Abstract
Limited presence of hCA IX in normal physiological tissues and their overexpression only in solid hypoxic tumors made this isoform excellent possible target for developing new anticancer agents. We reported designing and synthesis of two novel series of benzenesulfonamides derivatives as hCA IX inhibitors bearing rigid cyclic linkers (1,3,5-dihydrotriazine in series A and 1,3,5-triazine in series B) in replace of traditional linear linkers. Also, novel cyanoethenyl spacer was assembled next to the 1,3,5-triazine linker in series B. Target compounds of series (A) and (B) were screened against four hCA isoforms. Human CA IX efficiently inhibited in series (A) by compound 5a (KI = 134.8 nM). Meanwhile, in series (B) the most active inhibitor was 12i (KI = 38.8 nM). US-NCI protocol was followed to evaluate the anticancer activity of target compounds against panel of sixty cancer cell lines. Compound 12d, exposed the best activity towards breast cancer (MDA-MB-468) with GI% = 62%. The most active analogues, 12d and 12i were further screened for in vitro cytotoxic activity under hypoxic condition against breast cancer (MDA-MB-468) (IC50 = 3.99 ± 0.21 and 1.48 ± 0.08 µM, respectively) and leukemia (CCRF-CM) cell line (IC50 = 4.51 ± 0.24 and 9.83 ± 0.52 µM, respectively). In addition, 12d arrested breast cancer MDA-MB-468 cell cycle in G0-G1 and S phases and induced its apoptosis which indicated by increasing the level of cleaved caspases 3 and 9. Molecular docking was performed for selected analogues to understand their biological alterations. This study revealed that insertion of 1,3,5-triazines as cyclic linkers enhanced the significant anticancer and hCA IX inhibition activity of benzenesulfonamides.
Subject terms: Medicinal chemistry, Chemical synthesis, Cancer
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are well known family of zinc metalloenzymes recognized in all living organisms1. In humans, there are 15 isoforms of human CAs (hCAs) have been discovered until now. These isoforms can be classified relying on their localization into: cytosolic isoforms, (CA I, CA II, CA III, CAVII, CA XIII), transmembrane bound isoforms, (CA IV, CA IX, CA XII, CA XIV), mitochondrial isoforms (CA VA, CA VB), and CA VI, which found in body fluids like saliva2–4. CAs catalyzes the reversible hydration of CO2 and H2O to HCO3− and H+ ions and via this important catalytic activity, cells can regulate extracellular and intracellular pH. Accordingly, CAs role is critical in many physiological processes associated with CO2 hydration such as respiration, ureagenesis, pH regulation and bone resorption5. On the other hand, many pathological conditions, resembling cerebral edema, glaucoma, epilepsy and cancer, were reported owing to deregulated activity or overexpression of different carbonic anhydrase isoforms6–8. Transmembrane hCA IX and XII isoforms expression in normal physiological conditions is limited to few number of normal tissues such as the epithelium lining the gastrointestinal tract9. Even though, they are highly overexpressed in hypoxic tumors like colon, ovaries, breast, and lung cancer which sort them the tumor associated members of the carbonic anhydrase family10,11. Human CA IX plays significant role in survival and metastasis of tumor cells under hypoxic conditions and its protection from apoptosis due to its ability to stabilize the extracellular pH12. Because of its overexpression in many solid tumors, besides its restricted presence in normal tissue, inhibition of hCA IX has been attracted the attentions as excellent target to design new anti-proliferative agents, for various types of malignant solid tumors 13–15.
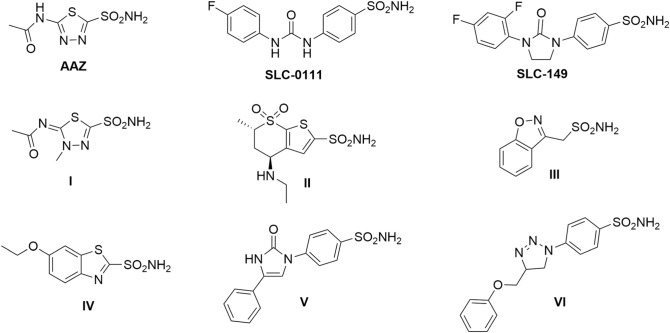
Sulfonamides are well known class of compounds having inhibitory effect on CAs. Acetazolamide (AAZ), methazolamide (I), dorzolamide (II), zonisamide (III) and ethoxzolamide (IV) are sulfonamide drugs used clinically in treatment of different pathological conditions related to CAs, for example ocular hypertension, glaucoma, edema and epilepsy, Fig. 116,17. Lacking of selectivity, is the main weakness of this classical class, particularly nonselective inhibition of hCA IX, hindered their applications as anticancer drugs 18. Thus, there was a need to design and pursue new sulfonamide derivatives selectively inhibiting hCA IX. Among many investigated scaffolds ureidobenzensulfonamide, SLC-0111, was the most successful selective hCA IX inhibitor. It reached phase I/II clinical trials for treatment of metastatic solid tumors (Fig. 1) 19,20. Chemical structure of SLC-0111, displayed the essential pharmacophores required to develop novel analogues selectively inhibit hCA IX which comprises; the benzenesulfonamide head as a zinc anchoring group, the hydrophobic tail, and urea as a linker 21. Oxoimidazolidine, imodazol-2-one and triazole rings, are reported as possible cyclic linkers in SLC-149 22, compounds V23 and VI24, respectively as a substitute of the urea linker in, SLC-0111, where, they disclosed strong hCA IX inhibiton in the nanomolar range, Fig. 120–22.
Figure 1.
Chemical structures of carbonic anhydrase inhibitors.
Recently, 1,3,5-triazine and its derivatives have raised a wide attention as potent anticancer scaffolds 25–27. Antineoplastic agents incorporating 1,3,5-triazine ring, such as altretamine28 and decitabine29, are used in treatment of recurrent ovarian cancer and chronic myelomonocytic leukemia respectively. Flexibility by which 1,3,5-triazine ring synthesized and subjected to substitution with different groups, made it proper nucleus to be incorporated as a rigid cyclic linker in our target compounds. Relying on various reported studies, we have designed, synthesized and biologically evaluate two novel series of 1,3,5-triazinyl benzenesulfonamides as anticancer agents aiming selective inhibition of hCA IX. The benzenesulfonamide moiety is retained in our target compounds as a zinc binding (ZBG) head while, dihydro-1,3,5-triazine and 1,3,5-triazine rings are constructed as bioisostaric replacement of previously reported cyclic linkers. Various lipophilic tails are assembled as well.
Rational design
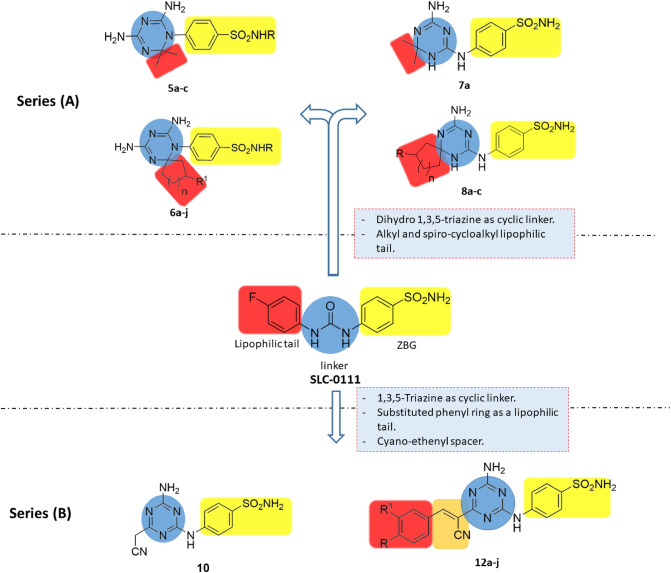
Two novel series of triazinyl benzenesulfonamides were designed depending on the structural features of SLC-0111 as selective inhibitors of tumor associated hCA IX. In series (A), seventeen compounds, 5a–c, 6a–j, 7a and 8a–c, were created enclosing the dihydro-1,3,5-triazine ring as a rigid cyclic linker between the ZBG head, benzenesulfonamide, and alkyl or spirocycloalkyl lipophilic tail. Regarding compounds, 5a–c, and 7a, the lipophilic tail were two methyl groups at 2 position of the dihydrotriazine ring. Target compounds 6a–j and 8a–c incorporated the dihydrotriazine spirocycloalkyl derivatives as lipophilic tails based on previous reported studies for spiro compounds exhibited antiproliferative activity30. Moreover, in analogues, 5b–c and 6d–j, secondary sulfonamides where N-substituted with pyridine and thiazole rings were explored. In series (B), eleven compounds, 10 and 12a–j, were designed comprising 1,3,5-triazine ring as a linker. Compound 10 had a cyanomethyl side chain. In analogues, 12a–j, two carbon spacer was constructed at 4 position of the triazine ring while, substituted phenyl rings were retained as the lipophilic tails as shown in Fig. 2.
Figure 2.
Rational design of target compounds in series (A) and (B) as novel hCA IX inhibitors.
Results and discussion
Chemistry
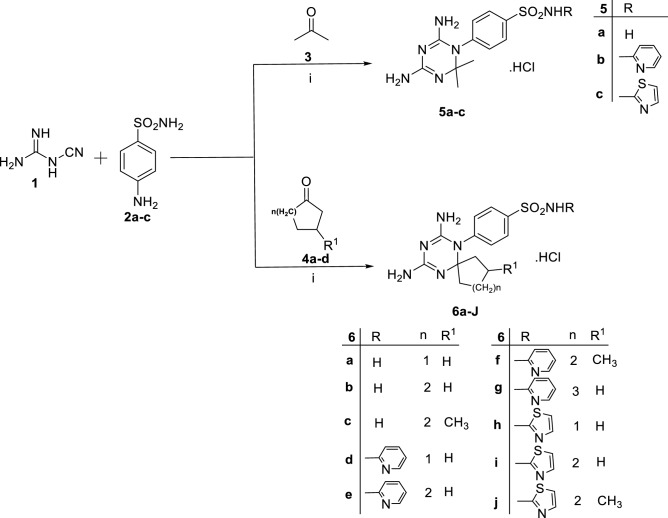
Synthesis of target compounds, 5a–c and 6a–j, in series (A) was accomplished by one pot condensation reaction of sulfanilamide, (or its derivatives, 2a–c), with dicynamide 1, and acetone 3, (or cyclic ketones, 4a–d), under acidic condition, conc. HCl, as outlined in Fig. 331,32. The chemical structures of achieved products were confirmed by spectral data and elemental analysis as reported in the experimental part (supplementary materials). The 1H NMR spectra of series (A) disclosed the characteristic aliphatic protons signals at δ = 0.84–1.90 ppm. The existence of two singlet signals at δ 7.57–7.60 and 7.67–7.72 ppm (D2O exchangeable) respectively, assigned to two (NH2) groups of 4,6-diamino-1,2-dihydrotriazine rings. Moreover, a downfield singlet signal at δ = 9.11–9.47 ppm was attributed to +NHCl− proton.
Figure 3.
Synthesis and chemical structures of compounds, 5a–c and 6a–j. Reagents and conditions: (i) absolute ethanol, conc. HCl, heat under reflux, 24 h.
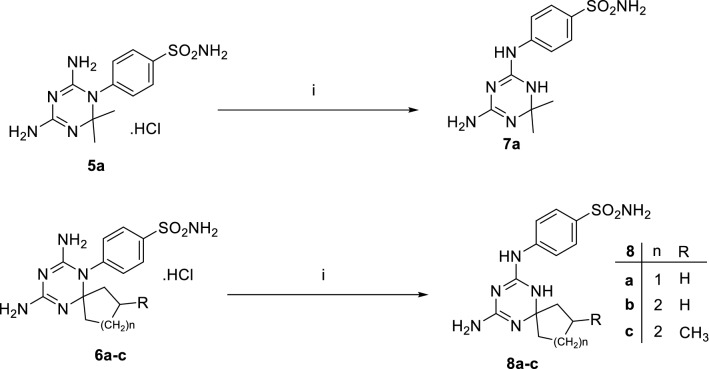
The 4,6-diamino-1,2-dihydrotriazinyl derivatives, 5a and 6a–c, were subjected to Dimroth rearrangement, where exocyclic and endocyclic nitrogen atoms switch places via heating under reflux in strong alkaline condition, 1N NaOH, to furnish the corresponding 4-amino-1,6-dihydrotriazine analogues, 7a and 8a–c, respectively as illustrated in Fig. 431. The 1H NMR spectra of analogues, 7a and 8a–c, were characterized by the disappearance of the downfield singlet signals of the, +NHCl−, protons where, Dimroth rearrangement reaction afforded the free amines, 7a and 8a–c. This rearrangement reaction was performed to transfer the ZBG, benzenesulfonamide, from 3 position of the endocyclic nitrogen of the 1,3,5-triazine linker to the exocyclic amino group at the 4 position of the linker. Accordingly, the distance between the ZBG and the lipophilic tail converted longer in analogues 7a & 8a–c than in compounds 5a–c & 6a–j, which may have an impact on hCA IX inhibition.
Figure 4.
Synthesis and chemical structures of compounds, 7a and 8a–c. Reagents and conditions: (i) 50% aqueous ethanol, 1N. NaOH, heat under reflux, 3 h.
Regarding synthesis of series (B), the starting benzenesulfonamide biguanide 9, was prepared via nucleophilic addition of sulfanilamide 2a, to the cyano group of dicynamide 1 under acidic condition, pH = 2. Afterward, cyclocondensation of the biguanide 9, with ethyl cyanoacetate yielded 4-amino-6-(cyanomethyl)-1,3,5-triazinylaminobenzenesulfonamide, 1033,34. Finally, Aldol condensation of the active methylene group in compound, 10 with different benzaldehydes, 11a–j, was accomplished via basic catalyst, triethylamine (TEA), to provide the ({4-amino-6-[1-cyano-2-phenylethenyl]-1,3,5-triazinyl}aminobenzenesulfonamide derivatives, 12a–j, as reported in Fig. 535,36.
Figure 5.
Synthesis and chemical structures of compounds 12a–j. Reagents and conditions: (i) absolute ethanol, conc. HCl, heating under reflux, 24 h. (ii) a: MeOH/MeONa, room temp., 3 h. b: ethyl cyanoacetate, DMF, heating under reflux, 6 h. (iii) absolute ethanol, DMF, TEA, heating under reflux, 2–5 h.
Structure elucidation of target compounds, 10 and 12a–j, was supported by spectroscopic data and elemental analysis (supplementary material). 1H NMR spectrum of compound 10 exposed a characteristic aliphatic singlet signal of methylene protons –CH2CN at δ = 4.03 ppm. The disappearance of that methylene singlet signal at δ 4.03 ppm and the appearance of singlet signal dispensed for the vinyl proton –C=C–H at δ = 8.46–8.62 ppm confirmed the establishment of the aldol condensation products, 12a–j. Whereas, 1H NMR spectra of 12c, 12d, 12e, 12g and 12j exhibited the presence of aliphatic signals corresponding to –OCH3 at δ = 3.89 ppm, –N(CH3)2 at δ = 3.09 ppm, –OCH2O– at δ = 6.21 ppm, –CH3 at δ = 2.43 ppm and –OCH3 at δ = 3.85 ppm, respectively.
Biological evaluation
Carbonic anhydrase inhibition
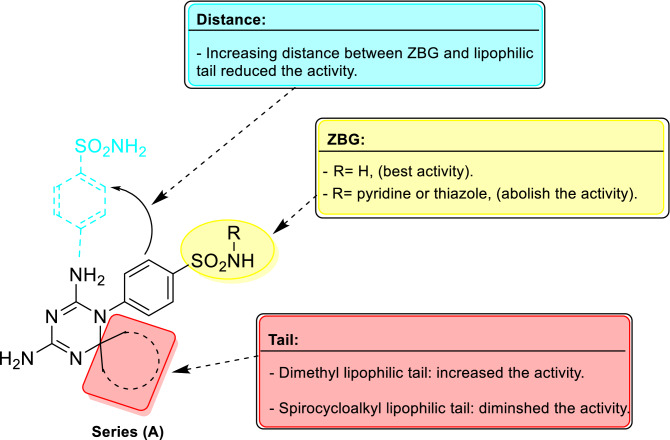
Target compounds of series (A) and (B) were screened as inhibitors concerning the physiologically relevant hCAs; cytosolic isoforms (hCA I and II) in addition to the transmembrane tumor related isoforms (hCA IX and XII) via a stopped-flow assay of carbonic anhydrase catalyzed CO2 hydration37. Acetazolamide (AAZ), CA I drug, was also incorporated in the assays as a standard drug. Human CAs I, II, IX and XII inhibition constants (KI) and the calculated selectivity indexes (SI) are reported in Tables 1 and 2. The subsequent structure activity relationship (SAR) of target compounds in series (A) and (B) were attained as following depending on the KI values revealed in Tables 1 and 2. Selectivity indexes were calculated and reported in Table 3 because selectivity is an important element in designing new hCA IX inhibitors to avoid classical side effects related to inhibition of cytosolic off-target isoforms, hCA I & II. Regarding, SAR and selectivity of series (A) compounds having the dihydrotriazine linker towards hCAs I, II, IX and XII:
-
(i)
Analogues with free sulfonamide group, eight compounds, were active and displayed variable inhibition potency against hCAs I, II, IX and XII. N-substituted sulphonamides, nine compounds, with either a pyridine or thiazole rings disclosed abolished hCAs inhibitory effect. Accordingly, the primary sulfonamide group is essential for activity as a zinc binding group.
-
(ii)
The active compounds reported inhibition of the cytosolic isoform hCA1 with KI in the range of 94.4–6844 nM. The most active compound, 8a, KI = 94.4 nM, was more active than standard inhibitor, AAZ, KI = 250 nM. The spirocycloalkyl tails in analogues 6a–c, KI = 628–145.5 nM, enhanced the activity compared to the dimethyl tail in compound 5a, KI = 884.3 nM. Increasing the size and lipophilicity of the tail in analogue 6c, bearing the 4-methylcyclohexane, enriched the potency, KI = 145.5 nM, compared to 6b, KI = 437.2 nM and 6a, KI = 628 nM having the cyclohexane and cyclopentane tails respectively.
Increasing the distance between the ZBG and the lipophilic tail improved the potency of analogues 7a and 8a–c, KI = 94.4–6844 nM. Within these compounds, the presence of spirocycloalkyl lipophilic tails in compounds, 8a–c, KI = 94.4–408 nM, strongly improved the activity relative to the dimethyl one, 7a, KI = 6844 nM. Regarding the size of the spirocycloalkyl tails, cyclopentane in 8a, KI = 94.4 nM, reported the best potency relative to the cyclohexane and 4-methylcyclohexane, 8b–c analogues, with KI = 353.7 and 408 nM respectively. Therefore, increasing the size of the lipophilic cyclic tails decrease the activity as exhibited in Table 1.
-
(iii)
Target compounds in series (A) displayed weaker inhibitory effects toward hCA II than the reference drug, AAZ, KI = 12.1 nM. Eight analogues were active inhibitors against hCA II with KI range from 179.4 to 3621 nM. The most active analogue, 6c, KI = 179.3 nM, incorporated the 4-methylcycolhexane as a lipophilic tail. Increasing the distance between the ZBG and this tail resulted in decreasing the potency as exposed in analogue 8c, KI = 591.4 nM.
-
(iv)
The tumor related isoform, hCA IX activity was inhibited by target compounds of series (A) with KI's within the range of 134.8 to 2280 nM, while standard drug AAZ, KI = 25.7 nM. Compound 5a, reported the best inhibitory effect with KI = 134.8 nM which was more active than analogue 7a, KI = 294.9 nM with longer distance between the ZBG and lipophilic dimethyl group at the dihydrotriazine linker. Incorporation of spirocycloalkyl lipophilic tails in compounds, 6a–c & 8a–c, KI = 1056–2280 nM dropped the potency, while increasing the distance between the ZBG and the lipophilic spirocycloalkyl tails diminished the activity as observed for 8a–b, KI = 1416–2280 nM, except for analogue 8c, KI = 1224 nM bearing the 4-methylcyclohexane.
-
(v)
Target compounds exhibited moderate inhibition to the tumor associated isoform, hCA XII, KI = 936.2–7423 nM compared to AAZ, KI = 5.7 nM. Analogue, 8c, having the 4-methylcyclohexane was the strongest inhibitor with KI = 936.2 nM.
-
(vi)
Concerning selectivity to the tumor associated isoform, hCA IX, active inhibitors of series (A) reported SI (I/IX) range from 23.2 to 0.1 relative to AAZ, SI (I/IX) = 9.7. Meanwhile, the SI (II/IX) ranged from 6.8 to 0.1 relative to AAZ, SI (II/IX) = 0.5. Inhibitor, 7a, revealed better selectivity profile, SI (I/IX) = 23.2 & SI (II/IX) = 1.3, than AAZ. The strongest inhibitor, 5a, showed good selectivity, relative to hCA I, SI (I/IX) = 6.7 and the best selectivity concerning hCA II, SI (II/IX) = 6.8 compared to the standard drug, AAZ. The SAR of series (A) can be summarized as shown in Fig. 6.
Table 1.
Inhibition data of carbonic anhydrase enzyme for series (A) target compounds and standard inhibitor, AAZ, against hCAs I, II, IX and XII via a stopped-flow CO2 hydrase assay37.
|
| |||||||
|---|---|---|---|---|---|---|---|
| Comp | R | n | R1 | KI (nM)* | |||
| hCA I | hCA II | hCA IX | hCA XII | ||||
| 5a | H | – | – | 884.3 | 913.4 | 134.8 | 3522 |
| 5b | 2-pyridinyl | – | – | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 5c | 2-(1,3thiazolyl) | – | – | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6a | H | 1 | H | 628.2 | 831.5 | 1056 | 5336 |
| 6b | H | 2 | H | 437.2 | 915.2 | 1163 | 4851 |
| 6c | H | 2 | CH3 | 145.5 | 179.4 | 1849 | 4910 |
| 6d | 2-pyridinyl | 1 | H | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6e | 2-pyridinyl | 2 | H | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6f. | 2-pyridinyl | 2 | CH3 | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6g | 2-pyridinyl | 3 | H | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6h | 2-(1,3thiazolyl) | 1 | H | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6i | 2-(1,3thiazolyl) | 2 | H | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 6j | 2-(1,3thiazolyl) | 2 | CH3 | > 10,000 | > 10,000 | > 10,000 | > 10,000 |
| 7a | – | – | – | 6844 | 383.9 | 294.9 | 4653 |
| 8a | H | 1 | – | 94.4 | 3621 | 1416 | 7423 |
| 8b | H | 2 | – | 353.7 | 3163 | 2280 | 5431 |
| 8c | CH3 | 2 | – | 408.0 | 591.4 | 1224 | 9362 |
| AAZ | – | – | – | 250 | 12.1 | 25.7 | 5.7 |
*Mean from 3 different assays, by a stopped flow technique (errors were in the range of ± 5–10% of the reported values).
Table 2.
Inhibition data of carbonic anhydrase enzyme for series (A) target compounds and standard inhibitor, AAZ, against hCAs I, II, IX and XII via a stopped-flow CO2 hydrase assay37.
|
| ||||||
|---|---|---|---|---|---|---|
| Comp | R | R1 | KI (nM)* | |||
| hCA I | hCA II | hCA IX | hCA XII | |||
| 10 | – | – | 72.3 | 49.2 | 2454 | 7633 |
| 12a | H | H | 3599 | 435.9 | 1536 | 8057 |
| 12b | H | Br | 2616 | 2002 | 1022 | 8208 |
| 12c | OCH3 | H | 961.9 | 1937 | 1393 | 8678 |
| 12d | N(CH3)2 | H | 577.3 | 363.6 | 190.0 | 187.9 |
| 12e | –OCH2O– | 4052 | 893.0 | 971.3 | 8537 | |
| 12f. | F | H | 589.6 | 493.4 | 194.8 | 4198 |
| 12g | CH3 | H | 158.7 | 696.9 | 194.8 | 2306 |
| 12h | H | Cl | 507.8 | 320.0 | 52.2 | 87.5 |
| 12i | H | F | 480.1 | 253.1 | 38.8 | 181.7 |
| 12j | H | OCH3 | 494.2 | 365.7 | 89.9 | 83.7 |
| AAZ | – | – | 250 | 12.1 | 25.7 | 5.7 |
*Mean from 3 different assays, by a stopped flow technique (errors were in the range of ± 5–10% of the reported values).
Table 3.
Selectivity index (SI) of series (A), (B) and AAZ toward tumor related hCA IX & XII over the off-target hCA I & II.
| Compound* | Selectivity index (SI) | |||
|---|---|---|---|---|
| I/IX | II/IX | I/XII | II/XII | |
| 5a | 6.7 | 6.8 | 0.3 | 0.2 |
| 6a | 0.6 | 0.8 | 0.1 | 0.2 |
| 6b | 0.4 | 0.8 | 0.1 | 0.2 |
| 6c | 0.1 | 0.1 | 0.02 | 0.04 |
| 7a | 23.2 | 1.3 | 1.5 | 0.1 |
| 8a | 0.1 | 2.5 | 0.01 | 0.5 |
| 8b | 0.2 | 1.4 | 0.1 | 0.6 |
| 8c | 0.3 | 0.5 | 0.4 | 0.6 |
| 10 | 0.03 | 0.02 | 0.01 | 0.006 |
| 12a | 2.3 | 0.3 | 0.4 | 0.05 |
| 12b | 2.5 | 2 | 0.3 | 0.2 |
| 12c | 0.7 | 1.4 | 0.1 | 0.2 |
| 12d | 3 | 2 | 3 | 2 |
| 12e | 4 | 1 | 0.5 | 0.1 |
| 12f. | 3 | 2.5 | 0.1 | 0.1 |
| 12g | 0.8 | 3.6 | 0.07 | 0.3 |
| 12h | 9.7 | 6.1 | 5.8 | 3.6 |
| 12i | 12.4 | 6.5 | 2.6 | 1.4 |
| 12j | 5.5 | 4.1 | 5.9 | 4.3 |
| AAZ | 9.7 | 0.5 | 43.9 | 2.1 |
*Compounds with Ki < 10,000 aren’t shown.
Figure 6.
Summary of SAR for series (A) compounds having the dihydrotriazine linker as inhibitors of the tumor associated, hCA IX.
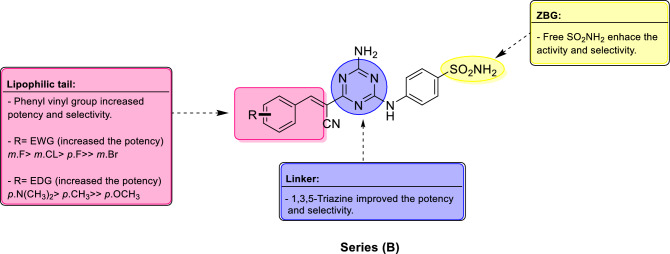
Series (B) compounds, integrated the primary sulfonamide as ZBG, 1,3,5-triazine aromatic linker, cyanoethenyl spacer, and substituted phenyls as lipophilic tails, were active inhibitors toward the four tested hCAs I, II, IX and XII. Target compounds of series (B), displayed higher potency against hCAs I, II, IX and XII compared to series (A). Moreover, they reported better selectivity towards the tumor associated isoforms, hCA IX and XII. Therefore, converting the dihydro1,3,5-triazine linker to the aromatic 1,3,5-triazine, constructing vinyl spacer in addition to the lipophilic phenyl tail were effective modifications that enhanced potency and selectivity of series (B). The SAR and selectivity of target compounds in series (B) against hCAs I, II, IX and XII were accomplished depending on the KI and SI values recorded in Tables 2 and 3 respectively, as following:
-
(i)
Compound 10, displayed relatively, strong inhibition to cytosolic isoforms; hCA 1 and II with KI = 72.3 and 49.2 nM respectively although, it exposed weak inhibition for the tumor associated isoforms; hCA IX, and XI; KI = 2454 and 7633 nM respectively. Condensation of, 10 with diverse aldehydes furnished analogues, 12a–j, having the vinyl linker and variety of lipophilic tails. Consequently, the potency of, 12a–j, towards tumor associated isoform; hCA IX was improved, while the inhibition of cytosolic isoforms; hCA 1 and II was diminished. Six analogues displayed higher potency toward tumor associated isoform; hCA IX than hCA I and II, while selectivity profiles of 12a–j were improved compared to 10.
-
(ii)
Target compounds in series (B) inhibited hCA I in the high nanomolar range reporting KI values ranging from 72.3 to 3599 nM, while AAZ, KI = 250 nM. They exposed weaker activity towards hCA I than AAZ, except compound 10 and 12g, which displayed KI = 72.3 and 158.7 nM respectively. The presence of aromatic lipophilic tails in analogues 12a–j, KI = 158.7–3599 nM, reduced the affinity to hCA I compared to compound 10, KI = 72.3 nM. The least active compound 12a, KI = 3599 nM, possessed non-substituted phenyl group as a lipophilic ail. The presence of the p-methyl group in 12g, enhanced the potency disclosing the best activity with KI = 158.7 nM, followed by 12i bearing m-fluoro, KI = 480.1 nM, while 12j with m-methoxy reported KI = 494.2 nM.
-
(iii)
Series (B) established lower inhibitory effect regarding, hCA II, KI = 49.2–2002 nM relative to the reference drug AAZ, KI = 12.1 nM. Compound 10, KI = 49.2 nM, demonstrated higher potency than target compounds, 12a–j, KI = 253.1–2002 nM, because of lacking the lipophilic aromatic tail. Substitution of phenyl group with electron with drawing groups such as m-fluoro group enhanced the inhibitory activity of analogue, 12i, KI = 253.1 nM, while m-chloro analogue, 12h, reported KI = 320.0 nM. Placement of fluoride atom from meta position, 12i, KI = 253.1 nM, to para position diminished potency as observed in analogue 12f., KI = 493.4 nM. Increasing the size of the halogen with lower electronegativity diminished the activity 8 folds as detected in m-bromo analogue, 12b, KI = 2002 nM. Electron donating groups improved the potency in the following order: p-dimethylamino >m-methoxy >p-methyl >> p-methoxy in analogues, 12d, KI = 363.6 nM, 12j, KI = 365.7 nM, 12g, KI = 696.9 nM and 12c, KI = 1937 nM respectively.
-
(iv)
The tumor associated isoform hCA IX was inhibited by series (B) in the high nanomolar range recording KI in the range from 38.8 to 2454 nM, while the reference drug AAZ, KI = 25.7 nM. Compound 10, KI = 2454 nM, revealed the lowest potency. The potency of analogues, 12a–j, KI = 38.8–1536 nM, was significantly improved upon the addition of the lipophilic phenyl tails. The non-substituted analogue, 12a, KI = 1536 nM, reported the worst effect while phenyl tail substitution with either electron donating or electron withdrawing groups enhanced the activity as displayed in analogues, 12b–j, which reported better KIs = 89.9–1393 nM. The best inhibitor 12i, KI = 38.8 nM (which is more potent than the lead compound SLC-0111, KI = 45.0 nM38) had the strongest and smallest electronegative halogen, fluoride atom, at the meta position of the phenyl tail. The presence of strong and small electron with drawing groups significantly enhanced the activity in the order; m-fluoro (12i) > m-chloro (12h), > p-fluoro (12f.) >> m-bromo (12b) which reported KI values = 38.8, 52.2, 194.8 and 1022 nM respectively. Electron donating groups improved the potency of analogue 12j, KI = 89.9 nM with m-methoxyphenyl tail more than the para substituted analogues in the order; 12d (p-N(CH3)2) > 12g (p-CH3) >> 12c (p-OCH3) with KI values = 190.0, 194.8, and 1393 nM respectively.
Concerning selectivity, compounds 12a–j with cyanoethenyl spacer and lipophilic tails, established variable selectivity profiles towards the hCA IX in respect to the cytosolic isoforms. They reported SI (I/IX) ranging from 0.7 to 12.4 and, SI (II/IX) = 0.3–6.5 compared to the reference drug, AAZ, SI (I/IX) = 9.7 and SI (II/IX) = 0.5. The most active analogue, 12i, displayed the best selectivity towards hCA IX with SI (I/IX) = 12.4 and SI (II/IX) = 6.5 which was 1.3 and 13 times the corresponding values for the reference drug AAZ.
-
(v)
Finally, hCA XII was inhibited by compounds of series (B) in the high nanomolar range reporting KI values from 83.7 to 8678 nM, while the reference drug, AAZ, KI = 5.7 nM. Incorporation of phenyl vinyl group to compound 10, KI = 7633 nM, enhanced the potency in six analogues with KI values ranged from 83.7 to 4198 nM. The most active compound 12j, KI = 83.6, displayed 93.6 times the potency of compound 10. Electron with drawing groups such as halogens on the phenyl tails significantly affected inhibition of hCA XII in the order: m-Cl > m-F >> p-F >> m-Br in analogues 12h, 12i, 12f. & 12b with KIs = 87.5, 181.7, 4198 and 8208 nM respectively. Electronegativity and orientation of halogen markedly affected the potency where moving the fluoride atom from meta position in 12i to para position in analogue 12b diminished the activity. Electron donating groups increased the potency in the following order: m-methoxy > p-dimethylamino >> p-methyl >> p-methoxy in analogues, 12j, 12d, 12g & 12c with KIs = 83.7, 187.9, 2306 and 8678 nM respectively.
-
(vi)
Analogues 12a–j revealed higher selectivity toward hCA XII over hCA I with SI (I/XII) = 0.07 to 5.9, which was better than compound 10, SI (I/XII) = 0.01 and lower than reference drug AAZ, SI (I/XII) = 43.9. In addition, 12a–j reported higher selectivity toward hCA XII over hCA II with SI (II/XII) from 0.05 to 4.3, which was significantly, higher than both compound 10, SI (II/XII) = 0.006 and reference drug AAZ, SI (II/XII) = 2.1. The best selectivity was reported for 12j, SI (I/XII) = 5.9 and SI (II/XII) = 4.3 followed by analogue 12h, SI (I/XII) = 5.8 and SI (II/XII) = 3.6. The SAR of series (B) is illustrated in Fig. 7.
Figure 7.
Summary of SAR for series (B) analogues, incorporating the 1,3,5-triazine linker, vinyl spacer and substituted phenyl tails towards selective inhibition of hCA IX.
Anticancer activity
In vitro antitumor activity towards 60 cancer cell lines (NCI, USA)
Target compounds in series (A) and (B), were submitted to the National Cancer Institute (NCI) Developmental Therapeutic Program (www.dtp.nci.nih.gov), where they have been assessed, in vitro for their anticancer activity. Two series (A) and (B), were screened in a single dose (10 µM) versus full panel composed of 60 cancer cell lines, in accordance with protocol of drug evaluation branch, NCI, Bethesda. Sulforhodamine B (SRB) colorimetric assay was used to determine cell growth and viability 39,40. The achieved data were described as mean-graph of treated cancer cell lines and their corresponding growth percentage (%G), (supplementary data), which, were then transformed to the percentage growth inhibition (GI%) caused by examined compounds as shown in Table 4, for the most active analogues against the most sensitive cancer cell lines.
Table 4.
Calculated GI% for target compounds, in series (A) and (B) at (10−5 M) concentration, concerning the most sensitive NCI cancer cell lines.
| Compounda | Subpanel/cell line | |||
|---|---|---|---|---|
| CNS cancer (SNB-75) | Renal cancer (UO-31) | Breast cancer (MDA-MB-468) | Leukemia (SR) | |
| 5a | 29 | – | – | – |
| 5b | 28 | 11 | – | – |
| 5c | 35 | 11 | – | – |
| 6a | – | 19 | 18 | 13 |
| 6b | 28 | – | – | – |
| 6c | 21 | 16 | – | – |
| 6d | 30 | 12 | – | – |
| 6e | – | 11 | – | – |
| 6f. | 23 | 11 | – | – |
| 6g | 33 | 14 | – | – |
| 6h | – | 17 | – | – |
| 6i | – | 14 | – | – |
| 6j | 29 | 17 | – | – |
| 7a | – | 12 | – | – |
| 8a | 14 | 17 | – | – |
| 8b | 21 | – | 11 | 15 |
| 8c | – | – | – | – |
| 10 | 33 | – | – | – |
| 12a | – | – | – | – |
| 12b | 14 | 16 | 16 | 14 |
| 12c | – | 14 | 22 | 16 |
| 12d | 12 | 14 | 62 | 51 |
| 12e | – | 13 | 27 | 11 |
| 12f. | 10 | 15 | 15 | 11 |
| 12g | 15 | 11 | 11 | – |
| 12h | – | – | 11 | 27 |
| 12i | – | 20 | 17 | – |
| 12j | 11 | 14 | – | – |
aOnly GI% ≥ 10% are shown.
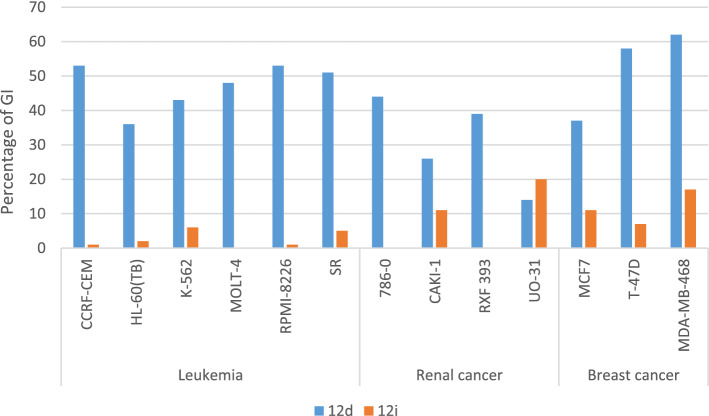
Exploration of the acquired data pointed out that, compounds of series (B) were more potent than series (A) against the most sensitive cancer cell lines. Regarding series (A), CNS cancer cell line, (SNB-75), was the most affected cells by compound 5c, (GI% = 35%), while renal, (UO-31), and breast (MDA-MB-468) cancers were inhibited by compound 6a, with GI% = 19% and 18% respectively. Analogue 8b reported the best activity against leukemia, (SR), (GI% = 15%), which was the least sensitive cancer cells to series (A) as reported in Table 4. Meanwhile, for series (B), breast cancer was the most sensitive cell lines followed by leukemia, then renal cancer and finally CNS cancer cell lines. Compound 12d, exposed the best activity towards breast cancer (MDA-MB-468) with GI% = 62%. Leukemia (CCRF-CEM) was inhibited by 12d, with GI% = 53%, while it affected renal (UO-31), and CNS (SNB-75), cancer cell lines with GI% = 44% and 29% respectively.
Compound 12d, the most potent derivative in this study, displayed wide antitumor efficacy against the majority of cancer cell lines examined. 12d Inhibited the growth of 44 cancer cell lines with GI% ≥ 10%. It showed GI% ≥ 50% against leukemia, (CCRF-CEM, RPMI-8226 and SR) and breast cancer cell lines, (T-47D and MDA-MB-468). Analogue, 12i, disclosed its best cytotoxicity towards renal cancer (UO-31), breast cancer (MDA-MB-468) and non-small cell lung cancer (HOP-92) cell lines, with GI% = 20%, 17% and 16% respectively, Fig. 8. Although, 12i demonstrated better inhibition of hCA IX with KI = 38.8 nM compared to 12d, KI = 190.0 nM.
Figure 8.
Assessment of anticancer activity of 12d and 12i relying on GI% towards the most sensitive NCI cancer cell lines.
In vitro anti-tumor activity on cancer cells under hypoxic condition
Enzyme inhibition assay on tumor associated hCA IX revealed that compound 12i was the most potent and selective inhibitor with (KI = 38.8 nM, SI (I/IX) = 12.4, SI (II/IX) = 6.5), while 12d reported lower values, (KI = 190 nM, SI (I/IX) = 3.0, SI (II/IX) = 2.0). Under normoxic condition, (5% CO2, 95% air at 37 °C), analogue 12d exhibited the best cytotoxic activity towards breast cancer cells (MDA-MB-468, GI% = 61.39) and leukemia (CCRF-CM, GI% = 52.61), although 12i displayed lower activity in both breast cancer cells, (MDA-MB-468, GI% = 16.58) and leukemia (CCRF-CM, GI% = 0.76) as depicted on Table 5 which may be explained by non-hypoxia cell culture conditions used for anti-tumor screening assay by NCI protocol.
Table 5.
Growth inhibition percentage (GI%) of compounds 12d and 12i on breast cancer (MDA-MB-468) and leukemia (CCRF-CM) cell lines under normoxic and hypoxic condition.
| Compound | GI% | |||
|---|---|---|---|---|
| Normoxia | Hypoxia | |||
| MDA-MB-468 | CCRF-CM | MDA-MB-468 | CCRF-CM | |
| 12d | 61.39 | 52.61 | 59.38 | 55.82 |
| 12i | 16.58 | 0.76 | 64.75 | 50.14 |
Accordingly, we have performed anticancer screening under hypoxic condition (1% O2, 5% CO2, and 94%N2 at 37 °C) for the most active compounds, 12d and 12i. It was observed that potency of analogue 12i was strongly enhanced against both breast cancer cells (MDA-MB-468, GI% = 64.75) and leukemia (CCRF-CM, GI% = 50.14) because of its high selectivity towards hCA IX which is over expressed under hypoxic condition. On the other hand, compound 12d displayed lower cytotoxic activity towards breast cancer cells (MDA-MB-468, GI% = 59.38) and slightly higher anticancer effect against leukemia (CCRF-CM, GI% = 55.82) under hypoxic condition relative to its activity under normoxic condition because of its lower selectivity towards hCA IX beside high probability for off-target mechanism (Table 5).
Furthermore, compounds 12d and 12i were evaluated quantitatively for their anti-proliferative activity under hypoxic condition against breast cancer (MDA-MB-468) and leukemia (CCRF-CM) cell lines, using staurosporine as a reference anticancer drug and following the MMT assay protocol (Table 6)41–43. The cytotoxic activity toward (MDA-MB-468) breast cancer cell line, were estimated under hypoxic condition which leading to over expression of hCA IX. The achieved IC50 values demonstrated that, compound 12i, IC50 = 1.48 ± 0.08 µM, is more potent and selective than 12d, IC50 = 3.99 ± 0.21 µM, consistent with the enzyme assay results. Analogues 12i and 12d were more potent than reference drug, staurosporine, IC50 = 6.07 ± 0.03 µM. Meanwhile, 12d, IC50 = 4.51 ± 0.24 µM, was more cytotoxic than compound 12i, IC50 = 9.83 ± 0.52 µM, against leukemia (CCRF-CM) cell line because, 12d may have another off target cytotoxic mechanism 43,44. Staurosporine, IC50 = 2.17 ± 0.11 µM, disclosed higher cytotoxic activity than 12d and 12i against leukemia.
Table 6.
In vitro cytotoxic activity of 12d and 12i against tumor cells, MDA-MB-468 and CCRF-CM under hypoxic conditions (1%O2, 5% CO2 and 94% N2) compared to staurosporine.
| Compound | IC50 (µM)* | |
|---|---|---|
| MDA-MB-468 | CCRF-CM | |
| 12d | 3.99 ± 0.21 | 4.51 ± 0.24 |
| 12i | 1.48 ± 0.08 | 9.83 ± 0.52 |
| Staurosporine | 6.07 ± 0.03 | 2.17 ± 0.11 |
*The mean ± S.D. of three experiments.
Cell cycle analysis
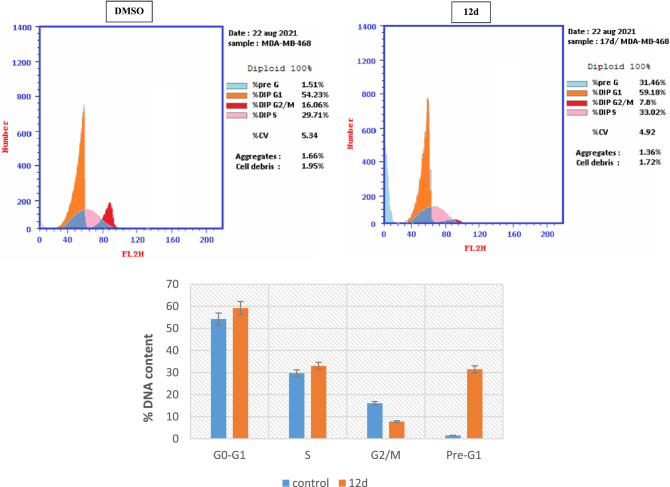
Cell cycle is composed of four distinct phases: G1, S, G2, and M45. Flow cytometry analysis of DNA ploidy in MDA-MB-468 breast cancer cells was used to evaluate the influence of compound 12d on normal cell cycle progression46. MDA-MB-468 cells, were incubated with 12d for 24h at its IC50 concentration (3.99 μM) to induce a significant disruption in the cell cycle profile (Fig. 9). Analogue 12d arrested cell growth in G0–G1 and S phases where it induced an increase in cells in G0-G1 and S phases with concurrent significant decrease in G2/M phase. Apoptosis and DNA fragmentation are possible mechanisms for 12d-induced cancer cell death based on the observed high increase in a pre-G1 cell population by 20 fold compared to the control.
Figure 9.
Impact of 12d and control (DMSO) on the cell cycle of breast cancer cells, (MDA-MB-468), error bars represent the ± SD for three biological replicates.
Impact of 12d on breast cancer cell (MDA-MB-468) apoptosis
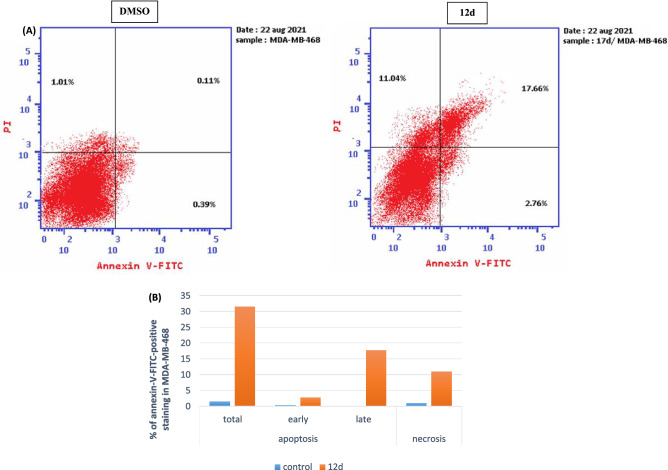
Apoptosis of tumor cells is the principal therapeutic objective of antiproliferative agents47. The apoptotic induction potential of 12d in MDA-MB-468 cells were assessed using the Annexin V/propidium iodide double staining assay 38,48. Study of flow cytometry data revealed that 12d differentiates between apoptotic (early and late phases) and necrotic cell populations compared to the control (DMSO). Compound 12d increased the percentage of apoptotic MDA-MB-468 cells in early stage (LR) from 0.39% in the control to 2.76% and in late apoptosis phase (UR) from 0.11% in control to 17.66% with total increase in the apoptotic cells by about 20 fold compared to the control (Fig. 10). These data support that compound 12d induce cell death by the apoptotic mechanism rather than the necrotic way.
Figure 10.
(A) Apoptosis assay in MDA-MB-468 breast cancer cells: effect of control (DMSO) and compound 12d on the percentage of annexin V-FITC-positive staining. LL stands for viable, LR for early apoptotic, UR for late apoptotic, and UL for necrotic, (B) influence of compound 12d on the percentage of annexin V-FITC positively stained apoptotic MDA-MB-468 cancer cells in comparison to the control (DMSO).
Western blot analysis of apoptotic markers, caspase 3 and caspase 9
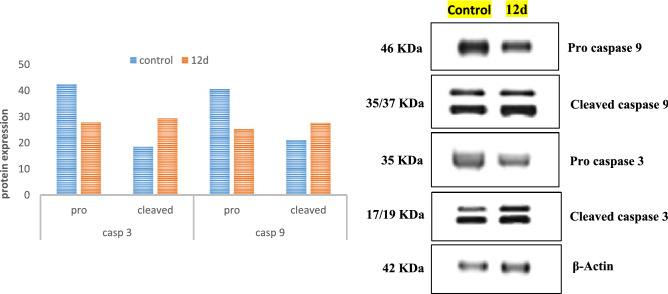
Caspases, cysteine-containing aspartic acid-specific proteases, afford essential factors in cell networks that control and regulate death of the cell. Caspase-3 is a vital executioner protease which is triggered by cascade of initiator caspases such as caspase-9 49. This study was further extended to explore the apoptosis mechanism of compound 12d upon breast cancer, MDA-MB-468, cells by measuring the expression of apoptosis biomarkers (cleaved caspase 3 and caspase 9) via Gel electrophoresis and immune-blot analysis of proteins (Western Blot) using β-actin to normalize the loading 50. Assay results are reported in Fig. 11. It revealed that 12d induced significant activation of both caspase 3 and caspase 9 which are the hallmarks of apoptosis relative to the control. Accordingly, compound 12d have the ability to induce apoptosis in MDA-MB-468 cancer cells probably by pathways involving both caspase3 and caspase9.
Figure 11.
Outcomes of compound 12d on expression of apoptotic biomarkers; cleaved caspase 3 and 9 evaluated via western blot analysis.
Normal cell line cytotoxicity
Cytotoxic activity of the most active analogue, 12d was evaluated against normal human embryonic kidney cells (HEK-293) via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MMT) assay to measure cells viability 51. Staurosporine was used as a reference anticancer drug. As reported in Table 7, 12d demonstrated lower cytotoxicity against normal cells HEK-293 with IC50 = 68.08 µM, compared to the reference, staurosporine, IC50 = 35.33 µM. Compound 12d disclosed better selectivity towards cancer than normal cells with higher safety index, about twofold, than staurosporine.
Table 7.
Cytotoxic activity of 12d, against normal human embryonic kidney cells (HEK-293).
| Compound | IC50 ± SD* µM |
|---|---|
| HEK-293 | |
| 12d | 68.08 ± 3.72 |
| Staurosporine | 35.33 ± 1.93 |
*Data are shown as mean ± SD of three independent experiments.
Molecular docking study
We have performed molecular docking for the most active analogues, 12d and 12i beside AAZ via Molecular Operating Environment 2020 (MOE) software package52. Target compounds, 12d, 12i and AAZ were docked in the active site of the crystal structure of hCA IX, (PDB: 3IAI)53 to understand their mechanism of action through studying pattern of interactions with amino acids and zinc ion incorporated in the active site. The tumor associated hCA IX is a transmembrane glycoprotein with an extracellular active site. The active site is found at the bottom of a cavity taking conical shape where three histidine residues (His 94, His96 and His119) coordinate the zinc ion at the base of the active site cleft 54.
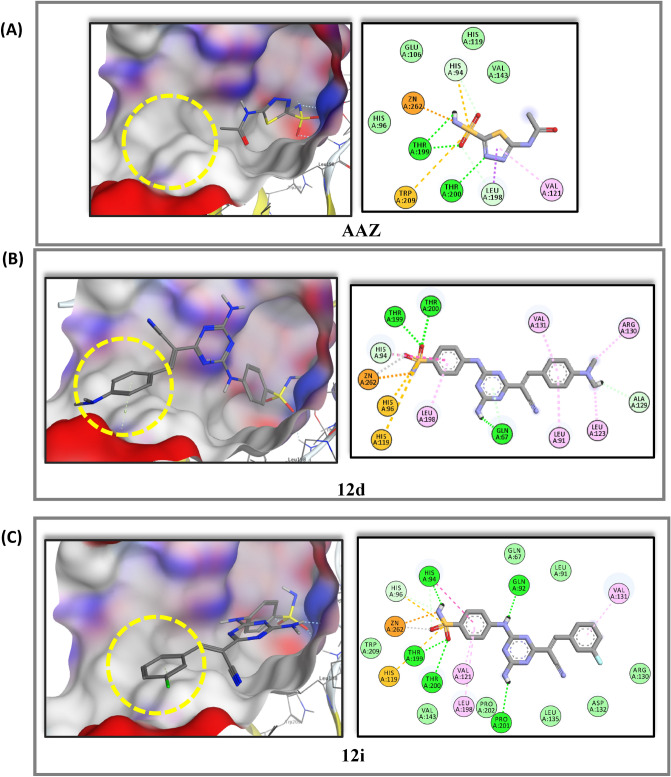
Docking study evidenced that benzenesulfonamide head (ZBG) accommodated deeply in the bottom of the active site by forming chelating bond between negatively charged NH− group and Zn2+. They formed H-bonds between SO2NH– group and amino acids, Thr199, Thr200, His94, His96, and His119. Benzene ring of ZBG displayed hydrophobic attraction force with Leu198 and Val121 mimic the reference drug Acetazolamide (AAZ) binding mode. 1,3,5-Triazine linker in 12d established H-bond through C4-amino group with Gln67, while in 12i C4-amino group formed H-bond with Pro201 beside additional H-bond between Gln92 and NH as illustrated in Fig. 12 and Table 8. Presence of triazine linker and cyanoethenyl spacer enabled the lipophilic tails in both compounds to accommodate accurately in the hydrophobic pocket (encircled in yellow) in the active site lined with Val131 which was inaccessible by AAZ. Dimethyl amino group in 12d additionally showed hydrophobic attraction force with Ala129 and Arg130, Fig. 12. The docking energy scores (S) and amino acid reported in interactions between the hCA IX and inhibitors (12d, 12i and AAZ) are summarized in Table 8.
Figure 12.
Binding modes of 12d, in 2D and 3D representation (B) and 12i in 2D and 3D representation (C) in comparison to AAZ (A), docked in the active site of the crystal structure of hCA IX, (PDB: 3IAI), blue and gray colors surfaces specify the hydrophilic and lipophilic rims respectively.
Table 8.
Docking data for analogues 12d, 12i and AAZ in the active site of hCA IX (PDB: 3IAI).
| Cpd | S, (Kcal/mol) | Amino acids that interact with inhibitors | ||
|---|---|---|---|---|
| Head, ZBG | Linker | Lipophilic tails | ||
| 12d | − 7.90 | His94, His96, His119, Thr199,Thr200, Val121, Leu198 (in addition to Zn) | Gln67 | Val131, Ala129, Arg130 |
| 12i | − 7.87 | His94, His96, His119, Thr199,Thr200, Leu198 (in addition to Zn) | Gln92, Pro201 | Val131 |
| AAZ | − 6.88 | His94, His96, His119, Thr199,Thr200, Val121, Leu198 (in addition to Zn) | – | – |
Conclusion
Twenty eight novel compounds of hCA IX inhibitors in series (A) and (B), were designed synthesized and evaluated biologically as anticancer agents. Target compounds enclosed benzenesulfonamide scaffold, as a zinc binding moiety. Series (A) integrated either 2,4-diamino-1,2-dihydro-1,3,5-triazine, (5a–c & 6a–j), or 4-amino-1,6-dihydro-1,3,5-triazine, (7a & 8a–c), as rigid cyclic linkers with dimethyl or spirocycloalkyls incorporated as lipophilic tails. Series (B) analogues, (10 and 12a–j), were constructed with aromatic cyclic linker, 4-amino-1,3,5-triazine, fused with cyanoethenyl group as a spacer, while substituted phenyls endured as the lipophilic tails. Target compounds were evaluated for their inhibition activity against physiologically relevant hCAs, (I & II) and transmembrane tumor associated hCAs (IX & XII). Regarding series (A), analogues, 5a and 7a exhibited promising inhibitory action towards hCA IX, KI = 134.8 and 294.9 nM, respectively while other members of this series reported moderate to weak inhibitory effect against hCA IX and XII. On the other hand, series (B), displayed better activity against hCA IX than series (A). The most active analogues, 12i, 12h, 12j, and 12d disclosed KI = 38.8, 52.2, 89.9, and 190 nM respectively. Concerning selectivity, target compounds; 5a, 12h, 12i and 12j showed good selectivity toward hCA IX over both off targets isoforms, SI (I/IX) = 6.7, 9.7, 12.4 and 5.5 respectively, SI (II/IX) = 6.8, 6.1, 6.5, and 4.1 respectively. Acetazolamide was used as a reference hCAs inhibitor, which revealed inhibition of hCA IX, with KI = 25.7 nM and selectivity values, SI ((I/IX) = 9.5, SI (II/IX) = 0.5. Moreover, target compounds were screened for their antiproliferative effect at single dose (10−5 M) assay against US-NCI panel composed of 60 cancer cell lines. Compound, 12d, demonstrated the best broad spectrum anticancer activity while, the most sensitive cancer cell line was the breast cancer cells, (MDA-MB-468). Accordingly, 12d was tested for cell cycle disturbance and apoptosis induction in MDA-MB-468 cancer cells. It was found that 12d, arrested cell cycle at G0-G1 and S phases. It initiated about 20-fold increase in the annexin V-FITC positive apoptotic cells in comparison to control. Apoptosis induction mechanism was achieved by western blot technique which exposed that upon treatment of MDA-MB-468 breast cancer cells with 12d, it produced threefold increase in apoptotic biomarkers, cleaved caspases 3 and 9 expression. Finally, molecular docking study was carried out for selected analogues and AAZ to investigate their possible binding modes within hCA IX active site.
Experimental
Chemistry
Melting points were determined via Stuart melting point apparatus (Stuart SMP10) and were reported uncorrected. Reactions progress were monitored using thin layer chromatography (TLC) using pre-coated sheets with silica gel 60 F254 obtained from Merk. System used was Chloroform: Methanol (9:1) and sheets were visualized with UV light (254 nm). Bruker FT-NMR spectrometer was used to generate 1H NMR spectra of all prepared compounds at 400 MHz, and to generate 13C NMR spectra of compounds 5a, 5b, 6a, 6b, 6c, 6d, 6e, 6f., 6g, 10, and 12e at 400 MHz using DMSO‑d6 as a solvent. While JOEL NMR spectrometer was used to obtain 13C NMR of compounds 5c, 6h, 6i, 6j, 7a, 8a, 8b, 8c, 12a, 12b, 12c, 12d, 12f., 12g, 12h, 12i, and 12j at 500 MHz using DMSO‑d6 as a solvent. All chemical shift values (δH) were reported in ppm. Coupling constants (J) and multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad) were given in Hz. 1H NMR spectra were performed by Faculty of Pharmacy, Mansoura University, Egypt. 13C NMR spectra were performed by Faculty of Pharmacy (Bruker FT-NMR spectrometer), and Faculty of Science (JOEL NMR spectrometer), Mansoura University, Egypt. Electron ionization mass spectra (EI-MS) was carried out using Thermo Scientific, ISQ Single Quadruple MS (ionization energy = 70 Ev). Elemental analysis for C, H, N, and S elements was performed by Perkin–Elmer 2400 CHNS analyzer and reported results were within ± 0.40 of the theoretically calculated values. Mass spectroscopy and Elemental analysis were performed in the regional center for mycology and biotechnology, Al-Azhar University, Nasr City, Cairo, Egypt. All reagents used were purchased from commercial companies Alfa Aesar, Sigma-Aldrich or Acros and were used as such without any purification.
General procedure for synthesis of 4-(4,6-diamino-1,2-dihydro-1,3,5-triazin-1-yl) benzene-1-sulfonamide HCl derivatives (5a-c & 6a-j)
Sulfanilamide or its derivatives (20 mmol), cyanoguanidine (1.68 g, 20 mmol) and ketone (25 mmol) were mixed in absolute ethanol (50 mL), then conc HCl (3.0 mL) was added. The reaction mixture was stirred and heated under reflux for several hours varied relying on the ketones used. The precipitated products were filtered and recrystallized from aqueous ethanol to afford products (5a–c, and 6a–j).
4-(4,6-Diamino-1-phenyl-1,2-dihydro-2,2-dimethyl-1,3,5-triazin-1-yl) benzene-1-sulfonamide HCl (5a). Yield (2.10 g, 35.4%) as white crystalline powder, m.p = 221–223 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.36 (6H, s, 2xCH3), 6.62 (1H, s, NH), 7.76 (1H, s, NH), 7.61 (2H, s, SO2NH2), 7.61 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 7.95 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 9.32 (1H, s, +NHCl−). 13C NMR (100 MHz, DMSO) δ (ppm): 24.38, 72.02, 128, 131.52, 138.09, 145.42, 157.64, 158.40, EI-MS: m/z: 297.2 [M+]. Anal. Calcd. For C11H16N6O2S: C, 44.58; H, 5.44; N, 28.36; S, 10.82. Found: C, 44.87; H, 5.66; N, 28.12; S, 11.10.
4-(4,6-Diamino-2,2-dimethyl-1,2-dihydro-1,3,5-triazin-1-yl)-N-(pyridin-2-yl)benzene-1-sulfonamide HCl (5b). Yield (2.75 g, 36.82%) as white crystalline powder with m.p = 209–211 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.32 (6H, s, 2xCH3), 6.60 (1H, s, NH), 6.86 (1H, t, J = 7.6 Hz, 4-H of pyridine), 7.28 (1H, d, J = 7.6 Hz, 6-H of pyridine) 7.55 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.79 (1H, t, J = 7.6 Hz, 5-H of pyridine), 7.85–7.87 (1H, m, 3-H of pyridine) 7.97 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.11 (1H, s, +NHCl−), 12.92 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 27.62, 70.26, 115.51, 128.58, 131.14, 138.18, 142.16, 144.28, 154.44 157.41, 158.13, EI-MS: m/z: 374.67 [M+]. Anal. Calcd. For C16H19N7O2S: C, 51.46; H, 5.13; N, 26.26; S, 8.59. Found: C, 51.68; H, 5.3; N, 26.42; S, 8.72.
4-(4,6-Diamino-2,2-dimethyl-1,2-dihydro-1,3,5-triazin-1-yl)-N-(thiazol-2-yl)benzene-1-sulfonamide.HCl (5c). Yield (2.95 g 38.8%) as white crystalline powder with m.p = 216–218 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.33 (6H, s, 2xCH3), 6.63 (1H, S, NH), 6.86 (1H, d, J = 4.4 Hz, 5-H of thiazole), 7.28 (1H, d, J = 4.4 Hz, 4-H of thiazole), 7.56 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7,57 (2H, s, NH2), 7.71(1H, s, NH), 7.91 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.31 (1H, s, +NHCl−), 12.98 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 21.18, 29.40, 30.99, 34.95, 72.02, 128, 131.52, 138.09, 145.42, 157.64, 158.40, EI-MS: m/z: 380.14 [M+]. Anal. Calcd. For C14H17N7O2S2: C, 44.31; H, 4.52; N, 25.84; S, 16.90. Found: C, 44.55; H, 4.71; N, 25.99; S, 16.70.
4-(7,9-Diamino-6,8,10-triazaspiro[4.5]deca-7,9-dien-6-yl) benzene-1-sulfonamide HCl (6a). Yield (2.92 g, 45.29%) as white crystalline powder with m.p = 240–241 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.51–1.54 (2H, m, CH2), 1.60–1.66 (2H, m, CH2), 1.71–1.74 (2H, m, CH2), 1.82–1.84 (2H, m, CH2), 6.67 (1H, s, NH), 7.35 (1H, s, NH), 7.59 (2H, s, SO2NH2), 7.60 (2H, d, J = 8.0 Hz, 3,5-H2 of sulfanilamide ring), 7.82 (1H, S, NH), 7.95 (2H, d, J = 8.0 Hz, 2,6-H2 of sulfanilamide ring), 9.47 (1H, s, +NHCl−). 13C NMR (100 MHz, DMSO) δ (ppm): 21.18, 35.22, 72.02, 128, 131.52, 138.09, 145.42, 157.64, 158.4, EI-MS: m/z: 323.2 [M+]. Anal. Calcd. For C13H18N6O2S: C, 48.43; H, 5.63; N, 26.07; S, 9.93. Found: C, 50.31; H, 6.26; N, 25.28; S, 9.81.
4-(2,4-Diamino-1,3,5-triazaspiro[5.5]undeca-2,4-dien-1-yl)benzene-1-sulfonamide.HCl (6b). Yield (3.10 g, 46.1%) as white crystalline powder with m.p = 257–259 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.91–0.94 (1H, m, CH), 1.22–1.30 (2H, m, CH2), 1.51–1.57 (3H, m, CH + CH2), 1.64–1.74 (2H, m, CH2), 1.87–1.90 (2H, m, CH2), 6.56 (1H, s, NH), 7.58 (2H, d, J = 8.0 Hz, 3,5-H2 of sulfanilamide ring), 7.59 (2H, s, SO2NH2), 7.61 (1H, s, NH), 7.94 (2H, d, J = 8.0 Hz, 2,6-H2 of sulfanilamide ring), 9.24 (1H, s, +NHCl−). 13C NMR (100 MHz, DMSO) δ (ppm): 21.18, 24.38, 35.22, 72.02, 128, 131.52, 138.09, 145.42, 157.64, 158.4, EI-MS: m/z: 337.01 [M+]. Anal. Calcd. For C14H20N6O2S: C, 49.98; H, 5.99; N, 24.98; S, 9.53. Found: C, 48.16; H, 5.91; N, 26.25; S, 10.2.
4-(2,4-Diamino-9-methyl-1,3,5-triazaspiro[5.5]undeca-2,4-dien-1-yl)benzene-1-sulfonamide.HCl (6c). Yield (3.0 g, 42.8%) as white crystalline powder with m.p = 245–247 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.85 (3H, d, J = 6.4 Hz CH3), 1.15–1.19 (1H, m, CH), 1.29–1.54 (6H, m, 3xCH2), 1.86–1.89 (2H, m, CH2) 6.56 (1H, s, NH), 7.58 (2H, d, J = 8.0 Hz, 3,5-H2 of sulfanilamide ring), 7.59 (2H, s, SO2NH2), 7.7 (1H, s, NH), 7.94 (2H, d, J = 8.0 Hz, 2,6-H2 of sulfanilamide ring), 9.24 (1H, s, +NHCl−). 13C NMR (100 MHz, DMSO) δ (ppm): 21.18, 29.4, 30.99, 34.95, 72.02, 128, 131.52, 138.09, 145.42, 157.64, 158.4, EI-MS: m/z: 352.36 [M+]. Anal. Calcd. For C15H23N6O2S: C, 51.26; H, 6.6; N, 23.91; S, 9.12. Found: C, 51.23; H, 6.54; N, 23.83; S, 9.39.
4-(7,9-Diamino-6,8,10-triazaspiro[4.5]deca-7,9-dien-6-yl}-N-(pyridin-2-yl)benzene-1-sulfonamide.HCl (6d). Yield (2.35 g, 58.7%) as white crystalline powder with m.p = 221–223 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.43–1.48 (2H, m, CH2), 1.59–1.70 (4H, m, 2xCH2), 1.79–1.81 (2H, m, CH2), 6.65 (1H, s, NH), 6.87(1H, t, J = 7.0 Hz, 4-H of pyridine), 7.17 (1H, d, J = 7.0 Hz, 6-H of pyridine), 7.55 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.75 (1H, t, J = 7.0 Hz, 5-H of pyridine), 7.88 (1H, d, J = 7.0 Hz, 3-H of pyridine) 7.98 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.37 (1H, s, +NHCl−), 12.61 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 20.99, 36.68, 79.51, 114.74, 115.47, 128.72, 131.1, 138.37, 142.1, 144.28, 154.39, 158.22, 158.62, EI-MS: m/z: 403.6 [M+]. Anal. Calcd. For C18H21N7O2S: C, 54.12; H, 5.3; N, 24.54; S, 8.03. Found: C, 54.00; H, 5.1; N, 24.66; S, 8.25.
4-(2,4-Diamino-1,3,5-triazaspiro[5.5]undeca-2,4-dien-1-yl)-N-(pyridin-2-yl)benzene-1-sulfonamide.HCl (6e). Yield (7.0 g, 84.6%) as white crystalline powder with m.p = 246–248 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.87–0.96 (1H, m, CH) 1.06 (2H, t, J = 6.8 Hz, CH2), 1.19–1.26 (3H, m, CH + CH2), 1.49–1.55 ( 4H, m, 2xCH2), 1.63–1.72 (2H, m, CH2), 1.84–1.87 (2H, m, CH2), 6.56 (1H, S, NH), 6.87 (1H, t, J = 7.6 Hz, 4-H of pyridine), 7.30 (1H, d, J = 7.6 Hz, 6-H of pyridine), 7.53 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.70 (2H, s, NH2) 7.80 (1H, t, J = 7.6 Hz, 5-H of pyridine), 7.88 (1H, d, J = 7.6 Hz, 3-H of pyridine), 7.98 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.23 (1H, s, +NHCl−), 12.68 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 21.16, 24.33, 35.18, 72.06, 114.62, 115.53, 128.57, 131.4, 138.05, 142.13, 144.34, 154.43, 157.16, 158.43, EI-MS: m/z: 413.6 [M+]. Anal. Calcd. For C19H23N7O2S: C, 55.19; H, 5.61; N, 23.71; S, 7.75. Found: C, 55.42; H, 5.82; N, 23.92; S, 7.89.
4-(2,4-Diamino-9-methyl-1,3,5-triazaspiro[5.5]undeca-2,4-dien-1-yl)-N-(pyridin-2-yl)benzene-1-sulfonamide.HCl (6f.). Yield (5.80 g, 67.83%) as white crystalline powder with m.p = 217–219 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.83 (3H, d, J = 6.4 Hz CH3), 1.13–1.17 (1H, m, CH), 1.25–1.32 (2H, m, CH2), 1.38–1.51(4H, m, 2xCH2), 1.82–1.85 ( 2H, m, CH2), 6.54 (1H, S, NH), 6.86 (1H, t, J = 7.6 Hz, 4-H of pyridine), 7.29 (1H, d, J = 7.6 Hz, 6-H of pyridine) 7.52 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.69 (2H, br, NH2) 7.79 (1H, t, J = 7.6 Hz, 5-H of pyridine), 7.96–7.98 (3H, m, 3-H of pyridine + 2,6-H2 of sulfanilamide ring) 9.15 (1H, s, +NHCl−), 12.68 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 21.7, 29.25, 30.9, 34.88, 71.9, 114.52, 115.47, 128.53, 131.29, 137.94, 141.95, 144.42, 154.6, 157.68, 158.39. EI-MS: m/z: 428.53 [M+]. Anal. Calcd. For C20H25N7O2S: C, 56.19; H, 5.89; N, 22.93; S, 7.50. Found: C, 56.33; H, 5.63; N, 23.2; S, 7.77.
4-(2,4-Diamino-1,3,5-triazaspiro[5.6]dodeca-2,4-dien-1-yl)-N-(pyridin-2-yl)benzene-1-sulfonamide.HCl (6g). Yield (1.40 g, 16.3%) as white crystalline powder with m.p = 239–241 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.6–1.06 (2H, m, CH2), 1.21–1.25 (4H, m, 2xCH2), 1.42 (2H, br, CH2), 1.77–1.83 ( 2H, m, CH2), 1.90–1.96 (2H, m, CH2), 6.52 (1H, s, NH), 6.82–6.88 (1H, m, 4-H of pyridine), 7.21 (1H, d, J = 7.6 Hz, 6-H of pyridine) 7.59 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.71 (2H, s, NH2), 7.74–7.78 (1H, m, 5-H of pyridine), 7.97–7.99 (3H, m, 3-H of pyridine + 2,6-H2 of sulfanilamide ring), 9.15 (1H, s, +NHCl−), 13C NMR (100 MHz, DMSO) δ (ppm): 21.07, 29.02, 75.54, 128.84, 131.74, 138.27, 157.41, 158.15. EI-MS: m/z: 428.99 [M+]. Anal. Calcd. For C20H25N7O2S: C, 56.19; H, 5.89; N, 22.93; S, 7.50. Found: C, 56.4; H, 6; N, 23.1; S, 7.81.
4-{7,9-Diamino-6,8,10-triazaspiro[4.5]deca-7,9-dien-6-yl}-N-(1,3-thiazol-2-yl)benzene-1-sulfonamide.HCl (6h). Yield (4.2 g 51.8%) as white crystalline powder with m.p = 216–218 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.50–1.80 (8H, m, 4xCH2), 6.67 (1H, s, NH), 6.90–6.92 (1H, m, 5-H of thiazole), 7.3 (1H, m, 4-H of thiazole), 7.54 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.70–7.80 (3H, br, NH2 + NH) 7.91 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.23 (1H, s, +NHCl−), 12.94 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 16.09, 20.56, 36.15, 79.04, 127.73, 130.94, 138.094, 143.01, 154.93, 157.80, 158.04, 168.24. EI-MS: m/z: 406.72 [M+]. Anal. Calcd. For C16H19N7O2S2: C, 47.39; H, 4.72; N, 24.18; S, 15.81. Found: C, 47.1; H, 4.93; N, 24.45; S, 15.99.
4-(2,4-Diamino-1,3,5-triazaspiro[5.5]undeca-2,4-dien-1-yl)-N-(1,3-thiazol-2-yl)benzene-1-sulfonamide.HCl (6i). Yield (4.90 g 58.4%) as white crystalline powder with m.p = 245–247 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.93–0.96 (1H, m, CH), 1.22–1.28 (2H, m, CH2) 1.48–1.56 (3H, m, CH2 + CH), 1.63–1.70 (2H, m, CH2), 1.84–1.87 (2H, m, CH2), 6.56 (1H, s, NH), 6.90 (1H, d, J = 4.4 Hz, 5-H of thiazole), 7.30 (1H, d, J = 4.4 Hz, 4-H of thiazole), 7.54 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.71 (3H, s, NH2 + NH) 7.91 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.23 (1H, s, +NHCl−), 12.94 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 20.74, 23.78, 34.68, 71.57, 108.58,124.55, 127.54, 131.05, 137.79, 143.57, 157.18, 157.90, 169.02, EI-MS: m/z: 420.03 [M+]. Anal. Calcd. For C17H21N7O2S2: C, 48.67; H, 5.05; N, 23.37; S, 15.28. Found: C, 48.87; H, 5.29; N, 23.61; S, 15.02.
4-(2,4-Diamino-9-methyl-1,3,5-triazaspiro[5.5]undeca-2,4-dien-1-yl)-N-(1,3-thiazol-2-yl)benzene-1-sulfonamide.HCl (6j). Yield (4.60 g, 53.1%) as white crystalline powder with m.p = 243–245 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.83 (3H, d, J = 6.4 Hz, CH3), 1.06 (1H, t, J = 7.2 Hz, CH), 1.17–1.20 (H, m, CH), 1.30 (2H, m, CH2), 1.40–1.49 (3H, m, CH2 + CH), 1.82–1.85 (2H, m,CH2), 6.57 (1H, s, NH), 6.90 (1H, d, J = 4.4 Hz, 5-H of thiazole), 7.30 (1H, d, J = 4.4 Hz, 4-H of thiazole) 7.53 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.71 (3H, s, NH2 + NH) 7.91 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring), 9.18 (1H, s, +NHCl−), 12.96 (1H, s, SO2NHR). 13C NMR (100 MHz, DMSO) δ (ppm): 55.32, 110.38, 114.73, 116.88, 118.38, 119.32, 122.99, 126.36, 130.46, 133.43, 137.20, 142.79, 149.43, 159.50, 163.99, 166.56, 167.55, EI-MS: m/z: 434.07 [M+]. Anal. Calcd. For C18H23N7O2S2: C, 49.87; H, 5.35; N, 22.62; S, 14.79. Found: C, 49.58; H, 5.56; N, 22.9; S, 14.97.
General procedure for synthesis of 4-[(4-amino-1,6-dihydro-1,3,5-triazin-2-yl)amino]benzene-1-sulfonamide derivatives by Dimroth rearrangement (7a, 8a-8c)
To a stirred solution of 5a and 6a–c (10 mmol) in 50% aqueous ethanol (20 mL), sodium hydroxide (1 N) was added until pH reach 11. The reaction mixture was heated under reflux for 3 h. The white solid precipitated after evaporation of solvent under vacuum, was filtered off, washed with water and then dried under vacuum.
4-[(4-Amino-6,6-dimethyl-1,6-dihydro-1,3,5-triazin-2-yl)amino]benzene-1-sulfonamide (7a). Yield (1.3 g, 43.9%) as white crystalline powder with m.p = 268–270 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.27 (6H, s, 2xCH3), 5.74 (2H, s, NH2), 6.75 (1H, s, NH), 7.37 (2H, s, NH2), 7.54 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.82 (2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring). 13C NMR (100 MHz, DMSO) δ (ppm): 31.45, 67.03, 117.13, 126.00, 133.61, 146.55, 154.22, 157.86, EI-MS: m/z: 297.26 [M+]. Anal. Calcd. C11H16N6O2S: C, 44.58; H, 5.44; N, 28.36; S, 10.82. Found: C, 44.66; H, 5.61; N, 28.17; S, 11.
4-({9-Amino-6,8,10-triazaspiro[4.5]deca-7,9-dien-7-yl}amino)benzene-1-sulfonamide (8a). Yield (1.8 g, 56.2%) as white crystalline powder with m.p = 264–266 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.58 – 1.70 (8H, m, 4xCH2), 5.72 (2H, s, NH2), 6.82 (1H, s, NH), 7.4 (2H, s, NH2), 7.52 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.85(2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring). 13C NMR (100 MHz, DMSO) δ (ppm): 22.11, 42.14, 77.49, 116.94, 126.05, 133.61, 146.37, 154.06, 158.07, EI-MS: m/z: 322.93 [M+]. Anal. Calcd. For C13H18N6O2S: C, 48.43; H, 5.63; N, 26.07; S, 9.94. Found: C, 48.62; H, 5.81; N, 26.3; S, 10.1.
4-({4-Amino-1,3,5-triazaspiro[5.5]undeca-2,4-dien-2-yl}amino)benzene-1-sulfonamide (8b). Yield (2.6 g, 77.3%) as white crystalline powder with m.p = 270–272 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 1.34–1.35 (2H, m, CH2), 1.42–1.49 (4H, m, 2xCH2), 1.59–1.62 (2H, m, CH2), 1.71 (2H, d, J = 8.4 Hz, CH2), 5.72 (2H, s, NH2), 6.82 (1H, s, NH), 7.4 (2H, s, NH2), 7.52 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.85(2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring). 13C NMR (100 MHz, DMSO) δ (ppm): 21.61, 25.23, 68.35, 116.92, 126.03, 133.61, 146.34, 153.72, 157.87, EI-MS: m/z: 336.96 [M+]. Anal. Calcd. For C14H20N6O2S: C, 49.98; H, 5.99; N, 24.98; S, 9.53. Found: C, 49.81; H, 6.2; N, 25.19; S, 9.77.
4-({4-Amino-9-methyl-1,3,5-triazaspiro[5.5]undeca-2,4-dien-2-yl}amino)benzene-1-sulfonamide (8c). Yield (2.80 g, 80%) as white crystalline powder with m.p = 264–266 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 0.95 (3H, d, J = 6.0 Hz, CH3), 1.34–1.35 (2H, m, CH2), 1.49–1.51 (6H, m, 3xCH2), 1.68–1.70 (2H, m, CH2), 5.68 (2H, s, NH2), 6.54 (1H, s, NH), 7.4 (2H, s, NH2), 7.57 (2H, d, J = 8.4 Hz, 3,5-H2 of sulfanilamide ring), 7.95(2H, d, J = 8.4 Hz, 2,6-H2 of sulfanilamide ring). 13C NMR (100 MHz, DMSO) δ (ppm): 22.42, 29.54, 30.48, 31.46, 38.74, 67.89, 68.32, 116.72, 117.16, 126.02, 133.58, 146.22, 153.33, 154.42, 158.09, EI-MS: m/z: 350.27 [M+]. Anal. Calcd. For C15H22N6O2S: C, 51.41; H, 6.33; N, 23.98; S, 9.15. Found: C, 51.68; H, 6.54; N, 23.79; S, 9.36.
4-{[4-amino-6-(cyanomethyl)-1,3,5-triazin-2-yl]amino}benzene-1-sulfonamide (10)
A mixture of the sulfanilamide (10 mmol) and cyanoguanidine (20 mmol), were stirred and heated under reflux in absolute ethanol, then conc. HCl was added until pH reach 2.6 followed by continuous addition of conc. HCl from time to time to maintain pH constant. The solution became clear and the white product started to precipitate after 3 h. The reaction mixture was left over night, followed by filtration to get white powder of benzenesulfonamide biguanide salt which then added to stirring solution of sodium methoxide in methanol. After few minutes, the white powder dissolved and the product precipitated after several minutes. The reaction mixture was stirred at room temperature overnight. The white powder of benzenesulfonamide biguanide was filtered, washed with methanol and dried under vacuum. The yielded white powder (30 mmol) was dissolved in DMF (20 mL) and stirred under reflux. Then ethylcyanoacetate (45 mmol) was added to reaction mixture dropwise over one hour. Reaction mixture was stirred for 6 h. The reaction solution was poured into ice water and let stand for one hour at room temperature. Pale red precipitate was formed, filtered out and washed with ethanol to give compound (10) in pure form. Yield (1.1 g, 36.1%) as pale red powder with m.p = 284–286 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 4.03 (2H, s, CH2) 7.25 (2H, s, NH2), 7.40 (1H, s. NH), 7.50 (1H, s. NH), 7.71 (2H, d, J = 8.8 Hz, 3,5-H2 of sulfanilamide ring), 7.98 (2H, d, J = 8.8 Hz, 2,6-H2 of sulfanilamide ring) 10.04 (1H, s, NH), 13C NMR (100 MHz, DMSO) δ (ppm): 27.45, 117.48, 119.73, 126.77, 137.64, 143.21, 164.52, 167.10, 170.07, EI-MS: m/z: 305.18 [M+]. Anal. Calcd. For C11H11N7O2S: C, 43.27; H, 3.63; N, 32.11; S, 10.5. Found: C, 43.04; H, 3.9; N, 32.4; S, 10.8.
General procedure for preparation 4-({4-amino-6-[(1E)-1-cyano-2-phenyleth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide derivatives (12a-j)
Compound 10, (1.0 mmol) and substituted benzaldehydes (1.0 mmol) were added to mixture of ethanol and DMF (1:1) in presence of catalytic amount of trimethylamine. Then the reaction mixture was heated under reflux with monitoring of the reaction using TLC (chloroform and methanol, 9:1) until completion of the reaction. The reaction mixture was poured into ice water to afford colored precipitate which filtered under vacuum and washed with methanol.
4-({4-Amino-6-[(1E)-1-cyano-2-phenyleth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12a). Yield (257 mg, 65.3%) as yellowish white powder with m.p = 174–176 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 7.26 (2H, s, NH2), 7.49 (1H, s, NH), 7.53 (1H, s, NH), 7.61–7.66 (3H, m,3,5-H2 + 4-H of phenyl ring), 7.75 (2H, d, J = 8.0 Hz, 3,5-H2 of sulfanilamide ring), 8.03–8.05 (4H, m, 2,6-H2 of sulfanilamide ring + 2,6-H2 of phenyl ring), 8.64 (1H, s, C=C–H), 10.09 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 30.79, 35.81, 110.20, 116.87, 119.33, 126.37, 129.37, 130.30, 132.22, 132.39, 137.20, 142.81, 149.56, 164.00, 166.57, 167.59, EI-MS: m/z: 395.55 [M+]. Anal. Calcd. For C18H15N7O2S: C, 54.95; H, 3.84; N, 24.92; S, 8.15. Found: C, 54.77; H, 3.61; N, 24.73; S, 8.4.
4-({4-Amino-6-[(1E)-2-(3-bromophenyl)-1-cyanoeth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12b). Yield (345 mg, 73.1%) as white powder with m.p = 234–236 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 7.27 (2H, s, NH2), 7.52–7.59 (3H, m, NH2 + 5-H of phenyl ring), 7.76–7.81 (3H, m, 3,5-H2 of sulfanilamide ring + 6-H of phenyl ring), 8.01–8.09 (3H, m, 2,6-H2 of sulfanilamide ring + 4-H of phenyl ring), 8.27 (1H, s, 2-H of phenyl ring), 8.59 (1H, s, C=C–H), 10.11 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 111.96, 116.51, 119.36, 122.34, 126.39, 129.44, 131.37, 132.11, 134.54, 134.66, 137.24, 142.73, 147.86, 163.99, 166.55, 167.26, EI-MS: m/z: 472.55 [M+]. Anal. Calcd. For C18H14N7O2S: C, 45.77; H, 2.99; N, 20.76; S, 6.79. Found: C, 54.99; H, 3.2; N, 21; S, 7.03.
4-({4-Amino-6-[(1E)-1-cyano-2-(4-methoxyphenyl)eth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12c). Yield (386 mg, 91.2%) as yellow powder with m.p = 281–283 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 2.52 (3H, s, CH3), 7.18 (2H, br, 3,5-H2 of phenyl ring), 7.25 (2H, s, NH2), 7.41 (1H, s, NH), 7.47 (1H, s, NH), 7.74–7.76 (2H, m, 3,5-H2 of sulfanilamide ring), 8.01–8.08 (4H, m, 2,6-H2 of sulfanilamide ring + 2,6-H2 of phenyl ring), 8.57 (1H, s, C=C–H), 10.03 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 55.69, 106.50, 113.55, 114.96, 117.48, 119.25, 124.75, 126.36, 132.76, 137.08, 142.89, 148.99, 162.64, 163.97, 166.53, 168.04, EI-MS: m/z: 423.41 [M+]. Anal. Calcd. For C19H17N7O3S: C, 53.89; H, 4.05; N, 23.15; S, 7.57. Found: C, 44.1; H, 4.3; N, 23.4; S, 7.79.
4-({4-Amino-6-[(1E)-1-cyano-2-[4-(dimethylamino)phenyl]eth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12d). Yield (160 mg, 36.7%) as orange powder with m.p = 239–241 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 3.09 (6H, s, 2xCH3), 6.87 (2H, d, J = 8.8 Hz, 3,5-H2 of phenyl ring) 7.26 (2H, s, NH2), 7.38 (1H, s, NH), 7.73 (2H, d, J = 8.8 Hz, 3,5-H2 of sulfanilamide ring), 7.95 (2H, d, J = 8.8 Hz, 2,6-H2 of phenyl ring), 8.05 (2H, d, J = 8.8 Hz, 2,6-H2 of sulfanilamide ring), 8.46 (1H, s, C=C–H), 9.95 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 93.74, 100.85, 111.69, 111.83, 118.61, 119.11, 119.23, 121.53, 126.32, 129.50, 132.97, 136.88, 137.15, 142.75, 143.06, 149.16, 151.96, 152.96, 159.86, 163.92, 166.45, 166.61, 168.87, 169.60. EI-MS: m/z: 436.06 [M+]. Anal. Calcd. For C20H20N8O2S: C, 55.03; H, 4.62; N, 25.67; S, 7.34. Found: C, 55.3; H, 4.88; N, 25.47; S, 7.55.
4-({4-Amino-6-[(1E)-2-(2H-1,3-benzodioxol-5-yl)-1-cyanoeth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12e). Yield (362 mg, 82.8%) as yellow powder with m.p = 269–271 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 6.20 (2H, s, CH2), 7.17 (1H, d, J = 8.0 Hz, 3-H of phenyl ring), 7.27 (2H, s, NH2), 7.48–7.54 (3H, m, NH2 + 2-H of phenyl ring), 7.75 (3H, s, 3,5-H2 of sulfanilamide ring + 6-H of phenyl ring), 8.03–8.05 (2H, m, 2,6-H2 of sulfanilamide ring), 8.52 (1H, s, C=C–H) 10.05 (1H, s, NH). Anal. 13C NMR (100 MHz, DMSO) δ (ppm): 102.87, 107.54, 108.54, 109.59, 117.83, 119.75, 126.83, 128.87, 137.59, 143.32, 148.66, 149.43, 151.54, 164.43, 166.98, 168.39. EI-MS: m/z: 437.12 [M+]. Calcd. For C19H15N7O4S: C, 52.17; H, 3.46; N, 22.41; S, 7.33. Found: C, 52.4; H, 3.17; N, 22.7; S, 7.09.
4-({4-Amino-6-[(1E)-1-cyano-2-(4-fluorophenyl)eth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12f.) Yield (388 mg, 94.3%) as yellow powder with m.p = 234–236 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 7.26 (2H, s, NH2), 7.48 (2H, t, J = 8.8 Hz, 3,5-H2 of phenyl ring), 7.54 (1H, s, NH), 7.74 (2H, d, J = 8.8 Hz, 3,5-H2 of sulfanilamide ring), 8.04 (2H, d, J = 8.8 Hz, 2,6-H2 of sulfanilamide ring), 8.13 (2H, t, J = 8.8 Hz, 2,6-H2 of phenyl ring), 8.62 (1H, s, C=C–H) 10.09 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 109.92, 116.50, 116.67, 116.86, 119.34, 126.39, 128.90, 128.92, 133.00, 133.08, 137.2, 142.80, 148.28, 162.35, 163.05, 164.00, 165.06, 166.57, 167.57. EI-MS: m/z: 394.64 [M+]. Anal. Calcd. For C18H14N7O2S: C, 52.55; H, 3.43; N, 23.83; S, 7.79. Found: C, 52.7; H, 3.09; N, 23.99; S, 8.01.
4-({4-Amino-6-[(1E)-1-cyano-2-(4-methylphenyl)eth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12g). Yield (274 mg, 67.3%) as yellow powder with m.p = 204–206 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 2.43 (3H, s, CH3) 7.26 (2H, s, NH2), 7.44 (3H, d, J = 8.0 Hz, 3,5-H2 of phenyl ring + NH), 7.53 (1H, s, NH), 7.75 (2H, d, J = 8.0 Hz, 3,5-H2 of sulfanilamide ring), 7.96 (2H, d, J = 8.0 Hz, 2,6-H2 of phenyl ring) 8.05 (2H, d, J = 8.0 Hz, 2,6-H2 of sulfanilamide ring), 8.60 (1H, s, C=C–H) 10.08 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 21.31, 108.81, 117.08, 119.29, 126.37, 129.51, 130.00, 130.44, 137.15, 142.83, 143.09, 149.45, 163.99, 166.56, 167.77. EI-MS: m/z: 403.39 [M+]. Anal. Calcd. For C19H17N7O2S: C, 56.01; H, 4.21; N, 24.06; S, 7.87. Found: C, 56.3; H, 4.5; N, 24.3; S, 7.98.
4-({4-Amino-6-[(1E)-2-(3-chlorophenyl)-1-cyanoeth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12h). Yield (315 mg, 73.7%) as yellowish white powder with m.p = 222–224 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 7.27 (2H, s, NH2), 7.57 (2H, s, NH2), 7.66–7.76 (4H, m, 2,5-H2 of phenyl ring + 3,5-H2 of sulfanilamide ring), 7.96 (1H, s, 4-H of phenyl ring) 8.03–8.05 (2H, m, 2,6-H2 of sulfanilamide ring), 8.12 (1H, s, 6-H of phenyl ring) 8.61 (1H, s, C=C–H) 10.11 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 112.01, 116.52, 119.37, 126.39, 129.07, 129.26, 131.16, 132.79, 133.85, 134.29, 137.24, 142.74, 147.92, 163.99, 166.55, 167.27, EI-MS: m/z: 427.09 [M+]. Anal. Calcd. For C18H14N7O2S: C, 50.53; H, 3.3; N, 22.92; S, 7.49. Found: C, 50.3; H, 3.2; N, 23.00; S, 7.72.
4-({4-Amino-6-[(1E)-1-cyano-2-(3-fluorophenyl)eth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12i). Yield (335 mg, 81.5%) as white powder with m.p = 243–245 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 7.27 (2H, s, NH2), 7.50–7.57 (3H, m, NH2 + 4-H of phenyl ring), 7.67–7.76 (3H, m, 5-H of phenyl ring + 3,5-H2 of sulfanilamide ring), 7.87 (2H, s, 2,6-H2 of phenyl ring), 8.04 (2H, s, 2,6-H2 of sulfanilamide ring), 8.62 (1H, s, C=C–H) 10.11 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 111.86, 116.14, 116.32, 116.57, 119.01, 119.17, 119.38, 126.41, 126.76, 131.41, 131.47, 134.40, 134.46, 137.25, 142.76, 148.09, EI-MS: m/z: 411.47 [M+]. Anal. Calcd. For C18H14N7O2S: C, 52.55; H, 3.43; N, 23.83; S, 7.79. Found: C, 52.81; H, 3.6; N, 23.99; S, 8.00.
4-({4-Amino-6-[(1E)-1-cyano-2-(3-methylphenyl)eth-1-en-1-yl]-1,3,5-triazin-2-yl}amino)benzene-1-sulfonamide (12j). Yield (283 mg, 69.5%) as pale red powder with m.p = 238–240 °C. 1H NMR (400 MHz, DMSO‑d6) δ (ppm): 2.52 (3H, s, CH3) 7.23–7.35 (3H, m, 4-H of phenyl ring + NH2), 7.56–7.65 (5H, m, 2,5-H2 + 6-H of phenyl ring + NH2), 7.75 (2H, s, 3,5-H2 of sulfanilamide ring), 8.05 (2H, s, 2,6-H2 of sulfanilamide ring), 8.62 (1H, s, C=C–H) 10.1 (1H, s, NH). 13C NMR (100 MHz, DMSO) δ (ppm): 110.38, 114.73, 116.88, 118.38, 119.32, 122.99, 126.36, 130.46, 133.43, 137.20, 142.79, 149.43, 159.50, 163.99, 166.56, 167.55, EI-MS: m/z: 403.39 [M+]. Anal. Calcd. For C19H17N7O3S: C, 53.89; H, 4.05; N, 23.15; S, 7.57. Found: C, 54.1; H, 4.32; N, 23.4; S, 7.78.
Biological evaluation
CA inhibitory assay
Four CA isoforms inhibition activity was measured by assaying CA catalyzed CO2 hydration via an applied photophysics stopped-flow instrument 37. PRISM 3 and the Cheng-prusoff equation were used to determine the inhibition constant by non-linear least square methods as reported 55, and represent the mean from three different determination at least. All tested carbonic anhydrase isoforms were recombinant proteins prepared in lab as described earlier 56,57.
In vitro anticancer screening on NCI 60 cancer cell lines
Protocol of Drug Evaluation Branch, NCI, Bethesda, was adopted in the antitumor assay 58. Assessment of cell growth and viability was carried out utilizing SRB assay and 48 h drug exposure protocol as previously reported 39,40.
Antiproliferative activity towards MDA-MB-468 and CCRF-CEM cell lines
Breast cancer MDA-MB-468 and leukemia CCRF-CEM cell lines were obtained from American Type Culture Collection (ATCC). All cells were incubated in hypoxic atmosphere (1% O2, 5% CO2, and 94%N2) and maintained at 37˚C. All procedures to determine compounds cytotoxicity were accomplished using MTT assay as reported 44,59,60.
Annexin V-FITC apoptosis assay
Cells apoptosis further analyzed by measuring phosphatidylserine externalization using V-FITC/PI apoptosis detection kit (BD Biosciences, USA) according manufacturer's instructions, as reported 61,62.
Cell cycle analysis
Breast cancer MDA-MB-468 cells were treated for 24 h with the IC50 concentrations of sulfonamide 12d, (3.99 µM) and then ice-cold phosphate buffered saline (PBS) was used to rinse the cells twice. The treated cells were centrifuged and maintained in ice-cold ethanol (70%) before being washed again with PBS and then re-suspended in RNase (100 mg/mL) followed by staining with 40 µg/mL PI, and analyzed by flow cytometry technique using a FACS Calibur (Becton Dickinson,BD, USA). Cell cycle distribution were calculated via CellQuest software 5.1 (Becton Dickinson) 44,63.
Western blot analysis
MDA-MB-468 cells, were harvested on dishes of cell culture and treated for 24 h with compound 12d. An equal amount of protein transferred to PVDF membranes after separated by SDS-PAGE (5–15%). Skim milk with concentration 5% was used to block the blotted membranes for 1 h before being incubated with the desired primary antibodies at 37 °C for 1 h. Colorimetric technique was used to visualize immune reactive bands using Geldoc-it, UVP, England. To quantify equal loading, membrane was reseeded with β-actin antibody 64.
HEK-293 cell growth inhibition assay
HEK 293 cell growth inhibition assay was performed according to the procedure previously published 42. All data was collected in triplicate, and IC50 values were calculated as the average of three separate trials.
Molecular docking
To investigate possible binding modes, carbonic anhydrase IX crystal structure (PDB: 3IAI)53, ligands preparation and the docking process were carried out using Molecular Operating Environment 2020 (MOE) software package52 and the binding mode for analogues 12d and 12i were visualized in 2D with the help of Discovery Studio v20.1.019295.
Supplementary Information
Author contributions
M.H.E.-H. designed the whole study and revised the manuscript. T.F.E.-M. and N.S. supervised the chemistry work, while A.I.Z.-A. synthesized the compounds and write the manuscript. C.T.S. and A.A. performed the biology experimental.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdelrahman I. Zain-Alabdeen, Email: abdelrahman.ibrahim@pharm.tanta.edu.eg
Claudiu T. Supuran, Email: claudiu.supuran@unifi.it
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21024-7.
References
- 1.Supuran CT. Carbonic anhydrases: From biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J. Enzyme Inhib. Med. Chem. 2013;28:229–230. doi: 10.3109/14756366.2013.761876. [DOI] [PubMed] [Google Scholar]
- 2.Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med. Res. Rev. 2020;40:2485–2565. doi: 10.1002/med.21713. [DOI] [PubMed] [Google Scholar]
- 3.Uslu AG, et al. Benzimidazole derivatives as potent and isoform selective tumor-associated carbonic anhydrase IX/XII inhibitors. Bioorg. Chem. 2020;95:103544. doi: 10.1016/j.bioorg.2019.103544. [DOI] [PubMed] [Google Scholar]
- 4.de Sousa ET, Lima-Holanda AT, Sales LS, Nobre-dos-Santos M. Combined effect of starch and sucrose on carbonic anhydrase VI activity in saliva and biofilm of children with early childhood caries. Exposure to starch and sucrose alters carbonic anhydrase VI activity in saliva and biofilm. Clin. Oral Investig. 2021;25:2555–2568. doi: 10.1007/s00784-020-03567-z. [DOI] [PubMed] [Google Scholar]
- 5.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 6.Supuran CT. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin. Ther. Pat. 2018;28:713–721. doi: 10.1080/13543776.2018.1519023. [DOI] [PubMed] [Google Scholar]
- 7.Fares M, et al. Discovery of potent dual-tailed benzenesulfonamide inhibitors of human carbonic anhydrases implicated in glaucoma and in vivo profiling of their intraocular pressure-lowering action. J. Med. Chem. 2020;63:3317–3326. doi: 10.1021/acs.jmedchem.9b02090. [DOI] [PubMed] [Google Scholar]
- 8.Mboge MY, Mahon BP, McKenna R, Frost SC. Carbonic anhydrases: role in pH control and cancer. Metabolites. 2018;8:19. doi: 10.3390/metabo8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastorek, J. & Pastorekova, S. in Seminars in Cancer Biology. 52–64 (Elsevier). [DOI] [PubMed]
- 10.Mboge MY, McKenna R, Frost SC. Advances in anti-cancer drug development targeting carbonic anhydrase IX and XII. Top. Anti-cancer Res. 2015;5:3. [PMC free article] [PubMed] [Google Scholar]
- 11.Supuran CT, et al. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med. Res. Rev. 2018;38:1799–1836. doi: 10.1002/med.21497. [DOI] [PubMed] [Google Scholar]
- 12.Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites. 2017;7:48. doi: 10.3390/metabo7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nocentini A, Supuran CT. Carbonic anhydrase inhibitors as antitumor/antimetastatic agents: A patent review (2008–2018) Expert Opin. Ther. Pat. 2018;28:729–740. doi: 10.1080/13543776.2018.1508453. [DOI] [PubMed] [Google Scholar]
- 14.De Simone G, Supuran CT. Carbonic anhydrase IX: Biochemical and crystallographic characterization of a novel antitumor target. Biochim. Biophys. Acta BBA Proteins Proteom. 2010;1804:404–409. doi: 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Ansari MF, et al. Design, synthesis and biological evaluation of novel pyridine-thiazolidinone derivatives as anticancer agents: Targeting human carbonic anhydrase IX. Eur. J. Med. Chem. 2018;144:544–556. doi: 10.1016/j.ejmech.2017.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Kumar S, Rulhania S, Jaswal S, Monga V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2021;209:112923. doi: 10.1016/j.ejmech.2020.112923. [DOI] [PubMed] [Google Scholar]
- 17.Manzoor S, Petreni A, Raza MK, Supuran CT, Hoda N. Novel triazole-sulfonamide bearing pyrimidine moieties with carbonic anhydrase inhibitory action: Design, synthesis, computational and enzyme inhibition studies. Bioorg. Med. Chem. Lett. 2021;48:128249. doi: 10.1016/j.bmcl.2021.128249. [DOI] [PubMed] [Google Scholar]
- 18.Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012;112:4421–4468. doi: 10.1021/cr200176r. [DOI] [PubMed] [Google Scholar]
- 19.Pacchiano F, et al. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J. Med. Chem. 2011;54:1896–1902. doi: 10.1021/jm101541x. [DOI] [PubMed] [Google Scholar]
- 20.Elimam DM, et al. Natural inspired ligustrazine-based SLC-0111 analogues as novel carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2022;228:114008. doi: 10.1016/j.ejmech.2021.114008. [DOI] [PubMed] [Google Scholar]
- 21.Akocak S, Lolak N, Bua S, Supuran CT. Discovery of novel 1, 3-diaryltriazene sulfonamides as carbonic anhydrase I, II, VII, and IX inhibitors. J. Enzyme Inhib. Med. Chem. 2018;33:1575–1580. doi: 10.1080/14756366.2018.1515933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mboge MY, et al. Inhibition of carbonic anhydrase using SLC-149: Support for a noncatalytic function of CAIX in breast cancer. J. Med. Chem. 2021;64:1713–1724. doi: 10.1021/acs.jmedchem.0c02077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Congiu C, Onnis V, Balboni G, Supuran CT. Synthesis and carbonic anhydrase I, II, IX and XII inhibition studies of 4-N, N-disubstituted sulfanilamides incorporating 4, 4, 4-trifluoro-3-oxo-but-1-enyl, phenacylthiourea and imidazol-2 (3H)-one/thione moieties. Bioorg. Med. Chem. Lett. 2014;24:1776–1779. doi: 10.1016/j.bmcl.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Nocentini A, et al. Benzenesulfonamides incorporating flexible triazole moieties are highly effective carbonic anhydrase inhibitors: synthesis and kinetic, crystallographic, computational, and intraocular pressure lowering investigations. J. Med. Chem. 2016;59:10692–10704. doi: 10.1021/acs.jmedchem.6b01389. [DOI] [PubMed] [Google Scholar]
- 25.Kothayer H, et al. Optimised synthesis of diamino-triazinylmethyl benzoates as inhibitors of Rad6B ubiquitin conjugating enzyme. Tetrahedron Lett. 2014;55:7015–7018. doi: 10.1016/j.tetlet.2014.10.122. [DOI] [Google Scholar]
- 26.Lolak N, Akocak S, Bua S, Supuran CT. Design, synthesis and biological evaluation of novel ureido benzenesulfonamides incorporating 1, 3, 5-triazine moieties as potent carbonic anhydrase IX inhibitors. Bioorg. Chem. 2019;82:117–122. doi: 10.1016/j.bioorg.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Mikuš P, et al. Novel sulfonamides incorporating 1, 3, 5-triazine and amino acid structural motifs as inhibitors of the physiological carbonic anhydrase isozymes I, II and IV and tumor-associated isozyme IX. Bioorg. Chem. 2018;81:241–252. doi: 10.1016/j.bioorg.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh R, Bharathkar SK, Kishore N. Anticancer altretamine recognition by bovine serum albumin and its role as inhibitor of fibril formation: Biophysical insights. Int. J. Biol. Macromol. 2019;138:359–369. doi: 10.1016/j.ijbiomac.2019.07.093. [DOI] [PubMed] [Google Scholar]
- 29.Montesinos P, et al. Safety and efficacy of talacotuzumab plus decitabine or decitabine alone in patients with acute myeloid leukemia not eligible for chemotherapy: results from a multicenter, randomized, phase 2/3 study. Leukemia. 2021;35:62–74. doi: 10.1038/s41375-020-0773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng H-L, Ma X, Chew E-H, Chui W-K. Design, synthesis, and biological evaluation of coupled bioactive scaffolds as potential anticancer agents for dual targeting of dihydrofolate reductase and thioredoxin reductase. J. Med. Chem. 2017;60:1734–1745. doi: 10.1021/acs.jmedchem.6b01253. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Poon T-Y, Wong PTH, Chui W-K. Synthesis and in vitro evaluation of 2, 4-diamino-1, 3, 5-triazine derivatives as neuronal voltage-gated sodium channel blockers. Bioorg. Med. Chem. Lett. 2009;19:5644–5647. doi: 10.1016/j.bmcl.2009.08.052. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, et al. Synthesis and antimicrobial activity of N1-benzyl or N1-benzyloxy-1, 6-dihydro-1, 3, 5-triazine-2, 4-diamines. Bioorg. Med. Chem. Lett. 2011;21:5428–5431. doi: 10.1016/j.bmcl.2011.06.125. [DOI] [PubMed] [Google Scholar]
- 33.Makowska A, Sączewski F, Bednarski PJ, Sączewski J, Balewski Ł. Hybrid molecules composed of 2, 4-diamino-1, 3, 5-triazines and 2-imino-coumarins and coumarins. Synthesis and cytotoxic properties. Molecules. 2018;23:1616. doi: 10.3390/molecules23071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furukawa M, Kojima Y, Hayashi S. Reaction of biguanides and related compounds. VII. The condensation of arylbiguanide with α, β-unsaturated carboxylic ester in dimethylformamide. Chem. Pharm. Bull. 1973;21:1126–1131. doi: 10.1248/cpb.21.1126. [DOI] [Google Scholar]
- 35.Pomarnacka E, Bednarski P, Grunert R, Reszka P. Synthesis and anticancer activity of novel 2-amino-4-(4-phenylpiperazino)-1, 3, 5-triazine derivatives. Acta Pol. Pharm. Drug Res. 2004;61:461–467. [PubMed] [Google Scholar]
- 36.Sączewski F, Bułakowska A, Bednarski P, Grunert R. Synthesis, structure and anticancer activity of novel 2, 4-diamino-1, 3, 5-triazine derivatives. Eur. J. Med. Chem. 2006;41:219–225. doi: 10.1016/j.ejmech.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase: I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971;246:2561–2573. doi: 10.1016/S0021-9258(18)62326-9. [DOI] [PubMed] [Google Scholar]
- 38.Eldehna WM, et al. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: Design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur. J. Med. Chem. 2019;162:147–160. doi: 10.1016/j.ejmech.2018.10.068. [DOI] [PubMed] [Google Scholar]
- 39.Abo-Ashour MF, et al. Novel indole-thiazolidinone conjugates: Design, synthesis and whole-cell phenotypic evaluation as a novel class of antimicrobial agents. Eur. J. Med. Chem. 2018;160:49–60. doi: 10.1016/j.ejmech.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Abdel-Aziz AA-M, et al. Synthesis and potential antitumor activity of 7-(4-substituted piperazin-1-yl)-4-oxoquinolines based on ciprofloxacin and norfloxacin scaffolds: in silico studies. J. Enzyme Inhib. Med. Chem. 2016;31:796–809. doi: 10.3109/14756366.2015.1069288. [DOI] [PubMed] [Google Scholar]
- 41.Shaldam M, et al. Development of novel benzofuran-based SLC-0111 analogs as selective cancer-associated carbonic anhydrase isoform IX inhibitors. Eur. J. Med. Chem. 2021;216:113283. doi: 10.1016/j.ejmech.2021.113283. [DOI] [PubMed] [Google Scholar]
- 42.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 43.Eldehna WM, et al. Synthesis, biological evaluation and in silico studies with 4-benzylidene-2-phenyl-5 (4H)-imidazolone-based benzenesulfonamides as novel selective carbonic anhydrase IX inhibitors endowed with anticancer activity. Bioorg. Chem. 2019;90:103102. doi: 10.1016/j.bioorg.2019.103102. [DOI] [PubMed] [Google Scholar]
- 44.Said MA, et al. Sulfonamide-based ring-fused analogues for CAN508 as novel carbonic anhydrase inhibitors endowed with antitumor activity: Design, synthesis, and in vitro biological evaluation. Eur. J. Med. Chem. 2020;189:112019. doi: 10.1016/j.ejmech.2019.112019. [DOI] [PubMed] [Google Scholar]
- 45.Bertoli C, Skotheim JM, De Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013;14:518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dokla EM, et al. Indolin-2-one derivatives as selective Aurora B kinase inhibitors targeting breast cancer. Bioorg. Chem. 2021;117:105451. doi: 10.1016/j.bioorg.2021.105451. [DOI] [PubMed] [Google Scholar]
- 47.Eldehna WM, et al. Development of isatin-thiazolo [3, 2-a] benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity: Synthesis, biological and molecular dynamics investigations. Bioorg. Chem. 2021;110:104748. doi: 10.1016/j.bioorg.2021.104748. [DOI] [PubMed] [Google Scholar]
- 48.Pietkiewicz S, Schmidt JH, Lavrik IN. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods. 2015;423:99–103. doi: 10.1016/j.jim.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 50.Gorr TA, Vogel J. Western blotting revisited: critical perusal of underappreciated technical issues. Proteom. Clin. Appl. 2015;9:396–405. doi: 10.1002/prca.201400118. [DOI] [PubMed] [Google Scholar]
- 51.Grela E, Kozłowska J, Grabowiecka A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018;120:303–311. doi: 10.1016/j.acthis.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Molecular Operating Environment (MOE), C. C. G. U., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2022.
- 53.Alterio V, et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc. Natl. Acad. Sci. 2009;106:16233–16238. doi: 10.1073/pnas.0908301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supuran CT. Carbonic anhydrases as drug targets-an overview. Curr. Top. Med. Chem. 2007;7:825–833. doi: 10.2174/156802607780636690. [DOI] [PubMed] [Google Scholar]
- 55.Fares M, et al. Synthesis of bulky-tailed sulfonamides incorporating pyrido [2, 3-d][1, 2, 4] triazolo [4, 3-a] pyrimidin-1 (5H)-yl) moieties and evaluation of their carbonic anhydrases I, II, IV and IX inhibitory effects. Bioorg. Med. Chem. 2017;25:2210–2217. doi: 10.1016/j.bmc.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 56.Eldehna WM, et al. Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in vitro antitumor activity towards colon cancer. Bioorg. Chem. 2018;81:425–432. doi: 10.1016/j.bioorg.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Leitans J, et al. Efficient expression and crystallization system of cancer-associated carbonic anhydrase isoform IX. J. Med. Chem. 2015;58:9004–9009. doi: 10.1021/acs.jmedchem.5b01343. [DOI] [PubMed] [Google Scholar]
- 58.Teicher BA. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval. Springer; 2013. [Google Scholar]
- 59.Tatiparti K, Sau S, Gawde KA, Iyer AK. Copper-free ‘click’chemistry-based synthesis and characterization of carbonic anhydrase-IX anchored albumin-paclitaxel nanoparticles for targeting tumor hypoxia. Int. J. Mol. Sci. 2018;19:838. doi: 10.3390/ijms19030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sagardoy AA, et al. Benzo [b] thiophene-6-carboxamide 1, 1-dioxides: Inhibitors of human cancer cell growth at nanomolar concentrations. Bioorg. Med. Chem. 2010;18:5701–5707. doi: 10.1016/j.bmc.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Eldehna WM, et al. Novel [(3-indolylmethylene) hydrazono] indolin-2-ones as apoptotic anti-proliferative agents: design, synthesis and in vitro biological evaluation. J. Enzyme Inhib. Med. Chem. 2018;33:686–700. doi: 10.1080/14756366.2017.1421181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eldehna WM, et al. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J. Enzyme Inhib. Med. Chem. 2017;32:600–613. doi: 10.1080/14756366.2017.1279155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sabt A, et al. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: synthesis, in vitro biological evaluation, and QSAR studies. J. Enzyme Inhib. Med. Chem. 2018;33:1095–1107. doi: 10.1080/14756366.2018.1477137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gul HI, et al. Anticancer effects of new dibenzenesulfonamides by inducing apoptosis and autophagy pathways and their carbonic anhydrase inhibitory effects on hCA I, hCA II, hCA IX, hCA XII isoenzymes. Bioorg. Chem. 2018;78:290–297. doi: 10.1016/j.bioorg.2018.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].