Abstract
A large area of the terrestrial land surface is used for livestock grazing. Trees on grazing lands provide and can enhance multiple ecosystem services such as provisioning, cultural and regulating, that include carbon sequestration. In this study, we assessed the above- and belowground carbon stocks across six different land-uses in livestock-dominated landscapes of Mexico. We measured tree biomass and soil organic carbon (SOC) stocks in fodder banks, live fences, pasturelands with dispersed trees, secondary forests, and primary forests from three different geographical regions and compared them with conventional open pasturelands respectively. We also calculated tree diversity indices for each land-use and their similarity with native primary forests. The aboveground woody biomass stocks differed significantly between land-uses and followed the gradient from less diverse conventional open pasturelands to silvopastoral systems and ecologically complex primary forests. The SOC stocks showed a differential response to the land-use gradient dependent on the study region. Multivariate analyses showed that woody biomass, fine root biomass, and SOC concentrations were positively related, while land-use history and soil bulk density showed an inverse relationship to these variables. Silvopastoral systems and forest remnants stored 27–163% more carbon compared to open pasturelands. Our results demonstrate the importance of promoting appropriate silvopastoral systems and conserving forest remnants within livestock-dominated landscapes as a land-based carbon mitigation strategy. Furthermore, our findings also have important implications to help better manage livestock-dominated landscapes and minimize pressures on natural protected areas and biodiversity in the hotspots of deforestation for grassland expansion.
Subject terms: Carbon cycle, Climate-change mitigation, Ecosystem services, Biodiversity
Introduction
Land-use change significantly alters the global biogeochemical cycles and contributes to climate change1. Between 1850 and 2015, global land-use change emitted an estimated 145 ± 16 Pg of carbon (C) to the atmosphere, counting the loss of biomass and soil organic carbon (SOC)2. Land conversion from forest to agriculture (i.e., crops and pasturelands) was one of the main drivers causing such C emissions3–5. Ongoing land-use change from forest to crop and grasslands not only emits C due to the loss of vegetation but also depletes up to 60% of SOC6,7. Estimate from historical land-use change accounts for a loss of 133–135 Pg SOC8,9. Between 2009 and 2018, land-use change contributed on average 14% (1.5 ± 0.7 Pg C year−1) of the total C emissions globally10. However, terrestrial ecosystems annually remove 3.2 ± 0.6 Pg of C from the atmosphere and store it in biomass and soils offsetting 29% of the anthropogenic C emissions10,11. Owing to the contribution, the implementation of agroforestry practices (e.g., silvopastoral systems) can enhance biomass as well as soil C storage in crop and pasturelands12–16.
Currently, about 37% of ice-free land surface worldwide is used for livestock grazing, which is three times larger than cropland area17,18. In Latin America alone, the area dedicated to pastureland increased nearly 2.5 times between 1850 and 2015, expanding from 229 to 564 million ha, while forest area decreased 25%, from 1248 to 932 million ha2. Additionally, it is estimated that more than one-third of the pastureland in Latin America is in a state of degradation and exhibits low resilience to climate variability19–21. In Mexico, 80.3 million ha of land are currently used as permanent and cultivated pasture lands17. Furthermore, during the years between 1985 and 2011, induced pastureland establishment accounted for 41% of the total native vegetation (mainly scrubland and tropical forest) loss, with the expansion of bovine livestock grazing currently considered one of the main drivers of deforestation in South and Southeastern Mexico22–25. Finally, conversion of native vegetation to pasturelands has caused the loss of 30 to 50% of the SOC stocks (0–60 cm depth) nationwide26.
Lands devoted to livestock production can be converted to treed ecosystems like silvopasture to augment C storage at regional and global scales27–30. Global soils have the potential to sequester an additional 1.62 Pg C year−1, of which 47% corresponds to SOC enhancement in agriculture and grasslands through a wide range of soil management practices14. Besides the increase in above- and belowground C storage, silvopastoral systems (SPS) create habitat for wildlife and promote biodiversity conservation through landscape connectivity31–33. Compared to conventional grass monoculture, SPS also ensures greater ecosystem resilience and decreases climate vulnerability34–36.
Extensive grass monoculture practices for animal production have high environmental footprints37–40. Inappropriate management and overgrazing lead to land degradation41–43. Globally, 93% of pasturelands have an aboveground C density of less than 5 Mg ha−128. Hence, C enhancement through the implementation of SPS, and conservation of forest remnants within farmlands are the most promising nature-based solutions to mitigate greenhouse gas (GHG) emissions and increase ecosystem services from the livestock farmlands27,44,45. Converting conventional open pasturelands to SPS increases the accrual of atmospheric CO2 while controlling deforestation for the expansion of pure grasslands reduces CO2 emissions through C retention within the landscape46,47. Furthermore, SPS also contributes to reducing or buffering CO2 emissions from soil respiration compared to open pasturelands48,49. Additional benefits of C enhancement through SPS include: nutrient cycling and soil fertility improvement, increasing biological diversity, reduce soil erosion, increase forage production, and regulating heat stress to grazing animals through tree shades50–52.
Silvopasture is an animal-agroforestry where woody perennials, grasses, and animals interact biologically and economically in the same land unit53,54. There are multiple combinations of woody perennials and grasses in space and time to optimize resources and diversify production as well as ecological benefits55–57. Dispersed trees in pastures, woody perennials with grasses in alleys, live fences (shelterbelts), fodder banks, and grasses under tree plantations are some examples of SPS53,58. In many parts of the world, forest browsing is also a common animal agroforestry practice. SPS has been promoted throughout Latin America and in other parts of the world due to its multiple benefits, including productivity increase, animal wellbeing, C sequestration, and biodiversity conservation59–62.
Earlier studies have shown that C storage in SPS is highly variable and depends greatly on local ecological conditions, land-use history, and management intensity41,63–65. Some have demonstrated the value of using land-use intensity gradients to show the impacts of forested ecosystems simplification on biodiversity56,66–68, fewer studies have used this approach to explore the variations in C storage in livestock-dominated landscapes, particularly comparing simplified open pasturelands with complex silvopasture and forested systems.
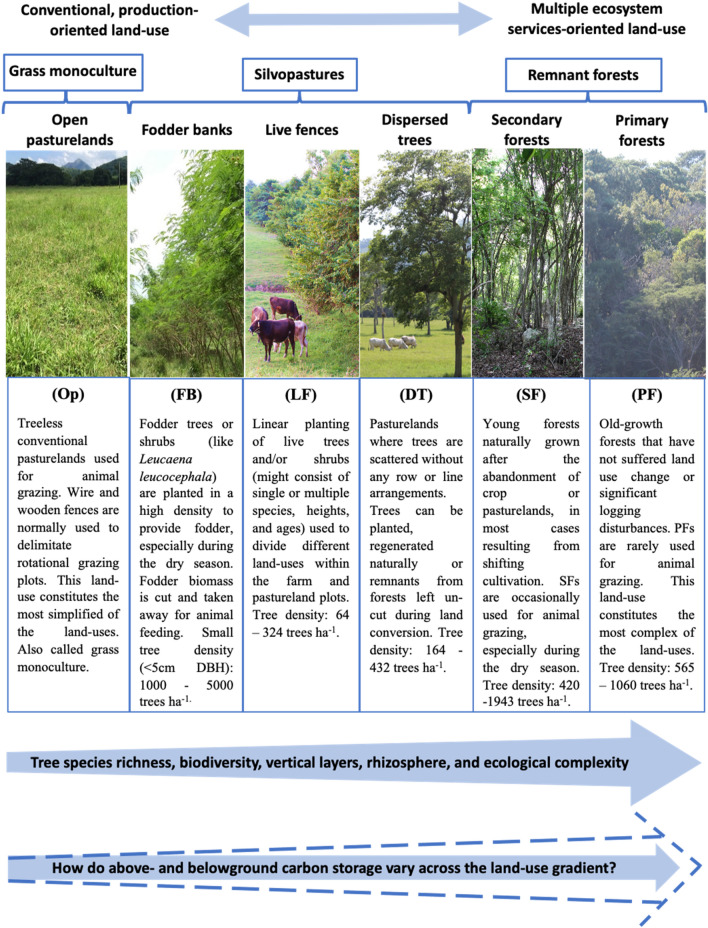
In this context, our understanding of the variations in C storage among different land-use systems in a gradient from open pasture to silvopasture and forest remnants is limited. Carbon estimations by ecological zone default values do not cover the finer scale differences between land-use within the same landscape28. Finer scale evaluations of C densities within the landscape are required to better understand the regional ecosystem change processes and articulate them to the land management decisions. Effective formulation and implementation of strategies to enhance C storage in lands used for livestock production require detailed knowledge regarding the quantities of C that can be stored across different land-uses including SPS and their relationship with land-use history and soil properties. In Mexico, studies related to C sequestration in livestock-dominated landscapes are mostly limited to only one geographical region or covering only one or a few land-use types69–71. Accounting C storage in a gradient of land-uses from structurally simple open pastures to ecologically more complex systems like multi-species silvopasture and native forests (Fig. 1) are important to better understand the ecological processes and GHG mitigation potential. Here, we evaluated the tree species richness, diversity, and C stocks across a land-use gradient, from conventional pasture monoculture and silvopasture to primary forest in livestock-dominated landscapes. We quantified C stocks in above and belowground pools in open pasturelands (OP), fodder banks (FB), live fences (LF) dispersed trees on pasturelands (DT), secondary forests (SF), and primary forests (PF) following a gradient of land-use in Jalisco, Chiapas, and Campeche, Mexico (the definitions and characteristics of sampled land-uses are presented in Fig. 1). We also explored the relationships between carbon storage, land-use history, and soil properties that can explain SOC variations in these land-uses. We hypothesized that C storage in biomass and soil increases with the gradient of land-use from ecologically simple open pasturelands to more diverse, ecologically complex SPS, secondary and primary forests.
Figure 1.
Illustration and description of the land-use gradient from conventional open pasturelands (OP), fodder banks, (FB) live fences (LF), pasturelands with dispersed trees (DT), secondary forests (SF) to primary forests (PF) selected for carbon monitoring. Land-uses followed an intensity gradient from ecologically simple to complex systems. The photographs were taken by Deb Raj Aryal from Chiapas (OP, FB, DT, PF) and Campeche (SF), Mexico. The animals in the images were to represent that the lands we sampled are used for animal grazing.
Results
Tree species richness and diversity
Tree species richness was higher in PF, followed by SF, DT, LF, and FB. OP have no trees, so tree richness values for this land-use is zero. The Sorenson´s similarity coefficient, which is a measure of the degree of similarity with our reference land-use (PF), ranged from 0.00 to 1.00, where 1.00 indicates that all the species are common between PF and the respective land-use and 0 indicates that no species are common (Table 1). The Shannon–Weaver biodiversity index ranged from 0 to 3.62, the lowest values corresponded to OP and the highest to the PF of the Campeche region. The highest values of the Shannon–Weaver (H´) index indicate a greater diversity of tree species. The general order of tree species diversity values was: OP < FB < LF < DT < SF < PF, except in Chiapas where PF showed a lower diversity index than SF and two of our silvopastoral systems (DT and LF). The Pilou´s evenness index (J) varied according to the region and land-uses. The J index can oscillate between 0.0 and 1.0, where the first indicates completely unequal while the last indicates total equality of abundance. The evenness index of primary forests in the Chiapas region was lower than other land-uses, indicating that a few species were more abundant than others (Table 1).
Table 1.
Tree species richness, Sorenson´s similarity coefficients with primary forest species, Shannon´s diversity index, and Pilou´s evenness indices between land-uses for three study regions.
| Land-use (from simplified to complex) | Tree species richness, N (Sorenson’s similarity coefficient, CC) | Shannon’s diversity index, H (Pilou’s evenness index, J) | ||||
|---|---|---|---|---|---|---|
| Jalisco | Campeche | Chiapas | Jalisco | Campeche | Chiapas | |
| Open pasturelands | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Fodder banks | 1 (0.00) | nd | 2 (0.00) | 0 (0.00) | nd | 0.53 (0.77) |
| Live fences | nd | 11 (0.16) | 16 (0.19) | nd | 1.44 (0.60) | 2.20 (0.79) |
| Dispersed trees | 13 (0.42) | 11 (0.26) | 16 (0.37) | 2.09 (0.82) | 1.56 (0.65) | 2.29 (0.83) |
| Secondary forest | 20 (1.00) | 35 (0.54) | 25 (0.27) | 2.37 (0.79) | 2.47 (0.69) | 2.46 (0.77) |
| Primary forests | nd | 65 (1.00) | 27 (1.00) | nd | 3.62 (0.86) | 1.80 (0.55) |
PF Primary forests, SF Secondary forests, DT Dispersed tree in pasturelands, LF Live fences, FB Fodder banks, OP Open pasturelands.
nd data not available.
Living tree biomass carbon stocks
The mean C stocks in tree AGB varied between 1.8 and 134.6 Mg C ha−1 with significant differences between land-uses, the lowest value corresponded to FB of Chiapas and the highest to PF of the same region (Table 2). Woody biomass was absent in OP due to the absence of trees. The AGB stock differences between study sites were not significant for FB, LF, DT, and PF. Only in SF, Campeche, and Chiapas sites stored higher C stocks compared to Jalisco. DT stored 12.6 Mg C ha−1 in Chiapas sites, 14.8 Mg C ha−1 in Jalisco, and 34.8 Mg C ha−1 Campeche with no significant differences between them. Average among three regions, DT stored 18.2 Mg C ha−1 in AGB and was significantly higher than LF (2.6 Mg C ha−1) and FB (2.2 Mg C ha−1) but did not differ statistically from SF (25.0 Mg C ha−1). Carbon stocks in root biomass ranged from 0.5 to 31.6 Mg C ha−1 and followed a similar pattern with AGB (Table 2). Averaged among regions, DT stored about 15% of PF biomass and 73% of SF tree biomass C stocks.
Table 2.
Back-transformed weighted means and respective 95% confidence intervals of carbon stocks in above-ground biomass (AGB), root biomass (RB), and total tree biomass (Mg C ha−1) between different land-uses in three regions of Mexico. Tree total biomass: AGB + RB. Superscript uppercase letters indicate significant differences between land-uses, while lowercase letters indicate statistical differences between geographical regions (Tukey HSD, p < 0.05).
| Land-use | Region | AGB (Mg C ha−1) | RB (Mg C ha−1) | Tree total (Mg C ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CI − 95% | CI + 95% | Mean | CI − 95% | CI + 95% | Mean | CI − 95% | CI + 95% | ||
| Fodder banks | Jalisco | 3.0a | 0.5 | 17.1 | 0.9a | 0.2 | 4.7 | 4.0a | 0.7 | 21.7 |
| Campeche | nd | nd | nd | nd | nd | nd | nd | nd | nd | |
| Chiapas | 1.8a | 0.7 | 3.4 | 0.5a | 0.2 | 1.1 | 2.2a | 0.9 | 4.5 | |
| All regions | 2.2A | 1.1 | 3.8 | 0.7A | 0.4 | 1.1 | 2.9A | 1.5 | 4.9 | |
| Live fences | Jalisco | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Campeche | 2.1a | 0.5 | 9.1 | 0.7a | 0.2 | 2.6 | 2.7a | 0.6 | 11.7 | |
| Chiapas | 3.1a | 1.9 | 5.1 | 1.0a | 0.6 | 1.5 | 4.1a | 2.5 | 6.6 | |
| All regions | 2.6A | 1.4 | 4.6 | 0.8A | 0.5 | 1.4 | 3.4A | 1.9 | 6 | |
| Dispersed trees | Jalisco | 14.8b | 9.2 | 23.8 | 4.1b | 2.6 | 6.4 | 18.9b | 11.8 | 30.2 |
| Campeche | 34.8b | 14.5 | 83.3 | 9.0b | 4 | 20.3 | 43.9b | 18.6 | 103.6 | |
| Chiapas | 12.6b | 5.7 | 27.7 | 3.5b | 1.7 | 7.3 | 16.1b | 7.4 | 35 | |
| All regions | 18.2B | 12.2 | 27.2 | 5.0B | 3.4 | 7.2 | 23.2B | 15.6 | 34.4 | |
| Secondary forests | Jalisco | 9.3a | 5.5 | 15.7 | 2.7a | 1.6 | 4.3 | 11.9a | 7.1 | 20 |
| Campeche | 55.0b | 40.4 | 74.9 | 13.8b | 10.4 | 18.4 | 68.8b | 50.8 | 93.3 | |
| Chiapas | 31.3b | 16.4 | 59.8 | 8.2b | 4.5 | 14.9 | 39.5b | 20.9 | 74.7 | |
| All regions | 25.0B | 16.2 | 38.8 | 6.7B | 4.4 | 10 | 31.7B | 20.6 | 48.8 | |
| Primary forests | Jalisco | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Campeche | 110.8c | 56.9 | 215.6 | 26.4c | 14.3 | 49 | 137.2c | 71.2 | 264.5 | |
| Chiapas | 134.6c | 99.6 | 181.8 | 31.6c | 23.9 | 41.8 | 166.2c | 123.5 | 223.6 | |
| All regions | 127.3C | 100.2 | 161.7 | 30.0C | 24.1 | 37.5 | 157.3C | 124.3 | 199.2 | |
nd data not available.
Aboveground grass biomass, surface litter, and deadwood carbon stocks
Land-uses differ significantly in the amount of C stored in aboveground grass biomass (Fig. 2). Back-transformed mean C stock in this pool ranged from 0.24 to 1.29 Mg C ha−1. In this pool, FB and OP hold the higher C stock compared to SF and PF. Mean C stock in surface litter pool ranged from 0.93 to 2.84 Mg C ha−1, PF stored significantly higher C stock compared to other land-uses (Fig. 2). Regarding C stocks in the deadwood pool, SF (4.79 Mg C ha−1) stored significantly higher than FB and DT but not statistically different from PF (3.02 Mg C ha−1) and LF (2.85 Mg C ha−1).
Figure 2.
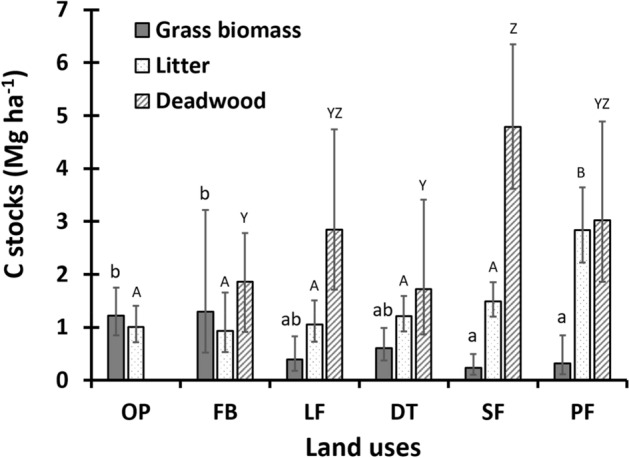
Average carbon stocks in grass biomass, ground litter, and deadwood material in different land-uses. OP = Open pastures, FB = Fodder banks, LF = Live fences, DT = Pasturelands with dispersed trees, SF = Secondary forests, PF = Primary forests. Error bars indicate the respective 95% confidence intervals. Different letters above the bars indicate significant differences between land-uses (p < 0.05).
Soil organic carbon stocks
The SOC was the biggest C pool across these land-uses, demonstrating the importance of conserving this C reservoir. The SOC stocks (0–30 cm) showed a differential response to land-uses according to the region of the study. In Jalisco region, SOC stocks varied from 130.3 to 145.9 Mg C ha−1, the lower value corresponded to OP and the upper value to SF. However, differences between land-uses were not significant (Table 3). In Campeche region, SOC stocks ranged between 108.7 and 152.5 Mg C ha−1, the lower value also corresponded to OP and the upper value to DT, with statistically significant differences. In Chiapas region, SOC stocks ranged from 152.5 to 227.5 Mg C ha−1, the lower value corresponded again to OP and the upper value to LF, with statistically significant differences (Table 3).
Table 3.
Average soil organic carbon (SOC) stocks (Mg C ha−1) to 30 cm depth by land-uses across regions.
| Land-use | Region | SOC stocks to 30 cm depth (Mg C ha−1) | ||
|---|---|---|---|---|
| Mean | Lower 95% CI | Upper 95% CI | ||
| Open pasturelands | Jalisco | 130.3x | 115.3 | 145.3 |
| Campeche | 108.7a | 92.3 | 125.1 | |
| Chiapas | 152.3p | 137.4 | 167.3 | |
| Fodder banks | Jalisco | 145.7x | 124.5 | 166.9 |
| Campeche | nd | nd | nd | |
| Chiapas | 175.5p | 160.5 | 190.5 | |
| Live fences | Jalisco | nd | nd | nd |
| Campeche | 126.6ab | 110.1 | 143.0 | |
| Chiapas | 227.5q | 212.5 | 242.5 | |
| Dispersed trees on pasturelands | Jalisco | 144.3x | 127.9 | 160.7 |
| Campeche | 152.3b | 135.9 | 168.7 | |
| Chiapas | 182.5pq | 167.6 | 197.5 | |
| Secondary forests | Jalisco | 145.9x | 130.9 | 160.9 |
| Campeche | 127.7ab | 111.3 | 144.1 | |
| Chiapas | 186.8pq | 165.6 | 208.0 | |
| Primary forests | Jalisco | nd | nd | nd |
| Campeche | 129.0ab | 112.6 | 145.4 | |
| Chiapas | 220.5q | 204.1 | 236.9 | |
Different letters indicate statistical differences between land-uses within the same region (Tukey HSD, p < 0.05).
nd: data not available.
Soil organic carbon concentration
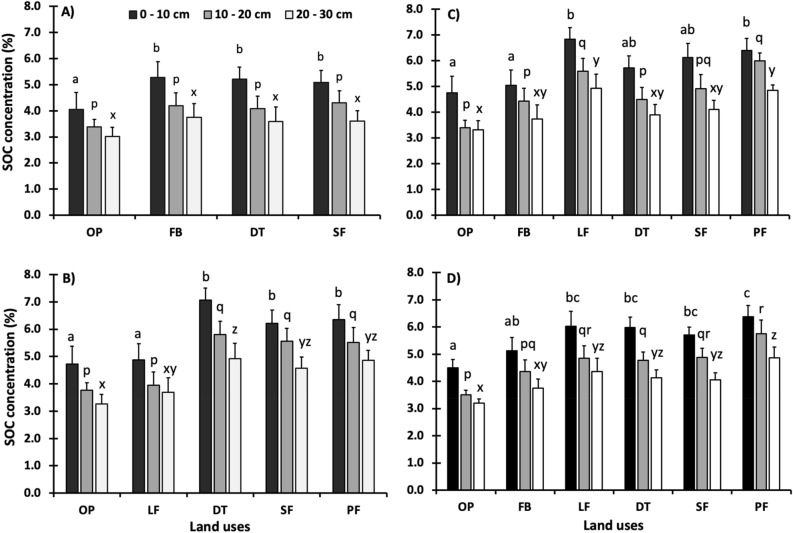
The SOC concentration (%) varied between land-uses and soil depth categories (p < 0.05) but without significant interactions between them in each region. In the Jalisco region, SOC concentrations in 0–10 cm depth, varied from 4.1 to 5.5%, the lower value corresponded to OP and the upper value to DT, with statistically significant differences. At 20–30 cm soil layer, it ranged from 3.2 to 3.8%, but no statistical differences were observed among land-uses (Fig. 3A). In the Campeche region, SOC content at 0–10 cm layer varied from 4.9 to 7.3%, the lower value corresponded to LF and the upper value to DT. A similar trend was observed at 10–20 cm depth. At 20–30 cm layer, it varied between 3.7 and 5.0%, where the lower value corresponded to OP and the higher value to SF (Fig. 3B). In the Chiapas region, SOC concentration at 0–10 cm layer ranged from 4.7 to 6.8%, the lower value corresponded to OP and the upper value to LF. At 10–20 cm layer, it differed from 3.4 to 6.0%, with the lowest value from OP and the highest from PF. At 20–30 cm layer, it ranged from 3.3 to 4.9%, with OP being the smallest (Fig. 3C). When averaged among regions, LF, DT, and SF SOC contents did not differ statistically from PF at 0–10 cm and 20–30 cm layers (Fig. 3D).
Figure 3.
Soil organic carbon (SOC) content (%) across sampled land uses by soil depth category. (A) Jalisco, (B) Campeche, (C) Chiapas, (D) All regions. Lowercase letters above the bars indicate significant differences between land-uses in the same soil depth category. The y-axis shows SOC content (%) and the x-axis shows the different land-uses sampled, where OP = Open pasturelands, FB = Fodder banks, DT = Pasturelands with dispersed trees, LF = Live fences, SF = Secondary forests, PF = Primary forests.
Fine root biomass (FRB)
The effect of land-uses on the average FRB varied by region. In the Jalisco region, FRB at 0 – 30 cm depth ranged from 2.10 to 5.80 Mg ha−1, where OP showed the smallest amount and SF the highest. In the Campeche region, FRB ranged from 3.04 to 9.82 Mg ha−1, where the smallest value corresponded to LF and the greatest to PF (Table 4). In the Chiapas region, FRB to 30 cm depth varied from 2.71 to 12.50 Mg ha−1, the smallest being the FB and the greatest the LF. Fine root biomass in OP, FB, and DT did not differ statistically (Table 4).
Table 4.
Fine root biomass (Mg ha−1) in the soils across different land-uses by depth classes and region.
| Land-use/soil depth | Fine root biomass stocks, Mg ha−1, mean (± 95% CI) | ||
|---|---|---|---|
| Jalisco | Campeche | Chiapas | |
| Open pasturelands | |||
| 0–10 cm | 1.28 (± 0.70)a | 3.00 (± 1.52)ab | 2.99 (± 1.27)a |
| 10–20 cm | 0.41 (0.25)p | 1.41 (± 1.03)p | 0.54 (± 0.23)p |
| 20–30 cm | 0.41 (± 0.28)x | 0.25 (± 0.15)x | 0.24 (± 0.07)x |
| Σ(0–30) cm | 2.10 (± 0.92)A | 4.66 (± 1.99)A | 3.77 (± 1.35)A |
| Fodder banks | |||
| 0–10 cm | 2.26 (± 1.11)a | nd | 1.66 (± 0.45)a |
| 10–20 cm | 0.95 (± 0.70)p | nd | 0.75 (± 0.31)p |
| 20–30 cm | 0.68 (± 0.50)x | nd | 0.29 (± 0.09)x |
| Σ(0 – 30) cm | 3.90 (± 1.92)AB | nd | 2.71 (± 0.64)A |
| Live fences | |||
| 0–10 cm | nd | 2.09 (± 1.08)a | 4.91 (± 2.41)a |
| 10–20 cm | nd | 0.57 (± 0.18)p | 4.87 (± 3.66)p |
| 20–30 cm | nd | 0.38 (± 0.16)x | 2.72 (± 3.51)x |
| Σ(0–30) cm | nd | 3.04 (± 1.13)A | 12.50 (± 7.74)B |
| Dispersed trees on pasturelands | |||
| 0–10 cm | 2.19 (± 1.96)a | 1.71 (± 0.78)a | 1.28 (± 0.47)a |
| 10–20 cm | 1.07 (± 0.90)p | 1.10 (± 0.65)p | 1.40 (± 0.70)p |
| 20–30 cm | 0.72 (± 0.51)x | 1.11 (± 0.67)xy | 0.96 (± 0.96)x |
| Σ(0–30) cm | 3.98 (± 2.46)AB | 3.92 (± 1.12)A | 3.64 (± 1.51)A |
| Secondary forests | |||
| 0–10 cm | 1.79 (± 0.65)a | 2.72 (± 1.69)ab | 1.68 (± 1.21)a |
| 10–20 cm | 2.20 (± 1.49)p | 1.88 (± 0.87)p | 1.97 (± 1.30)p |
| 20–30 cm | 2.48 (± 1.59)x | 1.73 (± 0.92)xy | 1.99 (± 1.44)x |
| Σ(0–30) cm | 5.80 (± 2.32)B | 6.33 (± 2.65)AB | 6.64 (± 2.08)AB |
| Primary forests | |||
| 0–10 cm | nd | 4.88 (± 1.63)b | 5.21 (± 3.00)a |
| 10–20 cm | nd | 2.72 (± 1.48)p | 2.69 (± 0.99)p |
| 20–30 cm | nd | 2.22 (± 1.01)y | 4.25 (± 2.93)x |
| Σ(0–30) cm | nd | 9.82 (± 1.96)B | 12.15 (± 4.44)B |
Different letters indicate statistical differences between land-uses (p < 0.05) within the same depth class. CI = Confidence intervals.
nd: data not available.
Soil bulk density
The average soil bulk density (BD) tends to be higher in OP compared to other land-uses, but this tendency differed by region. In the Jalisco region, BD ranged between 1.1 and 1.3 g cm−3 with OP being significantly higher than other land-uses. In the Campeche region, it ranged between 0.8 and 1.0 g cm−3, with PF and SF showing the lowest values. In the Chiapas region, BD ranged from 1.2 to 1.4 g cm−3, with OP presenting the greatest value (Table 5).
Table 5.
Soil bulk density (g cm−3) across land-uses by depth category and study region.
| Land-use | Soil depth | Soil bulk density, g cm−3, mean (± 95% CI) | ||
|---|---|---|---|---|
| Jalisco | Campeche | Chiapas | ||
| Open pasturelands | ||||
| 0–10 cm | 1.2 (± 0.07)a | 0.9 (± 0.10)ab | 1.3 (± 0.06)a | |
| 10–20 cm | 1.3 (± 0.08)p | 0.9 (± 0.14)pq | 1.4 (± 0.04)q | |
| 20–30 cm | 1.3 (± 0.08)y | 1.0 (± 0.11)x | 1.4 (± 0.04)x | |
| 0–30 cm | 1.3 (± 0.05)B | 1.0 (± 0.07)AB | 1.4 (± 0.03)B | |
| Fodder banks | ||||
| 0–10 cm | 1.1 (± 0.08)a | nd | 1.3 (± 0.05)a | |
| 10–20 cm | 1.1 (± 0.04)p | nd | 1.3 (± 0.05)pq | |
| 20–30 cm | 1.1 (± 0.09)xy | nd | 1.4 (± 0.04)x | |
| 0–30 cm | 1.1 (± 0.04)A | nd | 1.3 (± 0.03)AB | |
| Live fences | ||||
| 0–10 cm | nd | 1.0 (± 0.10)b | 1.3 (± 0.05)a | |
| 10–20 cm | nd | 1.1 (± 0.10)q | 1.3 (± 0.07)pq | |
| 20–30 cm | nd | 0.9 (c 0.12)x | 1.3 (± 0.06)x | |
| 0–30 cm | nd | 1.0 (± 0.06)B | 1.3 (± 0.03)AB | |
| Dispersed trees on pasturelands | ||||
| 0–10 cm | 1.1 (± 0.10)a | 0.9 (± 0.09)a | 1.3 (± 0.06)a | |
| 10–20 cm | 1.2 (± 0.10)p | 0.8 (± 0.09)p | 1.3(± 0.05)pq | |
| 20–30 cm | 1.2 (± 0.06)xy | 0.9 (± 0.07)x | 1.3(± 0.06)x | |
| 0–30 cm | 1.1 (± 0.05)A | 0.9 (± 0.05)A | 1.3 (± 0.03)AB | |
| Secondary forests | ||||
| 0–10 cm | 1.1 (± 0.07)a | 0.7 (± 0.12)a | 1.2 (± 0.05)a | |
| 10–20 cm | 1.2 (± 0.08)p | 0.8 (± 0.12)p | 1.2 (± 0.09)p | |
| 20–30 cm | 1.1 (± 0.12)x | 0.8 (± 0.09)x | 1.3 (± 0.07)x | |
| 0–30 cm | 1.1 (± 0.05)A | 0.8 (± 0.06)A | 1.2 (± 0.04)A | |
| Primary forests | ||||
| 0–10 cm | nd | 0.8 (± 0.14)a | 1.2 (± 0.05)a | |
| 10–20 cm | nd | 0.8 (± 0.10)p | 1.3 (± 0.07)pq | |
| 20–30 cm | nd | 0.8 (± 0.13)x | 1.3 (± 0.05)x | |
| 0–30 cm | nd | 0.8 (± 0.07)A | 1.3 (± 0.06)AB | |
Different letters indicate the statistical differences between land-uses (p < 0.05). CI = Confidence intervals.
nd: data not available.
Relationship between variables of land-use history, soil properties, and carbon storage
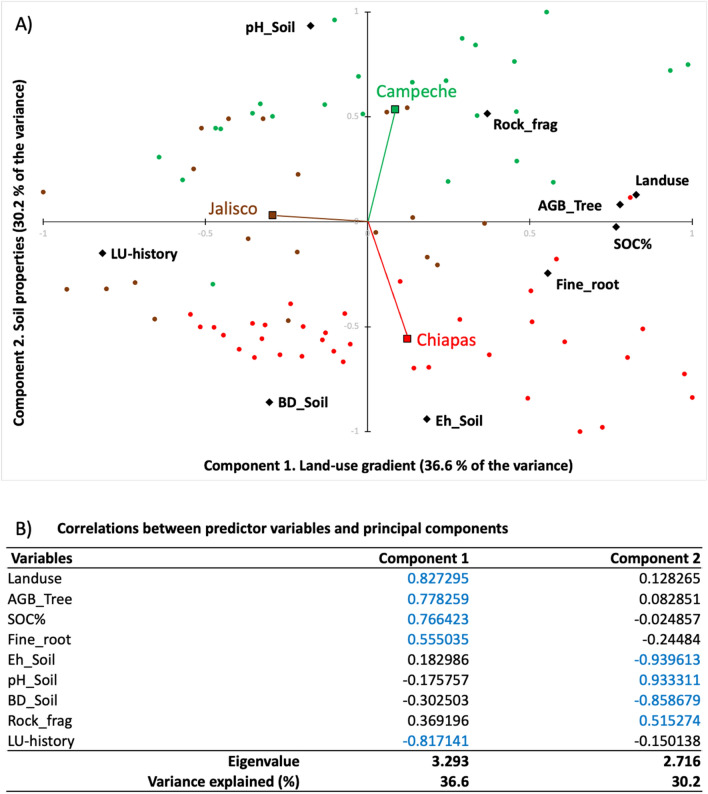
We observed that tree aboveground biomass, fine root biomass, and SOC concentrations were positively related variables, indicating that the incorporation of trees enhances SOC storage (Supplementary Table T1). The relationships among variables such as tree biomass, fine root biomass, SOC concentration (%), soil pH, oxidation–reduction potential (Eh), bulk density, land-use history (years after forest conversion), and presence of rock fragments are shown in a principal component (PC) analysis (Fig. 4). The first two principal components with eigenvalues > 1 explained 66.8% of the variance (Fig. 4A). We observed that land use-gradient, land-use history, tree biomass, SOC concentrations, and fine root biomass were correlated more to PC1. Hence, we named this component as land-use gradient. We named the component 2 as soil properties because soil bulk density, pH, redox potential (Eh), and rock contents correlated more to this PC. In the biplot, we observe that tree biomass, SOC concentrations, and fine root biomass were clustered horizontally while soil pH, Eh and bulk density clustered vertically. Carbon storage was inversely related to land-use history (years that the land was used for animal grazing). Observations of Campeche region were distributed more to upper part, characterized by the higher soil pH but diverse in SOC concentrations. Jalisco region was characterized by lower SOC concentration and the longer history of land use. Chiapas region was characterized by the lower soil pH but showed a wider range in SOC concentrations (Fig. 4).
Figure 4.
Component plotting of multiple variables including soil organic carbon content (%) based on the principal component analysis (PCA). (A) PCA plot with observations; (B) Correlations between principal components and predictor variables. AGB = aboveground biomass, LU = land use, BD = soil bulk density (g cm−3), Eh = soil oxidation–reduction potential (ORP, mV), SOC = soil organic carbon concentrations (%).
Discussion
Carbon sequestration through SPS can contribute significantly to mitigating greenhouse gas emissions from the livestock sector and reduce the environmental footprints of animal products37,40,62. Avoided conversion of forests to OP leads to higher C retention within the landscape, while the transformation of OP to SPS enhances C accrual from the atmosphere46. In addition to C stored in biomass, these systems contribute to avoiding the loss of SOC due to the possible conversion to OP systems. In this study, FB, LF, DT, SF, and PF stored 26.5, 39.8, 45.3, 54.2, and 163.1% higher total C stocks respectively compared to OP, highlighting the importance of SPS and forests in livestock-dominated landscapes. The size of different C pools differs across land-uses because of the functional, structural, and compositional differences11,72–74. Silvopastoral systems (SPS) and forests have more vertical layers due to the presence of trees, are richer in species diversity, and have deeper root systems than OP32,65,75–77. The amount of living biomass stocks across the different land-uses varied because of the tree density, vegetation structure, and species differences, between land-uses16,78.
For example, in the LF, trees are planted in rows on the borders of about one-ha plots, and the interior matrix of the land is covered with grasses (i.e., when separating paddocks)79. Hence, the total tree density per ha is lower in this system compared to DT and forests (PF and SF), which explains the lower carbon stocks in living tree biomass80. Besides, in some cases, trees are pruned once a year to provide animal fodder and maintain the accessible height of the fences. In contrast, in the DT land-use, trees are found scattered within the pastureland matrices without any strict distance or row arrangements. Such a random distribution leads to higher access to sunlight resulting in wider crown diameter and bigger trees81. The average tree basal area (ha−1) was also higher in DT compared to LF, which increased the living tree biomass stocks71. Some of the trees in the DT are remnants of the original PF left uncut during the land conversion which are relatively bigger than other trees grown after. Tree density is even higher in SF, but the average tree size is relatively smaller if compared to PF. Across the study areas, PFs are generally composed of bigger native trees with higher stand-level wood density compared to SF, resulting in higher carbon stocks in woody biomass82.
Another factor that affects C storage is the intensity of land-use and management. Open pasturelands and other SPS (LF and DT) were grazed continuously with the same intensity but the presence of trees and their arrangements make them different in terms of biomass growth and organic matter input to the system80,83,84. Fodder banks are harvested frequently to feed animals, but grazing is limited in these systems59,85. Secondary forests are occasionally grazed but with lower intensity where biomass removal is lower compared to OP and SPS. Biomass removal by cattle grazing in PF is very rare making it a low-intensity system. Such differences in grazing management partly explain the change in above- and belowground organic matter input leading to the differences in total carbon storage between land-uses within the same landscape57,86,87. Reduced organic matter input, C loss from soil erosion, continuous loss from heterotrophic soil respiration, and reduced soil biological activity for organic matter stabilization are some of the principal causes of SOC depletion in OP44,73,88,89.
SOC stocks (Mg ha−1) are principally affected by SOC concentrations (%) but also by soil bulk density. Higher SOC concentrations explain the increased SOC stocks in forests (PF and SF) and SPS but the higher. Soil bulk density leads to higher soil mass per hectare and apparently higher SOC stocks. However, SOC concentrations (%) were significantly lower in OP compared to SPS or forest land-uses (PF and SF).
Forests (PF and SF) and SPS, are generally composed of plant species with diverse rooting systems that promote the accumulation of root-derived organic matter and microbial detritus to deeper soils90,91. In Alberta Canada, SPS had 30% higher SOC compared to open herb systems92. Higher carbon sequestration in SPS is also reported in Latin America and other parts of the world64,93,94. In addition to the higher accumulation of organic matter, the loss of carbon by mineralization and heterotrophic respiration is lower in SPS due to micro-climatic buffering48,69,95. The integration of trees on pasturelands improves soil microbial and enzymatic activity with the potential to increase organic carbon storage36,96,97.
The principal component analysis showed that land-use history (the time after forest conversion, years) was another important variable and related inversely with SOC %, but positively to soil bulk density (Fig. 4). Such a relationship indicates that the longer the land is used for livestock farming, the lower the concentration of SOC. The positive relations between land-use history and soil bulk density indicate that soil compaction is increasing with the time that the land is used for livestock farming (Fig. 4). However, further studies are required to have a deeper understanding of the role of land-use history on SOC sequestration in these areas. The soils of the Campeche region are calcareous with high Ca and Mg cations with pH slightly higher than neutral due to the karstic parent material98. Independent of land-use, SOC concentrations % (but not SOC stocks) were slightly higher in the Campeche region. Among others, the sorption of negatively charged organic matter by cation bridging in Ca-rich soils could have increased the stabilization of SOC, leading to higher C accumulation91,97,99. However, the higher presence of rocks in the soil of this region reduced the SOC stocks (Mg ha−1) because of the lower soil mass equivalent.
Storing PF level C in grazing lands should be an ambitious goal because it can significantly lower grass availability and hence reduce livestock productivity. However, increasing C stocks to the level of LF and DT can be considered viable options. Evidence showed that better management of pasturelands by incorporating trees should increase animal production intensity by restoring soil fertility allowing farmers to spare areas for conservation and hence higher C storage at the landscape scale12,100–102. Besides, conserving forest fragments or increasing trees within the matrix of livestock-dominated landscapes is a dual strategy that not only helps C sequestration but also benefits biodiversity conservation, heat stress management, dry season feeding, and overall ecosystem resilience35,36,103. Conserving even a small area of PF can help farmers as a seed pool for the regeneration of native trees on the surrounding landscape104.
Integrating different silvopastoral and forested land-uses within the farmlands can be recommended as a good practice that promotes the synergies between climate change adaptation and mitigation benefits. This is especially important in the hotspots where livestock farming is often considered to be a threat to important natural protected areas such as La Sepultura Biosphere Reserve in Chiapas, Calakmul, and Balam Ku Biosphere Reserves in Campeche, and Chamela-Cuixmala and Sierra de Manantlán Biosphere Reserves in Jalisco.
Mexico in its Intended Nationally Determined Contributions (INDC) has proposed to reduce total GHG emissions by 22% and achieve zero deforestation by 2030105. It is also stipulated that net emissions from land-use change and the forestry sector would go negative (i.e., higher carbon capture than emissions). This means the Agriculture Forestry and Other Land use (AFOLU) sector should mitigate 53 Tg CO2e per year (7 Tg from agriculture + 46 Tg from land-use change) by 2030 as compared to the business-as-usual scenario105. However, land-use change from forestry to pastureland is still common because of the increasing interest in livestock farming106. As a consequence, strategic frameworks and actions are still not well articulated to promote SPS as a carbon mitigation strategy at the local level, even though there´s plenty of evidence of its potential. Currently, 80.3 million ha of land is used for livestock grazing in Mexico, most of them as OP17. If 25% of the current OP could be converted to SPS, reaching the total C stock level to DT or LF found in this study, an additional 815 Tg C could be captured in these lands. It would take 25–80 years to sequester this amount of C, taking into account the variation in C sequestration rates of SPS (0.5–1.8 Mg C ha−1 year−1) in the region80. Chiapas currently has about 3.79 million hectares of grasslands107. Considering that there would be no deforestation for extra grassland expansion, a 25% SPS adoption could mitigate 63% of the projected livestock sector GHG (3.41 Tg CO2e) by 2050 in Chiapas. Campeche currently has about 1.05 million hectares of grasslands107. Considering the scenario that there would be no further deforestation for grassland expansion, 25% adoption of SPS could mitigate 58% of the projected livestock sector GHG (1.01 Tg CO2e) by 2050 in Campeche. Jalisco currently has 1.11 million hectares of grasslands107. With the 25% SPS adoption, only 8% of the projected 2050 livestock sector GHG emissions (8.55 Tg CO2e) could be mitigated in Jalisco. This is attributed to the fact that livestock production in Jalisco is more intensive than in Chiapas and Campeche region, which requires additional technological measures to reduce livestock sector GHG.
At the farm level, restoring 25% of the degraded pasturelands to achieve C stock level to SPS and/or conserving high C density forest remnants may require good technical guidance and incentives. Such a change in land-use and management could contribute significantly to meeting the ambitious national goals of Mexico regarding mitigation and adaptation to climate change. However, the institutional framework, farmers´ capacity to adopt SPS, and the time required to sequester this amount of carbon should be evaluated. It requires multiple actions to strengthen farmers´ capacity and incentivize carbon resilient land-use practices for socio-economic benefits at the local level108. Avoided deforestation and inclusion of trees as SPS on livestock farmlands would mitigate net carbon emissions and achieve carbon–neutral livestock production109. On the global scale, significant carbon benefit can be obtained by converting pasture monocultures (OP) to SPS because a large extension (~ 3.3 billion hectares) of land is currently used for livestock grazing17,101.
Looking at the other perspective, the continuous reduction of SOC and soil fertility in OP can reduce grass productivity. As a consequence, farmers can opt to convert the adjacent PF to increase the area of pastureland. Unless intervened technically and institutionally, there exists a great danger of losing high carbon density ecosystems (like PF = 349.2 Mg C ha−1) to establish low carbon density ones (OP = 132.7 Mg C ha−1). From this study, we came to know that none of the current SPS evaluated, i.e., FB (167.9 Mg C ha−1), LF (185.5 Mg C ha−1), or DT (192.9 Mg C ha−1), can store the amount of C accumulated by PF (349.2 Mg C ha−1), being the main differences driven by the trees present in PF (Supplementary Fig. F1). Crop and grassland expansion in southern Mexico accelerated during the 60 s to 90 s when federal and local governments incentivized forest conversion to OP and croplands22,110. Farmers during that period were trained to maintain their grasslands without trees, looking like a snooker board. In recent decades, the most common trend is the conversion of croplands, especially maize + bean, to grasslands because of higher income from animal farming111. Longer-duration and frequent droughts in northern arid parts of Mexico have also triggered recent livestock expansion in the south-southwest of the country. In this context, we estimate that more than 90% of the current SPS in the region were derived from the inclusion (planting or natural regeneration) of trees in OP. Therefore, the major part of the differences in C stock between SPS and OP is considered as the true offset of emissions.
Efficient strategies are needed at the local and regional levels to protect such vulnerable carbon stocks by conserving high C density systems and increasing carbon stocks in the low C density ecosystems112. It is necessary to promote the intensification of livestock production through SPS and other good management practices to take better advantage of biomass for animal production and C storage in the deforestation and grassland expansion hotspots. The development of evidence-based mechanisms for higher production per unit area would compensate for the opportunity cost of further land conversion and promote the release of land for restoration to high C density ecosystems in these livestock-dominated landscapes.
These results have implications for designing and scaling up the adoption of climate-smart livestock farming systems. We highlight the importance of conserving remnant PF, restoring degraded OP through the establishment of SPS, and land sparing for the increase of SF. We showed that SPS enhances C storage contributing to mitigating GHG emissions from livestock farming, in addition to species and structural diversification. Knowledge of the region- and system-specific variations on C storage help identify appropriate SPS according to the soil, climate, and landscape characteristics. Furthermore, we highlighted the relationship between above and belowground C pools, soil properties, and land-use history to be considered while implementing and measuring the contribution of C-enhancement strategies in livestock-dominated landscapes. Our results can partly contribute to decisions regarding the inclusion of SPS in the development of Nationally Appropriate Mitigation Actions (NAMA) for the livestock sector in Mexico and other countries of similar contexts.
Materials and methods
Study sites
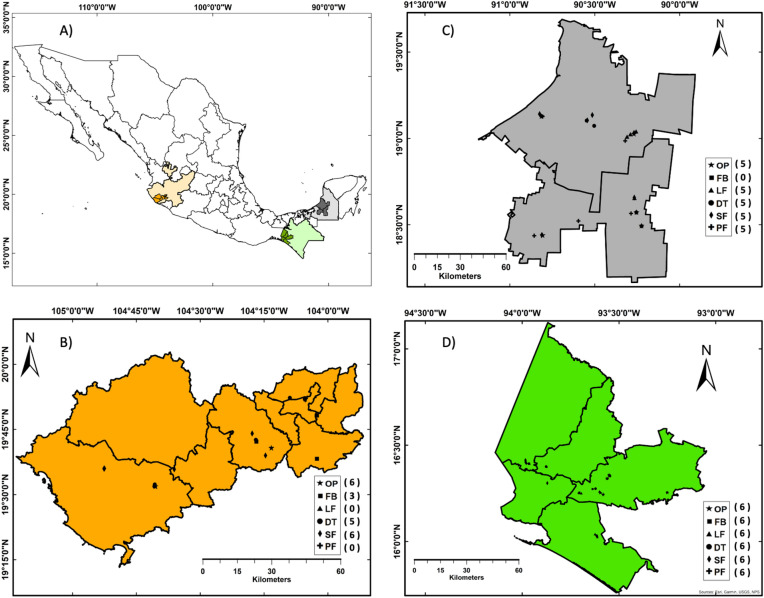
The study was carried out in three tropical livestock-dominated regions of Mexico: (1) Pacific coastal watershed in Jalisco, (2) La Sierra Madre in Chiapas, and (3) Southern Yucatan Peninsula in Campeche (Fig. 5). These regions have different soil pH varying between 6.0 and 7.0 in Jalisco, 4.5 and 5.5 in Chiapas, and 7.0 and 7.5 in Campeche, important biophysical characteristics of all regions are presented in Supplementary Table T2. Selected study regions contain important natural reserves and habitat conservation areas, some of them being part of the large Mesoamerican Biological Corridor initiative. However, the pressure on natural resources is increasing due to natural and anthropogenic factors113. Furthermore, these regions are prioritized for REDD + (reduction of emissions from deforestation and degradation + C enhancement) early actions in Mexico. The selected study sites correspond to areas of intervention of the project “Scaling up biodiversity conservation through climate-smart agrosilvopastoral practices in landscapes dominated by cattle-raising systems in three regions of Mexico”114.
Figure 5.
Study area location and distribution of sampling plots across landscapes. (A) States (light color) and study areas (darker color) where sampling was conducted. The letters and symbols correspond to the different types of land-uses, OP = Open pasturelands, FB = Fodder banks, LF = Live fences, DT = Pasturelands with dispersed trees, SF = Secondary forests, and PF = Primary forests. (B–D) Close-ups of the study sites in Jalisco (B), Campeche (C), and Chiapas (D). The values in the parenthesis show the number of plots sampled (n) for each land-use.
We selected the most common land-uses from each region where bovine cattle grazing is the principal farming activity. We conducted farmers´ interviews to learn about land-use history, stocking rates, and land management practices of each study site. Based on tree richness and diversity, vertical structure, and tree cover we identified a land-use gradient from simple to ecologically complex115 which is reflected as follows considering the six studied land-uses: open pasturelands (OP), fodder banks (FB), live fences (LF), pasturelands with dispersed trees (DT), secondary forests (SF), and primary forests (PF) (Fig. 5). Conventional OP was considered the simplest form of land-use due to the lack of trees, followed by FB which included one or two tree species, LF including multiple tree species, DP including multiple tree species, and greater tree cover (compared to previous land-uses), SF that share multiple native tree species with primary forests, and finally, PF (old-growth) which was considered the more complex form and was also used as a reference land-use115. We sampled a total of 79 plots across the three study areas (Fig. 5 shows the number of plots by land-use from each region). Sampled SF was occasionally used for livestock grazing, particularly during the dry season while PF was not grazed.
Measurement of carbon stocks in different pools
Tree biomass and diversity
We first delimited the sampling plots or fence distance to measure all the trees of ≥ 2.5 cm diameter at breast height (DBH). The size of each sample plot to measure tree biomass was 1000 m2 (40 m × 25 m) for FB, DT, SF, and PF. For LF, we measured the trees within a linear distance of 100 m. Since trees were absent in OP, we established similar plots for the nested sampling of other C pools.
We measured tree DBH (at 1.3 m from the ground level) by using diameter tapes and total height with the trigonometric approach using clinometers. All trees found within the plots were identified to the species level allotting the respective local and scientific names. We searched for wood density data in the global wood density database and other published literature116 and quantified the aboveground biomass (AGB) by using an allometric equation for tropical trees117.
where AGB is the aboveground biomass of the tree (kg), is the wood density of the species (g cm−3), D is the DBH (cm) and H is the total height (m). We used this equation (AIC = 3130, DF = 4002) because it covers a wide range of tropical trees including some species from southern Mexico.
For Leucaena leucocephala in FB, we developed allometric equations from the destructive sampling of 33 trees and used this equation to calculate biomass C stocks. Trees were measured in the field, cut at the ground level, and taken to the laboratory for oven drying. Dry biomass weights were then plotted to regression analysis with dasometric variables measured in the field118. In FB, tree heights were measured by measuring tapes or graduated sticks. Legume trees on FB are usually cut for fodder at about 1 m in height. Hence, the biomass model was developed with the base diameter (30 cm above ground level). An exponential model fitted best to our data with a determination coefficient (R2) of 0.95. The best fit equation is:
where AGBL is the aboveground biomass (g) for Leucaena, D is the tree diameter 30 cm above ground level (cm), and H is the height of the tree (cm). We used this equation to calculate the L. leucocephala biomass in the FB of this species.
Tree root biomass was quantified with the following allometric equation119
where RB is the root biomass of the tree (kg dry weight), AGB is the aboveground biomass (kg dry weight).
Individual tree biomass obtained from allometric equations were summed and converted to plot-level C stocks (Mg C ha−1) by using the sample area (1000 m2) and a C fraction of 0.47. The biomass stocks from the linear measurement of trees in LF were converted to per hectare considering that 200-m fences correspond to a one-hectare plot.
We also calculated tree species richness, Shannon–Weaver diversity index (H)120, Sorensen similarity coefficient (CC)121, and Pielou evenness index (J)122 for each land-use by study region.
where pi is the proportion (n/N) of individuals of one particular tree species (n) divided by the total number of individuals from all species found (N), lnPi is the natural logarithm of Pi and C is the sum of the S number of species.
where C is the number of species in common between PF and the respective land-use, S1 is the total number of species found in PF, and S2 is the total number of species found in the given land-use. CC gives a value between 0 and 1, the value closer to 1 denotes that the tree species composition is more similar to PF. CC was used to measure the degree to which selected land-uses share PF (native) species.
where H is the Shannon–Weaver index and S is the total number of tree species in the respective land-use. The result ranges from 0.0 to 1.0 with 1.0 reflecting total evenness. The evenness index (J) is low when only a few species are more abundant than others.
Grass biomass
In OP, FB, DT, SF, and PF, we collected the grass (or understory vegetation) samples within four square frames of 1 × 1 m2. The 1000 m2 plot was first divided into four quadrants and the frames were established randomly within each quadrant. In LF, grass biomass samples were collected from four quadrants distributed to both sides of the fences within the area of canopy coverage. Quadrants (square frames) were made of PVC tubes with an inner surface area of 1 m2. Once the sampling quadrants were located, we cut all the grass biomass at ground level within the quadrants. The grass biomass samples were put in paper bags, labeled accordingly, weighed, and transported to the laboratory for oven drying (70 °C). The oven-dried biomass was converted to C stocks (Mg C ha−1) by using a C fraction of 0.46 and the sampling area (4 m2)80.
Ground litter
The fallen leaves, small branches (≤ 1.0 cm), and their organic detritus were collected as litter mass within four 30 cm × 30 cm PVC quadrants distributed randomly within each C monitoring plot. Larger branches were sampled as deadwood material. Litter samples were collected separately in two categories depending upon the stage of decomposition. The litter samples were stored in paper bags, which were labeled with assigned personalized codes, then they were weighed, and transported to the laboratory for oven drying and chemical analysis. By using the C fraction and sample area, we extrapolated the litter C stock to Mg C per ha.
Deadwood carbon
We used the line intersection method sampling fallen dead wood materials (> 1 cm diameter) by measuring the diameter of wood pieces across four transects of 25 m each on the borderline of each 1000 m2 C monitoring plot123. The volume of wood was calculated as:
where, V is the volume of the wood (m3), di is the diameter (cm) of the piece of wood i, and L is the length (m) of the transect (25 m). In LF, only two transects were used. Besides, the volume of the deadwood fence (poles) was calculated by measuring the diameter and height. The deadwood mass was obtained by multiplying the volume with wood density according to the state of decomposition. A fraction of 0.47 was used to convert deadwood mass to C.
Fine root biomass
Fine root biomass was sampled by using a 5.5 cm diameter cylinder to the respective soil depth (0–10 cm, 10–20 cm, and 20–30 cm), separated from soil, washed, and oven-dried (70 °C) before weighing80,85. Root fragments greater than 2 mm in diameter were discarded from fine root biomass. Dry root biomass was then converted to per hectare value by using surface area and respective soil depth.
Soil properties
Soil samples were collected from four random locations within the 1000 m2 plot. To avoid the overlapping between litter and soil samples (within the organic horizon), we sampled within the same random quadrants where litter samples were collected. Soil samples were collected separately by depth (0–10 cm, 10–20 cm, and 20–30 cm) using a cylindrical metallic corer of 5.5 cm inner diameter and 10 cm height. The cylinder was struck manually until reaching the first 10 cm of soil and carefully removed and handled to obtain a complete sample. The same procedure was applied to collect consecutive soil samples from 10 to 20 and 20–30 cm depths. We collected 24 samples per plot, 12 for chemical analysis, and the parallel 12 for soil bulk density estimations.
Before chemical analysis, we separated root and rock fragments from the soil samples, dried them at room temperature, grounded, and sieved them using a 2 mm mesh. For C concentration (%), we used the potassium dichromate digestion method followed by sucrose calibrated spectrophotometry at 600 nm wavelength124. For soil pH and oxidation–reduction potential (Eh), we used the potentiometric method in a 1:2 soil water solution. For bulk density calculation, soil samples were oven-dried (105 °C) for 48 h, coarse fragments (roots and rocks) were separated and weighed. Soil bulk density was calculated as the ratio of dry weight to the volume of the cylinder used for sampling (g cm−3). Total bulk density was calculated without separating coarse fragments while soil bulk density was calculated with coarse fragment free soil mass to evaluate the effect of rock fragments. Soil bulk density is one of the important variables in the quantification of SOC stock since it is used to quantify the total soil mass per unit area to a certain depth. Using the oven-dry weight of the separated rocks, we quantified the amount of rock per unit area (Mg ha−1). Soil bulk density, corresponding SOC content (%), surface area, and soil depth values were used to calculate the SOC stocks (Mg ha−1) as follows:
where, SOCC is the soil organic carbon concentration (%); BD is the soil bulk density (g cm−3 or Mg m−3) after correcting coarse fragments; Depth is the depth of soil sample (m). SOC stocks were calculated according to the soil mass equivalent after subtracting the mass of rock fragments.
Data analysis
We first cross-checked all data from the three regions and six land-uses by creating a pivot table to visually explore the data. Any atypical value was subjected to further review and consultation with the field team. The verified data were tested for normality of distribution by the Shapiro–Wilk W test (p > 0.05). The non-normal data were transformed (log10). Log transformed data were used for one-way ANOVA to test the significant differences between sampled land-uses. We also used factorial ANOVA to test the effect of land-use and soil depth categories for SOC content and other soil properties. Back transformed weighted means and respective 95% confidence intervals are presented in the results. Furthermore, we applied a principal component analysis (PCA) based on correlations to reduce the dimensions and check the relationship between variables such as SOC concentrations, living tree biomass, soil properties, and land-use history. Variable groupings in PCA were based on correlation. We developed a correlation matrix among variables and their significance (Supplementary Table T1). To extract the principal components, we used eigenvalues greater than one. To associate variables with components, we considered those having a correlation higher than 0.5. We applied the Varimax rotation and clustered the variables in a biplot based on their loadings. The Kaiser–Meyer–Olkin measure of sampling adequacy was 0.685 and Bartlett's test of sphericity was significant (χ2 value = 597.5, p < 0.01) showing that the data contain enough variance to partition into components.
Ethics approval
This research did not use any methods that require licensing committee approval.
Supplementary Information
Acknowledgements
We acknowledge the collaborative project between CATIE (Centro Agronómico Tropical de Investigación y Enseñanza) and the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU), and the International Climate Initiative (IKI). We specifically thank the “Scaling-up Biodiversity Conservation through Climate-smart Agrosilvopastoral Practices in Landscapes dominated by Cattle-raising Systems in Three Regions of Mexico” project (16_IV_070_MEX_A_Agrosilvopastoral Systems), also known as BioPaSOS for supporting this research. CNTM and SCLC thank the Consejo Nacional de Ciencia y Tecnología for postgraduate scholarship support. We thank the landowners and ranchers of Jalisco, Chiapas, and Campeche for allowing us to carry out this research on their land. We thank the BioPaSOS technical team in their territories, particularly: Erika Hernández, Emma De Niz, Alenzy Chávez, Lourdes Pérez and Guadalupe Niño for supporting fieldwork activities. We also thank Jerzy Rzdoswky lab of El Colegio de la Frontera Sur Campeche and Animal nutrition lab of Universidad Autónoma de Chiapas (UNACH) for providing field equipment and lab facilities. We are thankful to Roldan Ruiz, Natalia Ysabel Labrín, Claudia Pinacho, Octavio Gómez, Juan Carlos Hernández and Beatriz Peña for field and laboratory assistance. We thank the CONANP Sepultura team of Chiapas and Juntas Intermunicipales de Medio Ambiente (JIMA) of Jalisco for providing logistic support during fieldwork. We acknowledge the anonymous reviewers and editors for their valuable comments and suggestions which significantly improved the quality of this paper.
Author contributions
D.R.A. and D.E.M.R. contributed to research design, data collection, analysis, and manuscript writing; S.L.C., C.N.T.M., A.L.N., J.A.J.T., E.P.S., J.E.B.S., R.R.D. contributed in field and laboratory work, data collection, and manuscript revision; F.C.C., A.M.S., C.J.S.L. contributed to research design, manuscript writing, and revision; M.L.A., F.G.H., R.P.R., and M.I. contributed to manuscript writing and revision.
Funding
German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety (BMU), through the International Climate Initiative (IKI), provided funding for this research (16_IV_070_MEX_A_Agrosilvopastoral Systems); Consejo Nacional de Ciencia y Tecnología, Mexico provided a postgraduate scholarship to CNTM and SCLC.
Data availability
Data can be obtained upon request from the corresponding author. Collection of plant biomass and soil samples were carried out with permissions from local authorities. We declare that this project was carried out in collaboration with the Mexican National Commission for Natural Protected Areas (CONANP, Spanish acronym), which is the local entity that regulate such permissions. We did not use any vertebrate animal for experimental purposes in this study. The animals in the images were to represent that the lands we sampled are used for animal grazing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21089-4.
References
- 1.Song X-P, et al. Global land change from 1982 to 2016. Nature. 2018;560:639–643. doi: 10.1038/s41586-018-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houghton RA, Nassikas AA. Global and regional fluxes of carbon from land use and land cover change 1850–2015. Glob. Biogeochem. Cycles. 2017;31:456–472. doi: 10.1002/2016GB005546. [DOI] [Google Scholar]
- 3.Phelps LN, Kaplan JO. Land use for animal production in global change studies: Defining and characterizing a framework. Glob. Change Biol. 2017;23:4457–4471. doi: 10.1111/gcb.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis PG, Slay CM, Harris NL, Tyukavina A, Hansen MC. Classifying drivers of global forest loss. Science. 2018;361:1108–1111. doi: 10.1126/science.aau3445. [DOI] [PubMed] [Google Scholar]
- 5.Hong C, et al. Global and regional drivers of land-use emissions in 1961–2017. Nature. 2021;589:554–561. doi: 10.1038/s41586-020-03138-y. [DOI] [PubMed] [Google Scholar]
- 6.Knorr W, Prentice IC, House J, Holland E. Long-term sensitivity of soil carbon turnover to warming. Nature. 2005;433:298–301. doi: 10.1038/nature03226. [DOI] [PubMed] [Google Scholar]
- 7.Shi Z, et al. The age distribution of global soil carbon inferred from radiocarbon measurements. Nat. Geosci. 2020;13:555–559. doi: 10.1038/s41561-020-0596-z. [DOI] [Google Scholar]
- 8.Sanderman J, Hengl T, Fiske GJ. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. 2017;114:9575–9580. doi: 10.1073/pnas.1706103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304:1623–1627. doi: 10.1126/science.1097396. [DOI] [PubMed] [Google Scholar]
- 10.Friedlingstein P, et al. Global carbon budget 2020. Earth Syst. Sci. Data. 2020;12:3269–3340. doi: 10.5194/essd-12-3269-2020. [DOI] [Google Scholar]
- 11.Yue C, Ciais P, Houghton RA, Nassikas AA. Contribution of land use to the interannual variability of the land carbon cycle. Nat. Commun. 2020;11:1–11. doi: 10.1038/s41467-020-16953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zomer RJ, et al. Global tree cover and biomass carbon on agricultural land: The contribution of agroforestry to global and national carbon budgets. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep29987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Stefano A, Jacobson MG. Soil carbon sequestration in agroforestry systems: a meta-analysis. Agrofor. Syst. 2018;92:285–299. [Google Scholar]
- 14.Bossio D, et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020;3:391–398. doi: 10.1038/s41893-020-0491-z. [DOI] [Google Scholar]
- 15.England JR, O’Grady AP, Fleming A, Marais Z, Mendham D. Trees on farms to support natural capital: An evidence-based review for grazed dairy systems. Sci. Total Environ. 2020;704:135345. doi: 10.1016/j.scitotenv.2019.135345. [DOI] [PubMed] [Google Scholar]
- 16.Ma Z, Chen HY, Bork EW, Carlyle CN, Chang SX. Carbon accumulation in agroforestry systems is affected by tree species diversity, age and regional climate: A global meta-analysis. Glob. Ecol. Biogeogr. 2020;29:1817–1828. doi: 10.1111/geb.13145. [DOI] [Google Scholar]
- 17.FAOSTAT. Data/Inputs/land use. In: Food Agriculture Organization. http://www.fao.org/faostat/en/#data/RL. (2020). Accessed 12 Sept 2020.
- 18.Shukla, P. R. et al. Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. (Intergovernmental Panel on Climate Change, 2019).
- 19.Galdino S, et al. Large-scale modeling of soil erosion with RUSLE for conservationist planning of degraded cultivated Brazilian pastures. Land Degrad. Dev. 2016;27:773–784. doi: 10.1002/ldr.2414. [DOI] [Google Scholar]
- 20.Stanimirova R, et al. Sensitivity of global pasturelands to climate variation. Earth’s Future. 2019;7:1353–1366. doi: 10.1029/2019EF001316. [DOI] [Google Scholar]
- 21.Tolimir M, et al. The conversion of forestland into agricultural land without appropriate measures to conserve SOM leads to the degradation of physical and rheological soil properties. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-70464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza-Ponce A, Corona-Núñez R, Kraxner F, Leduc S, Patrizio P. Identifying effects of land use cover changes and climate change on terrestrial ecosystems and carbon stocks in Mexico. Glob. Environ. Change. 2018;53:12–23. doi: 10.1016/j.gloenvcha.2018.08.004. [DOI] [Google Scholar]
- 23.Castillo-Santiago M, Hellier A, Tipper R, De Jong B. Carbon emissions from land-use change: An analysis of causal factors in Chiapas, Mexico. Mitig. Adapt. Strat. Glob. Change. 2007;12:1213–1235. doi: 10.1007/s11027-006-9060-7. [DOI] [Google Scholar]
- 24.Kolb M, Galicia L. Scenarios and story lines: drivers of land use change in southern Mexico. Environ. Dev. Sustain. 2018;20:681–702. doi: 10.1007/s10668-016-9905-5. [DOI] [Google Scholar]
- 25.Aryal DR, et al. Biomass accumulation in forests with high pressure of fuelwood extraction in Chiapas, Mexico. Revista Árvore. 2018;42:e420307. doi: 10.1590/1806-90882018000300007. [DOI] [Google Scholar]
- 26.Aryal DR, et al. Soil organic carbon depletion from forests to grasslands conversion in Mexico: A review. Agriculture. 2018;8:181. doi: 10.3390/agriculture8110181. [DOI] [Google Scholar]
- 27.Griscom BW, et al. Natural climate solutions. Proc. Natl. Acad. Sci. 2017;114:11645–11650. doi: 10.1073/pnas.1710465114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman M, et al. Large climate mitigation potential from adding trees to agricultural lands. Glob. Change Biol. 2020;26:4357–4365. doi: 10.1111/gcb.15121. [DOI] [PubMed] [Google Scholar]
- 29.Hayek MN, Harwatt H, Ripple WJ, Mueller ND. The carbon opportunity cost of animal-sourced food production on land. Nat. Sustain. 2021;4:21–24. doi: 10.1038/s41893-020-00603-4. [DOI] [Google Scholar]
- 30.Kothandaraman S, Dar JA, Sundarapandian S, Dayanandan S, Khan ML. Ecosystem-level carbon storage and its links to diversity, structural and environmental drivers in tropical forests of Western Ghats, India. Sci. Rep. 2020;10:1–15. doi: 10.1038/s41598-020-70313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Havlík P, et al. Climate change mitigation through livestock system transitions. Proc. Natl. Acad. Sci. 2014;111:3709–3714. doi: 10.1073/pnas.1308044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resende LO, et al. Silvopastoral management of beef cattle production for neutralizing the environmental impact of enteric methane emission. Agroforestry Syst. 2020;94:893–903. doi: 10.1007/s10457-019-00460-x. [DOI] [Google Scholar]
- 33.Sans GHC, Verón SR, Paruelo JM. Forest strips increase connectivity and modify forests’ functioning in a deforestation hotspot. J. Environ. Manage. 2021;290:112606. doi: 10.1016/j.jenvman.2021.112606. [DOI] [PubMed] [Google Scholar]
- 34.Searchinger TD, Wirsenius S, Beringer T, Dumas P. Assessing the efficiency of changes in land use for mitigating climate change. Nature. 2018;564:249–253. doi: 10.1038/s41586-018-0757-z. [DOI] [PubMed] [Google Scholar]
- 35.Lawson, G., Dupraz, C. & Watté, J. Can silvoarable systems maintain yield, resilience, and diversity in the face of changing environments? in Agroecosystem Diversity 145–168 (Elsevier, 2019).
- 36.Ramakrishnan S, et al. Silvopastoral system for resilience of key soil health indicators in semi-arid environment. Arch. Agron. Soil Sci. 2021;67:1834–1847. doi: 10.1080/03650340.2020.1814954. [DOI] [Google Scholar]
- 37.Gerber PJ, et al. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities. Food and Agriculture Organization of the United Nations (FAO); 2013. [Google Scholar]
- 38.Haberl H. Method précis: Human appropriation of net primary production (HANPP) In: Haberl H, Fischer-Kowalski M, Krausmann F, Winiwarter V, editors. Social Ecology. Society-Nature Relations across Time and Space. Springer Nature; 2016. pp. 332–334. [Google Scholar]
- 39.Smith P, et al. Global change pressures on soils from land use and management. Glob. Change Biol. 2016;22:1008–1028. doi: 10.1111/gcb.13068. [DOI] [PubMed] [Google Scholar]
- 40.Herrero M, et al. Greenhouse gas mitigation potentials in the livestock sector. Nat. Clim. Change. 2016;6:452–461. doi: 10.1038/nclimate2925. [DOI] [Google Scholar]
- 41.Lorenz K, Lal R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Develop. 2014;34:443–454. doi: 10.1007/s13593-014-0212-y. [DOI] [Google Scholar]
- 42.Michalk DL, et al. Sustainability and future food security—A global perspective for livestock production. Land Degrad. Dev. 2019;30:561–573. doi: 10.1002/ldr.3217. [DOI] [Google Scholar]
- 43.Bardgett RD, et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021;2:720–735. doi: 10.1038/s43017-021-00207-2. [DOI] [Google Scholar]
- 44.Pinheiro FM, Nair PR, Nair VD, Tonucci RG, Venturin RP. Soil carbon stock and stability under Eucalyptus-based silvopasture and other land-use systems in the Cerrado biodiversity hotspot. J. Environ. Manage. 2021;299:113676. doi: 10.1016/j.jenvman.2021.113676. [DOI] [PubMed] [Google Scholar]
- 45.Jose S, Walter D, Kumar BM. Ecological considerations in sustainable silvopasture design and management. Agrofor. Syst. 2019;93:317–331. doi: 10.1007/s10457-016-0065-2. [DOI] [Google Scholar]
- 46.Oldfield EE, et al. Crediting agricultural soil carbon sequestration. Science. 2022;375:1222–1225. doi: 10.1126/science.abl7991. [DOI] [PubMed] [Google Scholar]
- 47.Udawatta RP, Walter D, Jose S. Carbon sequestration by forests and agroforests: A reality check for the United States. Carbon Footprints. 2022;1:8. doi: 10.20517/cf.2022.06. [DOI] [Google Scholar]
- 48.Adame-Castro DE, et al. Diurnal and seasonal variations on soil CO2 fluxes in tropical silvopastoral systems. Soil Use Manag. 2020;36:671–681. doi: 10.1111/sum.12644. [DOI] [Google Scholar]
- 49.Contosta AR, Asbjornsen H, Orefice J, Perry A, Smith RG. Climate consequences of temperate forest conversion to open pasture or silvopasture. Agric. Ecosyst. Environ. 2022;333:107972. doi: 10.1016/j.agee.2022.107972. [DOI] [Google Scholar]
- 50.Vargas-Zeppetello LR, et al. Consistent cooling benefits of silvopasture in the tropics. Nat. Commun. 2022;13:1–9. doi: 10.1038/s41467-022-28388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casanova-Lugo F, et al. Effect of tree shade on the yield of Brachiaria brizantha grass in tropical livestock production systems in Mexico. Rangel. Ecol. Manage. 2022;80:31–38. doi: 10.1016/j.rama.2021.09.006. [DOI] [Google Scholar]
- 52.Valenzuela Que FG, et al. Silvopastoral systems improve carbon stocks at livestock ranches in Tabasco, Mexico. Soil Use Manag. 2022;38:1237–1249. doi: 10.1111/sum.12799. [DOI] [Google Scholar]
- 53.Nair PR. Classification of agroforestry systems. Agrofor. Syst. 1985;3:97–128. doi: 10.1007/BF00122638. [DOI] [Google Scholar]
- 54.Somarriba, E., Kass, D. & Ibrahim, M. Definition and classification of agroforestry systems. Agroforestry Prototypes for Belize. Agroforestry Project. CATIE (Tropical Agricultural Research and Higher Education Center), Costa rica 3 (1998).
- 55.Schroth G, et al. Agroforestry and Biodiversity Conservation in Tropical Landscapes. Island Press; 2004. [Google Scholar]
- 56.Harvey CA, et al. Patterns of animal diversity in different forms of tree cover in agricultural landscapes. Ecol. Appl. 2006;16:1986–1999. doi: 10.1890/1051-0761(2006)016[1986:POADID]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 57.Cardinael, R., Mao, Z., Chenu, C. & Hinsinger, P. Belowground functioning of agroforestry systems: Recent advances and perspectives. Plant Soil. 1–13 (2020).
- 58.Ibrahim M, Beer J. Agroforestry Prototypes for Belize. CATIE; 1998. [Google Scholar]
- 59.Ibrahim M, Villanueva C, Casasola F, Rojas J. Sistemas silvopastoriles como una herramienta para el mejoramiento de la productividad y restauración de la integridad ecológica de paisajes ganaderos. Pastos y Forrajes. 2006;29:383–419. [Google Scholar]
- 60.Phalan B, Onial M, Balmford A, Green RE. Reconciling food production and biodiversity conservation: Land sharing and land sparing compared. Science. 2011;333:1289–1291. doi: 10.1126/science.1208742. [DOI] [PubMed] [Google Scholar]
- 61.Van Zanten HH, et al. Defining a land boundary for sustainable livestock consumption. Glob. Change Biol. 2018;24:4185–4194. doi: 10.1111/gcb.14321. [DOI] [PubMed] [Google Scholar]
- 62.Torres CMME, et al. Greenhouse gas emissions and carbon sequestration by agroforestry systems in southeastern Brazil. Sci. Rep. 2017;7:1–7. doi: 10.1038/s41598-017-16821-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haile SG, Nair VD, Nair PR. Contribution of trees to carbon storage in soils of silvopastoral systems in Florida, USA. Glob. Change Biol. 2010;16:427–438. doi: 10.1111/j.1365-2486.2009.01981.x. [DOI] [Google Scholar]
- 64.Chatterjee N, Nair PR, Chakraborty S, Nair VD. Changes in soil carbon stocks across the Forest-Agroforest-Agriculture/Pasture continuum in various agroecological regions: A meta-analysis. Agric. Ecosyst. Environ. 2018;266:55–67. doi: 10.1016/j.agee.2018.07.014. [DOI] [Google Scholar]
- 65.Aynekulu E, et al. Carbon storage potential of silvopastoral systems of Colombia. Land. 2020;9:309. doi: 10.3390/land9090309. [DOI] [Google Scholar]
- 66.Birkhofer K, et al. Land-use type and intensity differentially filter traits in above-and below-ground arthropod communities. J. Anim. Ecol. 2017;86:511–520. doi: 10.1111/1365-2656.12641. [DOI] [PubMed] [Google Scholar]
- 67.Dahlsjö CA, et al. The local impact of macrofauna and land-use intensity on soil nutrient concentration and exchangeability in lowland tropical Peru. Biotropica. 2020;52:242–251. doi: 10.1111/btp.12676. [DOI] [Google Scholar]
- 68.Vizcaíno-Bravo Q, Williams-Linera G, Asbjornsen H. Biodiversity and carbon storage are correlated along a land use intensity gradient in a tropical montane forest watershed, Mexico. Basic Appl. Ecol. 2020;44:24–34. doi: 10.1016/j.baae.2019.12.004. [DOI] [Google Scholar]
- 69.Villanueva-López G, Martínez-Zurimendi P, Ramírez-Avilés L, Aryal DR, Casanova-Lugo F. Live fences reduce the diurnal and seasonal fluctuations of soil CO 2 emissions in livestock systems. Agron. Sustain. Dev. 2016;36:23. doi: 10.1007/s13593-016-0358-x. [DOI] [Google Scholar]
- 70.López-Santiago JG, et al. Carbon storage in a silvopastoral system compared to that in a deciduous dry forest in Michoacán, Mexico. Agroforestry Syst. 2019;93:199–211. doi: 10.1007/s10457-018-0259-x. [DOI] [Google Scholar]
- 71.Aryal DR, Gómez-González RR, Hernández-Nuriasmú R, Morales-Ruiz DE. Carbon stocks and tree diversity in scattered tree silvopastoral systems in Chiapas, Mexico. Agroforestry Syst. 2019;93:213–227. doi: 10.1007/s10457-018-0310-y. [DOI] [Google Scholar]
- 72.Beckert MR, Smith P, Lilly A, Chapman SJ. Soil and tree biomass carbon sequestration potential of silvopastoral and woodland-pasture systems in North East Scotland. Agrofor. Syst. 2016;90:371–383. doi: 10.1007/s10457-015-9860-4. [DOI] [Google Scholar]
- 73.Cárdenas A, Moliner A, Hontoria C, Ibrahim M. Ecological structure and carbon storage in traditional silvopastoral systems in Nicaragua. Agrofor. Syst. 2019;93:229–239. doi: 10.1007/s10457-018-0234-6. [DOI] [Google Scholar]
- 74.Lehmann J, et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020;13:529–534. doi: 10.1038/s41561-020-0612-3. [DOI] [Google Scholar]
- 75.Amézquita MC, Ibrahim M, Llanderal T, Buurman P, Amézquita E. Carbon sequestration in pastures, silvo-pastoral systems and forests in four regions of the Latin American tropics. J. Sustain. For. 2004;21:31–49. doi: 10.1300/J091v21n01_02. [DOI] [Google Scholar]
- 76.Rosenstock TS, et al. Making trees count: Measurement and reporting of agroforestry in UNFCCC national communications of non-Annex I countries. Agric. Ecosyst. Environ. 2019;284:106569. doi: 10.1016/j.agee.2019.106569. [DOI] [Google Scholar]
- 77.Junior MAL, Fracetto FJC, da Silva Ferreira J, Silva MB, Fracetto GGM. Legume-based silvopastoral systems drive C and N soil stocks in a subhumid tropical environment. CATENA. 2020;189:104508. doi: 10.1016/j.catena.2020.104508. [DOI] [Google Scholar]
- 78.Villanueva-Partida C, et al. Influence of the density of scattered trees in pastures on the structure and species composition of tree and grass cover in southern Tabasco, Mexico. Agric. Ecosyst. Environ. 2016;232:1–8. doi: 10.1016/j.agee.2016.07.020. [DOI] [Google Scholar]
- 79.Morantes-Toloza JL, Renjifo LM. Live fences in tropical production systems: A global review of uses and perceptions. Rev. Biol. Trop. 2018;66:739–753. doi: 10.15517/rbt.v66i2.33405. [DOI] [Google Scholar]
- 80.MoralesRuiz DE, et al. Carbon contents and fine root production in tropical silvopastoral systems. Land Degrad. Develop. 2021;32:738–756. doi: 10.1002/ldr.3761. [DOI] [Google Scholar]
- 81.Hoosbeek MR, Remme RP, Rusch GM. Trees enhance soil carbon sequestration and nutrient cycling in a silvopastoral system in south-western Nicaragua. Agrofor. Syst. 2018;92:263–273. [Google Scholar]
- 82.Aryal DR, et al. Fine wood decomposition rates decline with the sge of tropical successional forests in Southern Mexico: Implications to ecosystem carbon storage. Ecosystems. 2022;25:661–677. doi: 10.1007/s10021-021-00678-w. [DOI] [Google Scholar]
- 83.Dignac M-F, et al. Increasing soil carbon storage: Mechanisms, effects of agricultural practices and proxies. A review. Agron. Sustain. Develop. 2017;37:1–27. doi: 10.1007/s13593-017-0421-2. [DOI] [Google Scholar]
- 84.Sánchez-Silva S, et al. Fine root biomass stocks but not the production and turnover rates vary with the age of tropical successional forests in Southern Mexico. Rhizosphere. 2022;21:100474. doi: 10.1016/j.rhisph.2022.100474. [DOI] [Google Scholar]
- 85.Montejo-Martínez D, et al. Fine root density and vertical distribution of Leucaena leucocephala and grasses in silvopastoral systems under two harvest intervals. Agrofor. Syst. 2020;94:843–855. doi: 10.1007/s10457-019-00457-6. [DOI] [Google Scholar]
- 86.Sánchez-Silva S, De Jong BH, Aryal DR, Huerta-Lwanga E, Mendoza-Vega J. Trends in leaf traits, litter dynamics and associated nutrient cycling along a secondary successional chronosequence of semi-evergreen tropical forest in South-Eastern Mexico. J. Trop. Ecol. 2018;34:364–377. doi: 10.1017/S0266467418000366. [DOI] [Google Scholar]
- 87.Waters CM, Orgill SE, Melville GJ, Toole ID, Smith WJ. Management of grazing intensity in the semi-arid rangelands of Southern Australia: Effects on soil and biodiversity. Land Degrad. Dev. 2017;28:1363–1375. doi: 10.1002/ldr.2602. [DOI] [Google Scholar]
- 88.Baldassini P, Paruelo JM. Deforestation and current management practices reduce soil organic carbon in the semi-arid Chaco, Argentina. Agric. Syst. 2020;178:102749. doi: 10.1016/j.agsy.2019.102749. [DOI] [Google Scholar]
- 89.Abdalla M, et al. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018;253:62–81. doi: 10.1016/j.agee.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lange M, et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015;6:1–8. doi: 10.1038/ncomms7707. [DOI] [PubMed] [Google Scholar]
- 91.Wiesmeier M, et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma. 2019;333:149–162. doi: 10.1016/j.geoderma.2018.07.026. [DOI] [Google Scholar]
- 92.Lim S-S, et al. Soil organic carbon stocks in three Canadian agroforestry systems: From surface organic to deeper mineral soils. For. Ecol. Manage. 2018;417:103–109. doi: 10.1016/j.foreco.2018.02.050. [DOI] [Google Scholar]
- 93.Nair P. Carbon sequestration studies in agroforestry systems: A reality-check. Agrofor. Syst. 2012;86:243–253. doi: 10.1007/s10457-011-9434-z. [DOI] [Google Scholar]
- 94.Montagnini F, Ibrahim M, Murgueitio E. Silvopastoral systems and climate change mitigation in Latin America. Bois et forêts des tropiques. 2013;316:3–16. doi: 10.19182/bft2013.316.a20528. [DOI] [Google Scholar]
- 95.Allison SD, Wallenstein MD, Bradford MA. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010;3:336–340. doi: 10.1038/ngeo846. [DOI] [Google Scholar]
- 96.Sarto MV, et al. Soil microbial community and activity in a tropical integrated crop-livestock system. Appl. Soil. Ecol. 2020;145:103350. doi: 10.1016/j.apsoil.2019.08.012. [DOI] [Google Scholar]
- 97.Malik AA, et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-05980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bautista F, Palacio-Aponte G, Quintana P, Zinck JA. Spatial distribution and development of soils in tropical karst areas from the Peninsula of Yucatan, Mexico. Geomorphology. 2011;135:308–321. doi: 10.1016/j.geomorph.2011.02.014. [DOI] [Google Scholar]
- 99.Kaiser, M. et al. The influence of mineral characteristics on organic matter content, composition, and stability of topsoils under long‐term arable and forest land use. J. Geophys. Res. Biogeosci. 117, (2012).
- 100.Castillo MS, Tiezzi F, Franzluebbers AJ. Tree species effects on understory forage productivity and microclimate in a silvopasture of the Southeastern USA. Agric. Ecosyst. Environ. 2020;295:106917. doi: 10.1016/j.agee.2020.106917. [DOI] [Google Scholar]
- 101.Yang Y, Tilman D, Furey G, Lehman C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019;10:1–7. doi: 10.1038/s41467-019-08636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grass I, et al. Land-sharing/-sparing connectivity landscapes for ecosystem services and biodiversity conservation. People Nat. 2019;1:262–272. [Google Scholar]
- 103.Orefice J, Smith RG, Carroll J, Asbjornsen H, Howard T. Forage productivity and profitability in newly-established open pasture, silvopasture, and thinned forest production systems. Agrofor. Syst. 2019;93:51–65. doi: 10.1007/s10457-016-0052-7. [DOI] [Google Scholar]
- 104.Aryal DR, et al. Potencial de almacenamiento de carbono en áreas forestales en un sistema ganadero. Revista mexicana de ciencias forestales. 2018;9:150–180. doi: 10.29298/rmcf.v8i48.184. [DOI] [Google Scholar]
- 105.Gobierno de la Republica. Intended Nationally Determined Contribution, Mexico. (Instituto Nacional de Ecología y Cambio Climático, Mexico City, 2015).
- 106.Bonilla-Moheno M, Aide TM. Beyond deforestation: Land cover transitions in Mexico. Agric. Syst. 2020;178:102734. doi: 10.1016/j.agsy.2019.102734. [DOI] [Google Scholar]
- 107.INEGI. Mapa de uso de suelo y vegetación de México: Series I–VII. Instituto Nacional de Estadística y Geografía (INEGI), Aguascalientes, Mexico.https://www.inegi.org.mx/temas/usosuelo/#Map (2018). Accessed 17 Aug 2022.
- 108.Gosling E, Reith E, Knoke T, Paul C. A goal programming approach to evaluate agroforestry systems in Eastern Panama. J. Environ. Manage. 2020;261:110248. doi: 10.1016/j.jenvman.2020.110248. [DOI] [PubMed] [Google Scholar]
- 109.Bergier I, et al. Could bovine livestock intensification in Pantanal be neutral regarding enteric methane emissions? Sci. Total Environ. 2019;655:463–472. doi: 10.1016/j.scitotenv.2018.11.178. [DOI] [PubMed] [Google Scholar]
- 110.Barkin DE. uso de la tierra agrícola en Mexico. Problemas del Desarrollo. 1981;12:59–85. [Google Scholar]
- 111.Valdivieso-Pérez IA, García-Barrios LE, Álvarez-Solís D, Nahed-Toral J. From cornfields to grasslands: Change in the quality of soil. Terra Latinoamericana. 2012;30:363–374. [Google Scholar]
- 112.Goldstein A, et al. Protecting irrecoverable carbon in Earth’s ecosystems. Nat. Clim. Chang. 2020;10:287–295. doi: 10.1038/s41558-020-0738-8. [DOI] [Google Scholar]
- 113.CONAFOR. Acciones Tempranas REDD+ Mexico. https://www.gob.mx/conafor/documentos/acciones-tempranas-redd (2017). Accessed 04 Oct 2020.
- 114.CATIE. Bidiversidad y paisajes ganaderos agrosilvopastoriles sostenibles. https://www.biopasos.com (2020). Accessed 04 Oct 2020.
- 115.Freire-Santos PZF, Crouzeilles R, Sansevero JBB. Can agroforestry systems enhance biodiversity and ecosystem service provision in agricultural landscapes? A meta-analysis for the Brazilian Atlantic Forest. For. Ecol. Manage. 2019;433:140–145. doi: 10.1016/j.foreco.2018.10.064. [DOI] [Google Scholar]
- 116.Zanne, A. et al. Data from: Towards a worldwide wood economics spectrum. (2009). 10.5061/dryad.234. [DOI] [PubMed]
- 117.Chave J, et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 2014;20:3177–3190. doi: 10.1111/gcb.12629. [DOI] [PubMed] [Google Scholar]
- 118.Bojórquez A, et al. Improving the accuracy of aboveground biomass estimations in secondary tropical dry forests. For. Ecol. Manage. 2020;474:118384. doi: 10.1016/j.foreco.2020.118384. [DOI] [Google Scholar]
- 119.Cairns MA, Brown S, Helmer EH, Baumgardner GA. Root biomass allocation in the world’s upland forests. Oecologia. 1997;111:1–11. doi: 10.1007/s004420050201. [DOI] [PubMed] [Google Scholar]
- 120.Shannon, C.E., Weaver. A Mathematical Theory of Communication Vol. 27 (University of Illinois Press, 1964).
- 121.Sorensen TA. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. Biol. Skar. 1948;5:1–34. [Google Scholar]
- 122.Pielou EC. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966;13:131–144. doi: 10.1016/0022-5193(66)90013-0. [DOI] [Google Scholar]
- 123.Van Wagner C. Practical Aspects of the Line Intersect Method. Canadian Forestry Service; 1982. [Google Scholar]
- 124.Heanes D. Determination of total organic-C in soils by an improved chromic acid digestion and spectrophotometric procedure. Commun. Soil Sci. Plant Anal. 1984;15:1191–1213. doi: 10.1080/00103628409367551. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be obtained upon request from the corresponding author. Collection of plant biomass and soil samples were carried out with permissions from local authorities. We declare that this project was carried out in collaboration with the Mexican National Commission for Natural Protected Areas (CONANP, Spanish acronym), which is the local entity that regulate such permissions. We did not use any vertebrate animal for experimental purposes in this study. The animals in the images were to represent that the lands we sampled are used for animal grazing.