Abstract
Objectives:
While carotid artery intima media thickness (CIMT) is a widely used determinant of subclinical atherosclerosis, gray-scale median of intima-media complex (IM-GSM) is a relatively novel measure of echogenicity reflecting composition of the arterial wall. It is important to compare cardiovascular disease (CVD) risk factor correlates across CIMT and IM-GSM to determine whether these measures reflect distinct aspects of atherosclerosis.
Methods:
Baseline information from a completed randomized clinical trial of 643 healthy postmenopausal women without clinically apparent CVD were included in this cross-sectional study. The women were on average±SD 61±7 years old, and predominantly non-Hispanic White. CIMT and IM-GSM were measured by B-mode ultrasonogram in the far wall of the right common carotid artery. CVD risk factors including age, race, body mass index (BMI), smoking, weekly hours of physical activity, systolic (SBP) and diastolic blood pressure (DBP), lipids, glucose, and inflammatory markers were measured at baseline. Linear regression models were used to assess associations of CVD risk factors with CIMT and IM-GSM. Multivariable models included groups of risk factors added one at a time with and without basic demographic factors (age, race, BMI, physical activity) with model R2 values compared between CIMT and IM-GSM.
Results:
In multivariable analysis, Black race, BMI, SBP, and DBP were associated with CIMT (all p<0.04), whereas Hispanic race, physical activity, BMI, LDL-cholesterol, SBP, and leptin were correlates of IM-GSM (all p<0.05). Adjusted for age, race, BMI, and physical activity, the R2 value for SBP was greater for CIMT association, whereas R2 values for lipids, DBP, glucose, and inflammatory markers were greater for IM-GSM associations.
Conclusions:
CIMT and IM-GSM assess different attributes of subclinical atherosclerosis. Integrating both measures may provide improved assessment of atherosclerosis in asymptomatic individuals.
Keywords: CIMT, GSM, atherosclerosis, echogenicity, women, menopause
Introduction:
Atherosclerosis is a progressive inflammatory condition of the arterial wall, which is the primary underlying pathology for the majority of cardiovascular events1. Subclinical atherosclerosis is commonly assessed by carotid artery intima-media thickness (CIMT), a validated structural measure of the arterial wall obtained by B-mode ultrasound2. Gray-scale median of the intima-media complex (IM-GSM), determined from echogenicity of vascular wall ultrasonic images, was originally developed to assess arterial plaque composition. Echolucent (black) plaques are rich in lipids, associated with intraplaque hemorrhage and prone to rupture3,4 as well as predictive of future strokes5. Greater echolucency is indicated by lower GSM. Echogenic plaques, indicated by higher GSM, consist of more fibrous tissue and are stable.
Accumulating evidence suggests that echogenicity of plaque-free regions of arterial wall could be potentially useful for early detection of atherosclerosis6,7. The atherosclerosis process involves accumulation of lipids, inflammatory cells, cellular debris and hemorrhage in the arterial wall, which appear predominantly echolucent in ultrasound images8. Since lipid deposition in the arterial wall is one of the key early components of atherosclerosis9, it is suggested that IM-GSM may provide a sensitive non-invasive imaging approach for early detection of atherosclerosis7,10. Carotid artery wall echogenicity is related to cardiovascular disease (CVD) risk factors and is a predictor of mortality independent of CIMT11. Notably, correlation between CIMT and carotid artery IM-GSM is modestly and inversely statistically significant with correlation coefficients (r) ranging from −0.17 to −0.286,7,12, suggesting that these two non-invasive imaging approaches may reflect different aspects of atherosclerosis. To that end, it is important to compare CVD risk factor correlates of CIMT and IM-GSM.
Our objective for the current study is to compare CVD risk factors associated with CIMT and IM-GSM in postmenopausal women free of CVD, including a wide array of CVD risk factors using multiple statistical approaches.
Materials and Methods:
Study population:
This is a cross-sectional study among 643 healthy postmenopausal women who participated in the Early vs Late Intervention with Estradiol (ELITE). Details of ELITE design have been published13. Briefly, ELITE was a randomized, double-blinded, placebo-controlled trial designed to evaluate the effect of oral 17-β estradiol with or without vaginal progesterone (depending on hysterectomy) on subclinical atherosclerosis progression among women within 6 years of menopause (early postmenopause group) versus women more than 10 years after menopause (late postmenopause group) (ClinicalTrials.gov NCT00114517). Participating women were healthy with no history of CVD, diabetes, kidney or thyroid disease, or cancer and not taking hormone therapy 1 month prior to randomization. Demographic, physical, personal habits, medication use and clinical data collected at baseline were used for the current analysis. ELITE was approved by the Institutional Review Board of the University of Southern California. All participants provided written informed consent prior to trial-related procedures.
Carotid artery ultrasonogram:
Subclinical atherosclerosis was assessed from high-resolution B-mode ultrasound images of the far wall of the right distal common carotid artery obtained with a Siemens Acuson CV70 ultrasound system via L7–10 MHz linear-array transducer. A standardized protocol was used to obtain high-quality ultrasound images that were analyzed by in-house developed automated computerized edge detection software to measure atherosclerosis (Patents 2005, 2006, 2011)14,15. In brief, ultrasound images were acquired with jugular vein-carotid artery stacking to obtain well-defined images of the near and far wall intima-media complexes and internal anatomical landmarks for reproducing probe angulation. Instrumentation preset features enabled image capture with optimized configuration of imaging parameter settings including depth of field, gray maps, dynamic range, and persistence and edge enhancement levels. Gain and focal zone were adjusted to obtain optimum images at baseline. All baseline instrumentation settings including monitor intensity setting were maintained at follow-up examinations. A baseline image for each individual was used as a guide for reproducible image acquisition for longitudinal examinations.
Two measures of atherosclerosis derived from ultrasound images were determined: 1) CIMT; and 2) Gray-scale median of the intima-media complex (IM-GSM). In brief, using a dedicated workstation containing image processing software noted above, dual images of baseline and follow-up examinations were displayed simultaneously for standardized image processing. While a baseline image was displayed on the left-side of screen, follow-up images were displayed in real-time on the right-side of screen to permit accurate identification of the most suitable follow-up image that matched baseline image landmarks as precisely as possible. CIMT and IM-GSM were measured along a 1-cm segment demarcated by an electronic ruler, proximal to the carotid artery bulb in 10–15 sequential frames obtained on the R-wave of the simultaneously recorded ECG signal using frame-averaging (Figure 1). IM-GSM was determined from echogenicity of the same 1-cm segment using in-house developed software that determined pixel intensity (0–255; 0=black; 255=white) of each individual pixel in the intima-media complex. The median of all measured pixel intensities constituted the gray-scale median value. Whole images were digitized with a gray level resolution of 512 × 480 pixels and bit depth of 8 (256-degree gray scale per pixel) to standardize pixel density to 80 pixels per centimeter across images. Images were normalized by calibrating the gray level amplitude against the vessel lumen (blood) (gray value=0) and the adventitia (gray value=180) (Figure 2).
Figure 1. Example of carotid artery intima-media thickness measurement by computerized edge detection.
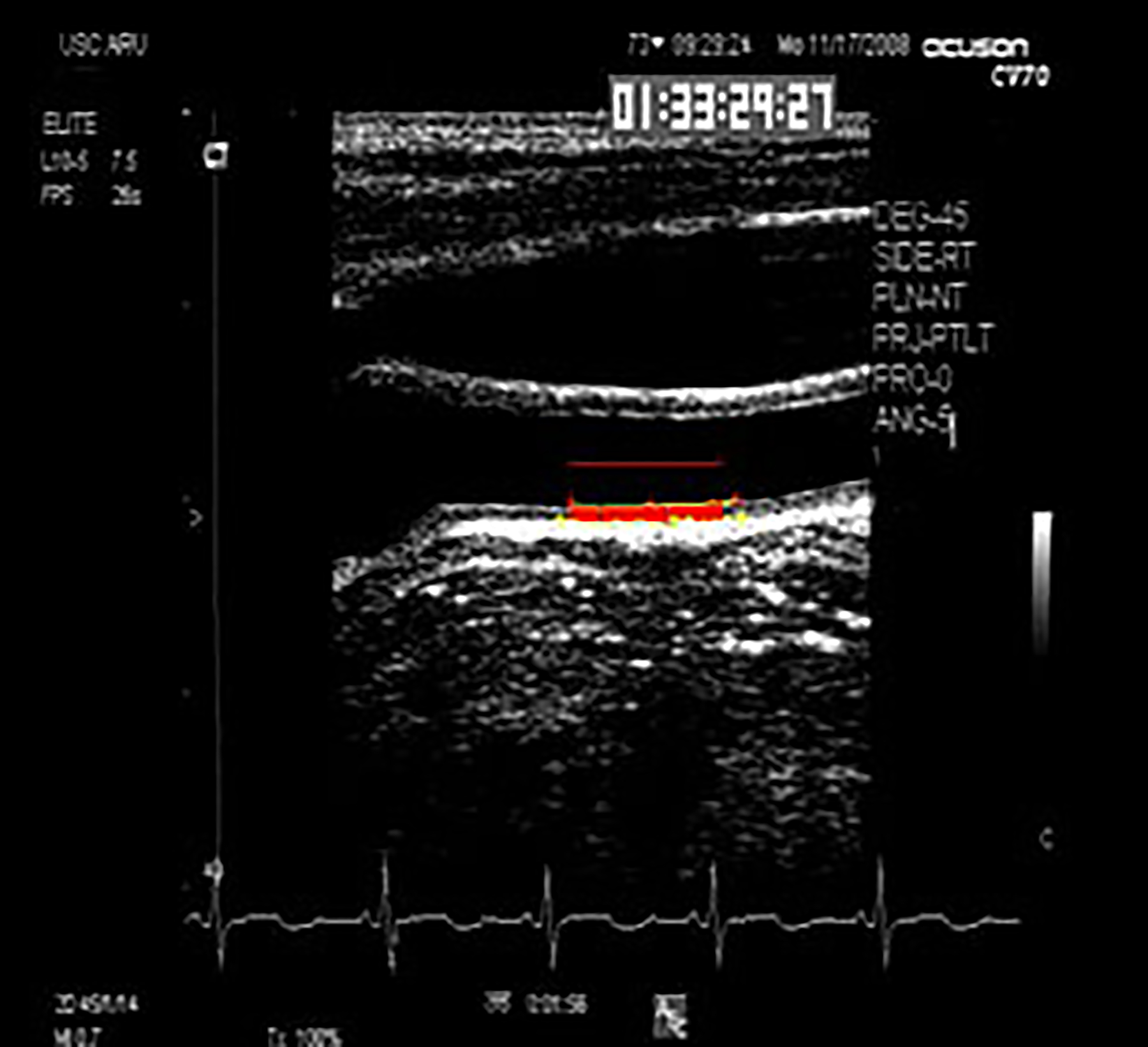
A computer-generated 1-cm electronic ruler is placed above far wall intima-media complex proximal to the carotid artery bulb. Automated computerized edge detection identifies lumen-intima and media-adventitia edges across which intima-media thickness is determined by averaged distances between paired pixels of computer-defined edges along span of electronic ruler.
Figure 2. Representative gray-scale of ultrasound images in the Early versus Late Intervention Trial of Estradiol.
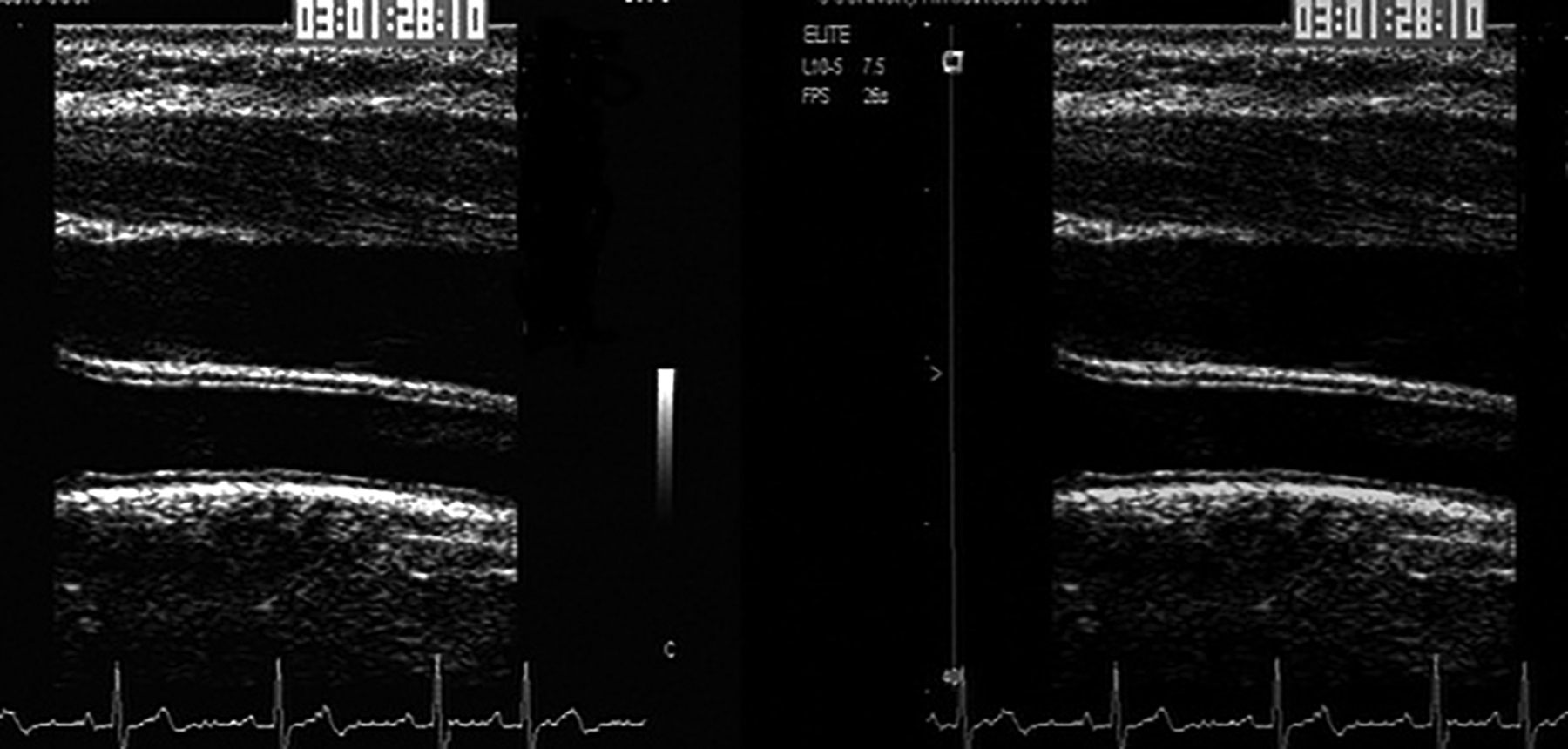
Image A (on left) is ultrasound image before normalization in which gray-scale across images subtly vary. Image B (on right) is ultrasound image after normalization in which gray-scale variations across ultrasound images are uniform.
Laboratory and clinical measurements:
Laboratory assessments were performed in overnight fasting blood samples collected at a baseline visit prior to randomization. Fasting serum glucose (FSG), triglycerides, and high-density lipoprotein- (HDL), low-density lipoprotein (LDL)-cholesterol, were measured as previously described13.
Assessment of physical activity:
The Stanford Seven-Day Physical Activity Recall questionnaire was used to collect physical activity information in the week prior to study visits16. Each reported activity was coded for its metabolic equivalents of energy expenditure (METs) using the coding scheme provided by Ainsworth, et al17. Energy expenditure of each activity over the week prior to each visit was calculated by multiplying hours of participation by the activity’s MET. Total energy expenditure was the sum of energy expenditure (MET-hours) during sleep, light, moderate and vigorous activities18.
Assay methods for biomarkers of inflammation and adipokines:
Serum levels of inflammatory biomarkers including ICAM-1, VCAM-1, E-selectin, P-selectin, MIP-1α, TNF- α, INF-γ, IL-1 α, IL-1β, IL-8, IL-10, MCP-1 were carried out on a multiplex platform (Simple Plx™ Ella, Protein Simple, CA) based on monoclonal antibody sandwich immunoassays using biotin-streptavidin conjugate chemistry. Fifty microliters of diluted serum was added to an appropriate cartridge, followed by placement into the Ella instrument. Each cartridge included a built-in lot-specific standard curve. Detection of monoclonal antibodies and streptavidin -DyLight650 conjugate as well as all washing steps were automatically performed by the instruments. Raw data were analyzed using simple plex Explorer software. The inter assay coefficients of variation ranged from 3.4% to 12.8% for all the assays. Serum leptin, adiponectin and ghrelin were measured by highly specific radioimmunoassay (RIA) and resistin by enzyme-linked immunosorbent assay (ELISA) using reagents from Linco Research (St. Charles, MO). Leptin assay sensitivity was 0.5 ng/ml and interassay coefficients of variation (CVs) were 6.2%, 4.7% and 3.6% at 4.9 ng/ml, 0.4 ng/ml and 25.6 ng/ml, respectively. Adiponectin assay sensitivity was 1 ng/ml, and interassay CVs were 9.2%, 6.9% and 9.2% at 1.5 ng/ml, 3.0 ng/ml and 7.5 ng/ml, respectively. Sensitivity of the total ghrelin assay was 93 pg/ml and interassay CVs were 14.7%, 16.0% and 16.7% at 1 ng/ml, 2 ng/ml and 3 ng/ml, respectively. IL-6 was measured by a highly specific direct ELISA using reagents obtained from R&D Systems (Minneapolis, MN). Minimal detectable level was 0.04 pg/ml, and interassay CVs were 9.6%, 7.2% and 6.5% at 0.5 pg/ml, 2.8 pg/ml and 5.6 pg/ml, respectively.
Statistical analysis:
Both outcome variables, CIMT and IM-GSM were analyzed as continuous variables. Other than race, all CVD risk factors were analyzed as continuous variables. Correlation between CIMT and IM-GSM was evaluated by Pearson’s correlation test. Linear regression models were fitted to evaluate associations between CVD risk factors and each atherosclerosis outcome variable. CVD risk factors associated with outcome variables with p<0.10 were included in the multivariable regression model. Due to very high correlation between SBP and DBP, separate multivariable regression models were run for SBP and DBP.
To compare relative contributions of each category of risk factors to each of the atherosclerosis outcomes, R2 from each regression model (including a particular set of risk factors such as lipids, blood pressure, glucose, and inflammatory markers) were compared between CIMT and IM-GSM outcomes. A basic demographic-risk factor model was first built including age, race, BMI and physical activity measured as total weekly MET. Subsequently, each set of risk factors was added to the regression models to compare the contribution of each particular risk factor category between CIMT and IM-GSM outcomes by calculating the R2 difference between R2 (basic demographic factors + additional risk factors) − R2 (basic demographic-risk factor model). BMI was excluded from the basic demographic risk factor model for assessing the contribution of adipokines on atherosclerosis markers to avoid collinearity between BMI and leptin (Pearson’s r = 0.76; p <0.0001). Analyses were performed using SAS version 9.2 (Cary, NC).
Results:
This post hoc analysis included baseline data from all 643 postmenopausal women who participated in ELITE. Women were on average±SD 61±7 years old, overweight (average BMI 27±5 kg/m2) with non-Hispanic White (68%) the majority race followed by Hispanic (14%), Black (9%) and Asian (8%) women (Table 1); 24% of women used antihypertensive medications and 20% used lipid-lowering medications. Average SBP and DBP, serum levels of triglycerides, HDL-cholesterol and FSG were within normal ranges. LDL-cholesterol level was slightly higher than a clinically normal level (mean 136±31 mg/dl).
Table 1.
Demographic and clinical variables (N = 643)
| Characteristics | |
|---|---|
| Age, years | 60.6 (6.9) |
| Race (%) | |
| White non-Hispanic | 68.43% |
| Black non-Hispanic | 9.33% |
| Hispanic | 14% |
| Asian | 8.24% |
| Body mass index, kg/m2 | 27.3 (5.4) |
| Physical activity, MET† hours/week | 245.8 (21.0) |
| Antihypertensive medication use (%) | 24% |
| Lipid lowering medication use (%) | 20% |
| Diastolic blood pressure, mmHg | 75.04 (7.1) |
| Systolic blood pressure, mmHg | 117.88 (12.4) |
| HDL cholesterol, mg/dl | 66.0 (17.6) |
| LDL cholesterol, mg/dl | 136.4 (31.4) |
| Triglycerides, mg/dl | 106.8 (12.6) |
| Glucose, mg/dl | 94.9 (10.3) |
| CIMT‡ (mm) | 0.77 (0.11) |
| IM-GSM¥ (unitless) | 59.9 (11.0) |
Mean (SD)
Metabolic equivalent of task
Carotid artery Intima-media thickness
Intima-media gray scale median
CIMT and IM-GSM were inversely correlated (Pearson’s r = −0.43, p <0.0001; Figure 3). When CVD risk factors of atherosclerosis were evaluated univariately for association, age, Black race, BMI, SBP, DBP, LDL-cholesterol, IL-6 and ICAM-1 were significantly directly and HDL-cholesterol significantly inversely associated with CIMT (Table 2, all p<0.04). Age, Black and Hispanic race, BMI, SBP, DBP, triglycerides, LDL-cholesterol, FSG, leptin, IL-6, ICAM-1, and E-selectin were significantly inversely associated with IM-GSM (indicating associations with greater echolucency; Table 2, all p<0.004); physical activity, HDL-cholesterol, adiponectin and ghrelin were significantly directly associated with IM-GSM (indicating association with less echolucency, all p<0.0003). In multivariable analysis, age, Black race, BMI, SBP and leptin were significantly independently associated with CIMT (Table 3). Age, Hispanic race, BMI, SBP, physical activity, LDL-cholesterol and leptin were significant independent correlates of IM-GSM (Table 3).
Figure 3. Correlation between CIMT and IM-GSM.
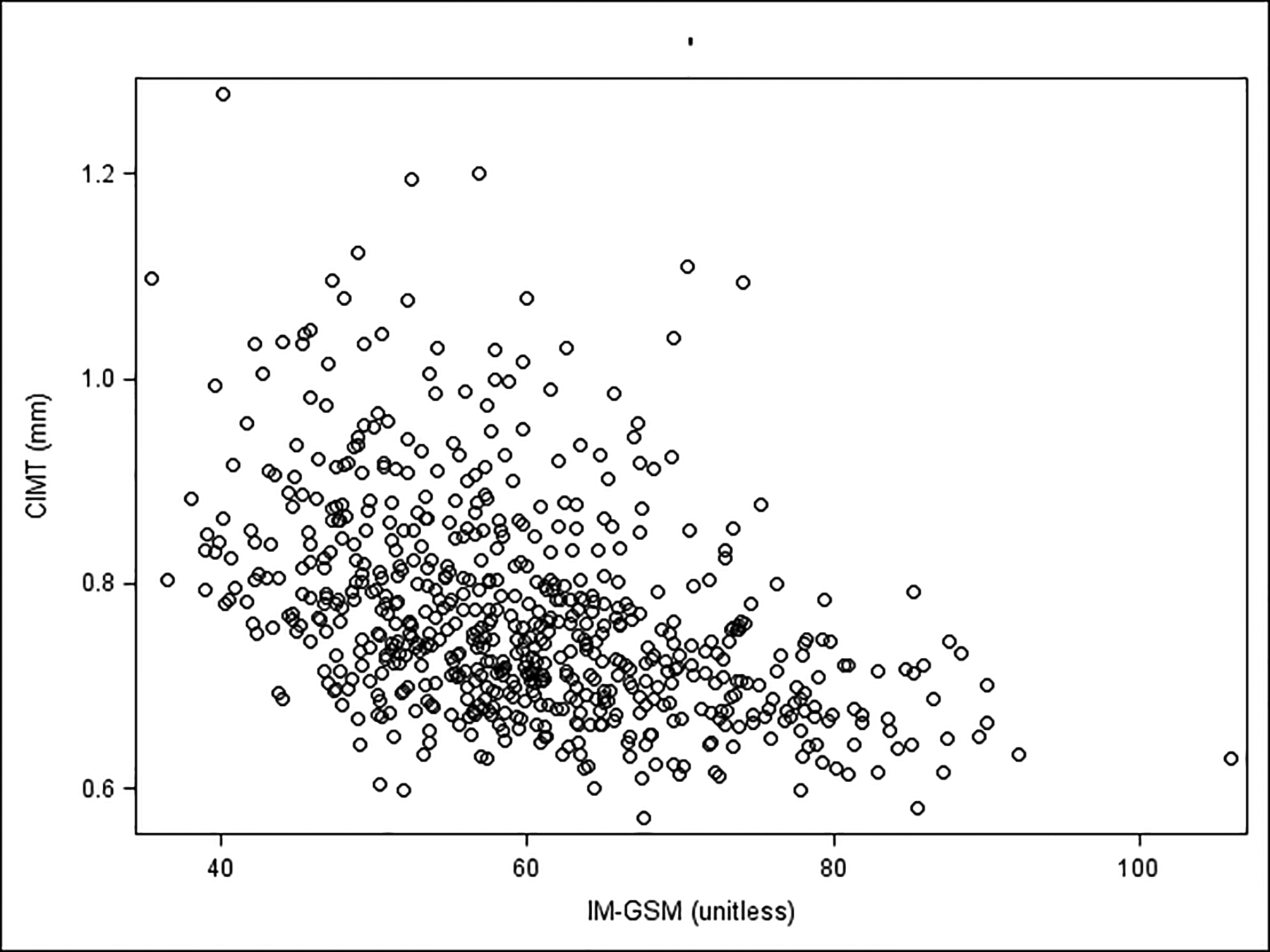
CIMT = Carotid artery intima-media thickness; IM-GSM = Carotid artery intima-media gray-scale median; Pearson’s r = −0.43; P-value<0.0001
Table 2:
Unadjusted associations of cardiovascular risk factors with CIMT and IM-GSM
| CIMT‡ (μm) | IM-GSM¥ (unitless) | |||||
|---|---|---|---|---|---|---|
| β-estimate | (SE) | p-value | β-estimate | (SE) | p-value | |
| Age, years | 5.288 | (0.565) | <.0001 | −0.235 | (0.062) | 0.0002 |
| Race | ||||||
| White non-Hispanic | ref | ref | ||||
| Black non-Hispanic | 43.33 | (14.35) | 0.003 | −4.54 | (1.48) | 0.002 |
| Hispanic | −21.46 | (12.06) | 0.08 | −5.50 | (1.25) | <.0001 |
| Asian | −14.78 | (15.16) | 0.33 | −0.99 | (1.57) | 0.53 |
| Body mass index, kg/m2 | 2.724 | (0.76) | 0.0004 | −0.853 | (0.07) | <.0001 |
| Physical activity, MET hours/week | 0.031 | (0.20) | 0.88 | 0.077 | (0.02) | 0.0002 |
| LDL cholesterol, mg/dl | 0.291 | (0.13) | 0.03 | −0.041 | (0.01) | 0.003 |
| HDL cholesterol, mg/dl | −0.501 | (0.24) | 0.03 | 0.147 | (0.02) | <.0001 |
| Triglycerides, mg/dl | 0.098 | (0.08) | 0.20 | −0.046 | (0.008) | <.0001 |
| Systolic blood pressure, mmHg | 2.609 | (0.32) | <.0001 | −0.198 | (0.03) | <.0001 |
| Diastolic blood pressure, mmHg | 1.489 | (0.59) | 0.01 | −0.285 | (0.06) | <.0001 |
| Antihypertensive medication use | 23.49 | (9.73) | 0.02 | −4.84 | (0.99) | <.0001 |
| Glucose, mg/dl | 0.682 | (0.40) | 0.09 | −0.209 | (0.04) | <.0001 |
| Leptin, ng/ml | 0.558 | (0.28) | 0.05 | −0.214 | (0.03) | <.0001 |
| Adiponectin, μg/ml | −0.909 | (0.61) | 0.13 | 0.272 | (0.06) | <.0001 |
| Ghrelin, pg/ml | −0.009 | (0.009) | 0.33 | 0.005 | (0.001) | <.0001 |
| IL-6, pg/ml | 8.967 | (3.40) | 0.009 | −1.283 | (0.35) | 0.0003 |
| ICAM-1, ng/ml | 0.148 | (0.06) | 0.01 | −0.021 | (0.006) | 0.0006 |
| VCAM-1, ng/ml | 0.025 | (0.025) | 0.31 | 0.0016 | (0.003) | 0.55 |
| E-selectin, ng/ml | 0.500 | (0.40) | 0.21 | −0.252 | (0.04) | <0.0001 |
| P-selectin, ng/ml | 0.120 | 0.144) | 0.40 | −0.008 | (0.015) | 0.57 |
| MIP-1α, pg/ml | −0.0001 | (0.013) | 0.99 | −0.0001 | (0.001) | 0.95 |
| TNF-α, pg/ml | 0.519 | (1.61) | 0.79 | −0.204 | (0.167) | 0.22 |
| INF-γ, pg/ml | −0.561 | (1.23) | 0.65 | −0.092 | (0.129) | 0.47 |
| IL-1α, pg/ml | 0.040 | (0.08) | 0.65 | −0.007 | (0.009) | 0.43 |
| IL-1β, pg/ml | −0.003 | (0.03) | 0.91 | −0.001 | (0.003) | 0.74 |
| IL-8, pg/ml | 0.004 | (0.22) | 0.83 | −0.003 | (0.002) | 0.21 |
| IL-10, pg/ml | −0.015 | (0.04) | 0.71 | −0.0008 | (0.004) | 0.85 |
| MCP-1, pg/ml | 0.041 | (0.028) | 0.15 | −0.004 | (0.003) | 0.20 |
Carotid artery Intima-media thickness
Intima-media gray scale median
Table 3:
Multivariable associations of cardiovascular risk factors with CIMT and IM-GSM
| Risk factors | CIMT (μm) | IM-GSM | ||||
|---|---|---|---|---|---|---|
| R2=0.21 | R2=0.26 | |||||
| β-estimate | (SE) | p-value | β-estimate | (SE) | p-value | |
| Age, years | 4.65 | (0.56) | <.0001 | −0.226 | (0.06) | 0.0001 |
| Race (%) | ||||||
| White non-Hispanic | ref | ref | ||||
| Black non-Hispanic | 27.78 | (13.54) | 0.04 | −1.715 | (1.437) | 0.23 |
| Hispanic | −20.99 | (11.31) | 0.06 | −2.802 | (1.197) | 0.02 |
| Asian | −3.831 | (14.13) | 0.79 | −2.690 | (1.461) | 0.07 |
| Body mass index, kg/m2 | 2.95 | 1(.109) | 0.008 | −0.815 | (0.116) | <.0001 |
| Physical activity, MET hours/week | 0.037 | (0.018) | 0.049 | |||
| LDL-cholesterol, mg/dl | 0.24 | (0.12) | 0.05 | −0.026 | (0.013) | 0.04 |
| HDL-cholesterol, mg/dl | −0.25 | (0.24) | 0.30 | 0.015 | 0.029) | 0.59 |
| Triglycerides, mg/dl | −0.005 | 0.009 | 0.60 | |||
| Systolic blood pressure, mmHg | 1.70 | (0.32) | <.0001 | −0.076 | 0.034 | 0.02 |
| †Diastolic blood pressure, mmHg | 1.17 | (0.58) | 0.04 | −0.075 | (0.06) | 0.19 |
| ‡Antihypertensive medication use | 0.82 | (9.59) | 0.93 | −1.78 | (0.10) | 0.07 |
| Glucose, mg/dl | −0.001 | (0.39) | 0.90 | −0.076 | (0.04) | 0.06 |
| ±Leptin, ng/ml | −0.11 | (0.29) | 0.70 | −0.099 | (0.03) | 0.002 |
| Adiponectin, μg/ml | 0.049 | (0.06) | 0.44 | |||
| Ghrelin, pg/ml | −0.0003 | (0.001) | 0.76 | |||
| IL-6, pg/ml | 5.72 | (3.12) | 0.07 | −0.59 | (0.32) | 0.07 |
| ICAM-1, ng/ml | 0.02 | (0.06) | 0.68 | −0.0004 | (0.006) | 0.95 |
| E-selectin, ng/ml | −0.075 | (0.04) | 0.08 | |||
Risk factors significantly (p-value<0.05) associated in the univariate analysis were included in the multivariate model for each outcome
Results from separate model replacing SBP with DBP, R2 = 0.26 for IM-GSM, R2 = 0.18 for CIMT model
Results from separate model not including SBP and DBP, R2 = 0.20 for IM-GSM, R2 = 0.18 for CIMT model
Results from separate model not including BMI, R2 = 0.20 for IM-GSM, R2 = 0.20 for CIMT model
Comparing contributions of additional risk factors beyond demographic risk factors in explaining variability of atherosclerosis outcomes, lipids (added R2 = 1% for CIMT and 2% for IM-GSM), FSG (added R2 = 0.1% for CIMT and 1% for IM-GSM), inflammatory biomarkers (added R2 = 0.5% for CIMT and 1.3% for IM-GSM), and adipokines (added R2 = 1% for CIMT and 7% for IM-GSM) explained less variability in CIMT than IM-GSM (Table 4). In contrast, only SBP contributed greater explanation for CIMT variation than IM-GSM (added R2 = 4% for CIMT and 2% for IM-GSM) in addition to age, race, and BMI (excluded from adipokines models), and physical activity MET.
Table 4:
Comparison of R2 for sets of cardiovascular risk factors between CIMT and IM-GSM
| CIMT (μm) | IM-GSM (unitless) | |||
|---|---|---|---|---|
| Beta (SE) | P-value | Beta (SE) | P-value | |
| Model 1: | ||||
| *Basic demographic model + lipids | R2difference =0.01 | †R2difference = 0.02 | ||
| HDL-cholesterol, mg/dl | −0.35 (0.27) | 0.19 | 0.02 (0.03) | 0.36 |
| LDL-Cholesterol, mg/dl | 0.33 (0.13) | 0.01 | −0.03 (0.01) | 0.01 |
| Triglycerides, mg/dl | −0.10 (0.09) | 0.24 | −0.01 (0.01) | 0.33 |
| Model 2: | R2difference = 0.01 | R2difference = 0.01 | ||
| Basic demographic model + diastolic blood pressure (DBP) | ||||
| DBP, mmHg | 1.61 (0.56) | 0.004 | −0.14 (0.06) | 0.01 |
| Model 3: | R2difference = 0.04 | R2difference = 0.02 | ||
| Basic demographic model + systolic blood pressure (SBP) | ||||
| SBP mmHg | 1.89 (0.32) | <0.0001 | −0.11 (0.03) | 0.0006 |
| Model 4: | R2difference = 0.001 | R2difference = 0.01 | ||
| Basic demographic model + Glucose | ||||
| Glucose, mg/dl | 0.27 (0.38) | 0.49 | −0.10 (0.04) | 0.008 |
| Model 5: | ||||
| Basic demographic model + Inflammatory markers | R2difference = 0.005 | R2difference = 0.013 | ||
| Il_6, pg/ml | 6.05 (3.21) | 0.06 | −0.53 (0.32) | 0.10 |
| ICAM-1, ng/ml | 0.05 (0.06) | 0.42 | −0.002 (0.006) | 0.74 |
| E-selectin, ng/ml | −0.16 (0.42) | 0.71 | −0.09 (0.04) | 0.03 |
| Model 6‡: | ||||
| Basic demographic model + Adipokines | R2difference = 0.01 | R2difference = 0.07 | ||
| Leptin, ng/ml | 0.32 (0.29) | 0.27 | −0.16 (0.003) | <.0001 |
| Adiponectin, μg/ml | −1.06 (0.58) | 0.07 | 0.16 (0.06) | 0.01 |
| Ghrelin, pg/ml | −0.005 (0.01) | 0.61 | 0.0002 (0.001) | 0.08 |
Basic demographic model includes age race, BMI, and MET (R2= 0.16 for CIMT and 0.22 for IM-GSM)
(R2 of each model – R2 of basic demographic model)
Basic demographic model includes age, race, and MET (R2= 0.14 for CIMT and 0.08 for IM-GSM)
Discussion:
In this cross-sectional analysis, data show a modest inverse correlation between CIMT and IM-GSM in healthy postmenopausal women. Comparing traditional cardiovascular risk factors univariately, the majority of risk factors (age, race, BMI, LDL- and HDL-cholesterol, SBP, and DBP) are shared between CIMT and IM-GSM. CVD risk factor correlates unique to IM-GSM included physical activity (measured by weekly metabolic equivalent-hours) and triglyceride and FSG levels. Additionally, compared with CIMT, IM-GSM was associated with a greater number of inflammation markers including IL-6, ICAM-1, E-selectin, ghrelin, leptin, and adiponectin, whereas CIMT was significantly associated with IL-6 and ICAM-1 only. Lipids, FSG, and inflammatory markers explained variation in IM-GSM more than variation in CIMT. SBP explained variation in CIMT more than variation in IM-GSM.
Since atherosclerosis is a driving force for CVD, assessment of subclinical atherosclerosis is of considerable importance for primary and secondary prevention of CVD. Determined from B-mode ultrasound images, CIMT is the most validated and widely used measure of subclinical atherosclerosis in research studies for over 3 decades. IM-GSM, also derived from B-mode ultrasound images, is based on the echogenicity of the intima-media complex of the common carotid artery. Previous studies measured echogenicity of plaques3–5,19. Use of echogenicity to evaluate plaque-free carotid artery segments as a correlate or predictor of CVD is less common6,7,11,12. Reports from the Prospective Study of the Vasculature in 1016 Uppsala Seniors (PIVUS) showed a strong correlation between carotid artery plaque GSM and IM-GSM (r=0.60, p<0.0001) independent of CIMT and plaque size20. A similar correlation between common carotid artery (CCA) IM-GSM and plaque GSM (r=0.76, p<0.001) independent of CIMT and plaque size was reported from another study among 87 asymptomatic patients with carotid artery disease21. The PIVUS study group subsequently reported IM-GSM as a new and independent predictor of all-cause mortality in the same Swedish elderly population of men12. IM-GSM has also been reported to be a strong correlate of stroke22. A case-cohort study from the Multi-Ethnic Study of Atherosclerosis (MESA) reported no significant association between IM-GSM and CHD, stroke, and CVD after controlling for CVD risk factors23. Taken together, data indicate that IM-GSM is a reliable indicator of subclinical atherosclerosis above and beyond CIMT and more importantly, IM-GSM is strongly associated with stroke and predictive of all-cause mortality.
Correlation (Pearson’s r) between CIMT and IM-GSM in the current study was −0.43. Consistent with this finding, other investigators have shown modest inverse correlations between CIMT and IM-GSM ranging from −0.17 to −0.516,7,12,22. In a pilot cross-sectional study among a convenience sample of 151 MESA participants, CIMT was not significantly associated IM-GSM24. The modest to low correlation between CIMT and IM-GSM is indicative of differences in pathophysiology of these two distinct measures. IM-GSM is a relatively novel measure of atherosclerosis and correlates of IM-GSM have been less studied compared with CIMT. In our population of 643 healthy postmenopausal women, IM-GSM was significantly univariately associated with a much wider array of CVD risk factors compared with CIMT. In multivariable analysis, Hispanic race, physical activity, LDL-cholesterol and leptin were only associated with IM-GSM, whereas Black race and DBP were discriminating risk factors for CIMT. Our results are consistent with the limited number of studies comparing CVD risk factor profile between CIMT and IM-GSM. In the MESA cross-sectional pilot study, lower IM-GSM was significantly associated with greater BMI24. Among PIVUS men and women, CIMT was associated with blood pressure, whereas IM-GSM was significantly related with HDL-cholesterol, markers of oxidative stress and inflammation11. A report from the METEOR study showed that SBP and male gender were significant correlates of CIMT, whereas lipid factors, in particular HDL-cholesterol and triglycerides were associated with IM-GSM in 984 healthy participants with a 10-year Framingham risk score < 10%7. Consistent with METEOR, another cross-sectional report from the Women’s Interagency HIV Study (WIHS) comparing correlates of CIMT and IM-GSM among HIV+ women and comparable HIV- controls showed that hypertension was associated with CIMT, whereas HDL- and LDL-cholesterol and triglycerides were associated with IM-GSM6.
Based on cumulated data, it is likely that carotid artery wall thickness and echogenicity reflect different pathogenic processes contributing to atherosclerosis, with CIMT reflecting structural aspects and echogenicity reflecting compositional features. The current study significantly contributes to limited data comparing CIMT with IM-GSM since a much wider range of CVD risk factors were included and an additional analytical approach comparing extent of contribution of risk factors to CIMT and IM-GSM by grouping them according to categories was conducted. As such, in addition to showing a wider range of CVD risk factors significantly associated with echogenicity, the current data also showed that serum levels of lipids, glucose, inflammatory markers, and adipocytokines contributed less to thickness than echogenicity of the carotid artery wall, whereas SPB explained thickness more than echogenicity of carotid artery wall. These data suggest that integration of both CIMT and IM-GSM measures would potentially be stronger in detection of subclinical atherosclerosis.
The current study population is limited to postmenopausal women, which could be a limitation with regards to generalizability of findings. However, postmenopausal women are a high risk population as risk of CVD increases after menopause25 and CVD is the leading cause of mortality in women in the US26. Although right CIMT used in this study is associated with coronary artery disease as assessed by serial quantitative coronary angiography27 and predicts clinical cardiovascular events28, echogenicity assessed unilaterally may underestimate atherosclerosis burden and CVD risk associations. Results of this study are cross-sectional in nature and should be evaluated longitudinally.
CIMT is a widely used measure of early subclinical atherosclerosis and has been shown predictive of future CVD independent of major risk factors28–30. Although recent reviews and meta-analyses confirm that CIMT is an independent predictor of cardiovascular events, further efforts are warranted to improve assessment of subclinical atherosclerosis31–33. Differential association of CVD risk factors with CIMT and IM-GSM shown in this study suggest that CIMT and IM-GSM reflect different atherosclerosis phenotypes. Further studies are needed to confirm these findings. Data from the current study have implications for primary prevention of CVD as study participants were healthy without clinically evident CVD, chronic diseases such as cancer, thyroid disease, and diabetes. Incorporating measurement of both CIMT and IM-GSM may yield a more comprehensive assessment of subclinical atherosclerosis in asymptomatic individuals. Future studies are needed to evaluate whether IM-GSM progression predicts cardiovascular events as does CIMT28,34.
Funding source:
This work was supported by The National Institute on Aging, National Institutes of Health (R01-AG024154 and R01-AG059690)
Footnotes
Disclosure: Authors have no disclosures.
References:
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. [DOI] [PubMed] [Google Scholar]
- 2.Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14(2):177–192. [DOI] [PubMed] [Google Scholar]
- 3.Gronholdt ML. Ultrasound and lipoproteins as predictors of lipid-rich, rupture-prone plaques in the carotid artery. Arterioscler Thromb Vasc Biol. 1999;19(1):2–13. [DOI] [PubMed] [Google Scholar]
- 4.Gronholdt ML, Wiebe BM, Laursen H, Nielsen TG, Schroeder TV, Sillesen H. Lipid-rich carotid artery plaques appear echolucent on ultrasound B-mode images and may be associated with intraplaque haemorrhage. Eur J Vasc Endovasc Surg. 1997;14(6):439–445. [DOI] [PubMed] [Google Scholar]
- 5.Gronholdt ML, Nordestgaard BG, Schroeder TV, Vorstrup S, Sillesen H. Ultrasonic echolucent carotid plaques predict future strokes. Circulation. 2001;104(1):68–73. [DOI] [PubMed] [Google Scholar]
- 6.Jung M, Parrinello CM, Xue X, et al. Echolucency of the carotid artery intima-media complex and intima-media thickness have different cardiovascular risk factor relationships: the Women’s Interagency HIV Study. J Am Heart Assoc. 2015;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters SA, Lind L, Palmer MK, et al. Increased age, high body mass index and low HDL-C levels are related to an echolucent carotid intima-media: the METEOR study. J Intern Med. 2012;272(3):257–266. [DOI] [PubMed] [Google Scholar]
- 8.Staub D, Partovi S, Schinkel AF, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. 2011;258(2):618–626. [DOI] [PubMed] [Google Scholar]
- 9.Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loizou CP, Pantziaris M, Pattichis MS, Kyriacou E, Pattichis CS. Ultrasound image texture analysis of the intima and media layers of the common carotid artery and its correlation with age and gender. Comput Med Imaging Graph. 2009;33(4):317–324. [DOI] [PubMed] [Google Scholar]
- 11.Andersson J, Sundstrom J, Gustavsson T, et al. Echogenecity of the carotid intima-media complex is related to cardiovascular risk factors, dyslipidemia, oxidative stress and inflammation: the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis. 2009;204(2):612–618. [DOI] [PubMed] [Google Scholar]
- 12.Wohlin M, Sundstrom J, Andren B, Larsson A, Lind L. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis. 2009;205(2):486–491. [DOI] [PubMed] [Google Scholar]
- 13.Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;135(11):939–953. [DOI] [PubMed] [Google Scholar]
- 15.Selzer RH, Hodis HN, Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111(1):1–11. [DOI] [PubMed] [Google Scholar]
- 16.Young DR, Haskell WL, Jatulis DE, Fortmann SP. Associations between changes in physical activity and risk factors for coronary heart disease in a community-based sample of men and women: the Stanford Five-City Project. Am J Epidemiol. 1993;138(4):205–216. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury F, Bernstein L, Hodis HN, Stanczyk FZ, Mack WJ. Physical activity and sex hormone levels in estradiol- and placebo-treated postmenopausal women. Menopause. 2011;18(10):1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ariyoshi K, Okuya S, Kunitsugu I, et al. Ultrasound analysis of gray-scale median value of carotid plaques is a useful reference index for cerebro-cardiovascular events in patients with type 2 diabetes. J Diabetes Investig. 2015;6(1):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind L, Andersson J, Ronn M, Gustavsson T. The echogenecity of the intima-media complex in the common carotid artery is closely related to the echogenecity in plaques. Atherosclerosis. 2007;195(2):411–414. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahimi P, Jashari F, Johansson E, et al. Common carotid intima-media features determine distal disease phenotype and vulnerability in asymptomatic patients. Int J Cardiol. 2015;196:22–28. [DOI] [PubMed] [Google Scholar]
- 22.Aizawa K, Elyas S, Adingupu DD, et al. Echogenicity of the Common Carotid Artery Intima-Media Complex in Stroke. Ultrasound Med Biol. 2016;42(5):1130–1137. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell CC, Korcarz CE, Gepner AD, et al. Carotid Artery Echolucency, Texture Features, and Incident Cardiovascular Disease Events: The MESA Study. J Am Heart Assoc. 2019;8(3):e010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell CC, Korcarz CE, Tattersall MC, et al. Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Br J Radiol. 2018;91(1084):20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114(2):413–419. [DOI] [PubMed] [Google Scholar]
- 26.Heron M. Deaths: Leading Causes for 2017. Natl Vital Stat Rep. 2019;68(6):1–77. [PubMed] [Google Scholar]
- 27.Mack WJ, LaBree L, Liu C, Selzer RH, Hodis HN. Correlations between measures of atherosclerosis change using carotid ultrasonography and coronary angiography. Atherosclerosis. 2000;150(2):371–379. [DOI] [PubMed] [Google Scholar]
- 28.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–269. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. [DOI] [PubMed] [Google Scholar]
- 30.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96(5):1432–1437. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Oord SC, Sijbrands EJ, ten Kate GL, et al. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis. 2013;228(1):1–11. [DOI] [PubMed] [Google Scholar]
- 33.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308(8):796–803. [DOI] [PubMed] [Google Scholar]
- 34.Willeit P, Tschiderer L, Allara E, et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation. 2020;142(7):621–642. [DOI] [PMC free article] [PubMed] [Google Scholar]


