Abstract
Background
Several studies have reported the efficacy of drug-coated balloons (DCB) for simple femoropopliteal (FP) lesions. However, the effectiveness of DCB for FP chronic total occlusive lesions (CTO) is controversial. The present study investigated the clinical outcomes of DCB for FP-CTO.
Materials and methods
We retrospectively analyzed 359 limbs of 318 patients who underwent endovascular therapy with DCB for FP-CTO between July 2017 and February 2021 at seven cardiovascular centers. The primary endpoint was 12-month primary patency. The secondary endpoints were the 12-month rates of freedom from: (1) clinically-driven target lesion revascularization (CD-TLR), and (2) re-occlusion. The association of baseline characteristics with the 12-month restenosis risk was investigated using the Cox proportional hazards regression model.
Results
The 12-month rate of primary patency was 79.8% (95% confidence interval [95%CI], 75.1% to 84.8%), whereas the corresponding rates of freedom from CD-TLR and re-occlusion were 86.4% (95%CI: 82.6% to 90.4%) and 88.5% (95%CI: 84.7% to 92.4%), respectively. The bailout stent rate was 8.9%. Independent risk factors for restenosis were hemodialysis (adjusted hazard ratio, 2.18 [1.39 to 3.45]; P = 0.001), chronic limb-threatening ischemia (CLTI) (2.02 [1.33 to 3.07]; P = 0.001), and restenosis lesion (2.02 [1.32 to 3.08]; P = 0.001). Use of dual antiplatelet therapy (DAPT) was identified as a protective factor for restenosis (0.54 [0.35 to 0.82]; P = 0.003).
Conclusions
Despite the low rate of bailout stent, DCB treatment for FP-CTO was effective in real-world clinical practice. Hemodialysis, CLTI, and restenosis lesion were independent risk factors for 12-month restenosis, and the use of DAPT significantly attenuated the risk of 12-month restenosis.
Keywords: Drug-coated balloon, Chronic total occlusive lesion, Endovascular therapy, Femoropopliteal occlusive disease
Introduction
Currently, the prevalence of peripheral artery disease (PAD) has dramatically increased in aging societies (Song et al. 2019). Endovascular therapy (EVT) is considered a first-line strategy for femoropopliteal (FP) occlusive disease because of its low invasiveness, fewer complications, and improved durability related to advances in devices and current techniques (Aboyans et al. 2018; Bailey et al. 2019). Favorable clinical results of drug-coated balloons (DCB) have been reported for simple FP lesions (Iida et al. 2018; Laird et al. 2019; Tepe et al. 2015). However, for complex FP lesions, its use is still controversial. In particular, for chronic total occlusive lesion (CTO), the most challenging situation regarding FP lesions, the clinical benefit of DCB has been variable; unfavorable and outstanding results have been reported (AbuRahma et al. 2019; Hayakawa et al. 2022a, 2022b). Previous studies of DCB treatment for FP-CTO or FP-complex lesions (including a high percentage of CTO) have reported an acceptable 12-month primary patency (78% to 85%) (Tepe et al. 2019; Liistro et al. 2019). However, in these studies, 20%–50% of cases required bailout stents indicating the findings were not related to the “pure” effect of DCB itself. The actual performance of DCB on complex FP lesions, especially on CTO, is unknown. Therefore, in the present study, we investigated the clinical outcomes of DCB for FP-CTO. We avoided using bailout stents after DCB where possible to utilize the advantage of the “leaving nothing behind” strategy.
Materials and methods
Study population and design
The EAGLE (Clinical result of EndovAscular treatment with druG-coated baLloon for fEmoropopliteal chronic total occlusion) study is a multicenter, retrospective analysis from the prospectively maintained database. Between April 2017 and February 2021, 3635 consecutive symptomatic PAD patients with FP lesions received EVT, and 1457 lesions had CTO segments. Of these, 359 lesions (318 patients) were treated with DCB and enrolled in this study. Patients with non-atherosclerotic disease including vasculitis or systemic inflammatory disease, life expectancy of less than 1 year, advanced malignancy, acute limb ischemia, and congenital anatomical abnormalities such as persistent sciatic artery, or aneurysmal lesions were excluded. The selection of DCB (high dose or low dose) was decided based on each operators’ decision.
The study protocol was approved by the local ethics committee at all participating centers, and the study was performed in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective study design, in which existing medical records were used. Alternatively, patients could opt out of the study. Relevant information regarding the study is available to the public in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Procedural protocol
Aspirin, clopidogrel, prasugrel, cilostazol, or oral anticoagulant drugs were started at least 24 hours before the procedure. Dual antiplatelet therapy (DAPT), defined as the administration of aspirin and clopidogrel or prasugrel, was used at least 1 month after EVT. After the insertion of a guiding sheath from the ipsilateral or contralateral femoral artery, 0.014-, 0.018-, or 0.035-in. guidewires were used with a back-up support catheter. A bi-directional approach was conducted as needed. The type of pre-dilation balloon used (semi-compliant, non-compliant, cutting, or scoring balloon) depended on the operator. DCB was used after confirming as much as possible that the residual stenosis was < 50% and the degree of dissection was less than grade D (Fujihara et al. 2017; Feldman et al. 2018). The evaluation of a pressure gradient was performed as required, and pressure gradients < 10 mmHg were defined as significant stenosis (Tepe et al. 2015). After successful lesion preparation, the target lesion was fully covered by the DCB (geographic mismatches were carefully avoided). If > 50% residual stenosis or The National Heart, Lung and Blood Institute (NHLBI) grade D or higher dissection was observed after using DCB, bailout stenting was considered (the necessity of stent use was finally judged by each operator). Atherectomy devices were not used in this study because they were not commercially available in our country during the study period. Procedures and measurements were performed in each institute by at least two or more specialists from the Japanese Association of Cardiovascular Intervention and Therapeutics.
Study endpoints and definitions
Procedural success was defined as < 50% residual stenosis without angiographic flow-limiting dissection. Calcification severity was evaluated by the Peripheral Arterial Calcium Scoring System (PACSS) grade (Rocha-Singh et al. 2014). The NHLBI and Kobayashi classifications were used to evaluate the severity of the dissection (Fujihara et al. 2017; Kobayashi et al. 2018). High-dose DCB (3.5 μg/mm2) was IN.PACT Admiral DCB (Medtronic Vascular, Santa Clara, CA, USA), and low-dose DCB (2.0 μg/mm2) was Lutonix RX DCB (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) or Ranger DCB (Boston Scientific Corporation, Marlborough, MA, USA).
The primary endpoint of this study was 12-month primary patency, defined as freedom from restenosis. We defined restenosis as 1) a peak systolic velocity ratio ≥ 2.5 by duplex ultrasound (DUS), or 2) a vessel with > 50% diameter stenosis by computed tomography angiography (CTA) or angiography. The secondary endpoints were 12-month freedom from 1) clinical driven target lesion revascularization (CD-TLR) and 2) re-occlusion. The rates of major amputation and all-cause mortality were also evaluated. Patency of the target vessel was evaluated by DUS, CTA, or angiography depending on the recommendations of the Society of Vascular Surgery: every 3 months during the first year, or every 6 months or annually thereafter (Stoner et al. 2016).
Statistical analysis
Data are presented as the mean ± standard deviation for continuous variables and as a percentage for categorical variables, unless otherwise indicated. A P-value < 0.05 was considered statistically significant, and 95% confidence intervals (CIs) were reported when appropriate. Time-to-events were estimated by the Kaplan-Meier method. The association of baseline characteristics with the 12-month restenosis risk was investigated using the Cox proportional hazards regression model. The variables with statistical significance in the univariate model were entered into the multivariate model. Missing data were addressed using the multiple imputation by chained equations method. In this procedure, we generated five imputed datasets and combined the analytic results based on Rubin’s rule. All statistical analyses were performed with R, version 4.1.1 (R Development Core Team, Vienna, Austria).
Results
Baseline characteristics
Clinical characteristics are summarized in Table 1. The mean age was 75 ± 9 years and 66.0% were male. The prevalences of diabetes mellitus and hemodialysis were 62.6% and 20.4%, respectively. Perioperative DAPT use was 66.4%, cilostazol use was 23.0%, and anticoagulant use was 17.9%. The percentages of CLTI, history of EVT, and popliteal involved lesions were 32.9%, 26.7%, and 47.6%, respectively. The reference vessel diameter was 5.1 ± 0.8 mm, the lesion length was 21.9 ± 10.0 cm, and the occlusion length was 14.5 ± 10.6 cm. PACCS grade 3 was observed in 10.0% of cases, and grade 4 in 23.1%. High-dose DCB use was 68.5% and intravascular ultrasound (IVUS) use was 77.2%. The rate of bailout stent was 8.9%.
Table 1.
Baseline characteristics
| Patient characteristics | (n = 318) | |
| Male sex | 210 (66.0%) | |
| Age (years) | 75 ± 9 | |
| Diabetes mellitus | 199 (62.6%) | |
| Smoking | 76 (23.9%) | |
| Renal failure on dialysis | 65 (20.4%) | |
| Cerebrovascular disease | 82 (25.8%) | |
| Coronary artery disease | 151 (47.5%) | |
| Chronic heart failure | 68 (21.4%) | |
| Dual antiplatelet therapy | 211 (66.4%) | |
| Cilostazol use | 73 (23.0%) | |
| Anticoagulant use | 57 (17.9%) | |
| β-blocker use | 120 (37.7%) | |
| Renin-angiotensin system inhibitor use | 169 (53.1%) | |
| Statin use | 207 (65.1%) | |
| Limb characteristics | (n = 359) | |
| Chronic limb-threatening ischemia | 118 (32.9%) | |
| Ankle brachial index | 0.60 ± 0.16 | (missing data, n = 64) |
| Restenosis lesion | 96 (26.7%) | |
| Lesion location | ||
| SFA | 142 (39.6%) | |
| SFA-Pop A | 155 (43.2%) | |
| SFA-BTK | 12 (3.3%) | |
| Pop A | 33 (9.2%) | |
| Pop A-BTK | 17 (4.7%) | |
| Popliteal involvement | 217 (60.4%) | |
| Reference vessel diameter (mm) | 5.1 ± 0.8 | |
| Lesion length (mm) | 218.8 ± 100.0 | |
| Length of chronic total occlusion (mm) | 145.3 ± 106.4 | |
| PACSS classification | ||
| Grade 0 | 173 (48.2%) | |
| Grade 1 | 48 (13.4%) | |
| Grade 2 | 19 (5.3%) | |
| Grade 3 | 36 (10.0%) | |
| Grade 4 | 83 (23.1%) | |
| Below-the-knee runoff | (missing data, n = 1) | |
| No runoff | 16 (4.5%) | |
| 1 runoff | 150 (41.9%) | |
| 2 runoffs | 131 (36.6%) | |
| 3 runoffs | 61 (17.0%) | |
| High-dose DCB use | 246 (68.5%) | |
| Mean diameter of DCB (mm) | 5.1 ± 0.7 | (missing data, n = 38) |
| Total length of DCB (mm) | 232.7 ± 102.1 | (missing data, n = 38) |
| Intravascular ultrasound use | 277 (77.2%) | |
| Stent implantation | 32 (8.9%) | |
| Dissection grade NHLBI ≥ C | 58 (16.2%) | |
| Dissection grade Kobayashi ≥1/3 | 46 (12.8%) | |
| Subintimal wire passage | 60 (21.7%) | (missing data, n = 83) |
| Degree of calcification | (missing data, n = 81) | |
| 0° | 85 (30.6%) | |
| 1 to 90° | 83 (29.9%) | |
| 91 to 180° | 29 (10.4%) | |
| 181 to 270° | 24 (8.6%) | |
| 271 to 360° | 57 (20.5%) | |
| Calcified nodule | 58 (20.8%) | (missing data, n = 80) |
| Degree of dissection after DCB | (missing data, n = 155) | |
| 0° | 62 (30.4%) | |
| 1 to 90° | 82 (40.2%) | |
| 91 to 180° | 52 (25.5%) | |
| 181 to 270° | 7 (3.4%) | |
| 271 to 360° | 1 (0.5%) | |
| Minimum lumen area (mm2) | 12.7 ± 5.1 | (missing data, n = 161) |
| Distal EEM area (mm2) | 29.8 ± 11.8 | (missing data, n = 92) |
| Distal lumen area (mm2) | 17.0 ± 6.9 | (missing data, n = 122) |
| Major amputation | 4 (1.3%) | |
| All-cause mortality | 45 (14.2%) | |
Outcome measures
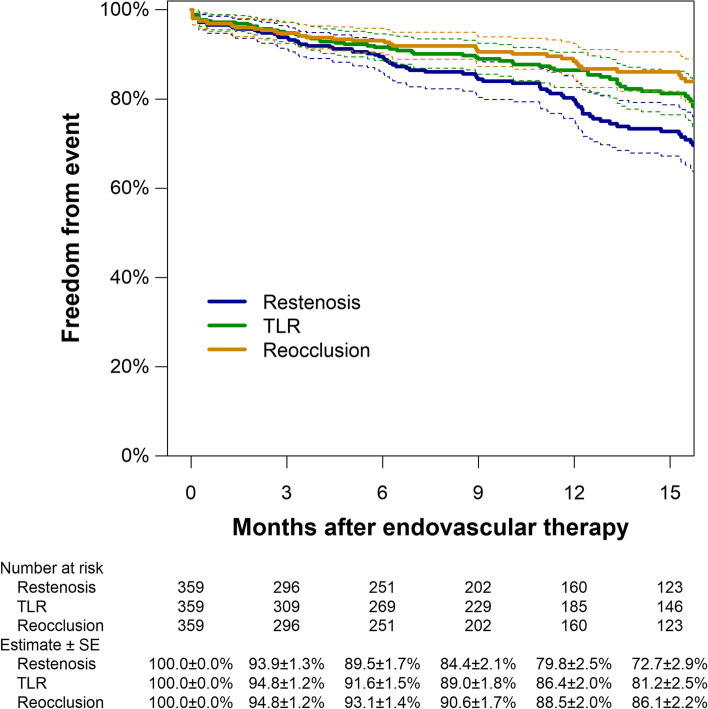
The median follow-up period was 10.9 months (interquartile range: 4.8–18.3). The rate of 12-month major amputation and all-cause mortality was 1.3% and 14.2%, respectively. During the study period, restenosis was detected in 93 cases. Figure 1 illustrates the Kaplan Meier estimates of primary patency and freedom from CD-TLR. The 12-month primary patency was 79.8% (95%CI: 75.1% to 84.8%), whereas the corresponding rates of freedom from CD-TLR and re-occlusion were 86.4% (95% CI: 82.6% to 90.4%) and 88.5% (95% CI: 84.7% to 92.4%), respectively. Table 2 shows the association of the clinical characteristics with the 12-month restenosis risk. Clinical features independently associated with restenosis risk were hemodialysis (adjusted hazard ratio, 2.18 [1.39 to 3.45]; P = 0.001), CLTI (2.02 [1.33 to 3.07]; P = 0.001), and restenosis lesion (2.02 [1.32 to 3.08]; P = 0.001). The administration of DAPT significantly attenuated the risk of 12-month restenosis (0.54 [0.35 to 0.82]; P = 0.003).
Fig. 1.
Kaplan Meier estimates of primary patency, freedom from TLR, and freedom from re-occlusion. Dotted lines indicate 95% confidence intervals. SE, standard error
Table 2.
Association of baseline characteristics with 1-year restenosis risk
| Unadjusted hazard ratio | Adjusted hazard ratio | |
|---|---|---|
| Male sex | 0.78 [0.51 to 1.18] (P = 0.24) | N/I |
| Age (years) | 0.98 [0.96 to 1.00] (P = 0.12) | N/I |
| Diabetes mellitus | 1.02 [0.67 to 1.55] (P = 0.94) | N/I |
| Smoking | 1.39 [0.89 to 2.20] (P = 0.15) | N/I |
| Renal failure on dialysis | 2.35 [1.50 to 3.66] (P < 0.001) | 2.18 [1.39 to 3.45] (P = 0.001) |
| Cerebrovascular disease | 1.49 [0.95 to 2.32] (P = 0.081) | N/I |
| Coronary artery disease | 0.78 [0.52 to 1.18] (P = 0.24) | N/I |
| Chronic heart failure | 0.84 [0.50 to 1.43] (P = 0.52) | N/I |
| Dual antiplatelet therapy | 0.62 [0.41 to 0.94] (P = 0.023) | 0.54 [0.35 to 0.82] (P = 0.003) |
| Cilostazol use | 1.11 [0.71 to 1.73] (P = 0.66) | N/I |
| Anticoagulant use | 1.23 [0.74 to 2.03] (P = 0.43) | N/I |
| β blocker use | 1.04 [0.68 to 1.59] (P = 0.86) | N/I |
| Renin-angiotensin system inhibitor use | 0.82 [0.54 to 1.23] (P = 0.33) | N/I |
| Statin use | 0.93 [0.61 to 1.43] (P = 0.75) | N/I |
| Chronic limb-threatening ischemia | 2.19 [1.45 to 3.29] (P < 0.001) | 2.02 [1.33 to 3.07] (P = 0.001) |
| Ankle brachial index | 1.30 [0.20 to 8.53] (P = 0.77) | N/I |
| Restenosis lesion | 1.98 [1.30 to 3.01] (P = 0.001) | 2.02 [1.32 to 3.08] (P = 0.001) |
| Popliteal involvement | 1.25 [0.83 to 1.88] (P = 0.28) | N/I |
| Reference vessel diameter (mm) | 0.81 [0.62 to 1.06] (P = 0.13) | N/I |
| Lesion length (mm) | 1.00 [1.00 to 1.00] (P = 0.61) | N/I |
| Length of chronic total occlusion (mm) | 1.00 [1.00 to 1.00] (P = 0.082) | N/I |
| PACSS classification | 1.05 [0.93 to 1.18] (P = 0.43) | N/I |
| Below-the-knee runoff | 1.99 [0.87 to 4.56] (P = 0.11) | N/I |
| High-dose DCB use | 0.71 [0.47 to 1.08] (P = 0.11) | N/I |
| Mean diameter of DCB (mm) | 0.74 [0.35 to 1.55] (P = 0.36) | N/I |
| Total length of DCB (mm) | 1.00 [1.00 to 1.00] (P = 0.99) | N/I |
| Intravascular ultrasound use | 0.85 [0.53 to 1.36] (P = 0.49) | N/I |
| Stent implantation | 1.17 [0.57 to 2.42] (P = 0.67) | N/I |
| Dissection grade NHLBI ≥ C | 1.40 [0.83 to 2.37] (P = 0.21) | N/I |
| Dissection grade Kobayashi ≥1/3 | 1.66 [0.94 to 2.94] (P = 0.081) | N/I |
| Subintimal wire passage | 1.00 [0.46 to 2.15] (P = 1.00) | N/I |
| Degree of calcification | 1.06 [0.78 to 1.43] (P = 0.67) | N/I |
| Calcified nodule | 1.07 [0.52 to 2.19] (P = 0.85) | N/I |
| Degree of dissection after DCB | 1.06 [0.64 to 1.74] (P = 0.79) | N/I |
| Minimum lumen area (mm2) | 0.95 [0.87 to 1.04] (P = 0.20) | N/I |
| Distal external elastic membrane area (mm2) | 1.01 [0.98 to 1.04] (P = 0.58) | N/I |
| Distal lumen area (mm2) | 0.98 [0.92 to 1.03] (P = 0.36) | N/I |
Data are hazard ratios [95% confidence intervals] (P values)
N/I not included
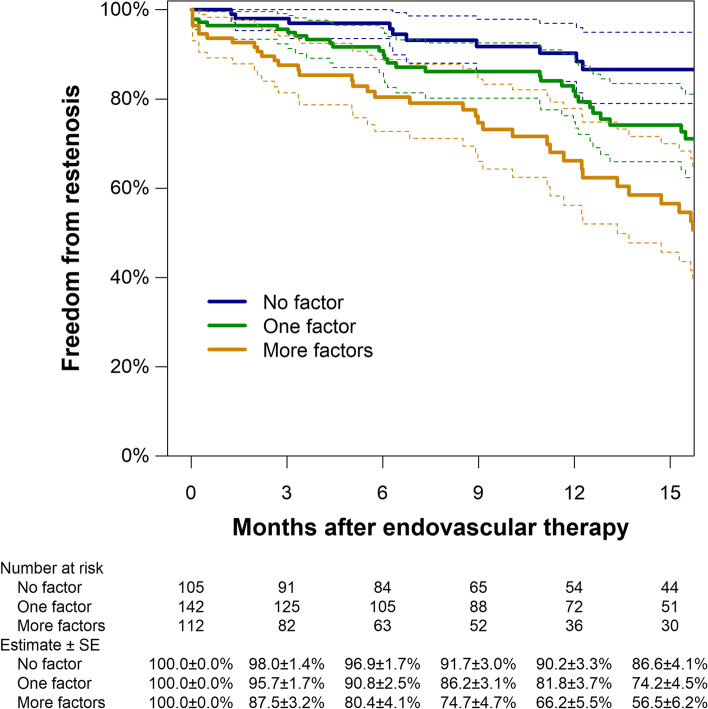
In summary, 1) hemodialysis, 2) no use of DAPT, 3) CLTI, and 4) restenosis lesion were risks for 12-month restenosis. The accumulation of these risks was associated with a lower rate of 12-month primary patency; however, in the absence of these risks, a higher rate of primary patency was observed (no risk factor: 90.2%, one risk factor: 81.8%, and two or more factors: 66.2%, respectively, Fig. 2).
Fig. 2.
Kaplan Meier estimates of primary patency based on the accumulation of restenosis risk factors. Dotted lines indicate 95% confidence intervals. SE, standard error
Discussion
The present study demonstrated the clinical effectiveness of DCB treatment for patients with FP-CTO diseases. Multivariate analysis demonstrated three independent risk factors: 1) hemodialysis, 2) CLTI, and 3) restenosis lesion. The administration of DAPT was a significant protective factor. To the best of our knowledge, this is the largest population studied regarding the relationship between DCB and FP-CTO in real-world target populations. Compared with previous studies, we report a favorable rate of 12-month primary patency (79.8%) and acceptable bailout stent rate (8.9%); therefore, DCB treatment is an acceptable strategy for FP-CTO (AbuRahma et al. 2019; Tepe et al. 2019; Liistro et al. 2019).
Successful DCB treatment requires the achievement of two conflicting factors: 1) adequate luminal gain and less severe dissection. To establish these contradictory factors, the presence of CTO is very challenging. As reported in a previous study, the presence of CTO is significantly associated with the incidence of severe dissection (Fujihara et al. 2017). A large volume of plaques in the CTO may lead to large dissections and significant recoil after balloon angioplasty, causing target lesion failure (or requiring bailout stents). Recent studies have reported acceptable results of DCB treatment for complex FP lesions; however, they had a high rate (21.0%–46.5%) of bailout stent (Tepe et al. 2019; Liistro et al. 2019; Bausback et al. 2019). Currently, there is no high-level evidence regarding the effectiveness of the “leaving nothing behind” strategy for patients with complex FP lesions. A previous single-center study reported outstanding 12-month primary patency (92.7%) of IVUS-guided DCB treatment for FP-CTO without bailout stent (Hayakawa et al. 2022a, 2022b). They performed all procedures using an IVUS-guided intraluminal approach, and DCB size was decided based on the IVUS findings. Furthermore, only cases with sufficient results regarding lesion preparation (without residual stenosis [> 50%] and severe dissection [NHLBI grade D or higher]) were enrolled in the DCB cohort. Although the level of evidence was insufficient, their important findings suggested that precise procedures and judgment may improve the clinical outcomes of DCB treatment for complex FP lesions. Furthermore, this might also reduce the incidence of bailout stent, maximizing the benefit of the “leaving nothing behind” strategy. In our multicenter study, lesion length (21.9 cm), occlusion length (14.5 cm), and PACCS grade 3 or 4 (33.1%), were more severe than in the IN.PACT global CTO imaging cohort (lesion length: 22.8 cm, CTO length: 11.9 cm, severe calcification: 3.2%). Despite the lesion severity, the bailout stent rate was notably lower (8.9%) than that of the IN.PACT global study (46.5%) (Tepe et al. 2019). The reason for this is unclear; however, we suspect that the high rate of IVUS-guided procedures (77.2%) avoided the need for subintimal crossing and selection of an appropriate device size. As a result, favorable primary patency and lower bailout stent rates were observed. The first randomized controlled trial (RCT) of IVUS-guided FP EVT (mainly DCB treatment) demonstrated the clinical benefit of IVUS (Allan et al. 2022). In future clinical trials, it will be necessary to clarify 1) what IVUS changes in the procedure and 2) how it has affected the outcomes.
This study demonstrated that 1) hemodialysis, 2) CLTI, and 3) restenosis lesions were independent risk factors and that the use of DAPT was a protective factor for 12-month restenosis after DCB treatment for FP-CTO. The clustering of these risk factors was associated with a lower primary patency rate (66.2%). However, when none of these risk factors were present, an outstanding primary patency rate (90.2%) was noted. This might be beneficial in daily clinical practice because it can easily predict the lesion prognosis and stratify the risk, resulting in a better strategy selection. The risk factors identified in our study overlapped with the predictors of restenosis after fluoropolymer-based drug-eluting stent (FP-DES) implantation. Iida et al. reported hemodialysis, CLTI, and history of revascularization were risk factors for restenosis in FP-DES. A smaller reference vessel diameter, CTO, and spot stenting were also risk factors for restenosis (Iida et al. 2022). Hemodialysis, CLTI, and restenosis lesion might be associated with lesion severity, and therefore, might negatively influence durability after DCB treatment.
In this study, the administration of DAPT was demonstrated to be a protective factor for restenosis. It is well known that cilostazol prevents neointimal hyperplasia, resulting in a reduced restenosis rate after EVT for FP lesions (Soga et al. 2018). It is unclear how DAPT reduces restenosis after DCB treatment; however, DAPT might reduce the incidence of thrombosis-related target lesion failure, resulting in a reduction of restenosis. In this study, the effects of DAPT between restenosis and re-occlusion were not evaluated separately. Data on DAPT after EVT are scarce, and it is not clear whether longer DAPT provides more benefit. A previous report showed that DAPT was associated with prolonged survival for patients with critical limb ischemia who underwent arterial revascularization; however, no benefit was shown in patients with claudication (Soden et al. 2016). An RCT of the efficacy of DAPT (aspirin plus clopidogrel) reported reduced peri-interventional platelet activation and a lower revascularization rate than aspirin plus placebo (Tepe et al. 2012). Our study suggests DCB treatment for FP-CTO is reasonable for patients without CLTI, hemodialysis, or a history of target lesion EVT. Furthermore, if the patient has sufficient tolerance to DAPT (equal to non-high bleeding risk), the prolongation of DAPT should be considered. However, the current study did not examine the duration, dose, or regimen of DAPT or platelet reactivity. Further investigation is needed in the future.
In this study, previously reported risk factors for restenosis, such as age, sex, vessel diameter, lesion length, severe calcification, and dissection were not revealed as risks for restenosis. Furthermore, angioplasty with DCB was performed for cases with successful lesion preparation; therefore, DCB may be effective even for those with long lesions, small vessels ≤4 mm (Hiramori et al. 2017) or severe calcification if successful lesion preparation (sufficient luminal gain and less dissection) can be obtained. In this study, previously reported risk factors did not have an elevated risk for restenosis possibly because of the unclear consensus on the definition of successful lesion preparation, which differed between the current study and previous studies. Therefore, better selection of the lesions might lead to better results where the IVUS has a positive impact on the selection process (Hayakawa et al. 2022a, 2022b). Achievement of sufficient luminal gain and less severe dissection are essential. Regarding the definition of “sufficient” luminal gain, Horie et al. proposed a cutoff value of postprocedural IVUS-evaluated minimum luminal area (MLA) of 12.7 mm2 (Horie et al. 2022). Angiographic evaluation is the gold standard for the evaluation of dissection. A previous study showed a significant relationship between non-stented moderate-to-severe (angiographic) dissections after DCB treatment and the incidence of major adverse limb events (Giannopoulos et al. 2021). We evaluated dissections mainly by the NHLBI classification. Only 16% of the subjects had a grade C or more dissection. Therefore, it was difficult to evaluate the dissection pattern and durability. Kozuki et al. reported the presence of IVUS-detected severe dissection (dissection angle > 63°) was significantly associated with 12-month restenosis (Kozuki et al. 2021). In this study, 77.2% of subjects received IVUS-guided DCB treatment, possibly resulting in favorable patency without the need for bailout stent. However, over 20% of patients still received angiographic-guided treatment. Furthermore, none of the IVUS parameters was significantly associated with factors of restenosis in the current study. However, there were many cases with missing data of IVUS parameters. Therefore, we could not adequately evaluate the association between clinical outcomes and IVUS parameters (such as post-procedural MLA and dissection angle). DCB and IVUS together might help us to achieve better clinical results in connection with the strategy of DCB in cases with longer, smaller, or heavily calcified vessels, although further studies are required. In addition, the size and type of DCB was not an independent factor for restenosis in the present study and the type and size of balloon used to achieve optimal lesion preparation were not examined. Therefore, these issues require further investigation.
Limitations
This study had several limitations. The present study was a retrospective, nonrandomized study with a small sample size; therefore, the evidence level is not high. In particular, the small sample size may be an important limitation with respect to the extraction of clinical events. In addition, patients with previous radiotherapy treatment or who had surgical femoropopliteal treatment could not be excluded in this study, so it is not known to what extent these patients were included. All clinical events were evaluated on-site and there was no independent clinical events committee. Furthermore, the lack of uniformity in follow-up modalities may have reduced the accuracy in terms of restenosis assessment. The application of DCB treatment was selected based on each operator’s decision without following any pre-established protocol. There may have been selection bias. The follow-up duration might have been too short for a thorough evaluation of the clinical outcome and complications. Moreover, the current study evaluated restenosis and re-occlusion but did not examine the details of complications such as aneurysmal degeneration after the DCB treatment. In the future, a well-designed, large-scale prospective study (with an independent event committee) will be required for equivalent evaluation. Because this was a single arm study of DCB, future studies should compare the results with other revascularization treatments.
Conclusions
The 12-month clinical outcome of DCB treatment for FP-CTO demonstrated the effectiveness of DCB in real-world clinical practice. Independent risk factors for restenosis were hemodialysis, CLTI, and restenosis lesion. Therefore, the use of DAPT may reduce the risk of restenosis.
Acknowledgements
We thank J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Abbreviations
- DCB
Drug-coated balloon
- FP
Femoropopliteal
- CTO
Chronic total occlusive lesions
- CD-TLR
Clinically-driven target lesion revascularization
- CLTI
Chronic limb-threatening ischemia
- DAPT
Dual antiplatelet therapy
- PAD
Peripheral artery disease
- EVT
Endovascular therapy
- NHLBI
The National Heart, Lung and Blood Institute
- PACSS
Peripheral Arterial Calcium Scoring System
- DUS
Duplex ultrasound
- CTA
Computed tomography angiography
- Cis
Confidence intervals
- IVUS
Intravascular ultrasound
- RCT
Randomized controlled trial
- FP-DES
Fluoropolymer-based drug-eluting stent
- MLA
Minimum luminal area
Authors’ contributions
NH is the corresponding author and wrote a paper, MT performed the statistical analysis for this study. TN, KH, KT, SM, YI, and KS designed the treatments, collected data at their respective sites, and were responsible for drafting the manuscript and critical revision for important intellectual content. KT, TK, SI, and MA performed the procedures and pre- and post- procedure follow-ups. JK gave final approval for the submitted manuscript. The author(s) read and approved the final manuscript.
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board, independent ethics committee, or research ethics board applicable to each study site, and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent for publication
For this type of study consent for publication is not required.
Competing interests
TN is a consultant of Boston Scientific, BD, Cordis, Century Medical Inc., COOK Medical, Kaneka Medix, NIPRO, OrbusNeichi, Medtronic, and TERUMO Corporation. KS received remuneration for lectures from Boston Scientific Japan, and consultant fees from Medtronic. KT is a consultant of Gore and received a speakers fee from Medtronic. The other authors report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aboyans V, Ricco J-B, Bartelink M-EL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European stroke organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- AbuRahma AF, AbuRahma ZT, Scott G, et al. Clinical outcome of drug-coated balloon angioplasty in patients with femoropopliteal disease: a real-world single-center experience. J Vasc Surg. 2019;70:1950–1959. doi: 10.1016/j.jvs.2019.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RB, Puckridge PJ, Spark JI, Delaney CL. The impact of intravascular ultrasound on femoropopliteal artery endovascular interventions: a randomized controlled trial. JACC Cardiovasc Interv. 2022;15:536–546. doi: 10.1016/j.jcin.2022.01.001. [DOI] [PubMed] [Google Scholar]
- Bailey SR, Beckman JA, Dao TD, et al. ACC/AHA/SCAI/SIR/SVM 2018 appropriate use criteria for peripheral artery intervention: a report of the American College of Cardiology Appropriate use Criteria Task Force, American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine. J Am Coll Cardiol. 2019;73:214–237. doi: 10.1016/j.jacc.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausback Y, Wittig T, Schmidt A, et al. Drug-eluting stent versus drug-coated balloon revascularization in patients with femoropopliteal arterial disease. J Am Coll Cardiol. 2019;73:667–679. doi: 10.1016/j.jacc.2018.11.039. [DOI] [PubMed] [Google Scholar]
- Feldman DN, Armstrong EJ, Aronow HD, et al. SCAI consensus guidelines for device selection in femoral-popliteal arterial interventions. Catheter Cardiovasc Interv. 2018;92:124–140. doi: 10.1002/ccd.27635. [DOI] [PubMed] [Google Scholar]
- Fujihara M, Takahara M, Sasaki S, et al. Angiographic dissection patterns and patency outcomes after balloon angioplasty for superficial femoral artery disease. J Endovasc Ther. 2017;24:367–375. doi: 10.1177/1526602817698634. [DOI] [PubMed] [Google Scholar]
- Giannopoulos S, Strobel A, Rudofker E, Kovach C, Schneider PA, Armstrong EJ. Association of postangioplasty femoropopliteal dissections with outcomes after drug-coated balloon angioplasty in the femoropopliteal arteries. J Endovasc Ther. 2021;28:593–603. doi: 10.1177/15266028211016441. [DOI] [PubMed] [Google Scholar]
- Hayakawa N, Kodera S, Arakawa M, Hirano S, Shakya S, Kanda J. Clinical outcome of drug-coated balloon vs scaffold device in patients with superficial femoral artery chronic total occlusion. Heart Vessel. 2022;37:282–290. doi: 10.1007/s00380-021-01912-0. [DOI] [PubMed] [Google Scholar]
- Hayakawa N, Kodera S, Takanashi K, et al. Optimal intraluminal drug-coated balloon versus drug-eluting stent in patients with chronic total occlusion of the superficial femoral artery: a retrospective analysis. Cardiovasc Revasc Med. 2022;S1553-8389(22):00175. doi: 10.1016/j.carrev.2022.04.002. [DOI] [PubMed] [Google Scholar]
- Hiramori S, Soga Y, Iida O, et al. Relationship between clinical outcomes and vessel size in endovascular therapy for femoropopliteal lesions. J Vasc Surg. 2017;65:1690–1697. doi: 10.1016/j.jvs.2016.12.128. [DOI] [PubMed] [Google Scholar]
- Horie K, Tanaka A, Taguri M, Inoue N. Impact of baseline and postprocedural intravascular ultrasound findings on 1-year primary patency after drug-coated balloon treatment of femoropopliteal lesions. J Endovasc Ther. 2022;29:66–75. doi: 10.1177/15266028211058683. [DOI] [PubMed] [Google Scholar]
- Iida O, Soga Y, Urasawa K, et al. Drug-coated balloon vs standard percutaneous transluminal angioplasty for the treatment of atherosclerotic lesions in the superficial femoral and proximal popliteal arteries: one-year results of the MDT-2113 SFA Japan randomized trial. J Endovasc Ther. 2018;25:109–117. doi: 10.1177/1526602817745565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida O, Takahara M, Soga Y, et al. 1-year outcomes of fluoropolymer-based drug-eluting stent in femoropopliteal practice: predictors of restenosis and aneurysmal degeneration. JACC Cardiovasc Interv. 2022;15:630–638. doi: 10.1016/j.jcin.2022.01.019. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Hirano K, Yamawaki M, et al. Simple classification and clinical outcomes of angiographic dissection after balloon angioplasty for femoropopliteal disease. J Vasc Surg. 2018;67:1151–1158. doi: 10.1016/j.jvs.2017.08.092. [DOI] [PubMed] [Google Scholar]
- Kozuki A, Takahara M, Shimizu M, et al. Outcomes of dissection angles as predictor of restenosis after drug-coated balloon treatment. J Atheroscler Thromb. 2021;28:954–962. doi: 10.5551/jat.59774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JA, Schneider PA, Jaff MR, et al. Long-term clinical effectiveness of a drug-coated balloon for the treatment of femoropopliteal lesions. Circ Cardiovasc Interv. 2019;12:e007702. doi: 10.1161/CIRCINTERVENTIONS.118.007702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liistro F, Angioli P, Porto I, et al. Drug-eluting balloon versus drug-eluting stent for complex femoropopliteal arterial lesions: the DRASTICO study. J Am Coll Cardiol. 2019;74:205–215. doi: 10.1016/j.jacc.2019.04.057. [DOI] [PubMed] [Google Scholar]
- Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv. 2014;83:E212–E220. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden PA, Zettervall SL, Ultee KH, et al. Dual antiplatelet therapy is associated with prolonged survival after lower extremity revascularization. J Vasc Surg. 2016;64:1633–44.e1. doi: 10.1016/j.jvs.2016.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga Y, Hamasaki T, Edahiro R, et al. Sustained effectiveness of cilostazol after endovascular treatment of femoropopliteal lesions: midterm follow-up from the sufficient treatment of peripheral intervention by cilostazol (STOP-IC) study. J Endovasc Ther. 2018;25:306–312. doi: 10.1177/1526602818771358. [DOI] [PubMed] [Google Scholar]
- Song P, Rudan D, Zhu Y, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- Stoner MC, Calligaro KD, Chaer RA, et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg. 2016;64:e1–e21. doi: 10.1016/j.jvs.2016.03.420. [DOI] [PubMed] [Google Scholar]
- Tepe G, Bantleon R, Brechtel K, et al. Management of peripheral arterial interventions with mono or dual antiplatelet therapy-the MIRROR study: a randomised and double-blinded clinical trial. Eur Radiol. 2012;22:1998–2006. doi: 10.1007/s00330-012-2441-2. [DOI] [PubMed] [Google Scholar]
- Tepe G, Laird J, Schneider P, et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepe G, Micari A, Keirse K, et al. Drug-coated balloon treatment for femoropopliteal artery disease: the chronic total occlusion cohort in the IN.PACT global study. J Am Coll Cardiol Intv. 2019;12:484–493. doi: 10.1016/j.jcin.2018.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.