Abstract
Thyroid storm occurs when thyroid hormones are released causing a hyperadrenergic state resulting in potentially life-threatening cardio-respiratory effects. The most common cause of thyroid storm is thyrotoxicosis secondary to Graves disease. Alternatively, subacute thyroiditis (SAT) is usually a self-limited condition consisting of painful inflammation of thyroid gland, often associated with viral illness. Transient hyperthyroidism is a common finding in SAT; however, thyroid storm is an extremely rare complication of SAT. We report the sixth recorded case of SAT associated with thyroid storm.
Keywords: subacute, thyroiditis, de quervain, thyroid storm, thyrotoxicosis
Case Presentation
A 70-year-old man presented to the emergency room with chronic diarrhea and confusion worsening over the previous 10 days. The patient had been admitted 2 months prior with gout and discharged on colchicine, allopurinol, and a prednisone taper. Diarrhea began shortly after his discharge. Family denied recent surgeries. The patient denied abdominal pain, hematochezia, melena, nausea, vomiting, fevers, chills, or night sweats. However, he was tremulous, weak, and experiencing worsening fatigue with 2 falls in the week prior to this admission. In addition, he required 2 L of oxygen on arrival. His past medical history was significant for sleep apnea on continuous positive airway pressure, heart failure on furosemide, non-alcoholic steatohepatitis status post liver transplant in 2013, hypertension, coronary artery disease status post drug eluting stent placement, and gout. He had a 60-pack-year smoking history, having quit 29 years prior. He denied the use of alcohol or drugs. His family history was positive for thyroid disease in his sister, although details were not known.
On admission, his pulse ranged between 117 and 135, temperature of 36.6°C (97.8°F), respirations 24, oxygen saturation 96% on 2 L NC, and blood pressure 96/72 mmHg. Physical examination demonstrated a morbidly obese man with tachycardia. He was lethargic, but arousable, and oriented only to person. Neurologic examination showed intact cranial nerves with normal motor and sensory examination (limited by patient’s cooperation). Physical examination was otherwise unremarkable, particularly demonstrating no enlargement or nodule of the thyroid, nor ophthalmopathy. Tenderness of the thyroid was unable to be determined due to the patient’s altered mental status.
Complete blood count showed white blood cells of 4.76 K/cumm, hemoglobin of 10.0 g/dL, and platelets of 106 K/cumm. The basic metabolic panel exhibited a sodium of 134.0 mmol/L, potassium of 5.20 mmol/L, BUN of 74.0 mg/dL, creatinine of 3.3 mg/dL, magnesium of 1.7 mg/dL, and phosphorus of 7.2 mg/dL. The INR was 1.54. Blood cultures, respiratory bacterial culture, gastrointestinal PCR, herpes simplex virus PCR, cytomegalovirus PCR, chest x-ray, and urinalysis did not demonstrate explanatory findings. VRE and MRSA cultures were negative. The patient’s computed tomography (CT) head was negative for an acute intracranial process. Urine drug screen was negative, and the patient’s tacrolimus levels returned within his goal range between 2 and 10 ng/mL. His hemoglobin A1c was 4.7. Glucose level was 286 mg/dL. Lithium level was less than 0.1 mmol/L, and his procalcitonin level was 0.09 ng/mL. The patient’s admission thyroid levels were consistent with significant thyrotoxicosis (TSH level of 0.01 mIU/L, Free T4 of 5.91 ng/dL, and Free T3 of 14.5 pg/mL). An ultrasound of the head and neck showed no masses or nodules with a diffusely mildly hypervascular thyroid. Given the unrevealing workup for other causes, his presenting symptoms of tachycardia, diarrhea, and delirium were attributed to the hyperadrenergic effects of thyroid storm. He and his family denied ingestion of any exogenous thyroid hormone. Other common precipitants of thyroid storm such as infection, myocardial infarction, and diabetic ketoacidosis had been excluded during his admission workup. A head and neck ultrasound was obtained and demonstrated a right thyroid lobe measuring 5.2 × 2.8 × 2.2 cm3 with no masses and a left thyroid lobe measuring 5.0 × 2.4 × 1.9 cm3 with no masses. He had an anti-thyroglobulin antibody of 2555.0 IU/mL. His Burch-Wartofsky score was 50 on admission (20 for AMS, 20 for HR >130, 10 for diarrhea), which was also highly suggestive of thyroid storm. 1
Endocrine was consulted, confirmed thyroid storm, and recommended he be transferred to the intensive care unit (ICU). He was started on propanol 40 mg every six hours, methimazole 20 mg three times a day (rather than propylthiouracil given concern for hepatoxicity), and prednisone 60 mg daily. His ICU course was complicated by a non-ST-elevation myocardial infarction, which was felt to be secondary to demand ischemia associated with his thyrotoxicosis. In addition, he suffered a right lower lobe pneumonia, which responded well to 7 days of piperacillin-tazobactam, and thrombocytopenia due to liver disease but exacerbated by sepsis.
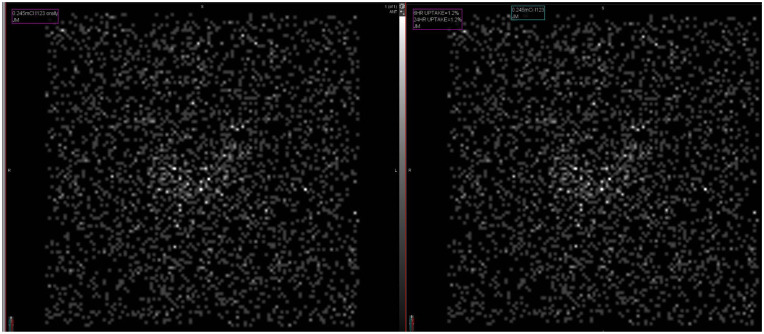
After aggressive treatment of his thyroid storm, he returned to his behavioral and cognitive baseline. The patient’s free T4 levels dropped into normal range after 9 days of treatment; however, on hospital day 21, his free T4 levels began to rise again hitting 1.64, 2.01, and 2.23 ng/dL. After a 6-day washout period of his methimazole, he had a thyroid uptake scan which was consistent with thyroiditis demonstrating a globally decreased uptake (1.2% at 6 hours and 1.2% at 24 hours) without active nodules (Figure 1). He restarted prednisone 40 mg daily at discharge. Three months later, his labs showed a Free T3 level of 2.1 pg/mL, T4 level of 0.82 ng/dL, and a TSH of 16.23 mIU/L. The patient denied any symptoms of hypothyroidism or hyperthyroidism. He began levothyroxine 50 mcg daily to treat hypothyroidism. At his next appointment, approximately a year later, he was found to be euthyroid with Free T3 level of 2.5 pg/mL, T4 level of 1.17 ng/dL, and a TSH of 1.38 mIU/L.
Figure 1.
Thyroid uptake scan. Radioactive iodine uptake scan of thyroid demonstrating globally decreased uptake (1.2%) at both 6 and 24 hours.
Literature Review
Thyroid storm is a rare but potentially life-threatening diagnosis, resulting from the exaggerated hypermetabolic effects of circulating thyroid hormones. Untreated, fatalities are common and frequently result from heart failure, arrhythmias, seizures, or coma. 1 Thyroid storm most frequently occurs in patients with thyrotoxicosis secondary to Graves disease, but may also be associated with thyroid adenoma, toxic multinodular goiter, and in rare cases, subacute thyroiditis (SAT).2,3 Thyroid storm more commonly develops in patients with underlying thyroid dysfunction and a triggering event such as infection, myocardial infarction, diabetic ketoacidosis, or pregnancy. 4 In the past, thyroid storm was sometimes precipitated by iatrogenic release of thyroid hormone during surgery on a hyperactive gland, but recent advances in surgical and pre-operative techniques have minimized this risk.4,5
Thyroiditis signifies inflammation of the thyroid gland, which can be related to infection, autoimmunity, trauma, radiation, or medications. Subacute thyroiditis, also known as de Quervain or granulomatous thyroiditis, is characterized by a painful, edematous thyroid gland with a predictable pattern of transient thyrotoxic state, followed by hypothyroid state, with resolution to euthyroidism. Subacute thyroiditis is more common in younger women, and often presents with neck pain, malaise, fatigue, and fever during the thyrotoxic phase. However, some authors report cases of nearly painless presentation.6,7 Anti-thyroglobulin antibodies are typically negative, although, there are reports of cases of SAT patients who have anti-thyroglobulin antibodies initially during the destructive phase, eventually decreasing during recovery. 8
Treatment of SAT is supportive, focusing on pain relief and treatment of thyrotoxic symptoms. Occasionally, treatment of thyroid inflammation with prednisone is necessary. Also, treatment of adrenergic symptoms with beta blockers is sometimes required. Patients typically exhibit a predictable self-limited clinical course of elevated thyroid hormone levels, which then dip to hypothyroid levels and ultimately return to euthyroid levels in most patients.9-11 While the underlying cause of SAT is not completely understood, viral infections are presumed to play a role in many cases. The incidence of SAT has been shown to have a seasonal pattern, and numerous viruses have been associated with SAT without a strict cause-effect being proven. 12 Furthermore, an immunological and genetic association has been established between SAT and the presence of HLA-B35 serotype, 13 and thus, when these individuals are exposed to a trigger, such as a viral illness, SAT can be induced. 14 The end result is destruction of the thyroid follicles and subsequent release of pre-stored thyroid hormones into the circulation. While thyrotoxic symptoms are common in SAT, thyroid storm has only rarely been associated with cases of SAT.2,3,15-17
Discussion
We present, to our knowledge, the sixth case in the literature of SAT leading to thyroid storm.2,3,15-17 In addition, our patient illustrated several atypical features for SAT compared with the previously reported cases. For example, our Doppler ultrasound with diffuse mild hypervascularity without goiter was not typical for SAT, showing no hypoechogenic areas, although it was also inconsistent with Graves disease. It has been suggested the ultrasound features of SAT may evolve over the course of the illness with the hypoechogenicity resolving and transitioning to transient mild increased vascularity, eventually returning to normal. 18 Although our patient did not complain of neck pain, he was encephalopathic on admission. In addition, a variant of SAT has been reported without neck pain.7,19,20 Our patient had globally low uptake of radioactive iodine, consistent with the expected findings for SAT and other case reports of SAT leading to thyroid storm.2,16 In addition, our patient showed the classic pattern of thyrotoxicosis followed by hypothyroidism which eventually resolved to euthyroidism on only 50 mcg daily (his weight-based replacement dose would be 200 mcg/day). He also had an elevated neutrophil to lymphocyte ratio of 5.27, which has been reported to be linked to SAT as opposed to Graves or toxic adenoma. 21 Workup did not demonstrate any findings particular to Graves disease (pretibial myxedema, thyroid bruit, ophthalmopathy, or palpable goiter), nor any thyroid nodules on palpation or imaging that could explain his storm. He had no history of amiodarone use and had received no iodinated contrast within the previous 6 months. As his condition improved and he was readied for discharge, a second rise in his thyroid hormones were noted, suggesting a relapse which is known to occur in a small number of cases of SAT, although some authors place the number as high as 20%.22,23
In a rare association, this patient’s SAT precipitated a thyroid storm, which was successfully treated with methimazole, steroids, and beta-blockade. When treating a patient suffering from SAT with prednisone, relapse may occur during the steroid taper necessitating a return to higher doses. In addition, patients who appear seriously ill with some combination of mental status changes, fever, cardiovascular excitation, and GI symptoms may be suffering from thyroid storm, even without a prior diagnosis of thyroid disease. This case illuminates the importance of including SAT in the differential diagnosis of causes of thyroid storm.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Katherine Newman  https://orcid.org/0000-0002-6004-9055
https://orcid.org/0000-0002-6004-9055
Leonidas Walthall  https://orcid.org/0000-0003-4782-0156
https://orcid.org/0000-0003-4782-0156
References
- 1. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am. 1993;22(2):263-277. [PubMed] [Google Scholar]
- 2. Swinburne JL, Kreisman SH. A rare case of subacute thyroiditis causing thyroid storm. Thyroid. 2007;17(1):73-76. doi: 10.1089/thy.2006.0140. [DOI] [PubMed] [Google Scholar]
- 3. Gaballa S, Hlaing KM, Bos N, et al. A rare case of subacute painful thyroiditis causing thyroid storm and a successful trial of propylthiouracil. Cureus. 2020;12(7):e9461. doi: 10.7759/cureus.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll R, Matfin G. Endocrine and metabolic emergencies: thyroid storm. Ther Adv Endocrinol Metab. 2010;1(3):139-145. doi: 10.1177/2042018810382481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pokhrel B, Aiman W, Bhusal K. Statpearls. Treasure Island, FL: StatPearls Publishing; 2022. [Google Scholar]
- 6. Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute thyroiditis: clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J Endocrinol Invest. 2007;30(8):631-635. doi: 10.1007/BF03347442. [DOI] [PubMed] [Google Scholar]
- 7. Daniels GH. Atypical subacute thyroiditis: preliminary observations. Thyroid. 2001;11(7):691-695. doi: 10.1089/105072501750362772. [DOI] [PubMed] [Google Scholar]
- 8. Nishihara E, Amino N, Kudo T, et al. Moderate frequency of anti-thyroglobulin antibodies in the early phase of subacute thyroiditis. Eur Thyroid J. 2019;8(5):268-272. doi: 10.1159/000501033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabassom A, Chippa V, Edens MA. Statpearls. Treasure Island, FL: StatPearls Publishing; 2022. [Google Scholar]
- 10. Hennessey JV. Subacute thyroiditis. In: Feingold KR, Anawalt B, Boyce A. eds. Endotext. Bethesda, MD: National Library of Medicine; 2018. https://www.ncbi.nlm.nih.gov/books/NBK279084/. Accessed February 22, 2022. [Google Scholar]
- 11. Nishihara E, Ohye H, Amino N, et al. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47(8):725-729. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]
- 12. Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohsako N, Tamai H, Sudo T, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80(12):3653-3656. doi: 10.1210/jcem.80.12.8530615. [DOI] [PubMed] [Google Scholar]
- 14. Volpé R, Row VV, Ezrin C. Circulating viral and thyroid antibodies in subacute thyroiditis. J Clin Endocrinol Metab. 1967;27(9):1275-1284. doi: 10.1210/jcem-27-9-1275. [DOI] [PubMed] [Google Scholar]
- 15. Salih AM, Kakamad FH, Rawezh QS, et al. Subacute thyroiditis causing thyrotoxic crisis; a case report with literature review. Int J Surg Case Rep. 2017;33:112-114. doi: 10.1016/j.ijscr.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sherman SI, Ladenson PW. Subacute thyroiditis causing thyroid storm. Thyroid. 2007;17(3):283. doi: 10.1089/thy.2007.0070. [DOI] [PubMed] [Google Scholar]
- 17. Assaad M, Zuhdi B, Gourbert M, et al. Thyroid storm caused by subacute thyroiditis in a patient with methicillin-resistant Staphylococcus aureus septicemia. J Endocr Soc. 2020;4(1):SUN-511. doi: 10.1210/jendso/bvaa046.709. [DOI] [Google Scholar]
- 18. Hiromatsu Y, Ishibashi M, Miyake I, et al. Color Doppler ultrasonography in patients with subacute thyroiditis. Thyroid. 1999;9(12):1189-1193. doi: 10.1089/thy.1999.9.1189. [DOI] [PubMed] [Google Scholar]
- 19. Carluccio AL, Sundaram NK, Yanagisawa RT, Tomer Y. Apathetic thyrotoxicosis secondary to atypical subacute thyroiditis. Endocr Pract. 2012;18(5):e127-e129. doi: 10.4158/EP11373.CR. [DOI] [PubMed] [Google Scholar]
- 20. de Bruin TW, Riekhoff FP, de Boer JJ. An outbreak of thyrotoxicosis due to atypical subacute thyroiditis. J Clin Endocrinol Metab. 1990;70(2):396-402. [DOI] [PubMed] [Google Scholar]
- 21. Taşkaldıran I, Omma T, Önder CE, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and platelet-to-lymphocyte ratio in different etiological causes of thyrotoxicosis. Turk J Med Sci. 2019;49(6):1687-1692. doi: 10.3906/sag-1901-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mizukoshi T, Noguchi S, Murakami T, Futata T, Yamashita H. Evaluation of recurrence in 36 subacute thyroiditis patients managed with prednisolone. Intern Med. 2001;40(4):292-295. doi: 10.2169/internalmedicine.40.292. [DOI] [PubMed] [Google Scholar]
- 23. Volpé R. The management of subacute (DeQuervain’s) thyroiditis. Thyroid. 1993;3(3):253-255. doi: 10.2169/internalmedicine.40.292. [DOI] [PubMed] [Google Scholar]