Abstract
Background:
The pathogenesis of bronchopulmonary dysplasia (BPD) is multifactorial, and there are limited data about prenatal exposures and risk of BPD.
Study Design:
Our study performed parallel analyses using a logistic regression model in a cohort of 4527 infants with data from a curated registry and using a phenome wide association study (PheWAS) based on ICD9/10-based phecodes. We examined 20 prenatal exposures from a neonatal intensive care unit (NICU) curated registry database related to pregnancy and maternal health as well as 94 maternal diagnosis phecodes with a PheWAS analysis.
Result:
In both the curated registry and PheWAS analyses, polyhydramnios was associated with an increased risk of BPD (OR 5.70, 95% CI 2.78–11.44, p = 1.37 × 10−6).
Conclusion:
Our data suggest that polyhydramnios may be a clinical indicator of premature infants at increased risk for bronchopulmonary dysplasia. Combining curated registry data with PheWAS analysis creates a valuable tool to generate hypotheses.
Introduction
Despite tremendous advances in neonatal care, bronchopulmonary dysplasia (BPD) remains a leading complication in survivors of preterm birth1,2. Even with the use of synchronized ventilation and postnatal surfactant therapy, BPD affects approximately half of all infants born extremely preterm (gestational age <28 weeks), with infants at higher risk for BPD the earlier their gestational age3,4. We have limited understanding of the role of many antenatal exposures in the pathophysiology of BPD. Antenatal factors such as chorioamnionitis, premature rupture of membranes, maternal diabetes, and others have been variably associated with increasing the risk of BPD5. The identification of antenatal risk factors prior to delivery could be used to identify infants at highest risk for BPD, with a goal of developing targeted therapies to mitigate this risk.
To study the effects of environmental exposures on the risk of BPD, we employed the clinical database from the Mildred Stahlman Division of Neonatology at Vanderbilt University Medical Center (VUMC) from infants born between 2005–2017, and integrated this database with the electronic health record (EHR) using a phenome-wide association study (PheWAS) approach6,7. PheWAS methodology was designed to study phenotype and genotype associations in genetic epidemiology studies, however recently the approach has expanded to evaluate associations between phenotypes and agentic clinical exposures8,9. This study represents a new application of PheWAS to evaluate the associations between antenatal exposures and maternal health factors with BPD. Our overall goal is to generate new hypotheses employing the robust nature of PheWAS methodology in order to identify modifiable factors that could be used to lower the incidence of BPD or translate into novel therapeutics.
Methods
Study Design for Curated Registry Model
We conducted a retrospective case-control observational study of preterm infants to evaluate the association of antenatal exposures with BPD. Inclusion criteria were infants born between 22–34 weeks gestation. This study was reviewed and approved by the institutional review board of VUMC, Nashville, Tennessee. For both cases and controls, we excluded infants who died prior to 28 days of age or who were transferred to VUMC after 14 days of life (due to high rates of incomplete maternal data). We defined BPD based on a modified National Institutes of Health consensus definition: requirement for supplemental oxygen at 28 days of life or at 36 weeks postmenstrual age4,10.
Study Population and Data Sources in the Registry Model
We used a prospectively ascertained database of 270 variables for all neonates admitted to the tertiary care neonatal intensive care unit (NICU) at Vanderbilt from 2005–2017, and for the purposes of this study, pulled out the 20 variables related to prenatal exposure. Data were collected by trained research coordinators, who manually gather maternal data and prenatal history for infants born at VUMC and the referring hospital records for transferred infants. We inspected the data for inconsistencies, and a minimum and maximum value was assigned to screen for each variable assigned based on physiologic plausibility, with values outside this range reconciled by manual chart review. We used the linked EHR data to reconcile or fill in data from the registry that was missing, conflicting, or implausible. All babies born before 34 weeks without BPD and babies without BPD who were sent home on oxygen were manually reviewed to validate BPD status. Gestational age and demographic information from the registry were cross checked with EHR forms. By combining the database with the EHR, there was a minimal amount of missing data. In total, 9 variables on which the model was based had some missing data, and the EHR search reconciled data in 5 of these 9 variables, including gestational age, birth weight, and maternal age (eTable 1 in Supplement). In addition, manual review identified 49 infants who died prior to 28 days of age but who were not flagged as deceased by the curated registry.
PheWAS Study Design and Data Sources
The PheWAS analysis was conducted on a subset of neonates who were born less than 34 weeks and survived more than 28 days, whose mothers received prenatal care at VUMC during the pregnancy (n=1961). We required that the mothers have at least 3 unique billing days at VUMC during the pregnancy on which the PheWAS was conducted, including the delivery encounter. Phenotypes were ascertained using phecodes, or validated phenotype definitions based on billing and diagnostic codes in the EHR, using an alpha version of the revised phecodes classification similar to the previously published version11. We used this alpha version because it includes more granular concepts for conditions diagnosed in pregnancy. Phecode definitions are available at the website phewascatalog.org. For each phecode, exposures of interest (e.g., maternal co-morbidities, pregnancy complications, etc.) were defined as those patients having at least one instance of that phecode during the pregnancy while unexposed patients were defined as those who had no instances of that phecode. Only phecodes with at least 100 exposed patients were used in the analysis (n=94), supplemental eTable 2.
Statistical Analysis
Data were summarized with descriptive statistics in Table 1. Twenty maternal factors pertaining to delivery, maternal diagnoses and maternal demographics were first evaluated for associations with BPD in univariable logistic regression models using registry data. We developed a base multivariable logistic regression model consisting of known associations with BPD, including: gestational age (days), birth weight (g), infant sex,12 as well as precision variables which included maternal race, birth year and birth center location (inborn vs outborn), to help improve the performance of the model, as shown by the ROC curve (eFigure 1 in Supplement). Each novel maternal factor was added independently to the base model and evaluated for statistical significance, defined as a Bonferroni-corrected p-value of < 0.002. Adjusted odds ratios (aOR) with 95% confidence intervals and p-values are reported. The PheWAS analysis11 used a logistic regression with BPD as the dependent variable and included covariates for gestational age, birth weight, sex, maternal race, and birth year. With 94 phenotypes included, we calculated the Bonferroni correction threshold for p<0.05 (5.3 × 10−4) and a false discovery rate (FDR) q<0.1 (9.9 × 10−4). PheWAS results of 94 phenotypes are in eTable 2 of the Supplement.
Table 1:
Descriptive statistics for infants in the study
| All subjects (N=4527) | ||
|---|---|---|
| Birthweight Mean (range)a | 1494g (340–3960g) | |
| Gestational age Mean (range)b | 30 weeks (22–33weeks) | |
| Sex | Male: 2440 (53.9%) Female: 2087 (46.1%) |
|
| Birth center Number (%) | Inborn: 3508 (77.5%) Outborn: 1019 (22.5%) |
|
| Birth year Mean (range) | 2011 (2005–2017) | |
| Maternal race/ethnicityc | 2713 (60.0%) 915 (20.2%) 382 (8.4%) 155 (3.4%) 362 (8.0%) |
|
25–75th percentile: 1080–1870g
weeks = weeks estimated post-menstrual age at birth
maternal race/ethnicity as self-reported
Results
Of 17077 infants in the NICU database (registry model), 4527 met inclusion criteria, including 1385 BPD cases and 3142 controls (Figure 1). Of the 4527 infants, 1961 were born to women with 3 or more billing encounters during the pregnancy, meeting inclusion criteria for PheWAS analysis. Of the 1961 infants in the PheWAS analysis, 531 had BPD. For the registry model, the mean birth weight and gestational age were 745 grams and 30 weeks, respectively (Table 1). Most of the infants were born at VUMC (77.5%), and 53.9% of the infants were male. The majority of mothers in this study were white (60.0%). Using a dichotomized diagnosis of BPD status, 1385 (30.6%) infants developed BPD, and 3142 (69.4%) infants did not develop BPD (Table 1). Multivariable logistic regression confirmed known significant associations with BPD, such as low gestational age, low birth weight and male sex. African American race showed a protective effect (Table 2). Consistent with prior studies, there was no association found for antenatal steroid use (Table 3)13. There was no association with birth year and the development of BPD (Table 2).
Figure 1.
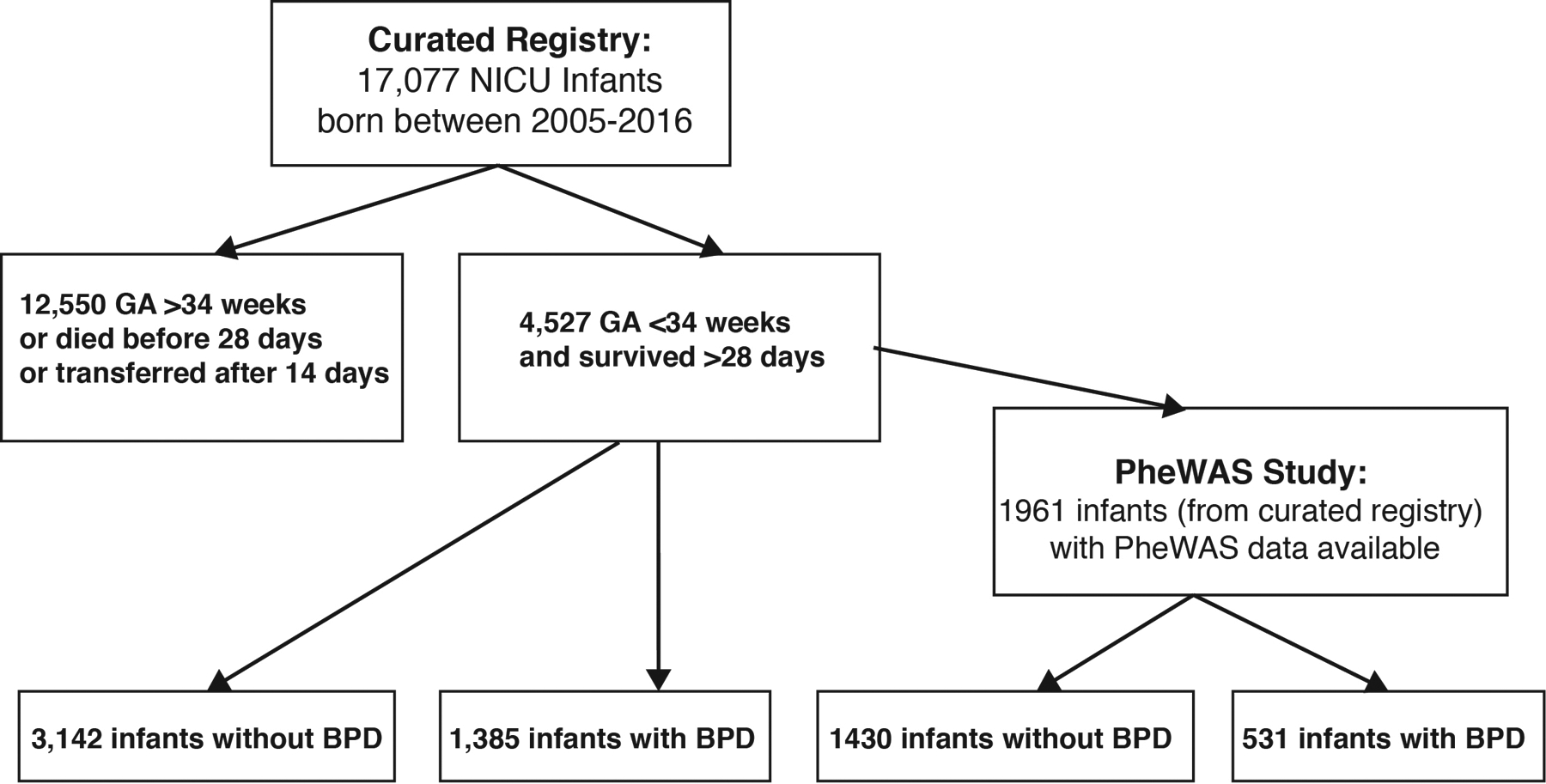
Schematic of how infants were enrolled in the study and how many had bronchopulmonary dysplasia (BPD).
Table 2:
Variables known to be associated with increased BPD risk or protective affect in curated registry
| BPD n=1385 |
Controls n=3142 |
aOR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Gestational age (days); mean (SD) | 27.5 (2.3) | 31.7 (1.7) | 0.90 | 0.89 – 0.91 | 2.6 × 10−86* |
| Birth weight (grams); mean (SD) | 1016 (396) | 1706 (433) | 0.37 | 0.26 – 0.54 | 1.2 × 10−7* |
| Male, N (%) | 758 (55) | 1682 (54) | 1.30 | 1.07 – 1.57 | 0.007 |
| Maternal Race-African American, N (%) | 293 (21) | 657 (21) | 0.54 | 0.43 – 0.68 | 2.3 × 10−3* |
| Birth year, mean (SD) | 2011 (3.8) | 2011 (3.9) | 1.01 | 0.99 – 1.04 | 0.34 |
indicates statistical significance based on Bonferroni threshold (with adjustment for gestational age, birthweight, sex, maternal race, and birth year)
Table 3:
Logistic Regression of Maternal Variables in NICU Registry with BPD Status
| Pregnancy Exposures and Complications | BPD | Controls | aOR | 95% CI | p-value |
|---|---|---|---|---|---|
| Multiple gestation, N (%) | 308 (22%) | 741 (24%) | 1.06 | 0.85 – 1.33 | 0.59 |
| Prenatal Care, N (%) | 1328 (96%) | 3013 (96%) | 1.84 | 1.14 – 3.01 | 0.01 |
| Vaginal Bleeding, N (%) | 322 (23%) | 432 (14%) | 1.04 | 0.81 – 1.34 | 0.73 |
| Chorioamnionitis, N (%) | 174 (13%) | 290 (9%) | 0.89 | 0.76 – 1.03 | 0.13 |
| Oligohydramnios, N (%) | 79 (6%) | 119 (4%) | 2.19 | 1.42 – 3.34 | 3.3 × 10−4* |
| Polyhydramnios, N (%) | 23 (2%) | 40 (1%) | 5.70 | 2.78 – 11.44 | 1.4 × 10−6* |
| Diabetes (all types), N (%) | 142 (10%) | 385 (11%) | 1.69 | 1.25 – 2.27 | 5.8 × 10−4* |
| Chronic Hypertension, N (%) | 174 (13%) | 290 (9%) | 1.14 | 0.86 – 1.51 | 0.37 |
| Maternal Drug Use, N (%) | 369 (27%) | 820 (24%) | 0.85 | 0.68 – 1.05 | 0.123 |
| Antenatal Steroids, N (%) | 1162 (84%) | 2523 (80%) | 1.14 | 0.85 – 1.51 | 0.39 |
| C-section, N (%) | 1024 (74%) | 2001 (64%) | 1.81 | 1.46 – 2.26 | 1.1 × 10−7* |
| Vertex Presentation, N (%) | 832 (60%) | 2369 (75%) | 0.77 | 0.63 – 0.94 | 0.01 |
| Anesthesia used in labor, N (%) | 1213 (88%) | 2830 (83%) | 1.31 | 0.96 – 1.81 | 0.09 |
| Pre-eclampsia, N (%) | 317 (23%) | 819 (26%) | 1.07 | 0.85 – 1.34 | 0.59 |
| Placenta abruption, N (%) | 218 (16%) | 271 (9) | 1.14 | 0.85 – 1.52 | 0.38 |
| Meconium, N (%) | 55 (4%) | 130 (4%) | 0.97 | 0.61 – 1.53 | 0.90 |
| Mom Age, mean (SD) | 26.8 (6.18) | 26.7 (5.94) | 1.02 | 1.00 – 1.03 | 0.22 |
| Gravida, mean (SD) | 2.7 (1.9) | 2.6 (1.8) | 1.00 | 0.97 – 1.01 | 0.98 |
| Preterm Premature Rupture of Membranes, (N (%) | 143 (10) | 426 (12) | 1.01 | 0.74 – 1.37 | 0.94 |
| Rupture of membranes (days), mean (SD) | 2.3 (8.5) | 1.4 (4.9) | 1.03 | 1.02 – 1.05 | 5.4 × 10−7* |
indicates statistical significance based on Bonferroni threshold (with adjustment for gestational age, birthweight, sex, maternal race, birth year and birth location)
Of the 20 antenatal exposures examined in logistic regression models (Supplement eTable 3), we found a strong association for the relationship between BPD and the following known risk factors for BPD: oligohydramnios, diabetes, cesarean section, and duration of rupture of membranes (Table 3)14,15,16,17. We also identified a new association between polyhydramnios and the risk of developing BPD (aOR 5.70, p = 1 × 10−6). While diabetes is associated with polyhydramnios in the third trimester, the association between polyhydramnios and BPD persisted even when we controlled for maternal diabetes (Supplement eTable 4), and after controlling separately for other congenital anomalies (Supplement eTable 5). Likewise, the significant association between BPD and polyhydramnios remained when we applied robust standard error estimation to account for correlations between births in multiple gestations (Supplement eTable 6).
PheWAS analysis of 94 maternal diagnoses revealed significant associations of BPD with polyhydramnios, problems associated with the amniotic cavity or membranes, and known or suspected fetal abnormality affecting management of the mother (Bonferroni corrected p < 0.05) (Figure 2). While not crossing the Bonferroni threshold, both maternal history of asthma and oligohydramnios were significant with false discovery ratio (FDR) q < 0.1 (Figure 2). Polyhydramnios had the strongest association with BPD of all of the prenatal factors tested. The diagnosis “problems associated with the amniotic cavity” includes the diagnoses of polyhydramnios and oligohydramnios. Six of the variables tested in registry-based analysis were also tested using EHR based phecodes (Supplement eTable 7). Polyhydramnios and oligohydramnios were significantly associated with BPD using both modeling approaches, and odds ratios from the PheWAS analysis were within the 95% confidence intervals of the registry analysis. The remaining four associations were non-significant (p > 0.05) in both analyses (Supplement eTable 7).
Figure 2.
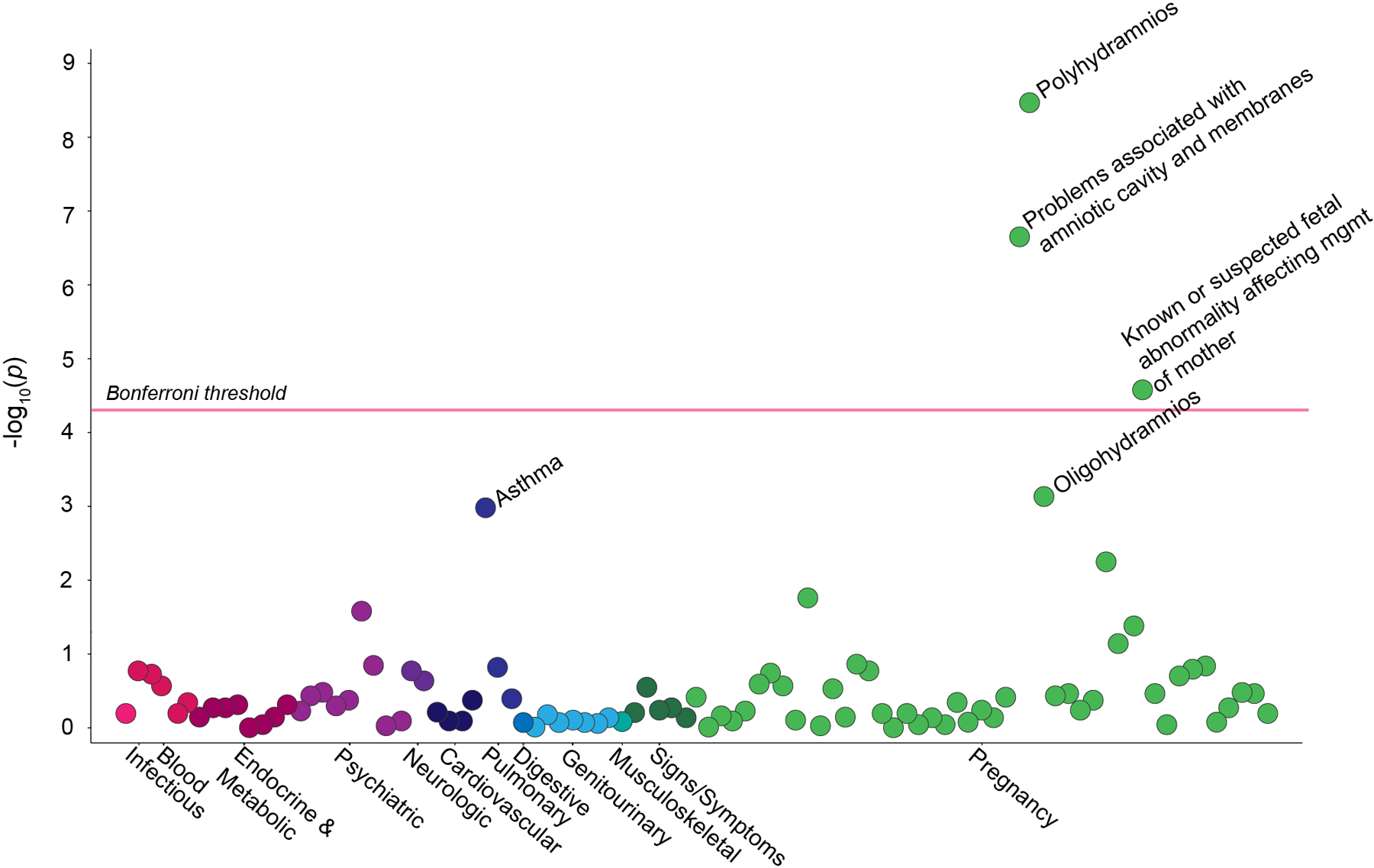
This Manhattan plot shows the significance of association between bronchopulmonary dysplasia and 94 different maternal phenotypes. The Phecode “problems associated with the amniotic cavity and membranes” includes polyhydramnios, oligohydramnios, premature rupture of membranes, and infection of the amniotic cavity. The Phecode “known or suspected fetal abnormality affecting management of the mother” includes the following diagnoses: 1) abnormality in fetal heart rate or rhythm, 2) central nervous system malformation affecting management of the mother, 3) chromosomal abnormality in fetus affecting management of the mother, 4) decreased fetal movements affecting management of the mother. Clusters of maternal phenotypes are shown on the x-axis. The y-axis displays the −log10(p)-value for each phenotype. The red horizontal line indicates a threshold the Bonferroni corrected p-value < 0.000532.
Discussion
PheWAS is a method first described in 2010 to look at the relationships between phenotypes in large human datasets18. We have adapted this approach to our own large registry of neonatal and perinatal data, the first use of a PheWAS approach in this population. We used a large curated cohort combined with maternal EHR data to generate hypotheses regarding the relationship between perinatal factors and BPD. This study demonstrates that registries and EHRs, both common sources of data for observational studies, are stronger when they are used together. The EHR data allowed us to fill in data that were missing from the registry and validate our outcome variable of BPD through manual review. Additionally, using EHR data, we were able to expand the number of maternal phenotypes beyond what was available in the registry. The PheWAS analysis leveraged billing code data with ICD-9 and ICD-10 codes. As such, it is likely less precise than the manually curated data in the registry7. Nevertheless, our results from this high throughput PheWAS analysis were strikingly similar to what we found with the registry data. Of the six variables available in both the registry and as phecodes, the associations results were consistent: both significant associations from the registry (polyhydramnios and oligohydramnios) were confirmed in the PheWAS with consistent effect sizes, and the four remaining non-significant associations (p > 0.05) were similarly non-significant in the PheWAS. The only difference in significance between the registry model and the PheWAS is with maternal diabetes, which may be explained by the registry grouping all diabetes together, whereas the phecodes used have small numbers of patients in specific diabetes subtypes. Our study suggests in large datasets, PheWAS could be used to look for associations of perinatal exposures with other neonatal morbidities.
Our logistic regression and PheWAS analysis both identify polyhydramnios as a perinatal exposure strongly associated with BPD, and our registry model confirmed prior known associations with BPD including sex, gestational age, birthweight, and maternal diabetes. While the association of BPD with oligohydramnios16, cesarean section, duration of membrane rupture, and diabetes15 has been described in prior datasets with smaller numbers, the finding of an association of BPD with polyhydramnios is novel. The finding of polyhydramnios suggests new hypotheses about the role of excess amniotic fluid in lung development. Low levels of lung fluid and prolonged membrane rupture have been associated with pulmonary hypoplasia in both animal models and human studies17,19, however, the relationship between excess amniotic fluid and abnormal lung development has received less attention. In our dataset, the relationship between polyhydramnios and BPD persisted even after controlling for major congenital anomalies and maternal diabetes, known causes of polyhydramnios20 (eTable 4 and eTable 5 in Supplement).
Prior work in term infants have shown that infections can result in polyhydramnios21,22. Based upon this work, we speculate that polyhydramnios may be a marker of subclinical inflammation in the second trimester. Work in animal models has shown that inflammation alone is sufficient to perturb normal lung development, and the creation of intrauterine inflammation via lipopolysaccharide (LPS) injection is used to model BPD in vivo23. While inflammation may lead to both polyhydramnios and abnormal lung development, it is also possible that the excess amniotic fluid alone results in primary lung injury due to abnormal hydrostatic pressure in the lung. Indeed, previous work in an animal model of fetal tracheal occlusion has shown that increased pressure in the lung results in fewer type 2 pneumocytes and decreased surfactant production24. Additional work in preclinical models is needed to test the speculation that both oligo- and polyhydramnios result in abnormal lung development. In addition, future studies to assess the content of the amniotic fluid in infants with second-trimester polyhydramnios and association with placental pathology may also provide insight into the pathophysiology linking polyhydramnios with the development of BPD.
Twin studies estimate that between 50–80% of the variance in BPD risk is genetic25,26 with the understanding that many of the genetic risk factors are only manifest in the setting of preterm birth. The link between a maternal history of asthma and BPD is interesting as it suggests a potentially heritable relationship between one form of chronic lung disease and the risk of developing BPD after preterm birth. Notably, a previous study in California with a larger cohort of preterm infants showed no relationship between maternal asthma and BPD risk27, however this study looked at asthma diagnoses coded at the time of hospital discharge rather than at any time during the pregnancy, as was the case with our PheWAS analysis. Further, only 2.5% of mothers in this study were coded as having asthma, whereas 7.5% of the mothers in our population had a history of asthma, a percentage more consistent with the described 7.7% incidence of asthma among adults in the United States28. Additional detailed EHR data with a larger cohort could also explore the relationship between maternal history of chronic lung diseases and asthma with the abnormal lung development seen in infants with BPD. Prospective studies of high-risk pregnancies could also correlate maternal asthma status during pregnancy and other indicators of maternal lung function and disease with infant risk of BPD.
While this study contains a large group of preterm infants and uses a richly curated clinical database combined with EHR and PheWAS analysis, there are some limitations. All of the infants in this study were cared for at a single center, and the definition of BPD did not stratify the outcome into mild, moderate, and severe forms of the disease. In addition, this is a retrospective study that relies on EHR data from one institution without an independent validation cohort. This study, however, does generate the hypothesis that polyhydramnios may be associated with the development of BPD. Future work will use a more granular definition of the staging of BPD based on degree of respiratory support. A larger sample size would also allow for the study of whether infants with polyhydramnios have a greater clinical response to postnatal corticosteroids.
Taken together, our data suggest that the PheWAS method can be applied to this population to look for links between maternal exposures and neonatal outcomes. We speculate that preterm polyhydramnios may be a clinical indicator of placental inflammation (even in the absence of obvious infection), and that polyhydramnios could be used to identify which infants are at the highest risk of developing BPD after preterm birth. The data from these analyses has generated a new hypothesis that can be tested by future mechanistic studies into the role of polyhydramnios and lung development after preterm birth. Additional studies in preclinical animal models and in observational-cohort clinical studies are needed to test these hypotheses, with a goal of identifying infants at highest risk for BPD and in identifying infants who may respond to targeted treatments after birth.
Supplementary Material
Impact:
Polyhydramnios was significantly associated with bronchopulmonary dysplasia in both a curated registry and by ICD coding analysis with a phenome wide association study (PheWAS).
Preterm polyhydramnios may be a clinical indicator of infants at increased risk for developing bronchopulmonary dysplasia after preterm birth.
Combining curated registry with PheWAS analysis creates a valuable tool to generate hypotheses about perinatal risk factors and morbidities associated with preterm birth.
Acknowledgements
The NICU Clinical Database is supported by the Mildred Stahlman Division of Neonatology at Vanderbilt University Medical Center. The authors are grateful for the stewardship of this database provided by Susan H. Guttentag MD, Steven D. Steele RN, and Theresa J. Rogers, RN. We also acknowledge the Vanderbilt Institute for Clinical and Translational Research (VICTR) for use of its operational infrastructure and resources.
Statement of financial support:
This work was supported by K08HL143051 (JMSS), the Parker B. Francis Fellowship (JMSS), T32HD060554 (MSC), R01LM010685 (LAB), and the John and Leslie Hooper Neonatal-Perinatal Endowment Fund (MSC).
Footnotes
Disclosure statement: The authors have no conflicts of interest relevant to this article to disclose.
Category of study: Clinical research article
Consent statement: Patient consent not required
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Martinez FD Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med 375, 871–878, doi: 10.1056/NEJMra1603287 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Goss K Long-term pulmonary vascular consequences of perinatal insults. J Physiol, doi: 10.1113/JP275859 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoll BJ et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 314, 1039–1051, doi: 10.1001/jama.2015.10244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins RD et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J Pediatr 197, 300–308, doi: 10.1016/j.jpeds.2018.01.043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson M et al. Association of Maternal Diabetes With Neonatal Outcomes of Very Preterm and Very Low-Birth-Weight Infants: An International Cohort Study. JAMA Pediatr 172, 867–875, doi: 10.1001/jamapediatrics.2018.1811 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi L et al. Evaluating statistical approaches to leverage large clinical datasets for uncovering therapeutic and adverse medication effects. Bioinformatics 34, 2988–2996, doi: 10.1093/bioinformatics/bty306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denny JC et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat Biotechnol 31, 1102–1110, doi: 10.1038/nbt.2749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roden DM Phenome-wide association studies: a new method for functional genomics in humans. J Physiol 595, 4109–4115, doi: 10.1113/JP273122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schraw JM, Langlois PH & Lupo PJ Comprehensive assessment of the associations between maternal diabetes and structural birth defects in offspring: a phenome-wide association study. Ann Epidemiol 53, 14–20 e18, doi: 10.1016/j.annepidem.2020.08.006 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH & Bancalari E Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163, 1723–1729, doi: 10.1164/ajrccm.163.7.2011060 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Wei WQ et al. Evaluating phecodes, clinical classification software, and ICD-9-CM codes for phenome-wide association studies in the electronic health record. PLoS One 12, e0175508, doi: 10.1371/journal.pone.0175508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abman SH et al. Interdisciplinary Care of Children with Severe Bronchopulmonary Dysplasia. J Pediatr 181, 12–28 e11, doi: 10.1016/j.jpeds.2016.10.082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts D, Brown J, Medley N & Dalziel SR Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3, CD004454, doi: 10.1002/14651858.CD004454.pub3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera L, Siddaiah R, Oji-Mmuo C, Silveyra GR & Silveyra P Biomarkers for Bronchopulmonary Dysplasia in the Preterm Infant. Front Pediatr 4, 33, doi: 10.3389/fped.2016.00033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheth S, Goto L, Bhandari V, Abraham B & Mowes A Factors associated with development of early and late pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. J Perinatol 40, 138–148, doi: 10.1038/s41372-019-0549-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanke K et al. Preterm prelabor rupture of membranes and outcome of very-low-birth-weight infants in the German Neonatal Network. PLoS One 10, e0122564, doi: 10.1371/journal.pone.0122564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adzick NS, Harrison MR, Glick PL, Villa RL & Finkbeiner W Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg 19, 658–665, doi: 10.1016/s0022-3468(84)80349-8 (1984). [DOI] [PubMed] [Google Scholar]
- 18.Denny JC et al. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics 26, 1205–1210, doi: 10.1093/bioinformatics/btq126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolini U, Fisk NM, Rodeck CH, Talbert DG & Wigglesworth JS Low amniotic pressure in oligohydramnios--is this the cause of pulmonary hypoplasia? Am J Obstet Gynecol 161, 1098–1101, doi: 10.1016/0002-9378(89)90641-8 (1989). [DOI] [PubMed] [Google Scholar]
- 20.Hamza A, Herr D, Solomayer EF & Meyberg-Solomayer G Polyhydramnios: Causes, Diagnosis and Therapy. Geburtshilfe Frauenheilkd 73, 1241–1246, doi: 10.1055/s-0033-1360163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fayyaz H & Rafi J TORCH screening in polyhydramnios: an observational study. J Matern Fetal Neonatal Med 25, 1069–1072, doi: 10.3109/14767058.2011.622002 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Mladina N, Mehikic G & Pasic A [TORCH infections in mothers as a cause of neonatal morbidity]. Med Arh 54, 273–276 (2000). [PubMed] [Google Scholar]
- 23.Benjamin JT et al. NF-kappaB activation limits airway branching through inhibition of Sp1-mediated fibroblast growth factor-10 expression. J Immunol 185, 4896–4903, doi: 10.4049/jimmunol.1001857 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piedboeuf B et al. Deleterious effect of tracheal obstruction on type II pneumocytes in fetal sheep. Pediatr Res 41, 473–479, doi: 10.1203/00006450-199704000-00004 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Bhandari V & Gruen JR The genetics of bronchopulmonary dysplasia. Semin Perinatol 30, 185–191, doi: 10.1053/j.semperi.2006.05.005 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Lavoie PM, Pham C & Jang KL Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics 122, 479–485, doi: 10.1542/peds.2007-2313 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gage S et al. Maternal Asthma, Preterm Birth, and Risk of Bronchopulmonary Dysplasia. J Pediatr 167, 875–880 e871, doi: 10.1016/j.jpeds.2015.06.048 (2015). [DOI] [PubMed] [Google Scholar]
- 28.CDC.gov. CDC - Asthma - Data and Surveillance - Asthma Surveillance Data. [online] Available at: http://www.cdc.gov/asthma/asthmadata.htm [Accessed 8 July 2020]. 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


