Subject terms: Chemical engineering, Environmental sciences, Materials science
Introduction
Wastewater treatment plants use activated sludge systems as a secondary treatment to remove organics, suspended solids, and nutrients1; membrane filtrations are conducted as a tertiary treatment to produce high-quality water for reuse and reclamation for various purposes2. The most advanced wastewater plants employ tertiary treatments based on membrane technologies, which mainly consist of pressure-driven membrane-like ultrafiltration (UF) and reverse osmosis (RO)3–9. Membrane technologies, particularly pressure-driven membranes, are considered the most promising approaches for reusing water9. However, microorganism colloids, dissolved organic matter, and suspended solids in the wastewater effluent.
cause membrane fouling on the surface or within the membranes’ pores. Approximately 10% of the effluent dissolved organic carbon (DOC) contributes to membrane fouling10,11, which decreases the membrane performance productivity, increases backwashing, and increases the costs of both membrane replacement and general treatment12. Decreasing fouling is of fundamental concern in membrane processes because it can increase the membranes’ operational life and decrease the membrane cleaning operations13. Numerous approaches, such as adsorption14–17, coagulation18,19, oxidation20, and ionic exchange21, have been investigated to reduce organic fouling, prevent fouling on the membranes, and improve the membrane’s filtration performance. Among these, advanced oxidation processes (AOPs) are some of the most promising22. The destructive process of AOPs is highly effective at removing organic compounds, especially from water. AOPs are characterized by the generation of highly reactive species, such as hydroxyl radicals (OH), which have a very high redox potential (2.8 V)21–24. Advanced oxidation processes can consist of chemical processes, including ozonation, H2O2 oxidation, the Fenton reaction, electrochemical or photochemical oxidation, and photochemical processes (e.g., photocatalysis, photolysis, the photo-Fenton reaction, solar heterogeneous photocatalytic oxidation, and combined UV/TiO2/O3 and UV/O3)24,25. Simultaneously using two or more types of AOPs has proven more effective in removing organic pollutants than using a single method alone24,26,27. Because of its nontoxicity, physical and optical properties, high stability, and high photocatalytic activity, titanium dioxide (TiO2) is the most commonly used and investigated catalyst25. It has numerous ideal properties (e.g., eco-friendly, low energy bandgap, resistance to photo-corrosion, and high UV absorption) and can be used without additives26. Further, TiO2 can only operate in the UV spectrum27–29. Advantageously, solar heterogeneous photocatalytic oxidation does not rely on lamps or LEDs29–31. This wavelength is controlled by the bandgap of the photocatalyst, which produces hydroxyl radicals and holes. The most frequently investigated photocatalyst, TiO2, exhibits a bandgap of 3.0 eV for the rutile modification and 3.2 eV for the anatase modification. The TiO2 material can only absorb wavelengths below 400 nm. Approximately 5% of solar radiation comes from this spectral range29,32–34. TiO2 particles entrapped in membranes or having titanium deposition on their surfaces may exhibit improved hydrophilicity, thereby reducing fouling30,31.
In this research, a commercial powder catalyst of TiO2 was employed and characterized for the photocatalytic degradation of municipal contaminants by adding UV/H2O2. The structure and performance of TiO2 were determined by various characterizations using scanning electron microscopy (SEM), X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) surface area analysis, and Fourier-transform infrared spectra (FT-IR). The study aimed to evaluate the feasibility and efficiency of using UV, UV/H2O2, and UV/TiO2 as a pretreatment step to control fouling in UF membranes while using the solar photooxidation process for the tertiary treatment of secondary effluent from municipal wastewater treatment plants.
Materials and methods
Materials
During the present study, wastewater from a wastewater treatment plant in Baghdad, Iraq, was collected and analyzed. Titanium dioxide (TiO2, purity ≥ 99%) and hydrogen peroxide (H2O2, 30% solution [w/w] in H2O) were used, with all chemicals purchased from Thomas Baker (India).
Characterization
X-ray powder diffraction (XRD) tests were conducted with a diffraction unit (Shimadzu-6000, Japan) at the Nanotechnology and Advanced Research Materials Center/University of Technology (Baghdad). A scanning electron microscope (SEM) (VEGA 3 LM, Germany) available at the Central Service Laboratory (College of Education for Pure Sciences/Ibn Al Haitham/Baghdad University) was used to perform morphological analysis of the catalyst TiO2. The total pore volume and specific surface area of the catalyst TiO2 were measured utilizing a Brunauer–Emmett–Teller (BET) surface area analyzer (Q-surf 9600, USA) from the Petroleum Research and Development Center (Baghdad). A Fourier-transform infrared (FT-IR) spectrophotometer (Bruker Tensor 27, Germany) recorded the FT-IR spectra ranging from 500 to 4000 cm−1.
Pretreatment process setup
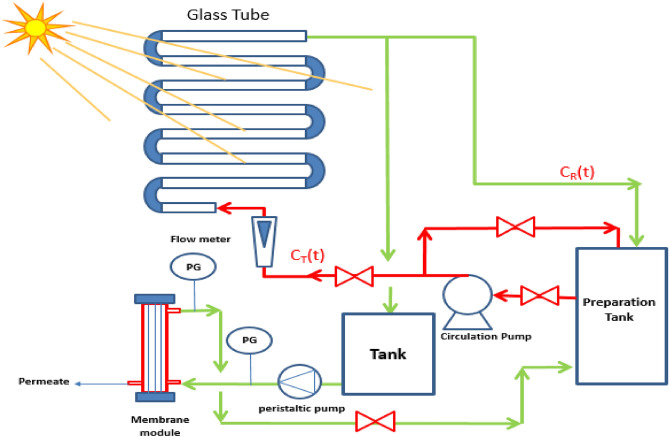
The solar photooxidation process of wastewater pretreatments was carried out with sunlight using a solar reactor system consisting of eight connected tubular glass pipes (0.022 m inside diameter and 0.55 m long). These pipes were supported with steel construction and sheltered via a reflective surface constructed from aluminum foil, as shown in Fig. 1. To mix and circulate the water at different flow rates; the photo-reactor was equipped with a pump with a capacity of 10–100 mL/min to ensure wastewater homogeneity in the glass tubes. The photo reactor was mounted and tilted at a 45° angle.
Figure 1.
Schematic representation of the solar photocatalytic reactor and membrane filtration systems.
Ultrafiltration system
The experimental arrangement of the ultrafiltration system, shown in Fig. 1, involved a membranes module, pressure gauge, and peristaltic pump. The UF module was connected to an influent tank with 5 L of wastewater collected from the photo-reactor tank. The peristaltic pump was operated at different flow rates. The pressure gauge continuously measured the transmembrane pressure (TMP).
Effluent wastewater
The wastewater sample was collected and analyzed from a wastewater treatment plant in Baghdad city. It was taken from the secondary clarifiers without nutrient removal and saved at 4 °C in a refrigerator throughout this study. Table 1 shows the physicochemical properties of wastewater influent used in this experiment without any other pretreatment.
Table 1.
Main Characteristics of the secondary wastewater.
| No | Parameter | Unit | Value |
|---|---|---|---|
| 1 | BOD5 | mg/L | 15–20 |
| 2 | COD | mg/L | 10–20 |
| 3 | NTU | Mg/L | 5–12 |
| 4 | NH3-N | mg/L | 4–5 |
| 5 | TOC | mg/L | 13–16 |
| 6 | NO2-N | mg/L | 0.6–0.8 |
| 7 | TN | mg/L | 33–36 |
| 8 | PO43−3 | mg/L | 18–20 |
| 9 | TDS | mg/L | 1200 |
| 10 | pH | – | 7–7.5 |
All experiments were conducted in two parts. First, wastewater was pretreated using a solar reactor with UV sunlight, UV sunlight/H2O2, and UV sunlight/TiO2 at a constant flow rate of 50 mL/min for 6 h. At 30-min intervals, the transmembrane pressure (TMP) was recorded to indicate membrane fouling. At the same intervals, the feed and permeate samples were also analyzed. The initial H2O2 concentration in UV sunlight/H2O2 experiments was 15 mg/L. This H2O2 concentration was carefully chosen according to the typical range (5–50 mg/L) used in other works related to the UV/H2O2 treatment indicated by Zhang et al.6. On the other hand, UV sunlight/TiO2, with a catalyst concentration of 0.75 g/L, was used in our experiments. Based on the results indicated by Ghaly et al.33.
Second, a cleaning process was conducted at the end of the experimental run. According to the subsequent cleaning procedure, the membrane was aerated with air bubbles for 20 min to remove most of the cake layer. After that, a 0.5 g/L surfactant solution was prepared, and the membrane was soaked, followed by bleach cleaning.
Analytical methods
UV254 absorption was measured using a Shimadzu UV visible spectrophotometer as an indicator of the total organic carbon (TOC). The measurement of the UV radiation was conducted at the “Center of Solar Energy Research—Ministry of Science and Technology” using Davis 6152 C Vantage Pro 2 Weather Station radiometer. The following equation was used to calculate the percentage removal of wastewater16,35–39:
| 1 |
where Ci = initial concentration of wastewater, and Co = final concentration.
Results and discussion
Characterization of the catalyst
Figure 2 displays the spectroscopic structures of TiO2 that were analyzed by X-ray diffraction (XRD). The crystal planes [(101), (004), (200), (105), (211), and (204)] appeared in the powder catalyst of TiO231,40. The general morphologies and microstructures of TiO2 were investigated by SEM analysis, as shown in Fig. 3. The surface morphology of TiO2 is also displayed in Fig. 3. Spherical nanoparticles with diameters mainly ranging from 14 to 20 nm were revealed in the SEM image of TiO2 in Fig. 3, echoing the observations of TiO2 morphology reported by Jin et al.36. However, the results of Chong et al. agree with these conclusions41. Brunauer investigated the pore volume and surface area–Emmett–Teller (BET) surface area analysis to understand the roles of TiO2. The specific surface area (SBET) of the TiO2 was 290 m2/g, and the total pore volume was 0.64 cm3/g; the homogeneous distribution of nano-TiO2 particles and the unique creation of a kaolin-layered structure could explain the huge surface area and total pore volume of the TiO2. The FT-IR spectra of the samples, shown in Fig. 4, were used to analyze the vibrational bands and interface interactions. The range of 699–732 cm−1, representing the obvious stretching vibration of Ti–O–Ti, was displayed by all three samples42, while the stretching vibration of the hydroxyl bonds appeared on the region of the broad peaks within the range from 3100 to 3600 cm−1. Due to the surface-adsorbed water molecules, an H–O–H bending vibration can be assigned at the peak of 1630 cm−1. Hydroxyl bonds cause improved photocatalytic activity through the adsorbed water molecules and lead to the formation of the hydroxyl radical (OH•), which can be classified as an oxidant reacting with oxygen (O2) or a photo-induced hole (h+)42.
Figure 2.
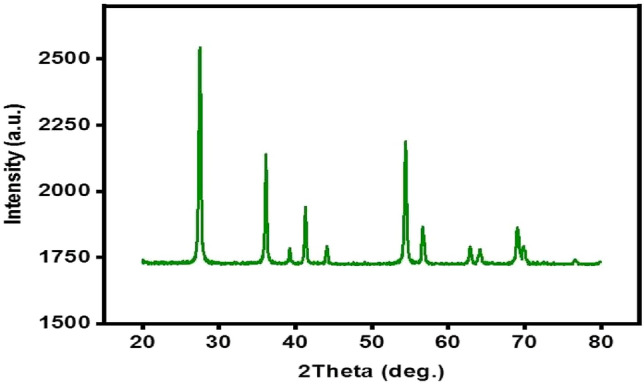
XRD images of TiO2.
Figure 3.

SEM images of TiO2.
Figure 4.
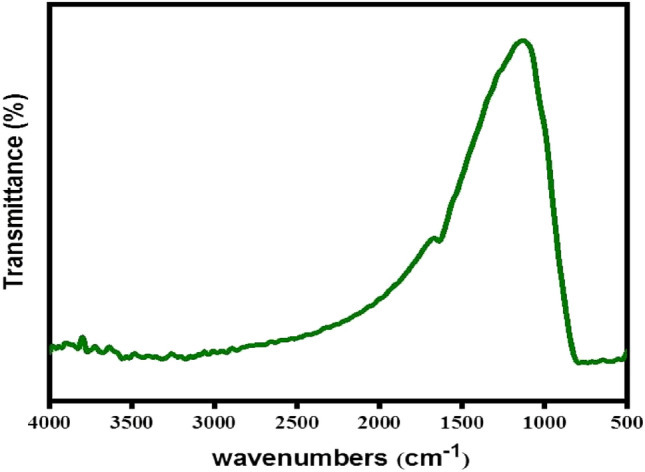
FTIR images of TiO2.
Influence of radiant flux
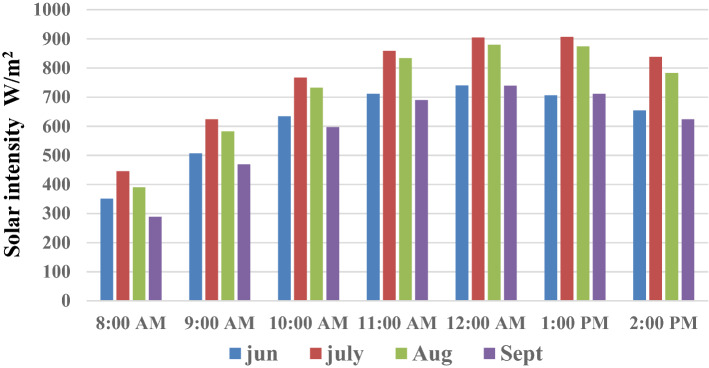
The chosen UV dosages were obtained from natural solar irradiation. The time of illumination vs. solar intensity is plotted in Fig. 5. All experiments were conducted between 8 a.m. and 2 p.m. local time, at a mean irradiance of 763 W/m2. The mean UV intensity for the complete experiment changed depending on the solar intensity. The UV intensity was recorded between 14.5 and 15.66 W/m2 from 8:00 a.m. to 2:00 p.m., corresponding to 2% of the power of the solar irradiation. The maximum solar intensity was 900 W/m2 at noon, with a UV intensity of 17.6 W/m2. In most common cases for disinfection, UV dosages do not exceed the value of 0.5 J cm−2, but the UV dosages obtained from natural solar irradiation were relatively higher than 0.5 J cm−243.
Figure 5.
The variation of solar intensity with the experimental time at several months.
Fouling reduction using UV-based pretreatment
Figure 6 shows the transmembrane pressure (TMP) improvement through the ultrafiltration system’s pretreated water using different UV intensity doses from the solar irradiation over 6 h. Through the first 60 min, the transmembrane pressure increased rapidly after filtration and reached a maximum after 6 h, after which the TMP decreased. However, without pretreatment, the TMP was at its highest, recording around 0.59 bar at the end of the run. In contrast, at the end of the run where UV sunlight was used, the reach was lower and reduced, indicating that pretreatment with UV sunlight dosages had a positive effect, yielding a TMP of approximately 0.42 bar. After pretreating the water with UV sunlight/H2O2 with an initial H2O2 concentration of 15 mg/L, the TMP was around 0.35 bar. In a simultaneous solar irradiation experiment, the TMP was recorded at approximately 0.32 bar at a catalyst concentration of 0.75 g/L. The reduction in the TMP in all previous experiments in this study has a similar trend.
Figure 6.
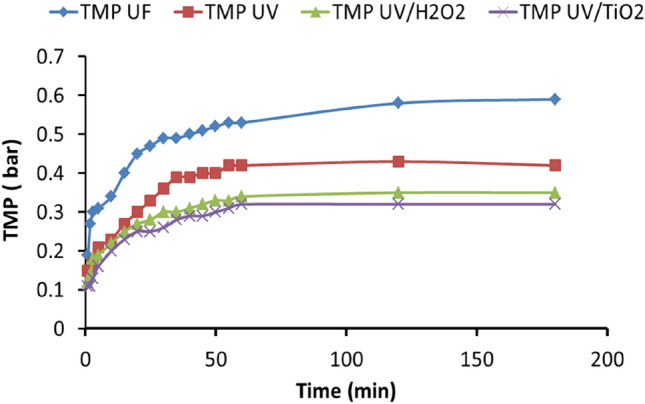
The effect of the time on TMP by UF, UV, UV/H2O2, and UV/TiO2.
Figure 7 shows that the maximum TMP peaked after 6 h using the UF system with the water treated with or without UV sunlight. It can be observed that the maximum TMP found in all experiments with UV sunlight, UV sunlight /H2O2, and UV sunlight/TiO2 was reduced by about 29, 41, and 45.8%, respectively, compared with those without pretreatment43.
Figure 7.
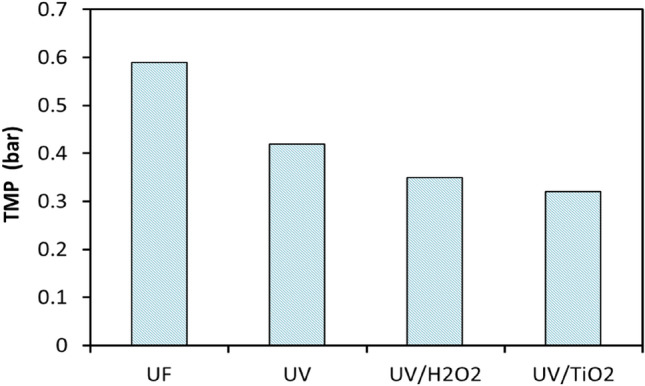
The effect of using UF, UV, UV/H2O2, and UV/TiO2 on TMP.
UV sunlight, UV sun/H2O2, and UV sun/TiO2 as pretreatments to enhance water quality
Removal efficiency of turbidity and TOC using the ultrafiltration (UF) process
The removal efficiency was reduced by 25%, with an initial value of 8 NTU and a final value of 6 NTU, while the removal efficiency for TOC was about 10%, with an initial value of 14 mg/L and a final value of 12.6 mg/L as shown in Fig. 8.
Figure 8.
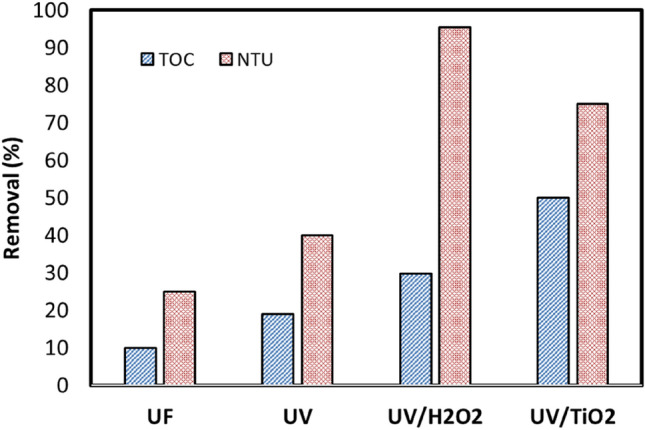
The effect of using UF, UV, UV/H2O2, and UV/TiO2 on removal efficiency of TOC and turbidity.
Removal efficiency of TOC and turbidity after the (UF) process using UV sunlight
The removal efficiency was increased up to 20%. It enhanced the removal efficiency of the turbidity to 40% as shown in Fig. 8. The results obtained appeared that UV irradiation is an effective technology for the removal of total organic carbon (TOC) and turbidity from municipal wastewater during the post-treatment of secondary effluents. Still, its efficiency depends on the type of organic compound and secondary effluent quality. In general, no influence of the kind of effluent was noticed for organic compounds with very slow or fast photo transformation kinetics. In contrast, for those compounds with intermediate kinetics, their photo transformation would be enhanced in effluents of better quality. Therefore, despite UV treatment being an efficient technology to photo transform organic compounds, small development or modifications such as; increasing UV dose, using oxidant agents such as; H2O2, and using catalysts to enhance the reduction of total organic carbon (TOC) and turbidity in UV systems44.
Removal efficiency of TOC and turbidity after the (UF) process Using UV sunlight/H2O2
The turbidity removal efficiency after UF improved by about 95%; in the experiments using UV sunlight/H2O2 at an H2O2 concentration of 15 mg/L within a 6-h period of solar irradiation, and the TOC removal efficiency increased up to 30%, as shown in Fig. 8.
The higher TOC removal in the UV/H2O2 pretreatment process can be explained by the fact that the ⋅OH radicals generated by the process are highly reactive and oxidize the organic substances45. The higher removal efficiency of the turbidity of the pretreated wastewater was associated with the UV or UV/H2O2 pretreatments affecting the TOC and likely affecting the suspended solids’ size.
Removal efficiency of TOC and turbidity after the (UF) process Using UV sunlight/TiO2
In the case of using TiO2 with UV sunlight, degradation experiments require a specific amount of catalyst, so the optimum catalyst loading for removing TOC from wastewater must be determined to avoid using the excess catalyst. Several authors have investigated the photocatalytic oxidation process as a function of catalyst loading with different semiconductor copmpounds33,46,47. Since TiO2 scatters light, excess TiO2 in suspensions will prevent sunlight from penetrating47,48. Therefore, the dosage of TiO2 in the photoreactor needs to be optimized, resulting in lower photocatalyst costs. Based on the results above, the optimum TiO2 concentration in this study was 0.75 g/L, the exact dosage of catalysts used by Ghaly et al.33 in their experiments.
In a simultaneous solar irradiation experiment, the TOC was removed from the wastewater by 50% at a catalyst concentration of 0.75 g/L. According to the results, the high decomposition observed under both solar light and TiO2 was solely due to the photocatalytic reaction of the semiconductor particles. The wastewater degradation was induced by the photoexcitation of semiconductors to electron–hole pairs on the catalyst’s surface49. At an optimum TiO2 loading of 0.75 g/L, the photocatalytic oxidation of the treated wastewater also showed a removal efficiency of 87.5% NTU, as shown in Fig. 8.
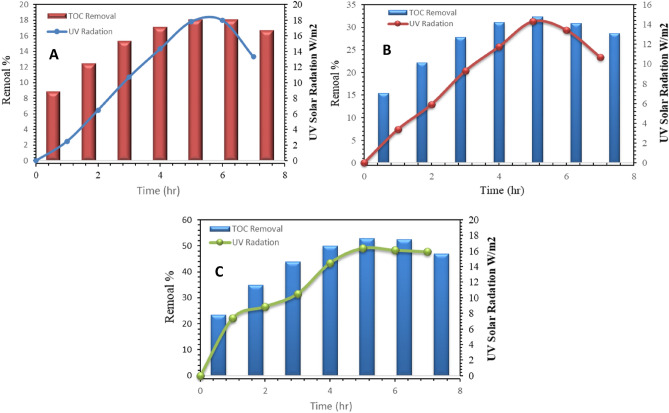
The removal efficiency of TOC as a function of solar UV intensity
The removal efficiency increased as the solar UV intensity increased in all experiments. Figure 9 shows that the TOC content decreased gradually as the UV intensity increased. In the case of UV sunlight alone, the removal of TOC increased and caused removals of from 2.49 to 18%, with UV intensity between 8.9 and 18.14 W/m2. Simultaneous UV intensity caused 7.4 to 31.3% TOC removal at an H2O2 concentration of 15 mg/L within a 6-h period of solar irradiation. Instantaneous solar irradiation with a UV intensity between 7.8 and 15.66 W/m2 caused 50% TOC removal at a catalyst dosage of 0.75 g/L of TiO2 within 6 h of solar irradiation. According to the results, high decomposition under both UV intensity from solar light and TiO2 processes was exclusively due to the photocatalytic reaction of the semiconductor particles. Furthermore, these experiments showed that UV intensity and TiO2 were necessary to treat wastewater effectively49.
Figure 9.
The effect of time and UV intensity on removal efficiency of TOC by using UF,(A): UV, (B): UV/H2O2, and (C): UV/TiO2.
Comparative study
This study dealt with treating municipal wastewater to remove TOC and turbidity by using UV, UV/H2O2, and UV/TiO2 in the solar photooxidation process through a batch system and using ultrafiltration membranes to control the membrane fouling process. Table 2 compares this study and others for the removal of TOC and turbidity. This table presents that the performance of a solar photocatalysis reactor as pretreatment for wastewater in an integrated system was a promising process for removing total organic carbon (TOC) and turbidity from municipal wastewater by implementing an integrated system as tertiary treatment.
Table 2.
Comparison between this study and other studies.
| No. | Process | Removal | Process conditions | References |
|---|---|---|---|---|
| 1 | Microfiltration and visible-light-driven photocatalysison g-C3N4nanosheet/reduced graphene oxide membrane | TOC = 21 %, Turbidity = 84% by memberance TOC= 42%, Turbidity = 90% by integrated process | Time = 1 h. For the g-C3N4NS/RGO/CA (100 gm in 200 mL) membrane under visible light irradiation, the stable permeation flux = 140 L m−2h−1, pH = 7.1. | 50 |
| 2 | Solar photocatalysis using TiO2/ZnO/H2O2 to pretreat reverse osmosis (membrane fouling) | TOC = 76.5% | Reaction time = 179 min, TiO2 =,0.51 g/L, ZnO = 0.46 g/L, pH= 6.9 and H2O2 = 0.89 mL/L | 51 |
| 3 | Photocatalytic by bi-polymer electrospun nanofibers embedding Ag3PO4/P25 composite | TOC = 86 %, turbidity = 50% | Reaction time = 150 min, flow rate (5 mL/h), pH = 7 | 52 |
| 4 | photocatalysis, Fenton-based processes and ozonation | TOC < 20%, TOC = 74% by ozonation combined with H2O2 | Reaction time = 4 h, H2O2/Fe = 0.5O3= 4 g h−1 , H2O2 = 1500 mg L−1 , pH =10, reaction time = 2 h , temperature= 20 °C | 53 |
| 5 | hybrid ultrafiltration/ reverse osmosis (UF/RO) systeme | TOC = 98% | TMP = 3 bar, CFV =1 m/s, temperature = 40 °C, and pH = 9 | 54 |
| 6 | Solar photocatalysis reactor by UV, UV/H2O2, and UV/TiO2 and ultrafiltration membrane fouling | By UV TOC = 20%, Turbidity = 40% by UV/H2O2 TOC= 30%, Turbidity= 95% by UV/TiO2 TOC = 50%, Turbidity = 87.8% by | Reaction time of 6 hr., solar radiation of 14.8–18.4 w/m2.hr, pH = 7.0, H2O2 = 15 mg/L, and flow rate = 50 mL/min | This study |
Conclusions
This solar photooxidation process and membrane filtration system study investigated the efficiency and performance evaluation for TOC and turbidity removal from municipal wastewater at a reaction time of 6 h. Using a solar photooxidation process, experiments were conducted using UV, UV/H2O2, and UV/TiO2. The UV intensity of 18.41 w/m2.hr achieved its highest reduction of TOC and turbidity, which was 20 and 40%, respectively. The UV intensity of 14.8 w/m2.hr with a 15 mg/L concentration of H2O2 at a pH of 7.0 achieved its highest reduction in the TOC and turbidity, 30 and 95%, respectively. The UV intensity of 17.6 w/m2.hr with a catalyst concentration of 0.75 g/L of TiO2 achieved its highest reduction of TOC and turbidity, 50 and 87.8%, respectively. In the membrane fouling process using ultrafiltration, the TMP with ultrafiltration combined with UV, UV/H2O2, and UV/TiO2 versus ultrafiltration alone was reduced by about 29.41, 41, and 45.8%, respectively, after 6 h, with a constant flow rate of 50 mL/min, with the highest removal of TOC and turbidity being 50% and 95%, respectively. It might be concluded from this study that the processes of UV, UV/H2O2, and UV/TiO2 using the solar photooxidation process prevented the UF membrane fouling with higher removal of TOC and turbidity.
Acknowledgements
We gratefully acknowledge the scientific support of the Department of Chemical Engineering, University of Technology-Iraq; Environment and Water Directorate, Ministry of Science and Technology, Iraq; and the University of Mustansiriyah, College of Engineering, Department of Materials Engineering/Baghdad-Iraq.
Author contributions
S.A., K.R.K., A.N.A. and T.M.A. wrote the main manuscript text and prepared all figures. All authors reviewed the manuscript."
Data availability
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalash KR, Kadhom MA, Al-Furaiji MH. Short-cut nitrification of Iraqi municipal wastewater for nitrogen removal in a single reactor. IOP Conf. Ser. Mater. Sci. Eng. 2019 doi: 10.1088/1757-899X/518/2/022024. [DOI] [Google Scholar]
- 2.Alardhi, S. M., Alrubaye, J. M.& Albayati, T.M. Removal of methyl green dye from simulated waste water using hollow fiber ultrafiltration membrane,In 2nd International Scientific Conference of Al-Ayen University (ISCAU-2020), IOP Conf. Series: Materials Science and Engineering, 928, 052020 10.1088/1757-899X/928/5/052020(2020).
- 3.Jeong K, Lee D-S, Kim D-G, Ko S-O. Effects of ozonation and coagulation on effluent organic matter characteristics and ultrafiltration membrane fouling. J. Environ. Sci. 2014;26:1325–1331. doi: 10.1016/S1001-0742(13)60607-5. [DOI] [PubMed] [Google Scholar]
- 4.Genz C, Miehe U, Gnirß R, Jekel M. The effect of pre-ozonation and subsequent coagulation on the filtration of WWTP effluent with low-pressure membranes. Water Sci. Technol. 2011;64:1270–1276. doi: 10.2166/wst.2011.724. [DOI] [PubMed] [Google Scholar]
- 5.Mozia S, Janus M, Brożek P, Bering S, Tarnowski K, Mazur J, Morawski AW. A system coupling hybrid biological method with UV/O3 oxidation and membrane separation for treatment and reuse of industrial laundry wastewater. Environ. Sci. Pollut. Res. 2016;23:19145–19155. doi: 10.1007/s11356-016-7111-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Fan L, Roddick FA. Effect of feedwater pre-treatment using UV/H2O2 for mitigating the fouling of a ceramic MF membrane caused by soluble algal organic matter. J. Memb. Sci. 2015;493:683–689. doi: 10.1016/j.memsci.2015.07.024. [DOI] [Google Scholar]
- 7.Koutahzadeh N, Esfahani MR, Arce PE. Sequential use of UV/H2O2-(PSF/TiO2/MWCNT) mixed matrix membranes for dye removal in water purification: Membrane permeation, fouling, rejection, and decolorization. Environ. Eng. Sci. 2016;33:430–440. doi: 10.1089/ees.2016.0023. [DOI] [Google Scholar]
- 8.Yu W, Campos LC, Graham N. Application of pulsed UV-irradiation and pre-coagulation to control ultrafiltration membrane fouling in the treatment of micro-polluted surface water. Water Res. 2016;107(83):92. doi: 10.1016/j.watres.2016.10.058. [DOI] [PubMed] [Google Scholar]
- 9.Kalash K, Kadhom M, Al-Furaiji M. Thin film nanocomposite membranes filled with MCM-41 and SBA-15 nanoparticles for brackish water desalination via reverse osmosis. Environ. Technol. Innov. 2020;20:101101. doi: 10.1016/j.eti.2020.101101. [DOI] [Google Scholar]
- 10.Laabs CN, Amy GL, Jekel M. Understanding the size and character of fouling-causing substances from effluent organic matter (EfOM) in low-pressure membrane filtration. Environ. Sci. Technol. 2006;40:4495–4499. doi: 10.1021/es060070r. [DOI] [PubMed] [Google Scholar]
- 11.Kastl G, Sathasivan A, Fisher I, Van Leeuwen J. Modeling DOC Removal by Enhanced Coagulation. J. Am. Water Work. Assoc. 2004 doi: 10.1002/j.1551-8833.2004.tb10557.x. [DOI] [Google Scholar]
- 12.Le-Clech P, Chen V, Fane TAG. Fouling in membrane bioreactors used in wastewater treatment. J. Memb. Sci. 2006;284:17–53. doi: 10.1016/j.memsci.2006.08.019. [DOI] [Google Scholar]
- 13.Mozia S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010;73:71–91. doi: 10.1016/j.seppur.2010.03.021. [DOI] [Google Scholar]
- 14.Kadhom M, Kalash K, Al-Furaiji M. Performance of 2D MXene as an adsorbent for malachite green removal. Chemosphere. 2022;290:133256. doi: 10.1016/j.chemosphere.2021.133256. [DOI] [PubMed] [Google Scholar]
- 15.Ali NS, Jabbar NM, Alardhi SM, Majdi SH, Albayati TM. Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: Isotherm, kinetics, and thermodynamic studies. Heliyon. 2022;8:10276. doi: 10.1016/j.heliyon.2022.e10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalash KR, Al-furaiji MH, Waisi BI, Ali RA. Evaluation of adsorption performance of phenol using non-calcined Mobil composition of matter no 41 particles. Desalin Water Treat. 2020;198:26018. doi: 10.5004/dwt.2020.26018. [DOI] [Google Scholar]
- 17.Khader EH, Mohammed TJ, Albayati TM. Comparative performance between rice husk and granular activated carbon for the removal of azo tartrazine dye from aqueous solution Desalin. Water Treat. 2021;229:372–383. doi: 10.5004/dwt.2021.27374. [DOI] [Google Scholar]
- 18.Jeong K, Lee DS, Kim DG, Ko SO. Effects of ozonation and coagulation on effluent organic matter characteristics and ultrafiltration membrane fouling. J. Environ. Sci. (China) 2014;26:1325–1331. doi: 10.1016/S1001-0742(13)60607-5. [DOI] [PubMed] [Google Scholar]
- 19.Eh K, Thj M, N M. Use of natural coagulants for removal of COD, Oil and turbidity from produced waters in the petroleum industry. J. Pet. Environ. Biotechnol. 2018 doi: 10.4172/2157-7463.1000374. [DOI] [Google Scholar]
- 20.Benito A, Garcia G, Gonzalez-Olmos R. Fouling reduction by UV-based pretreatment in hollow fiber ultrafiltration membranes for urban wastewater reuse. J. Memb. Sci. 2017;536:141–147. doi: 10.1016/j.memsci.2017.04.070. [DOI] [Google Scholar]
- 21.Faouzi M, Cañizares P, Gadri A, Lobato J, Nasr B, Paz R, Rodrigo MA, Saez C. Advanced oxidation processes for the treatment of wastes polluted with azoic dyes. Electrochim. Acta. 2006;52:325–331. doi: 10.1016/j.electacta.2006.05.011. [DOI] [Google Scholar]
- 22.Kern DI, de Schwaickhardt RO, Mohr G, Lobo EA, Kist LT, Machado ÊL. Toxicity and genotoxicity of hospital laundry wastewaters treated with photocatalytic ozonation. Sci. Total Environ. 2013;443:566–572. doi: 10.1016/j.scitotenv.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Rivas FJ, Carbajo M, Beltrán F, Gimeno O, Frades J. Comparison of different advanced oxidation processes (AOPs) in the presence of perovskites. J. Hazard. Mater. 2008;155:407–414. doi: 10.1016/j.jhazmat.2007.11.081. [DOI] [PubMed] [Google Scholar]
- 24.Agustina TE, Ang HM, Vareek VK. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C Photochem. Rev. 2005;6:264–273. doi: 10.1016/j.jphotochemrev.2005.12.003. [DOI] [Google Scholar]
- 25.Akpan UG, Hameed BH. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009;170:520–529. doi: 10.1016/j.jhazmat.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 26.Lee CM, Palaniandy P, Dahlan I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: A review. Environ. Earth Sci. 2017;76:611. doi: 10.1007/s12665-017-6924-y. [DOI] [Google Scholar]
- 27.Aziz NAA, Palaniandy P, Aziz HA, Dahlan I. Review of the mechanism and operational factors influencing the degradation process of contaminants in heterogenous photocatalysis. J. Chem. Res. 2016;40:704–712. doi: 10.3184/174751916X14769685673665. [DOI] [Google Scholar]
- 28.Esplugas S, Giménez J, Contreras S, Pascual E, Rodríguez M. Comparison of different advanced oxidation processes for phenol degradation. Water Res. 2002;36:1034–1042. doi: 10.1016/S0043-1354(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Ji M, Zhao Y, Wang L. Kinetics of aqueous photocatalytic oxidation of fulvic acids in a photocatalysis–ultrafiltration reactor (PUR) Sep. Purif. Technol. 2006;50:107–113. doi: 10.1016/j.seppur.2005.11.017. [DOI] [Google Scholar]
- 30.Zhang T, Wang X, Zhang X. Recent progress in TiO2-mediated solar photocatalysis for industrial wastewater treatment. Int. J. Photoenergy. 2014 doi: 10.1155/2014/607954. [DOI] [Google Scholar]
- 31.Ghaly MY, Jamil TS, El-Seesy IE, Souaya ER, Nasr RA. Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2. Chem. Eng. J. 2011;168:446–454. doi: 10.1016/j.cej.2011.01.028. [DOI] [Google Scholar]
- 32.Neppolian B, Choi HC, Sakthivel S, Arabindoo B, Murugesan V. Solar light induced and TiO2 assisted degradation of textile dye reactive blue 4. Chemosphere. 2002;46:1173–1181. doi: 10.1016/S0045-6535(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 33.Chan AHC, Chan CK, Barford JP, Porter JF. Solar photocatalytic thin film cascade reactor for treatment of benzoic acid containing wastewater. Water Res. 2003;37:1125–1135. doi: 10.1016/S0043-1354(02)00465-7. [DOI] [PubMed] [Google Scholar]
- 34.Kuo WS, Ho PH. Solar photocatalytic decolorization of methylene blue in water. Chemosphere. 2001;45(77):83. doi: 10.1016/S0045-6535(01)00008-X. [DOI] [PubMed] [Google Scholar]
- 35.Al-Bayati MT. Removal of aniline and nitrosubstituted aniline from wastewater by particulate nanoporous MCM48. Part. Sci. Technol. Int. J. 2014;32(6):616–623. doi: 10.1080/02726351.2014.948973. [DOI] [Google Scholar]
- 36.Kadhum ST, Alkindi GY, Albayati TM. Remediation of phenolic wastewater implementing nano zerovalent iron as a granular third electrode in an electrochemical reactor. Int. J. Environ. Sci. Technol. 2022;19(3):1383–1392. doi: 10.1007/s13762-021-03205-5. [DOI] [Google Scholar]
- 37.Khadim AT, Albayati TM, Saady NMC. Desulfurization of actual diesel fuel onto modified mesoporous material Co/MCM-41. Environ. Nanotechnol. Monit. Manag. 2022;17:100635. doi: 10.1016/j.enmm.2021.100635. [DOI] [Google Scholar]
- 38.Kadhum Shaimaa T, Alkindi Ghayda Yassen, Albayati Talib M. Determination of chemical oxygen demand for phenolic compounds from oil refinery wastewater implementing different methods. Desalin. Water Treat. 2021;231:44–53. doi: 10.5004/dwt.2021.27443. [DOI] [Google Scholar]
- 39.Al-Jaaf HJ, Ali NS, Alardhi SM, Albayati TM. Implementing eggplant peels as an efficient bio-adsorbent for treatment of oily domestic wastewater. Desalin. Water Treat. 2022;245:226–237. doi: 10.5004/dwt.2022.27986. [DOI] [Google Scholar]
- 40.Jin Z, Duan W, Duan W, Liu B, Chen X, Yang F, Guo J. Indium doped and carbon modified P25 nanocomposites with high visible-light sensitivity for the photocatalytic degradation of organic dyes. Appl. Catal. A Gen. 2016;517:129–140. doi: 10.1016/j.apcata.2016.02.022. [DOI] [Google Scholar]
- 41.Chong MN, Vimonses V, Lei S, Jin B, Chow C, Saint C. Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater. 2009;117:233–242. doi: 10.1016/j.micromeso.2008.06.039. [DOI] [Google Scholar]
- 42.Yen Y-C, Ou S, Lin K-J. One-Pot synthesis of nitrogen-doped TiO2 nanowires with enhanced photocurrent generation. J. Chinese Chem. Soc. 2017;64:1392–1398. doi: 10.1002/jccs.201700226. [DOI] [Google Scholar]
- 43.Dotson AD, Keen VS, Metz D, Linden KG. UV/H2O2 treatment of drinking water increases post-chlorination DBP formation. Water Res. 2010;44:3703–3713. doi: 10.1016/j.watres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Lakshmanan R, Sanchez-Dominguez M, Matutes-Aquino JA, Wennmalm S, Rajarao GK. Removal of total organic carbon from sewage wastewater using poly(ethylenimine)-functionalized magnetic nanoparticles. Langmuir. 2014;30(4):1036–1044. doi: 10.1021/la404076n. [DOI] [PubMed] [Google Scholar]
- 45.Aurelius Can solar energy take part in fighting against COVID-19 pandemic? A review on inactivation of SARS-CoV-2 in water and air using solar energy. Duke Law J. 2019;1:1–13. doi: 10.13140/RG.2.2.31215.66723/1. [DOI] [Google Scholar]
- 46.Addamo M, Augugliaro V, Paola A, García-López Di E, Loddo V, Marcì G, Molinari R, Palmisano L, Schiavello M. Preparation, characterization, and photoactivity of polycrystalline nanostructured TiO2 catalysts. J. Phys. Chem. B. 2004;108:3303–3310. doi: 10.1021/jp0312924. [DOI] [Google Scholar]
- 47.Pera-Titus M, García-Molina V, Baños MA, Giménez J, Esplugas S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004;47:219–256. doi: 10.1016/j.apcatb.2003.09.010. [DOI] [Google Scholar]
- 48.Gogate PR, Pandit AB. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv Environ. Res. 2004;8:501–551. doi: 10.1016/S1093-0191(03)00032-7. [DOI] [Google Scholar]
- 49.Vilhunen S, Sillanpää M. Recent developments in photochemical and chemical AOPs in water treatment: A mini-review. Rev. Environ. Sci. Biotechnol. 2010;9:323–330. doi: 10.1007/s11157-010-9216-5. [DOI] [Google Scholar]
- 50.Zhao H, Chen S, Quan X, Yu H, Zhao H. Integration of microfiltration and visible-light-driven photocatalysis on g-C 3 N 4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B Environ. 2016;194:134–140. doi: 10.1016/j.apcatb.2016.04.042. [DOI] [Google Scholar]
- 51.Joy VM, Feroz S, Dutta S. Solar nanophotocatalytic pretreatment of seawater: Process optimization and performance evaluation using response surface methodology and genetic algorithm. Appl. Water Sci. 2021;11:18. doi: 10.1007/s13201-020-01353-6. [DOI] [Google Scholar]
- 52.Habib Z, Lee C-G, Li Q, Khan SJ, Ahmad NM, Jamal Y, Huang X, Javed H. Bi-Polymer electrospun nanofibers embedding Ag3PO4/P25 composite for efficient photocatalytic degradation and anti-microbial activity. Catalysts. 2020;10:7–784. doi: 10.3390/catal10070784. [DOI] [Google Scholar]
- 53.Jiménez S, Andreozzi M, Micó MM, Álvarez MG, Contreras S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019;666:12–21. doi: 10.1016/j.scitotenv.2019.02.128. [DOI] [PubMed] [Google Scholar]
- 54.Salahi A, Badrnezhad R, Abbasi M, Mohammadi T, Rekabdar F. oily wastewater treatment using a hybrid UF/RO system Desalin. Water Treat. 2011;28:75–82. doi: 10.5004/dwt.2011.2204. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.