Abstract
In the yeast Saccharomyces cerevisiae, IRE1 encodes a bifunctional protein with transmembrane kinase and endoribonuclease activities. HAC1 encodes a transcription factor which has a basic leucine zipper domain. Both gene products play a crucial role in the unfolded protein response. Mutants in which one of these genes is defective also show the inositol-auxotrophic (Ino−) phenotype, but the reason for this has not been clear. To investigate the mechanism underlying the Ino− phenotype, we screened a multicopy suppressor gene which can suppress the Ino− phenotype of the Δhac1 strain. We obtained a truncated form of the ITC1 gene that has a defect in its 3′ region. Although the truncated form of ITC1 clearly suppressed the Ino− phenotype of the Δhac1 strain, the full-length ITC1 had a moderate effect. The gene products of ITC1 and ISW2 are known to constitute a chromatin-remodeling complex (T. Tsukiyama, J. Palmer, C. C. Landel, J. Shiloach, and C. Wu, Genes Dev. 13:686–697, 1999). Surprisingly, the deletion of either ITC1 or ISW2 in the Δhac1 strain circumvented the inositol requirement and caused derepression of INO1 even under repression conditions, i.e., in inositol-containing medium. These data indicate that the Isw2p-Itc1p complex usually represses INO1 expression and that overexpression of the truncated form of ITC1 functions in a dominant negative manner in INO1 repression. It is conceivable that the repressor function of this complex is regulated by the C-terminal region of Itc1p.
It is well known that the accumulation of an unfolded protein in the endoplasmic reticulum (ER) initiates the unfolded protein response (UPR). The UPR induces the transcriptional upregulation of multiple ER resident proteins involved in protein folding (for reviews, see references 20, 23, and 44). BiP/GRP78 is an abundant protein residing in the ER and essential for protein folding and protein sorting as a molecular chaperone. The structure of BiP is highly conserved from higher eukaryotes to yeast. In the yeast Saccharomyces cerevisiae, the BiP protein is encoded by KAR2. As in mammalian cells, the expression of KAR2 in yeast cells is induced by a variety of treatments, such as the addition of tunicamycin, which causes the accumulation of the unfolded protein in the ER. IRE1 encodes a bifunctional protein with transmembrane kinase and endoribonuclease activities that transmits the stress signal from the ER to the nucleus. The accumulation of the unfolded protein triggers Ire1p oligomerization, thereby inducing autophosphorylation, resulting in subsequent elicitation of the kinase and RNase activities. Activated Ire1p, together with the tRNA ligase encoded by RLG1 and Ada5p, causes unconventional splicing of HAC1 mRNA. HAC1 mRNA splicing allows efficient translation of Hac1p, which has a basic leucine zipper domain and functions as a transcriptional factor for genes regulated by the UPR, such as KAR2, PDI1, and FKB2. Since Hac1p is necessary for IRE1-mediated KAR2 induction as a positive transcription factor, mutants having a defect in ire1 or hac1 are unable to induce the transcription of KAR2, resulting in an inability of yeast cells to grow under stress conditions such as with the addition of tunicamycin.
The IRE1 gene was first identified as the gene for inositol prototrophy (Ino+) of S. cerevisiae (29), and the HAC1 gene was isolated as a multicopy suppressor gene for the ire1 mutation (26). Mutants having a defect in ire1 or hac1 show inositol auxotrophy (Ino−) due to an inability to fully induce the expression of the INO1 gene, which encodes a rate-limiting enzyme for inositol synthesis (4, 24, 26). In S. cerevisiae, inositol is synthesized de novo in cells through the conversion of glucose 6-phosphate to inositol 1-phosphate, followed by dephosphorylation (5). The former reaction is mediated by inositol 1-phosphate synthase encoded by INO1 (6). Inositol is also taken up into cells in a carrier-mediated manner. S. cerevisiae possesses two distinct inositol transport systems. The major transport system is encoded by ITR1, and the minor one is encoded by ITR2 (30, 31). It is known that the expression of INO1 and ITR1, as well as a number of genes for enzymes involved in the synthesis of phospholipids in S. cerevisiae, is repressed in cells grown in the presence of inositol and derepressed in cells grown in the absence of inositol (32). INO1 and other coregulated genes of phospholipid biosynthesis contain one or two stretches of a conserved cis-acting promoter element, termed the inositol-choline-responsive element (ICRE). The INO2 and INO4 genes encode basic helix-loop-helix proteins that form a heterodimer and function as a transcriptional factor through binding to the ICRE (1, 41). Mutants having a defect in not only ino1 but also ino2 or ino4 exhibit the Ino− phenotype (10, 12). Several other mutants also show the Ino− phenotype. For example, mutations in the large subunit of RNA polymerase II (40) and the TATA binding protein (2, 43) lead to the Ino− phenotype due to an inability to express the INO1 gene. Depletion of the general transcription factor TFIIA also impairs INO1 activation (21). Cells having defects in the SWI1, SWI2, and SWI3 genes, which encode components of the SWI-SNF chromatin-remodeling complex, exhibit a derepression defect of INO1 (33–35). Furthermore, deletion of the INO80 gene, which is an SNF2-SWI2 paralogue and encodes a component of the INO80 chromatin-remodeling complex, prevents the efficient expression of INO1 (7, 42). On the other hand, mutations in the SIN3 and UME6 genes lead to high-level INO1 expression (15, 16). The SIN3 and UME6 gene products are components of a large complex that contains the RPD3 gene product, a histone deacetylase (17, 18, 39). Deletion of the RPD3 gene also leads to high-level INO1 expression. Additionally, a mutation in the OPI1 gene that encodes a protein containing leucine zipper and polyglutamine stretch motifs leads to an inositol overproduction phenotype (47).
Little is known about the mechanism by which defects of the IRE1 or HAC1 gene lead to a decrease in INO1 expression or about the mechanism by which inositol regulates INO1 expression. In this study, we attempted to isolate and characterize the yeast gene that can suppress the Ino− phenotype of the Δhac1 strain when present in multiple copies. Here, we show that multiple copies of truncated ITC1 can suppress the Ino− phenotype of the Δire1 and Δhac1 strains and that the Isw2p-Itc1p complex usually represses INO1 expression.
MATERIALS AND METHODS
Yeast strains and culture.
S. cerevisiae strains D452-2 (MATα leu2 his3 ura3), as the wild-type strain, YF4 (MATα leu2 his3 ura3 ire1::URA3), and HU1 (MATα leu2 his3 ura3 hac1::URA3) were described previously (26). Yeast cells were cultured aerobically in either yeast-peptone-dextrose or synthetic minimal medium with shaking at 30°C. The compositions of the yeast-peptone-dextrose and inositol-free minimal medium were as described previously (48). Inositol was added to the minimal medium at a concentration of 20 μg/ml. When necessary, l-leucine, l-histidine, and uracil were each added to the culture media at 20 μg/ml. Tunicamycin was added to the culture media at 0.5 μg/ml.
Plasmid construction.
YCp50 (38) and YCpL2 (22) are centromere-based vectors with the URA3 and LEU2 genes as selectable markers, respectively. YCpH2 is a centromere-based vector with the HIS3 gene as a selectable marker and was constructed as follows. The 1.8-kbp BamHI fragment of the HIS3 gene was inserted into the BamHI site of pUC19 to yield pUC-HIS3. The 2.3-kbp EcoRI/SmaI fragment of YCp50 was replaced with the 1.8-kbp EcoRI/HincII fragment of pUC-HIS3 to yield YCpH2. YEpM4 (28) and pHV-1 (37) are 2μm DNA-based vectors with the LEU2 and HIS3 genes as selectable markers, respectively. pADANS is a 2μm DNA-based vector with the yeast ADH1 promoter, the following small part of the coding region, the ADH1 terminator, and the yeast LEU2 gene (3). The LEU2 gene of pADANS was replaced with the LEU2 gene of pGAD424 (Clontech). The LEU2 gene in pADANSΔE thus obtained has no EcoRI site. Plasmid pIR42, harboring HAC1, was described previously (25). To construct a single-copy plasmid carrying the HAC1 gene, pIR42 was digested with BamHI and SmaI. An approximately 3.5-kbp fragment containing the HAC1 gene was ligated between the BamHI and SmaI sites of YCpL2 to yield YCpL2-42. Plasmid YEp133t containing a truncated form of ITC1 was originally isolated from a yeast genomic library which was described previously (28). To construct a multicopy plasmid carrying a truncated form of PCL10, an approximately 1.8-kbp HindIII/ScaI fragment from YEp133t was ligated between the HindIII and ScaI sites of YEpM4 to yield YEpPCL10. To construct a single-copy plasmid containing the truncated form of ITC1, an approximately 4.2-kbp BamHI/XbaI fragment from YEp133t was ligated between the BamHI and XbaI sites of YCpL2 to yield YCpL2-133t.
To construct a multicopy plasmid containing full-length ITC1, ITC1 was amplified by PCR with chromosomal DNA as a template. The PCR primers used were 5′-CAATGGTGTTATATAAAAGG-3′ and 5′-GTATGGTCCAATCTTGCGCG-3′. An approximately 3.9-kbp PCR product was inserted into vector pCR2.1-TOPO (Invitrogen) to yield pTA-ITC1. pTA-ITC1 was digested with NcoI and SacI, and an approximately 1.7-kbp fragment containing the 3′ region of ITC1 was separated by electrophoresis. YEp133t was digested with NcoI and SacI and subjected to electrophoresis to remove the small fragment, and then the fragment containing the 3′ region of ITC1 described above was inserted between the NcoI and SacI sites of YEp133t to yield YEp133w. The sequence of the 3′ region of ITC1 amplified by PCR was verified. To construct a single-copy plasmid containing full-length ITC1, YEp133w was digested with BamHI and XbaI. An approximately 5.2-kbp fragment containing full-length ITC1 was ligated between the BamHI and XbaI sites of YCpL2 to yield YCpL2-133w. To construct a multicopy plasmid containing ITC1 deletion derivatives, an approximately 3.6-kbp HindIII/EcoRV fragment of YEp133w was inserted between the HindIII and SmaI sites of YEpM4 to yield YEp133Δ1. An approximately 3.9-kbp HindIII fragment of YEp133w was inserted into the HindIII site of YEpM4 to yield YEp133Δ2. To construct single-copy plasmids containing the INO1-lacZ fusion gene, YEpINO1Z (13) was digested with HindIII and SmaI. An approximately 4-kbp HindIII/SmaI fragment containing the INO1-lacZ fusion gene was treated with Klenow large fragment and then inserted into the SmaI sites of YCpH2 and YCpL2 to yield YCpH2-INO1Z and YCpL2-INO1Z, respectively. To construct a single-copy plasmid containing the ISW2 gene, pISW2w (see below) was digested with SpeI. An approximately 6.1-kbp fragment containing the ISW2 gene was inserted into the XbaI site of YCpL2 to yield YCpL2-ISW2. To construct a multicopy plasmid that expresses Isw2p under the control of the ADH1 promoter, the ISW2 gene was amplified by PCR with chromosomal DNA as a template. The PCR primers used were 5′-TCATGACAGCCCAGCAAG-3′ and 5′-GCTTCTTGATCAATTTTG-3′. An approximately 3.3-kbp PCR product was inserted into pCR2.1-TOPO to yield pTA-ISW2. pTA-ISW2 was digested with SpeI and XhoI, and an approximately 3.3-kbp SpeI/XhoI fragment containing the ISW2 gene was inserted between the SpeI and XhoI sites of pBluescript II KS(+) to yield pKS+ISW2. pKS+ISW2 was digested with NotI, and an approximately 3.3-kbp NotI fragment containing the ISW2 gene was inserted into the NotI site of pADANSΔE to yield pAD-ISW2. pAD-ISW2 was digested with BamHI, and an approximately 5.3-kbp BamHI fragment containing the ADH1-ISW2 fusion gene was inserted into the BamHI site of pUC18 to yield pUC18-ISW2. An approximately 5.3-kbp SalI/SmaI fragment of pUC18-ISW2 containing the ADH1-ISW2 fusion gene was inserted between the SalI and SmaI sites of pHV1 to yield pHV-ADISW2.
Isolation of the wild-type ISW2 gene.
For isolation of the wild-type ISW2 gene, genomic DNA from D452-2 was digested with BglII and XhoI, and then approximately 8-kbp fragments were separated by gel electrophoresis and inserted between the BamHI and XhoI sites of pBluescript II KS(+). Escherichia coli cells were transformed with the ligation mixture. A transformant harboring the yeast ISW2-containing plasmid was detected by the PCR method using the synthetic primers described above, and the plasmid pISW2w was recovered from the transformant.
Construction of gene-disrupted strains.
To construct the Δitc1, Δisw2, Δhac1 Δitc1, and Δhac1 Δisw2 strains, HIS3-disrupted ITC1 and HIS3-disrupted ISW2 gene fragments were constructed by the method for the synthesis of marker-disrupted alleles of yeast genes (27). The PCR primers used for ITC1 disruption were 5′-CAATGGTGTTATATAAAAGG-3′, 5′-GTGTCTCCTCACTATCCAG-3′, 5′-CTGGTTAGATAATTGGGG-3′, and 5′-CCTCGCGCCTGGCCTCTG-3′. The PCR primers used for ISW2 disruption were 5′-TCATGACGACCCAGCAAG-3′, 5′-GTACGTATCGGACTTGTC-3′, 5′-GAGGCAGAAAATCGAACAG-3′, and 5′-GCTTCTTGATCAATTTTG-3′. The HIS3-disrupted gene fragments were used for the transformation of D452-2 and HU1. His+ colonies were selected, and gene disruption was confirmed by PCR with their chromosomal DNA as a template. The Δitc1, Δisw2, Δhac1 Δitc1, and Δhac1 Δisw2 strains thus obtained were designated IH-1, SH-1, HIW, and HSW, respectively.
β-Galactosidase assay.
β-Galactosidase was assayed at 37°C by measuring the increase in the absorbance at 420 nm with o-nitrophenyl-β-d-galactoside as the substrate after cells had been permeabilized with chloroform and sodium dodecyl sulfate as described previously (14).
Northern blot analysis.
For Northern blot analysis, total RNA was isolated from yeast cells as described by Kataoka et al. (19). Samples were subjected to electrophoresis in a 1% agarose gel containing formaldehyde, blotted onto a Biodyne A membrane (Pall BioSupport), and then hybridized. The probes used were 32P-labeled DNA fragments (with BcaBEST Labeling Kit [Takara Biochemicals]) of the entire coding regions of INO1 and ACT1, which were prepared by PCR. Hybridization and detection were carried out according to the manufacturer's manual.
Microscopic analysis.
To investigate cell morphology, cells were grown to the early log phase in minimal medium containing inositol. Images were taken under a confocal laser scanning microscope (LSM510; Carl Zeiss).
RESULTS
Isolation of the suppressor gene.
Disruption of IRE1 or HAC1 in S. cerevisiae results in the Ino− phenotype (4, 26). To elucidate the mechanism underlying the Ino− phenotype caused by ire1 or hac1 disruption, we attempted to isolate yeast suppressor genes that, when present in multiple copies, can suppress the Ino− phenotype of the Δhac1 strain. The Δhac1 strain, HU1, was transformed with a yeast genomic library constructed on a multicopy vector, with transformants that grew on inositol-free minimal medium being selected. Plasmids were isolated from the independent transformants. After retransformation of plasmids into yeast cells, four plasmids were found to be able to complement the Δhac1 strain. Restriction endonuclease analysis and sequence analysis revealed that all four plasmids were identical and contained 4.2-kbp inserts. We designated these plasmids YEp133t and used them for further analysis. As shown in Fig. 1A, the insert fragment in plasmid YEp133t was derived from chromosome VII and included two truncated open reading frames (ORFs), PCL10 (YGL132w) and ITC1 (YGL133w). However, the coding region of PCL10 is incomplete and its promoter region is missing in plasmid YEp133t. The subcloning study indicated that the Δhac1 strain transformed with multicopy plasmid YEpPCL10 (Fig. 1A), which contains the 1.8-kbp HindIII/ScaI fragment derived from the insert fragment of YEp133t, exhibited the Ino− phenotype. Therefore, we concluded that ITC1 but not PCL10 suppresses the Ino− phenotype of the Δhac1 strain. The ITC1 present in plasmid YEp133t is the truncated form and lacks the 3′ region of the ORF. We designated this truncated gene ITC1Δ. Plasmid YEp133t clearly suppressed the Ino− phenotype of the Δhac1 strain on inositol-free minimal medium (Fig. 1B). Overexpression of ITC1Δ also suppressed the Ino− phenotype of the Δire1 strain. However, overexpression of ITC1Δ could not restore the tunicamycin sensitivity of the Δire1 and Δhac1 strains (data not shown). To determine whether a single copy of ITC1Δ could also suppress the Ino− phenotype of the Δire1 and Δhac1 strains, a CEN4-based plasmid harboring ITC1Δ, YCpL2-133t, was constructed and introduced into the Δire1 and Δhac1 strains. The transformants obtained showed the Ino− phenotype, indicating that the suppression of the Ino− phenotype of the Δire1 and Δhac1 strains is caused by the gene dosage effect of ITC1Δ (data not shown).
FIG. 1.
Cloning of the suppressor gene. (A) Restriction map of the genomic region of S. cerevisiae containing the suppressor gene. The size and transcriptional orientation of each ORF are shown by arrows. Solid bars indicate the inserts of originally isolated plasmid YEp133t and its subclone, YEpPCL10. +, ability to complement the Ino− phenotype of the Δhac1 strain; −, inability to complement it. Abbreviations: E, EcoT22I; H, HindIII; N, NcoI; S, ScaI; V, EcoRV. (B) Suppression of the Ino− phenotype of the Δhac1 strain by ITC1Δ. The Δhac1 strain, HU1, harboring YCpL2-42 (HAC1), YEp133t (ITC1Δ), or vector YEpM4 alone was streaked onto minimal medium containing histidine with or without inositol. The transformants were grown at 30°C for 3 days.
Overexpression of full-length ITC1 in a Δhac1 strain.
ITC1 encodes a protein of 1,264 amino acid residues. The ITC1 product, Itc1p, contains a nuclear localization signal and a leucine zipper domain, a motif frequently found in DNA-binding proteins (8). The originally isolated ITC1Δ encodes a truncated protein, Itc1Δp, which lacks 299 amino acids at the C-terminal end (Fig. 2A). We next examined whether full-length ITC1 can suppress the Ino− phenotype of the Δhac1 strain. For this purpose, we constructed multicopy plasmid YEp133w carrying full-length ITC1 (see Materials and Methods) and introduced it into the Δhac1 strain, HU1. Interestingly, full-length ITC1 suppressed the Ino− phenotype of the Δhac1 strain much more weakly than ITC1Δ (Fig. 2B). This revealed that deletion of the C-terminal region of Itc1p is important for efficient suppression of the Ino− phenotype of the Δhac1 strain.
FIG. 2.
Deletion analysis of ITC1. (A) Linear diagram of Itc1p with a schematic representation of the deletion derivatives. The nuclear localization signal and leucine zipper domain within Itc1p are shown as solid and hatched boxes, respectively. Amino acid numbers from the initiation methionine are shown at the top. The bars show the approximate sizes of the truncated forms of Itc1p, with the number of amino acids (aa) at the right of each bar. (B) Suppression of the Ino− phenotype of the Δhac1 strain by ITC1 deletion derivatives. HU1 harboring YCpL2-42 (HAC1), YEp133Δ1 (ITC1Δ1), YEp133Δ2 (ITC1Δ2), YEp133t (ITC1Δ), YEp133w (ITC1), or vector YEpM4 alone was 10-fold serially diluted and then spotted onto minimal medium containing histidine with or without inositol. The transformants were grown at 30°C for 3 days.
Deletion analysis of ITC1.
The C-terminal region of Itc1p missing in Itc1Δp does not contain any obvious amino acid motif. To investigate the functional significance of the C-terminal region of Itc1p in suppression of the Ino− phenotype of the Δhac1 strain, we next constructed multicopy plasmids carrying a series of ITC1 deletion derivatives (Fig. 2A). Plasmids YEp133Δ1 and YEp133Δ2 carry ITC1Δ1 and ITC1Δ2, respectively. ITC1Δ1 and ITC1Δ2 encode truncated proteins comprising amino acid residues 1 to 757 and 1 to 850, respectively. After transformation of these plasmids into the Δhac1 strain, HU1, the transformants were examined for their ability to suppress the Ino− phenotype of this strain. As shown Fig. 2B, all the transformants grew at approximately the same rate on inositol-containing medium. However, on inositol-free medium, only the transformant having multiple copies of ITC1Δ exhibited the Ino+ phenotype similarly to the transformant having a single copy of HAC1. In contrast, ITC1Δ1 and ITC1Δ2 suppressed the Ino− phenotype of the Δhac1 strain much more weakly than ITC1Δ did. This indicates that the deletion of about 299 amino acids at the C-terminal end of Itc1p is critical for suppression of the Ino− phenotype of the Δhac1 strain, but further deletion abolished the ability of the suppression. Taken together, the above results suggest that the C-terminal region of Itc1p has an inhibitory effect on suppression of the Ino− phenotype of the Δhac1 strain and that the function of Itc1p might be regulated by its C-terminal region.
Effect of overexpression of ITC1Δ on INO1 expression.
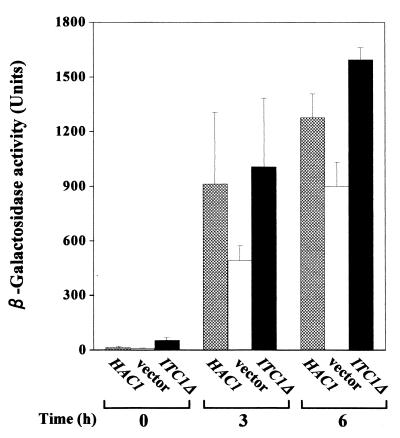
We next determined the effect of multiple copies of ITC1Δ on INO1 expression using the INO1-lacZ fusion gene as a reporter gene. Plasmid YCpH2-INO1Z carrying the INO1-lacZ fusion gene was introduced into the Δhac1 strain, HU1, together with a single copy of HAC1, multiple copies of ITC1Δ, or vector plasmid YEpM4. The transformants were grown in the absence of inositol, and then β-galactosidase activity was measured (Fig. 3). Consistent with a previous report (24), the level of β-galactosidase activity in the Δhac1 strain containing the vector alone was lower than that observed in the Δhac1 strain containing a single copy of HAC1. In contrast, the level of β-galactosidase activity in the Δhac1 strain transformed with multiple copies of ITC1Δ was higher than that observed in the Δhac1 strain transformed with the vector alone. These results indicate that the overexpression of ITC1Δ induces the transcriptional upregulation of INO1. This is why the Δhac1 strain transformed with YEp133t shows the Ino+ phenotype.
FIG. 3.
Effect of ITC1Δ on INO1 expression. The Δhac1 strain harboring YCpH2-INO1Z carrying the INO1-lacZ fusion gene together with YCpL2-42 (HAC1), YEp133t (ITC1Δ), or vector YEpM4 was precultured in minimal medium containing inositol for 12 h. The cells were then washed twice with water and cultured further in fresh minimal medium without inositol. At the indicated times, cells were removed from the cultures and β-galactosidase activity was measured. Data are means of three independent transformants. Error bars indicate standard deviations.
Phenotype of the Δhac1 Δitc1 strain.
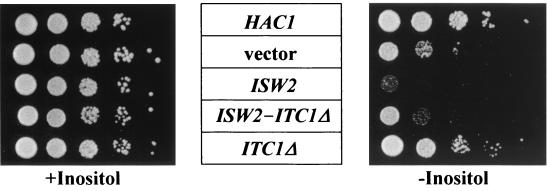
We next determined the effect of the deletion of ITC1 on cell growth. We first constructed a Δitc1 strain, IH-1, and its phenotype was examined. The Δitc1 strain did not show the Ino− phenotype and, as reported previously (8), exhibited aberrant morphology (see below). We next constructed a Δhac1 Δitc1 strain, HIW. The Δhac1 Δitc1 strain also exhibited aberrant morphology (data not shown). Surprisingly, the Δhac1 Δitc1 strain did not show the Ino− phenotype (Fig. 4A). To confirm this, a single copy of ITC1 on a CEN4-based vector was introduced into the Δhac1 Δitc1 strain and then its phenotype was examined. The transformants clearly exhibited the Ino− phenotype, similar to the Δhac1 strain (Fig. 4A). This indicates that the deletion of ITC1 can suppress the Ino− phenotype of the Δhac1 strain, suggesting that Itc1p is a negative regulator for the expression of INO1. However, the Δhac1 Δitc1 strain carrying a single copy of ITC1Δ did not exhibit the Ino− phenotype (Fig. 4A), suggesting that only full-length ITC1 can function as a negative regulator for the expression of INO1.
FIG. 4.
Phenotypes of the Δhac1 Δitc1 and Δhac1 Δisw2 strains. (A) Ino+ phenotype of the Δhac1 Δitc1 strain. The Δhac1 Δitc1 strain, HIW, harboring YCpL2-42 (HAC1), YCpL2-133t (ITC1Δ), YCpL2-133w (ITC1), or vector YCpL2 alone was grown on minimal medium with or without inositol at 30°C for 3 days. (B) Ino+ phenotype of the Δhac1 Δisw2 strain. The Δhac1 Δisw2 strain, HSW, harboring YCpL2-42 (HAC1), YCpL2-ISW2 (ISW2), or vector YCpL2 alone was grown on minimal medium with or without inositol at 30°C for 3 days. In both panels, the Δhac1 strain, HU1, harboring vector YCpH2 together with YCpL2-42 or vector YCpL2 is also shown, indicated by Δhac1 (HAC1) and Δhac1 (vector), respectively.
Phenotype of the Δhac1 Δisw2 strain.
Recently, it was reported that Itc1p, together with a gene product of ISW2 (45), constitutes a chromatin-remodeling complex (http://genome-www.stanford.edu/Saccharomyces/). Therefore, we next determined the effect of Isw2p on the expression of INO1. For this purpose, we constructed a Δhac1 Δisw2 strain, HSW. This strain exhibited aberrant morphology, similar to that of the Δhac1 Δitc1 strain (data not shown), and also exhibited the Ino+ phenotype. To confirm this more exactly, the Δhac1 Δisw2 strain was transformed with a single copy of HAC1, a single copy of ISW2, or vector plasmid YCpL2. The transformants were grown on minimal medium with or without inositol, and then the phenotype was examined (Fig. 4B). The Δhac1 Δisw2 strain carrying the vector alone exhibited the Ino+ phenotype. In contrast, the Ino− phenotype reappeared when a single copy of ISW2 was introduced into the Δhac1 Δisw2 strain. This clearly suggests that Itc1p, together with Isw2p, functions as a negative regulator of INO1. It should be noted that the Δisw2 strain we constructed also exhibited the Ino+ phenotype and aberrant morphology, similar to the Δitc1 strain (see below).
The Isw2p-Itc1p complex represses INO1 expression.
The data presented above strongly suggest that the Isw2p-Itc1p chromatin-remodeling complex represses the expression of INO1. To examine this possibility further, we introduced single-copy plasmid YCpL2-INO1Z carrying the INO1-lacZ fusion gene into strains HU1 (Δhac1), IH-1 (Δitc1), HIW (Δhac1 Δitc1), SH-1 (Δisw2), HSW (Δhac1 Δisw2), and D452-2 (wild type). The transformants were cultured in inositol-containing minimal medium, and then β-galactosidase activity was measured. As shown in Fig. 5A, the wild-type and Δhac1 cells exhibited low levels of β-galactosidase activity, indicating that the expression of INO1 is repressed by inositol in the medium. In contrast, the levels of β-galactosidase activity in the Δitc1, Δhac1 Δitc1, Δisw2, and Δhac1 Δisw2 cells were much higher than those observed in the wild-type and Δhac1 cells, strongly suggesting that the expression of INO1 is derepressed in these cells even in the presence of inositol in the medium. To confirm this, we next determined the abundance of mRNA of INO1 in disruptants by Northern blot analysis. The wild-type, Δhac1, Δitc1, Δhac1 Δitc1, Δisw2, and Δhac1 Δisw2 strains were grown to the early log phase in minimal medium containing inositol and then subjected to analysis (Fig. 5B). Consistent with the results obtained for the INO1-lacZ fusion gene, the amount of INO1 mRNA was low in the wild-type and Δhac1 cells, whereas the Δitc1, Δhac1 Δitc1, Δisw2, and Δhac1 Δisw2 cells showed high levels of INO1 mRNA. These data indicate that the Isw2p-Itc1p chromatin-remodeling complex represses the INO1 expression in the wild-type and Δhac1 cells, and the disruption of either ITC1 or ISW2 abolishes the function of the chromatin-remodeling complex, resulting in derepression of the INO1 expression. This is why the Δhac1 Δitc1 and Δhac1 Δisw2 strains can grow on inositol-free medium.
FIG. 5.
INO1 expression in Δitc1, Δisw2, Δhac1 Δitc1, and Δhac1 Δisw2 strains. (A) Reporter gene analysis of INO1 expression. Strains D452-2 (WT), HU1 (Δhac1), IH-1 (Δitc1), HIW (Δhac1 Δitc1), SH-1 (Δisw2), and HSW (Δhac1 Δisw2) were transformed with YCpL2-INO1Z carrying the INO1-lacZ fusion gene. The transformants were precultured in minimal medium containing inositol for 12 h. The cells were then washed twice with water and cultured further in fresh minimal medium containing inositol. After the cells had reached the early log phase, β-galactosidase activity was measured. Data are means for four independent transformants. Error bars indicate standard deviations. (B) Northern blot analysis of INO1 mRNA. Strains D452-2 (WT), HU1 (Δhac1), IH-1 (Δitc1), HIW (Δhac1 Δitc1), SH-1 (Δisw2), and HSW (Δhac1 Δisw2) were grown to the early log phase in minimal medium with inositol. Total RNA was isolated and used for Northern blot analysis (25 μg per lane).
Effects of overexpression of ITC1Δ and ISW2 on the Ino− phenotype.
We next determined the effects of overexpression of ITC1 and ISW2 on the Ino− phenotype of the Δhac1 strain. The Δhac1 strain was transformed with multiple copies of ISW2, multiple copies of ITC1Δ, or both. In the case of multiple copies of ISW2, we used a construct in which the expression of ISW2 is under the control of the ADH1 promoter. The transformants were grown on minimal medium with or without inositol (Fig. 6). As shown above, growth of the Δhac1 strain carrying multiple copies of ITC1Δ was similar to that of the strain having the HAC1 gene. However, the Δhac1 strain carrying multiple copies of both ISW2 and ITC1Δ showed the Ino− phenotype, similar to the Δhac1 strain carrying vectors. Furthermore, the introduction of multiple copies of ISW2 into the Δhac1 strain resulted in a stronger Ino− phenotype, suggesting that the overexpression of ISW2 causes severe repression of INO1. On the other hand, introduction of multiple copies of ITC1 had little effect on the Ino− phenotype of the Δhac1 strain, as described above. Multiple copies of ITC1, whose expression is under the control of the ADH1 promoter, also had little effect (data not shown). It seems likely that the activity of the Isw2p-Itc1p complex is regulated by the amount of Isw2p.
FIG. 6.
Effects of overexpression of ITC1 and ISW2 on INO1 expression. The Δhac1 strain, HU1, harboring pHV-ADISW2 plus vector YEpM4 (ISW2), vector pHV-1 plus YEp133t (ITC1Δ), pHV-ADISW2 plus YEp133t (ISW2-ITC1Δ), vector pHV-1 plus YCpL2-42 (HAC1), or vector pHV-1 plus vector YEpM4 (vector) was 10-fold serially diluted and then spotted onto minimal medium with or without inositol. The transformants were grown at 30°C for 3 days.
Aberrant morphology of the Δitc1 and Δisw2 strains.
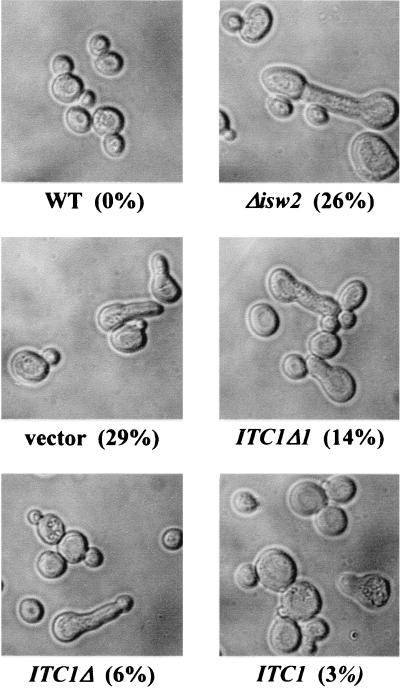
It has been reported that the deletion of ITC1 in α-type cells causes aberrant morphology resembling that of cells exposed to mating factors. This phenotype is not observed for MATa cells (8). This aberrant morphology is confirmed by the data shown in Fig. 7. Furthermore, we also found that the Δisw2 strain shows a similar aberrant morphology. We confirmed that a single copy of ISW2 could reverse the aberrant morphology of the Δisw2 strain. This strongly suggests that the Isw2p-Itc1p chromatin-remodeling complex plays a critical role in maintenance of the morphology of cells. We next examined the effect of the truncated form of ITC1 on cell morphology. The Δitc1 strain, IH-1, was transformed with a series of ITC1 deletion derivatives constructed on a multicopy plasmid (Fig. 2), and the cell morphology of the transformants was observed. As shown in Fig. 7, the Δitc1 and Δisw2 cells exhibited aberrant morphology (29 and 26%, respectively). A small number of the Δitc1 cells exhibited the aberrant morphology when transformed with full-length ITC1 (3%). On the other hand, the transformants of ITC1Δ1 and ITC1Δ each included a moderate number of the aberrant cells (14 and 6%, respectively). It seems likely that the C-terminal region of Itc1p is critical for the repression of INO1 but not for maintenance of morphology.
FIG. 7.
Aberrant morphology of Δitc1 and Δisw2 strains. The Δitc1 strain, IH-1, transformed with either YEp133Δ1 (ITC1Δ1), YEp133t (ITC1Δ), YEp133w (ITC1), or vector YEpM4 alone and strains D452-2 (WT) and SH-1 (Δisw2) were grown to the early log phase in minimal medium with inositol. Cell morphology was observed microscopically. Values in parentheses are percentages of morphologically aberrant cells determined under a microscope.
DISCUSSION
In this study, we isolated a truncated form of ITC1, ITC1Δ, as a suppressor gene for the Ino− phenotype of the Δhac1 strain. From the results of the reporter gene assay (Fig. 3), multiple copies of ITC1Δ were found to be able to derepress INO1 expression and circumvent the inositol requirement for growth of the Δhac1 strain (Fig. 2B). However, overexpression of the full-length ITC1 had a moderate effect on the growth of the Δhac1 strain. ITC1 encodes a protein of 1,264 amino acids which is a component of the chromatin-remodeling complex (45). The gene product of ITC1Δ we obtained lacks 299 amino acids at the C-terminal end. The truncated form further truncated at the C terminus suppressed the Ino− phenotype of the Δhac1 strain more weakly than ITC1Δ, suggesting that the length of the C-terminal region of Itc1p is critical for the derepression of INO1 expression. Furthermore, deletion of the chromosomal ITC1 gene in the Δhac1 strain also suppressed the Ino− phenotype of the Δhac1 strain (Fig. 4A). Introduction of a single copy of ITC1 into the Δhac1 Δitc1 strain gave it the Ino− phenotype again, but ITC1Δ did not. These results indicate that Itc1p is a negative regulator for INO1 expression and that the truncated form of Itc1p, Itc1Δp, competes with the effect of full-length Itc1p.
Itc1p and Isw2p form a chromatin-remodeling complex (45). This complex possesses nucleosome-stimulated ATPase and ATP-dependent nucleosome spacing activities. Isw2p exhibits ATPase activity. Therefore, we predicted that the disruption of ISW2 could also derepress the expression of INO1 and suppress the Ino− phenotype of the Δhac1 strain, and we found that this is the case. As shown in this study, deletion of not only ITC1 but also ISW2 in the wild-type and Δhac1 strains caused the derepression of INO1 expression even in the presence of inositol in the culture medium (Fig. 5). Inositol is well known to repress the expression of INO1. The Δhac1 Δisw2 strain can grow on inositol-free minimal medium (Fig. 4B). Conversely, introduction of multiple copies of ISW2, whose expression is under the control of the ADH1 promoter, into the Δhac1 strain caused a severer Ino− phenotype. Multiple copies of ITC1 of similar construction had no effect (data not shown). These results indicate that the amount of Isw2p limits the formation of the Isw2p-Itc1p complex.
During the preparation of this paper, data showing that the Isw2p-Itc1p chromatin-remodeling complex represses the expression of INO1 were reported by Goldmark et al. (9). By determining the levels of INO1 mRNA, they showed that the expression of INO1 is partially derepressed in the Δisw2 strain. They also revealed that the Isw2p-Itc1p chromatin-remodeling complex represses early meiotic genes during mitotic growth in a pathway parallel to that of the Rpd3p-Sin3p histone deacetylase complex and that the repressor function of the Isw2p-Itc1p complex is largely dependent on Ume6p, a sequence-specific DNA-binding protein. Their findings that the Isw2p-Itc1p chromatin-remodeling complex represses INO1 expression and that the disruption of ISW2 abolishes the function of the remodeling complex are consistent with the results we obtained in this study.
Taken together, these results suggest that the Isw2p-Itc1p chromatin-remodeling complex usually represses INO1 expression (Fig. 8). The amount of Isw2p is limited. Overexpression of ITC1Δ leads to the accumulation of the truncated form of Itc1p, Itc1Δp. Hence, a large amount of Itc1Δp deprives the Isw2-Itc1p chromatin-remodeling complex of Isw2p, resulting in derepression of the INO1 expression. In this model, it is strongly suggested that the C-terminal region of Itc1p has a regulatory effect on the Itc1p function. Itc1Δp behaves in a dominant negative manner toward Itc1p. Consistent with this idea, Goldmark et al. noticed that there are two types of Itc1p in yeast cells and that the electrophoretically slow-migrating one preferentially interacts with Ume6p. These two species might be generated through modification of the C-terminal region of Itc1p. The truncated form of Itc1p encoded by ITC1Δ that we obtained might have no ability to bind to Ume6p, so the chromatin-remodeling function was lost. According to their data, the derepression of INO1 caused by the deletion of ISW2 is moderate compared to that caused by the deletion of RPD3, SIN3, or UME6 (2.8-, 17-, 48-, and 117-fold, respectively). As described above, we showed that the disruption of not only ISW2 but also ITC1 restores the cell growth of the Δhac1 strain, which otherwise cannot grow on inositol-free medium. Therefore, our data clearly suggest that a decrease in the activity of the Isw2p-Itc1p chromatin-remodeling complex is sufficient to overcome the Ino− phenotype caused by the defect of IRE1-HAC1-mediated signaling. It is conceivable that the level of INO1 expression is positively and negatively regulated through the IRE1-HAC1-mediated activation pathway and the Isw2p-Itc1p complex-mediated repression pathway, respectively. However, the relationship between these positive and negative pathways is still unknown. The expression of the INO1 gene is known to be facilitated by the SWI-SNF and ADA-GCN5 complexes (35, 36). The gene product of IRE1 interacts with some component of the ADA-GCN5 complex and thereby regulates the function of HAC1 (20, 46). But so far, the mechanisms by which these complexes regulate the INO1 expression have not been elucidated precisely.
FIG. 8.
Model of Itc1Δp-mediated INO1 derepression. The Isw2p-Itc1p chromatin-remodeling complex represses the expression of INO1. However, overexpression of Itc1Δp causes depletion of the active form of Isw2p from the Isw2p-Itc1p complex responsible for the INO1 repression, resulting in derepression of INO1. Although the Isw2p-Itc1Δp complex cannot repress the INO1 expression, the Isw2p-Itc1p chromatin-remodeling complex with additionally overexpressed Isw2p restores the INO1 repression. In this model, the C-terminal region of Itc1p plays a crucial role in the INO1 repression.
It has been reported that Δitc1 cells show aberrant morphology (8). We also found that the deletion of ISW2 results in morphologic changes similar to those observed in the Δitc1 strain (Fig. 7). This clearly suggests that the Isw2p-Itc1p complex is responsible for maintaining the normal morphology of yeast cells. It has been reported that Δino2 cells exhibit aberrant morphology, similar to Δitc1 and Δisw2 cells (11). The expression of INO2 is known to be regulated similarly to that of INO1. The Isw2p-Itc1p complex might regulate INO2 expression and maintain the morphology of the yeast cells via Ino2p. Judging from the results obtained here with the ITC1 deletion derivatives, the morphologic abnormality was reversed upon the introduction of a series of the ITC1 gene, depending upon its length. However, in contrast to the effect on the INO1 expression, the overexpression of ITC1Δ, and even ITC1Δ1, partially but moderately suppressed the aberrant morphology of the Δitc1 strain. This suggests that the mechanism for the suppression of the aberrant morphology is different from that for the suppression of INO1 expression and that the regulatory function of the C-terminal region of Itc1p is different in these two forms of suppression. As described above, early meiotic genes are known to be derepressed in the Δisw2 strain during mitotic growth. However, the relationship between the mating type-specific morphologic change observed here and the derepression of early meiotic genes is unknown.
We show here that the expression of INO1 in Δisw2 or Δitc1 strains is partially derepressed even in the presence of inositol in the culture medium. Preliminary experiments revealed that the level of derepression increases further when inositol is removed from the culture medium. These results indicate that the repression of INO1 by inositol is partly mediated through the Isw2p-Itc1p chromatin-remodeling complex but that there could still be a sensory machinery for detecting the level of inositol and for regulating the expression of genes which are regulated by inositol via the ICRE. To elucidate the nature of this machinery and the functions of the Hac1p, further detailed analyses with mutants of ISW2 and ITC1 are necessary.
REFERENCES
- 1.Ambroziak J, Henry S A. INO2 and INO4 gene products, positive regulators of phospholipid biosynthesis in Saccharomyces cerevisiae, form a complex that binds to the INO1 promoter. J Biol Chem. 1994;269:15344–15349. [PubMed] [Google Scholar]
- 2.Arndt K M, Ricupero-Hovasse S, Winston F. TBP mutants defective in activated transcription in vivo. EMBO J. 1995;14:1490–1497. doi: 10.1002/j.1460-2075.1995.tb07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colicelli J, Nicolette C, Birchmeier C, Rodgers L, Riggs M, Wigler M. Expression of three mammalian cDNAs that interfere with RAS function in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:2913–2917. doi: 10.1073/pnas.88.7.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox J S, Chapman R E, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culbertson M R, Donahue T F, Henry S A. Control of inositol biosynthesis in Saccharomyces cerevisiae: properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J Bacteriol. 1976;126:232–242. doi: 10.1128/jb.126.1.232-242.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Johnson M, Henry S. Biosynthesis of inositol in yeast: primary structure of myo-inositol-1-phosphate synthase (EC 5.5.1.4) and functional analysis of its structural gene, the INO1 locus. J Biol Chem. 1989;264:1274–1283. [PubMed] [Google Scholar]
- 7.Ebbert R, Birkmann A, Schüller H-J. The product of the SNF2/SWI2 paralogue INO80 of Saccharomyces cerevisiae required for efficient expression of various yeast structural genes is part of a high-molecular-weight protein complex. Mol Microbiol. 1999;32:741–751. doi: 10.1046/j.1365-2958.1999.01390.x. [DOI] [PubMed] [Google Scholar]
- 8.Escribano M V, Mazón M J. Disruption of six novel ORFs from Saccharomyces cerevisiae chromosome VII and phenotypic analysis of the deletants. Yeast. 2000;16:621–630. doi: 10.1002/(SICI)1097-0061(200005)16:7<621::AID-YEA557>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Goldmark J P, Fazzio T G, Estep P W, Church G M, Tsukiyama T. The ISW2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell. 2000;103:423–433. doi: 10.1016/s0092-8674(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg M L, Lopes J M. Genetic regulation of phospholipid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1996;60:1–20. doi: 10.1128/mr.60.1.1-20.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond C I, Romano P, Roe S, Tontonoz P. INO2, a regulatory gene in yeast phospholipid biosynthesis, affects nuclear segregation and bud pattern formation. Cell Mol Biol Res. 1993;39:561–577. [PubMed] [Google Scholar]
- 12.Henry S A, Patton-Vogt J L. Genetic regulation of phospholipid metabolism: yeast as a model eukaryote. Prog Nucleic Acid Res Mol Biol. 1998;61:133–179. doi: 10.1016/s0079-6603(08)60826-0. [DOI] [PubMed] [Google Scholar]
- 13.Hosaka K, Nikawa J, Kodaki T, Yamashita S. A dominant mutation that alters the regulation of INO1 expression in Saccharomyces cerevisiae. J Biochem. 1992;111:352–358. doi: 10.1093/oxfordjournals.jbchem.a123761. [DOI] [PubMed] [Google Scholar]
- 14.Hosaka K, Murakami T, Kodaki T, Nikawa J, Yamashita S. Repression of choline kinase by inositol and choline in Saccharomyces cerevisiae. J Bacteriol. 1990;172:2005–2012. doi: 10.1128/jb.172.4.2005-2012.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudak K A, Lopes J M, Henry S A. A pleiotropic phospholipid biosynthetic regulatory mutation in Saccharomyces cerevisiae is allelic to sin3 (sdi1, ume4, rpd1) Genetics. 1994;136:475–483. doi: 10.1093/genetics/136.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson J C, Lopes J M. The yeast UME6 gene is required for both negative and positive transcriptional regulation of phospholipid biosynthetic gene expression. Nucleic Acids Res. 1996;24:1322–1329. doi: 10.1093/nar/24.7.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadosh D, Struhl K. Targeted recruitment of the Sin3-Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol Cell Biol. 1998;18:5121–5127. doi: 10.1128/mcb.18.9.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasten M M, Dorland S, Stillman D J. A large protein complex containing the yeast Sin3p and Rpd3p transcriptional regulators. Mol Cell Biol. 1997;17:4852–4858. doi: 10.1128/mcb.17.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kataoka T, Broek D, Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985;43:493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman R J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Gabriel S E, Roinick K L, Ward R D, Arndt K M. Analysis of TFIIA function in vivo: evidence for a role in TATA-binding protein recruitment and gene-specific activation. Mol Cell Biol. 1999;19:8673–8685. doi: 10.1128/mcb.19.12.8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushita M, Nikawa J. Isolation and characterization of a SCT1 gene which can suppress a choline-transport mutant of Saccharomyces cerevisiae. J Biochem. 1995;117:447–451. doi: 10.1093/jb/117.2.447. [DOI] [PubMed] [Google Scholar]
- 23.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 24.Nikawa J. A cDNA encoding the human transforming growth factor β receptor suppresses the growth defect of a yeast mutant. Gene. 1994;149:367–372. doi: 10.1016/0378-1119(94)90178-3. [DOI] [PubMed] [Google Scholar]
- 25.Nikawa J, Murakami A, Esumi E, Hosaka K. Cloning and sequence of the SCS2 gene, which can suppress the defect of INO1 expression in an inositol auxotrophic mutant of Saccharomyces cerevisiae. J Biochem. 1995;118:39–45. doi: 10.1093/oxfordjournals.jbchem.a124889. [DOI] [PubMed] [Google Scholar]
- 26.Nikawa J, Akiyoshi M, Hirata S, Fukuda T. Saccharomyces cerevisiae IRE2/HAC1 is involved in IRE1-mediated KAR2 expression. Nucleic Acids Res. 1996;24:4222–4226. doi: 10.1093/nar/24.21.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikawa J, Kawabata M. PCR- and ligation-mediated synthesis of marker cassettes with long flanking homology regions for gene disruption in Saccharomyces cerevisiae. Nucleic Acids Res. 1998;26:860–861. doi: 10.1093/nar/26.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikawa J, Sass P, Wigler M. Cloning and characterization of the low-affinity cyclic AMP phosphodiesterase gene of Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:3629–3636. doi: 10.1128/mcb.7.10.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikawa J, Yamashita S. IRE1 encodes a putative protein kinase containing a membrane-spanning domain and is required for inositol prototrophy in Saccharomyces cerevisiae. Mol Microbiol. 1992;6:1441–1446. doi: 10.1111/j.1365-2958.1992.tb00864.x. [DOI] [PubMed] [Google Scholar]
- 30.Nikawa J, Nagumo T, Yamashita S. myo-Inositol transport in Saccharomyces cerevisiae. J Bacteriol. 1982;150:441–446. doi: 10.1128/jb.150.2.441-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikawa J, Tsukagoshi Y, Yamashita S. Isolation and characterization of two distinct myo-inositol transport genes of Saccharomyces cerevisiae. J Biol Chem. 1991;266:11184–11191. [PubMed] [Google Scholar]
- 32.Paltauf F, Kohlwein S D, Henry S A. Regulation and compartmentalization of lipid synthesis in yeast. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 415–500. [Google Scholar]
- 33.Peterson C L, Dingwall A, Scott M P. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc Natl Acad Sci USA. 1994;91:2905–2908. doi: 10.1073/pnas.91.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson C L, Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992;68:573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 35.Peterson C L, Kruger W, Herskowitz I. A functional interaction between the C-terminal domain of RNA polymerase II and the negative regulator SIN1. Cell. 1991;64:1135–1143. doi: 10.1016/0092-8674(91)90268-4. [DOI] [PubMed] [Google Scholar]
- 36.Pollard K J, Peterson C L. Role for ADA/GCN5 products in antagonizing chromatin-mediated transcriptional repression. Mol Cell Biol. 1997;17:6212–6222. doi: 10.1128/mcb.17.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose M D, Broach J R. Cloning genes by complementation in yeast. Methods Enzymol. 1991;194:195–230. doi: 10.1016/0076-6879(91)94017-7. [DOI] [PubMed] [Google Scholar]
- 38.Rose M D, Novick P, Thomas J H, Botstein D, Fink G R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 39.Rundlett S E, Carmen A A, Suka N, Turner B M, Grunstein M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392:831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- 40.Scafe C, Chao D, Lopes J, Hirsch J P, Henry S, Young R A. RNA polymerase II C-terminal repeat influences response to transcriptional enhancer signals. Nature. 1990;347:491–494. doi: 10.1038/347491a0. [DOI] [PubMed] [Google Scholar]
- 41.Schwank S, Ebbert R, Rautenstrauβ K, Schweizer E, Schüller H-J. Yeast transcriptional activator INO2 interacts as an Ino2p/Ino4p basic helix-loop-helix heteromeric complex with the inositol/choline-responsive element necessary for expression of phospholipid biosynthetic genes in Saccharomyces cerevisiae. Nucleic Acids Res. 1995;23:230–237. doi: 10.1093/nar/23.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 43.Shirra M K, Arndt K M. Evidence for the involvement of the Glc7-Reg1 phosphatase and the Snf1-Snf4 kinase in the regulation of INO1 transcription in Saccharomyces cerevisiae. Genetics. 1999;152:73–87. doi: 10.1093/genetics/152.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidrauski C, Chapman R, Walter P. The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends Cell Biol. 1998;8:245–249. doi: 10.1016/s0962-8924(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 45.Tsukiyama T, Palmer J, Landel C C, Shiloach J, Wu C. Characterization of the imitation switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes Dev. 1999;13:686–697. doi: 10.1101/gad.13.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welihinda A A, Tirasophon W, Kaufman R J. The transcriptional co-activator ADA5 is required for HAC1 mRNA processing in vivo. J Biol Chem. 2000;275:3377–3381. doi: 10.1074/jbc.275.5.3377. [DOI] [PubMed] [Google Scholar]
- 47.White M J, Hirsch J P, Henry S A. The OPI1 gene of Saccharomyces cerevisiae, a negative regulator of phospholipid biosynthesis, encodes a protein containing polyglutamine tracts and a leucine zipper. J Biol Chem. 1991;266:863–872. [PubMed] [Google Scholar]
- 48.Yamashita S, Oshima A. Regulation of phosphatidylethanolamine methyltransferase level by myo-inositol in Saccharomyces cerevisiae. Eur J Biochem. 1980;104:611–616. doi: 10.1111/j.1432-1033.1980.tb04465.x. [DOI] [PubMed] [Google Scholar]