Abstract
Emergence of radioresistance in prostate cancer (PCa) cells is a major obstacle in cancer therapy and contributes to the relapse of the disease. EGF receptor (EGFR) signaling plays an important role in the development of radioresistance. Herein, we have assessed the modulatory effects of silibinin on radiation-induced resistance via DNA repair pathways in EGFR-knockdown DU145 cells. shRNA-based silencing of EGFR was done in radioresistant human PCa DU145 cells and effects of ionizing radiation (IR) and silibinin were assessed using clonogenic and trypan blue assays. Furthermore, radiosensitizing effects of silibinin on PCa in context with EGFR were analyzed using flow cytometry, comet assay, and immunoblotting. Silibinin decreased the colony formation ability with an increased death of DU145 cells exposed to IR (5 Gray), with a concomitant decrease in Rad51 protein expression. Silibinin (25 μM) augmented the IR-induced cytotoxic effect in EGFR-knockdown PCa cells, along with induction of G2/M phase cell cycle arrest. Further, we studied homologous recombination (HR) and non-homologous end joining (NHEJ) pathways in silibinin-induced DNA double-strand breaks in EGFR-knockdown DU145 cells. Silibinin down-regulated the expression of Rad51 and DNA-dependent protein kinase proteins without any considerable effect on Ku70 and Ku80 in IR-exposed EGFR-knockdown PCa cells. The pro-survival signaling proteins, phospho-extracellular signal-regulated kinases (ERK)1/2, phospho-Akt and phospho-STAT3 were decreased by silibinin in EGFR-deficient PCa cells. These findings suggest a novel mechanism of silibinin-induced radiosensitization of PCa cells by targeting DNA repair pathways, HR and NHEJ, and suppressing the pro-survival signaling pathways, ERK1/2, Akt and STAT3, in EGFR-knockdown PCa cells.
Keywords: Prostatic neoplasms, Silybin, Radiosensitization, Epidermal growth factor receptor, DNA repair pathways
INTRODUCTION
Prostate cancer (PCa) usually originates from epithelial cells in prostate gland, a major exocrine gland having functional association with reproduction in men. PCa is the second most commonly diagnosed cancer in men after the lung cancer with higher prevalence in developed countries. Since the incidence of PCa is rising [1,2], the improvement in its management including active surveillance, chemotherapy and radiation therapy (RT) is desired [3]. Over the years, RT is pivotal in management of PCa with curative and palliative intent [4-6]. However, development of therapeutic resistance to the RT is still one of the major stumbles while treating PCa patients.
Emergence of therapeutic resistance to RT in tumor cells is a complex process which is regulated by several factors including DNA damage and repair, mutations, chromosomal instability, activation of signaling pathways to adapt to radiotherapy-induced changes leading to development of radioresistance [7]. Ionizing radiation (IR)-induced DNA strand breaks can trigger a cascade of signaling activation resulting in DNA damage responses (DDRs) which rescue the cancer cells from radiation injuries via inducing DNA repair activation and cell cycle arrest [8]. The homologous recombination (HR) and non-homologous end joining (NHEJ) repair pathways mediate the activation of double strand breaks (DSBs) repair genes and were found to be altered and associated with development of radioresistance in cancer [9]. Studies have also highlighted the importance of targeting NHEJ and HR pathways in radiosensitizing the tumor cells [10]. Therefore, the understanding of mechanistic insights of regulation of these major DNA repair pathways in PCa is warranted.
Studies have suggested the role of membrane receptor tyrosine kinase, EGF receptor (EGFR), in regulating DNA repair machinery through HR and NHEJ pathways. Recently, we reported the key role of EGFR in regulation of intrinsic radioresistance in PCa by targeting DNA DSBs repair proteins, DNA-dependent protein kinase (PK) and Rad51 [11]. Some plant-derived small molecules have gained significant attention due to their lower toxicity to normal tissues and capability to sensitize the cancer cells to the radiation [12-14]. Our previous study has shown the role of small molecule, silibinin in radiosensitizing PCa cells by inhibiting the radiation-induced nuclear translocation of EGFR [15].
The mechanistic insights of silibinin-mediated radiosensitizing effect by regulating major DNA DSBs repair pathways, HR and NHEJ, through EGFR signaling have not been studied in PCa. In the present study, we investigated the effect of silibinin on the IR-induced biological and molecular events in EGFR-knockdown radioresistant PCa cells. Our findings suggested Rad51 and DNA-PK as molecular targets of silibinin in radiosensitizing the EGFR-deficient PCa cells by attenuating HR and NHEJ pathways.
MATERIALS AND METHODS
Chemicals and reagents
Antibodies for Rad51, Ku70, Ku80, p53, Akt, extracellular signal-regulated kinase (ERK)1/2, STAT3, proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase 1 (CDK1), Cdc25c, and phosphoproteins (p-CDK1-Tyr15, p-p53-Ser15, p-Akt-Ser473, p-ERK1/2-Thr202/Tyr204, p-STAT3-Tyr705) were purchased from Cell Signaling Technology (Beverly, MA, USA). DNA-PK and cyclin B1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). β-actin antibody was from Sigma Aldrich (St. Louis, MO, USA). Silibinin (S0417) was purchased from Sigma Aldrich.
Mammalian cell culture
Prostate carcinoma DU145 cells (NCCS, Pune, India) were cultured in RPMI-1640 media supplemented with 10% FBS (Gibco, Mumbai, India) and 1× Penicillin-Streptomycin-Amphotericin antibiotic. Cell cultures were maintained in 5% CO2 humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C temperature.
Short hairpin RNA based silencing of EGFR in DU145 cells
The recptor tyrosine kinase, EGFR, was knockdown in radioresistant DU145 PCa cells using lentiviral-based system and the cloning vector, pLKO.1. Experimentally validated shEGFR sequence for knockdown of the kinase was retrieved from https://www.sigmaaldrich.com/IN/en/semi-configurators/shrna?activeLink=selectClones and cloned in lentiviral vector, pLKO.1. Further, lentiviral particles were generated with the help of helper plasmids (psPAX2 and pMD2.G) and particles were transduced to generate stable EGFR-knockdown DU145 cells as detailed in the previous study [16].
Colony formation assay
Briefly, DU145 vector control (pLKO.1) and EGFR knockdown cells (shEGFR) were harvested and seeded at an appropriate density in a 6-well plate. After 24 hours, treatments of silibinin (25 µM) and/or IR (5 Gray [Gy]) were given and cells were maintained at 37°C in an incubator for 8 days. At the end of the experiment, cells were fixed with 4% formaldehyde and stained with 0.05% crystal violet as described in the previous study [15], and colonies containing more than 50 cells were counted.
Trypan blue dye exclusion assay
DU145 vector control (pLKO.1) and EGFR knockdown cells (shEGFR) were used for the trypan blue dye exclusion assay. In brief, after trypsinization, cells were counted and seeded at a density of 4×104 cells/well in a 12-well plate and treated with silibinin (25 µM) and/or IR (5 Gy). After the 48-hour treatments, cells were trypsinized, collected and processed for trypan blue staining and counted for live and dead cells as described earlier [17]. The coefficient of drug interaction (CDI) value was calculated as detailed in earlier studies [18,19]. The CDI value < 1 and > 1 indicates synergistic and antagonistic effects, respectively, while CDI value = 1 means the effect in combination is additive [18,19].
Cell cycle analysis using flow cytometry
Briefly, DU145 vector control (pLKO.1) and EGFR knockdown cells (shEGFR) were seeded at a density of 4×104 cells/well and after 24 hours, treated with silibinin (25 µM) and/or IR (5 Gy). After the 48-hour treatment, cells were harvested and resuspended in FACS cocktail solution [RNaseA (10 µg/mL), propidium iodide (25 µg/mL), saponin (0.2%) and Ethylenediaminetetraacetic acid (0.1 mM)] and kept overnight in dark at 4°C [20]. The cells were analyzed for cell cycle phase distribution by FACS (BD FACSAria, Franklin Lakes, NJ, USA) and data was analyzed using FACS Diva Software (Becton Dickinson, Franklin Lakes, NJ, USA).
Alkaline comet assay for DNA damage
DNA strand breaks were analyzed using the alkaline comet assay as described earlier [21]. Briefly, cells were trypsinized, counted and seeded at a density of 4×104 cells/well in 12-well plate and after 24 hours, treated with silibinin (25 µM) and/or IR (5 Gy). At the end of the treatments, cells were trypsinized briefly and 15 × 103 cells/0.5 mL in low melting point agarose was coated on the surface of a microscopic slide. Slides were then processed in single cell gel electrophoresis and stained with ethidium bromide (4 µg/mL) and subsequently visualized in fluorescent microscopy for the images. CometScore 2.0 software (http://rexhoover.com/index.php?id=cometscore) was used for scoring comet tail length and tail DNA percentage [21].
Western blotting analysis
Cells were seeded and treated with silibinin and IR as desired. After respective treatment time points, cells were harvested and whole cell lysate preparation, protein quantification and immunoblotting were performed as detailed in the earlier study [22].
Gamma irradiation protocol
Cells were irradiated at 5 Gy dose in 1× PBS followed by treatment with low dose of silibinin (25 µM). Cells were irradiated in common instrument fac60Co gamma chamber (Model 5000A; Bhabha Atomic Research Centre, Mumbai, India) at a dose rate 0.5 Gy/second.
Statistical analysis
All data were statistically analyzed using GraphPad Prism (version 5.0.0; GraphPad Software, San Diego, CA, USA). The analysis for comet assay data was done using CometScore 2.0 software (http://rexhoover.com/index.php?id=cometscore). One-way ANOVA test was used for analysis of the statistical significance and P-value less than 0.05 considered significant. Fold change values of the immunoblots were calculated using Image J software (https://imagej.nih.gov/ij/).
RESULTS
Silibinin radiosensitized human PCa DU145 radioresistant cells via Rad51
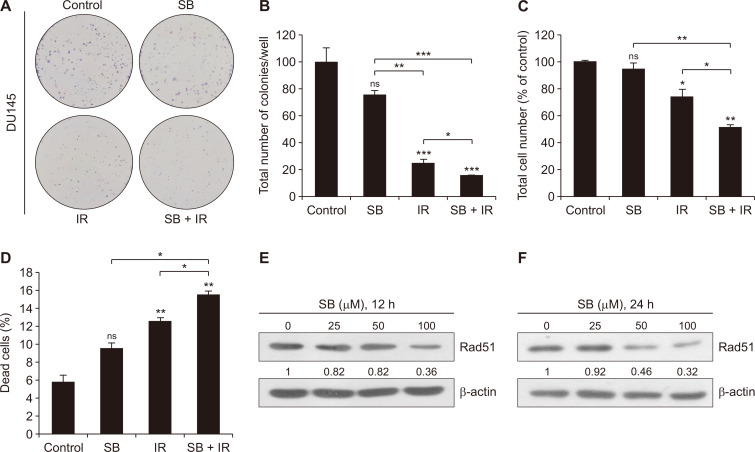
We studied the effect of a low dose of silibinin on modulating the IR effects in PCa cells. We treated radioresistant DU145 cells with silibinin (25 µM) and/or IR (5 Gy) for the colony formation assay. We observed the synergistic effect (CDI value = 0.83) of combined treatment with silibinin and IR on the reduction in the clonogenic potential of DU145 cells (Fig. 1A and 1B). Treatment with silibin alone induced moderate (24.79%) inhibition of colony formation ability; however, IR alone treatment strongly inhibited (74.4%, P < 0.001) the clonogenic potential in DU145 cells, which was further enhanced by 84.4% (P < 0.001, CDI < 1) following combined treatment as compared to control (Fig. 1A and 1B).
Figure 1. Combinatorial effects of a low dose of SB and IR on clonogenic potential and proliferation of DU145 cells.
(A) Human PCa DU145 cells were seeded in a 6-well culture plate at a density of 600 cells/well and treated with either SB (25 µM) or IR (5 Gy) or in combination and were maintained in a humidified CO2 incubator. After 10 days, plates were processed for the clonogenic assay as described in MATERIALS AND METHODS. Representative images for each treatment group and (B) quantitative data are represented as the total number of colonies/well. (C, D) Fourty thousand cells/well seeded in a 12-well plate were treated with SB (25 µM) and IR (5 Gy). After the 48-hour treatments, cells were trypsinized, harvested and processed for trypan blue staining and live and dead cells were counted using haemocytometer. (E, F) At ~70% confluency, DU145 cells were treated with SB (25, 50, and 100 µM) and harvested after 12 and 24 hours. Whole cell lysates were prepared as described in MATERIALS AND METHODS, and immunoblotting was done for Rad51 protein expression and β-actin was used as loading control. Data are presented as mean ± SE of triplicate samples for each treatment. Results are representative of three sets of independent experiments. Gy, gray; SB, silibinin; IR, ionizing radiation; PCa, prostate cancer; SE, standard error; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Further, the effect of silibinin and IR on cell proliferation was assessed by the trypan blue assay after the 48-hour treatment. Silibinin and IR reduced the total number of cells by 5.4% and 26.3% (P < 0.05), respectively, however, the combination of both significantly (P < 0.01) decreased the cell number by 49.0% (P < 0.01) as compared to control. Similar to the colony formation assay, the combined treatment synergistically (CDI value = 0.74) reduced the proliferation rate in DU145 cells (Fig. 1C). Under similar conditions, the silibinin and IR combination caused 15.4% (P < 0.01) cell death as compared to 9.4% and 12.5% (P < 0.01), in silibinin and IR alone treatments, respectively (Fig. 1D).
In an attempt to gain mechanistic insights, we assessed the effect of silibinin on the expression of Rad51, a key protein involved in the HR pathway in DU145 cells. Silibinin (25-100 µM) showed a decreased expression of Rad51 protein in concentration- as well as time-dependent manner after the 12- and 24-hour of treatments (Fig. 1E and 1F). Collectively, these results suggested that a non-toxic low dose of silibinin enhanced the inhibitory effects of IR on cell proliferation and clonogenicity, and also downregulated the expression of Rad51 in radioresistant DU145 cells.
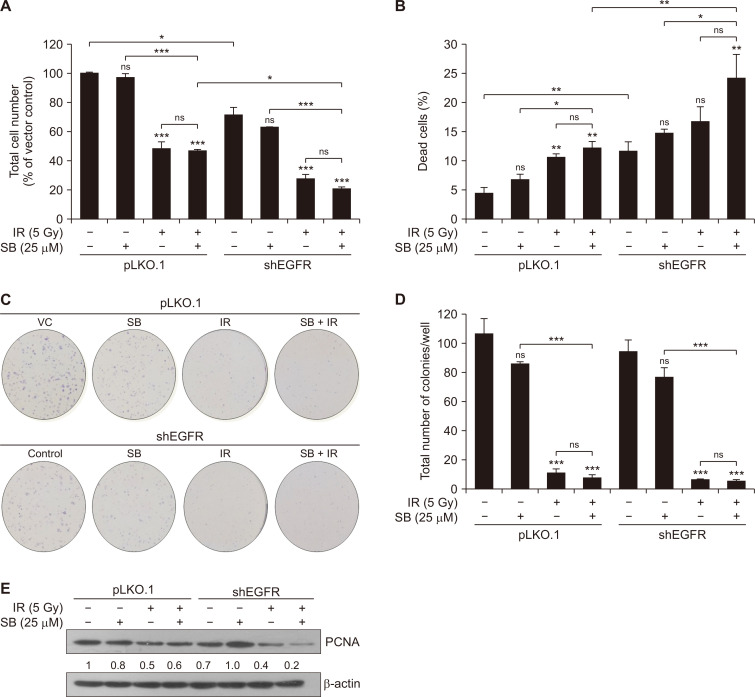
Silibinin augmented IR-induced cytotoxicity and inhibition of clonogenic potential in EGFR-knockdown PCa cells
We assessed the effect of silibinin on the biological effects of IR in EGFR-knockdown DU145 cells. We used pLKO.1 and EGFR-knockdown DU145 cells to assess the effect of silibinin after IR exposure on cell proliferation and clonogenic potential. EGFR-knockdown decreased the total cell number in all the treatments. Further, EGFR-knockdown decreased the survival rate by 29% (P < 0.05) in DU145 cells after 48 hours as compared to control. Furthermore, a low dose of silibinin treatment caused a no significant decrease in the total cell count in pLKO.1 (2.9%) and EGFR-knockdown (8%) DU145 cells at 48 hours when compared to their respective control (Fig. 2A). Concurrently IR treatment alone strongly decreased the total cell count in pLKO.1 and shEGFR cells by 51.6% (P < 0.001) and 44% (P < 0.001), respectively, as compared to their respective control. Combined treatment treatment of IR and silibinin in pLKO.1 and EGFR-knockdown cells reduced the cell survival by 52.9% (P < 0.001) and 50.9% (P < 0.001), respectively when compared to respective controls (Fig. 2A). Notably, we observed that silibinin potentiated the IR effect by 6.9% when compared to IR treatment alone in EGFR-knockdown cells; however, we did not observe any considerable change in vector control in similar treatment conditions (Fig. 2A). Combined treatment of silibinin and IR showed synergistic (CDI value = 0.84) anti-proliferation effects in EGFR-knockdown DU145 cells. Likewise, EGFR knockdown induced 11.6% (P < 0.01) cell death as compared to pLKO.1 control (4.3%) at 48 hours. A low, non-toxic dose of silibinin caused non-significant (3.3%) cell death in pLKO.1 and shEGFR cells when compared to their respective control. The IR treatment alone slightly improved the cell death by 7.3% (P < 0.01) and 5% in pLKO.1 and EGFR-knockdown cells, respectively with their respective control, however, co-treatment enhanced the IR-induced cytotoxicity by 7.8% (P < 0.01) and 12.5% (P < 0.01) in pLKO.1 and EGFR DU145 cells, respectively, under similar conditions (Fig. 2B).
Figure 2. SB enhanced IR-induced cytotoxicity in EGFR-knockdown DU145 cells.
Briefly, pLKO.1 (vector control) and shEGFR DU145 cells were seeded at a density of 4 × 104 cells/well and treated with either SB (25 µM) or IR (5 Gy) or their combination. After the 48-hour treatments, cells were harvested and counted by using the trypan blue assay. Data were quantified and represented as the total number of cells (A) and percent cell death (B). Briefly, 600 cells/well were seeded in a 6-well plate for pLKO.1 and shEGFR DU145 cells and after 24 hours, treated with silibinin and/or IR for the clonogenic assay. (C) Representatives images of the colonies assesed through crystal violet (0.05%) staining at 10 days for various treatment groups. (D) Quantitative data represented as the total number of colonies per well. (E) DU145 knockdown cells were treated with SB (25 µM) and/or IR (5 Gy) and harvested after 48 hours. Cell lysates were prepared and Immunoblotting was done for PCNA and β-actin was used as loading control. Data are presented as means ± SE of triplicate samples for each treatment. Results are representative of three independent experiments. VC, vector control; SB, silibinin; IR, ionizing radiation; EGFR, EGF receptor; Gy, gray; ns, not significant; shEGFR, short hairpin EGFR; PCNA, proliferating cell nuclear antigen; SE, standard error. *P < 0.05, **P < 0.01, *** P < 0.001.
Further, silibinin alone was found to decrease the colony formation irrespective of EGFR status of the cells. However, this effect was strongly reduced in EGFR-knockdown cells. Similar effects were observed in cells with IR alone or silibinin + IR treatments with respect to EGFR status of the cells. Silibinin slightly increased the effect of IR in reduction of colony formation ability in EGFR-knockdown cells (Fig. 2C). EGFR-knockdown alone reduced the total number of colonies by 12.3% when compared to vector control. Silibinin augmented the IR-induced effect and decreased the total number of colonies moderately by 16% in EGFR-knockdown cells when compared to IR alone treatment (Fig. 2D). Further, the decrease in cell growth and proliferation in the combined treatment of silibinin and IR in EGFR-knockdown cells was supported by the strong reduction in PCNA protein expression level (Fig. 2E). Collectively, a low dose of silibinin enhanced the IR-induced inhibition of cell growth, cytotoxicity and reduction in clonogenic potential. Moreover, both silibinin and IR were able to exert anticancer effects in EGFR-deficient PCa cells.
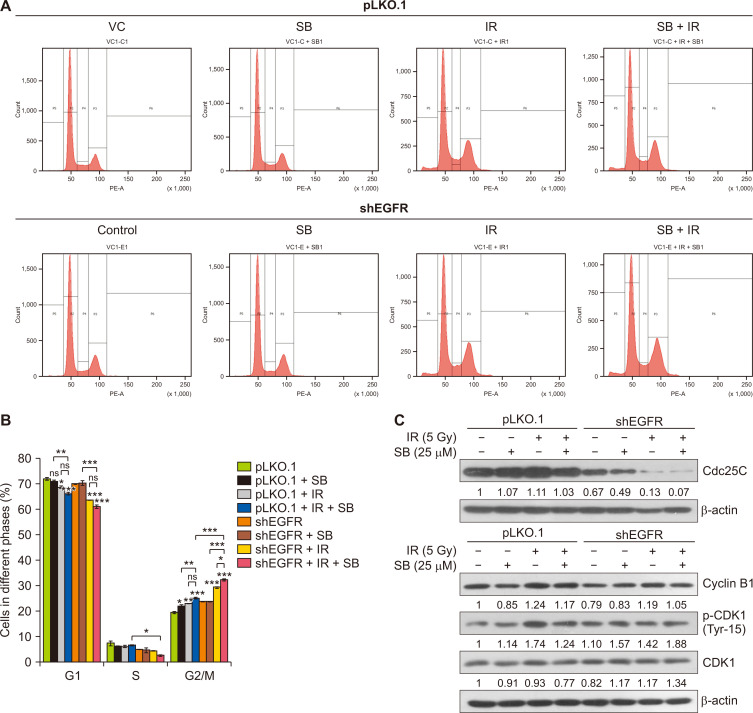
Silibinin enhanced IR-induced G2/M arrest in EGFR-knockdown PCa cells
Radiotherapy halts the cell cycle progression in tumor cells, and hence IR-induced cell cycle arrest is critical to therapeutic responses. Our study has shown that EGFR-knockdown in DU145 cells increased the G2/M phase cell population moderately. The IR treatment increased the G2/M phase cell population from 23.6% in vector control to 29.2% (P < 0.001) in EGFR-knockdown DU145 at 48 hours (Fig. 3A and 3B). When EGFR-knockdown cells were treated a low low dose of silibinin (25 µM), IR-induced G2/M phase arrest increased from 29.2% to 32.1% (P < 0.05) (Fig. 3B). The effect of cell cycle arrest was validated at a molecular level by evaluating the protein expression of CDK1 and Cdc25C in similar treatment conditions. The EGFR-knockdown alone reduced the level of Cdc25C which was further decreased by either silibinin or IR treatment (Fig. 3C). The combination of silibinin and IR strongly decreased the protein levels of Cdc25C as compared to control or alone treatments, but no significant reduction in cyclin B1 protein expression was observed in EGFR-knockdown DU145 cells in these treatments (Fig. 3C). We observed an increased phosphorylation level of CDK1 protein (at Tyr15, the inactivating phosphorylation) in EGFR-knockdown DU145 cells which was found to be correlated with strong reduction in the expression of Cdc25C protein in knockdown cancer cells (Fig. 3C). Hence, silibinin enhanced the radiation-induced G2/M phase of cell cycle arrest in EGFR-knockdown radioresistant PCa cells through targeting the phosphatase Cdc25C which is required to activate the CDK1-cyclin B1 complex.
Figure 3. SB augments IR-induced G2/M arrest in EGFR-knockdown DU145 cells.
pLKO.1 and EGFR-knockdown DU145 cells were seeded at a density of 4×104 cells/well in 12-well culture plates and treated with SB (25 µM) and/or IR (5 Gy). After the 48-hour treatments, cells were harvested and processed for cell cycle analysis by flow cytometry as described in MATERIALS AND METHODS. (A) Representative histogram showing cell cycle phase distribution in various treatments. (B) Quantitative data represented as a percent cell cycle distribution of different phases of cell cycle in various treatments. (C) pLKO.1 and EGFR-knockdown DU145 cells were seeded and treated with a low dose of SB (25 µM) and/or IR (5 Gy), and whole cell lysates were prepared and analyzed for the expression of Cdc25C, CDK1, p-CDK1 (Tyr15) and Cyclin B1 proteins. β-actin was used as a loading control. Data are presented as mean ± SE of duplicate independent wells and are representative of three independent sets of experiments. SB, silibinin; IR, ionizing radiation; EGFR, EGF receptor; VC, vector control; S, synthesis; G1, first gap; G2, second gap, M, Mitosis phases of the cell cycle; shEGFR, short hairpin EGF receptor; CDK1, cyclin-dependent kinase 1; p-CDK1, phospho-CDK1; Gy, gray; SE, standard error; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
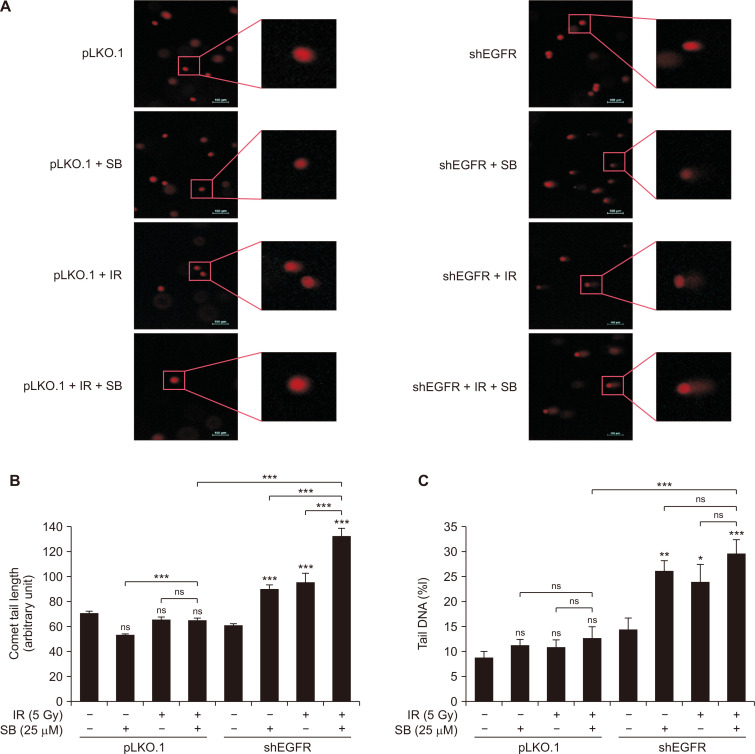
Low dose of silibinin enhanced DNA damaging efficacy of IR in EGFR-knockdown PCa cells
Next, we evaluated the effect of combination of a low dose of silibinin and IR on DNA damage in EGFR-knockdown DU145 cells using an alkaline comet assay. We observed that EGFR-knockdown sensitized the radioresistant PCa cells for DNA damage upon treatment with a low dose of silibinin (25 µM) as well as IR (5 Gy) (Fig. 4A). IR-induced comet tail length in EGFR-knockdown cells increased significantly (P < 0.001) by 35.7% as compared to vector control (Fig. 4B). Further, silibinin strongly increased the IR-induced comet tail length by 39% (P < 0.001) in these cells as compared to IR treatment alone (Fig. 4B). Likewise, tail DNA content was also increased in cells treated with silibinin alone (P < 0.01) or IR (P < 0.05) which was further enhanced by their combined treatment (P < 0.001) in EGFR-knockdown radioresistant PCa cells (Fig. 4C). EGFR-knockdown also increased the tail DNA content. These findings are indicative of accelerated IR-induced DNA damage upon treatment with silibinin in EGFR-knockdown radioresistant PCa cells.
Figure 4. Effect of SB and IR on induction of DNA damage in EGFR-knockdown DU145 cells.
At the end of the treatments, cells were trypsinized briefly and fifteen thousand cells/0.5 mL in low melting point agarose were coated on the surface of a microscopic slide and processed in single cell gel electrophoresis. Slides were further stained with ethidium bromide (4 µg/mL) and subsequently visualized with fluorescent microscope for the images. (A) Representative fluorescent images for various treatments taken at ×100 magnification. Quantitative data represented as (B) comet tail length, and (C) percent content of DNA in tail in respective treatments. Quantitative data presented as mean ± SE of triplicate for each treatment group. Results are representative of three independent experiments. SB, silibinin; IR, ionizing radiation; EGFR, EGF receptor; shEGFR, short hairpin EGF receptor; Gy, gray; SE, standard error; ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Silibinin suppressed the IR-induced expression of critical DSBs repair proteins in EGFR-knockdown PCa cells
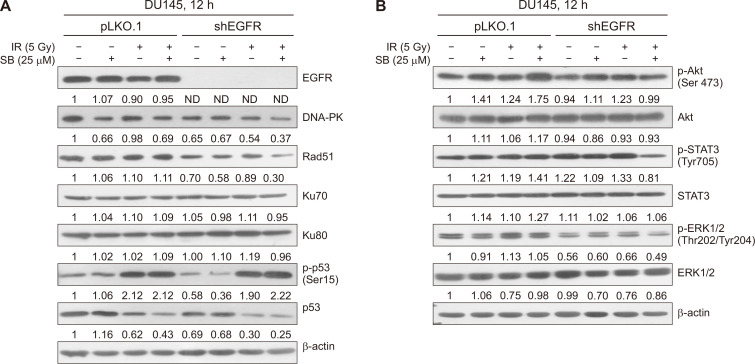
Further, we assessed the role of key molecular proteins involved in DNA DSBs repair pathways, HR and NHEJ. We observed that the silibinin treatment alone suppressed the expression of DNA-PK, a critical protein involved in the NHEJ pathway, in parent DU145 cells when compared to control (Fig. 5A). Silibinin also decreased the protein levels of DNA-PK and Rad51 in EGFR-knockdown cells; however, the levels of Ku70 and Ku80 proteins remained unchanged (Fig. 5A). The combination of silibinin and IR caused a strong decrease in the levels of DNA-PK and Rad51 in EGFR-knockdown cells. Further, silibinin enhanced the IR-induced phosphorylation of p53 protein at serine 15 in EGFR-knockdown DU145 cells, indicating the augmented DNA damage and cell cycle arrest in EGFR-knockdown cells (Fig. 5A). The observed decrease in the total p53 protein level in IR and combination treatments may be indicative of its degradation after the phosphorylation. Hence, in the absence of EGFR signaling, the lower dose of silibinin can enhance the IR-induced DNA damage by blocking HR and NHEJ repair pathways.
Figure 5. Effect of SB and IR on expression of DNA repair and pro-survival signaling proteins in EGFR-knockdown PCa cells.
At ~70% confluency, pLKO.1 and EGFR knockdown DU145 cells were treated with SB (25 µM) and/or IR (5 Gy) for 12 hours and cells were harvested and whole cell lysate was prepared. (A) Whole cell lysates were analyzed for the protein expression of EGFR, DNA-PK, Rad51, Ku70, Ku80, p-p53 and p53 by immunoblotting. β-actin was used as a loading control. (B) Cell lysates were analyzed for p-Akt, Akt, p-STAT3, STAT3, p-ERK1/2, ERK and β-actin proteins. Bands were quantitated using Image J software and represented as fold change with respect to control below each respective band. All the experiments were repeated at least three times. SB, silibinin; IR, ionizing radiation; EGFR, EGF receptor; PCa, prostate cancer; DNA-PK, DNA-dependent protein kinase; p-Akt, phospho-Akt; ERK1/2, extracellular signal-regulated kinases1/2; p-ERK1/2, phospho-extracellular signal-regulated kinases1/2; ND, not detected.
Silibinin inhibited IR-induced pro-survival signaling in EGFR-knockdown PCa cells
Next, we assessed the effect of silibinin on the expression of IR-induced pro-survival signaling molecules, Akt, ERK1/2, and STAT3, involved in initiating the survival response in tumor cells in response to radiotherapy which contributes to the development of radioresistance. Treatment with a low dose of silibinin did not show any considerable effect on the activation of Akt, ERK1/2, and STAT3 in parent or EGFR-knockdown DU145 cells (Fig. 5B). Notably, EGFR-knockdown itself decreased the expression of p-ERK1/2 and p-Akt proteins as compared to vector control. The combined treatment of silibinin and IR in EGFR-knockdown cells strongly decreased the levels of p-STAT3 and p-ERK1/2 without any considerable change in their total protein levels (Fig. 5B). These results suggested that a low dose of silibinin potentially attenuated the survival signaling response in cancer cells exposed to radiation which otherwise would have caused a cytoprotective effect in response to radiotherapy.
DISCUSSION
The central findings in the present study are that silibinin (a) enhanced the anticancer efficacy of IR in EGFR-knockdown DU145 radioresistant PCa cells, (b) targeted the HR pathway by suppressing Rad51 expression, (c) enhanced IR-induced G2/M arrest in EGFR-knockdown cells, (d) augmented IR-induced DNA damage by (e) attenuating the expression of IR-induced critical DSBs DNA repair proteins including Rad51 and DNA-PK, and (f) in combination with IR inhibited pro-survival signaling molecules including ERK1/2, Akt and STAT3 in EGFR signaling deficient radioresistant PCa cells.
This study deciphered the molecular alterations involved in silibinin-mediated radiosensitizing effects in EGFR-deficient radioresistant PCa cells which was mediated through the suppression of DNA repair pathways. As the role EGFR in promoting of IR-induced DNA repair pathways is known, we studied the potential of silibinin in sensitizing the PCa cells deficient in EGFR signaling. In this study, metastatic prostate carcinoma cells, DU145, were used to delineate the mechanisms associated with phytochemical-induced sensitizing effects on PCa owing to high radioresistant trait as compared to PC-3 and LNCaP cells as reported earlier [15,23].
Our previous study has shown that silibinin, at a low dose, potentially radiosensitized the PCa cells to the ionizing radiation [15]. In agreement with these findings, we observed the augmentation in reduction of clonogenicity in DU145 cells in combination of a low dose of silibinin and IR. Concurrently, silibinin enhanced the IR-induced cytotoxicity in radioresistant PCa cells, and decreased the cell proliferation. Further, we investigated the biological event as how silibinin executes its radiosensitizing effects in PCa cells. IR-mediated DNA damage induction is an integral mechanism for its anticancer effects, as effectiveness of radiotherapy mainly lies on its ability to cause lethal DNA DSBs damage in cancer cells; however, IR-induced DDR machinery activation is a major limitation attributed to the efficient DNA damage repair system possessed by cancer cells [24]. Many studies have revealed the role of HR DNA repair pathway in the development of radioresistance in tumor cells because fast proliferating cancer cells are more dependent on this pathway for survival [25,26].
Further, studies have also defined the role of Rad51, a key homologous recombination protein, in therapeutic resistance associated with poor patient survival to chemo- and radiotherapy and advocated that targeting Rad51 could potentially radiosensitized tumor cells [27-29]. In the present study, we observed that lower doses of silibinin reduced the expression of Rad51 protein in radioresistant DU145 cells. Hence, our study suggested the novel role of silibinin in blocking the Rad51-mediated HR pathway in radioresistant PCa cells.
Our recent study has shown that EGFR regulates intrinsic radioresistance in PCa cells via regulation of DNA repair pathway proteins, such as Rad51 and DNA-PK [11]. Therefore, we delved into the mechanistic aspects of modulation of radiation response by silibinin in the context of EGFR signaling. We found that silibinin synergistically inhibited the cell proliferation and clonogenic potential in EGFR-knockdown DU145 cells along with further augmentation of IR-induced cytotoxicity in cells. This was supported by the decreased expression of PCNA in silibinin-treated EGFR-knockdown PCa cells. It is well established fact that the outcome of the radiotherapy or radiosensitivity of the cancer cells may vary in the different phases of the cell cycle [30]. Silibinin was found to enhance the IR-induced G2/M arrest in EGFR-knockdown DU145 cells, which is clinically relevant as blocking cell cycle in G2/M phase will enhance IR-induced cytotoxic effects in a subsequent cycle of radiation owing to most radiosensitive phase of the cell cycle [30].
IR-induced EGFR signaling mediates the DNA DSBs damage repair pathways, HR and NHEJ, as well as pro-survival pathways in tumor cells which confers cytoprotective advantages and thus reduces the efficacy of clinical radiotherapy [31-33]. Consistent with these reports, our findings championed the role of receptor tyrosine kinase by regulating DNA repair pathways as blocking EGFR signaling increased the IR-induced DNA damage in radioresistant PCa cells. Nevertheless, silibinin further increased the IR-induced DNA damage in PCa cells in the absence of EGFR signaling. This observation suggested that silibinin can also alter pathways other than EGFR in sensitizing the PCa cells for IR-induced DNA damage. It may be noted that silibinin suppressed the IR-induced expression of critical DSBs repair proteins, Rad51 and DNA-PK which may be regulated by many upstream molecules including EGFR. This observation suggested that silibinin also has the capability of blocking HR and NHEJ pathways independently of EGFR in response to IR-induced DNA damage in radioresistant PCa cells. Concurrently, we did not observe any change in the expression of Ku70 and Ku80 proteins in our experimental conditions. Our study has indicated that the regulation of Ku proteins is independent of EGFR signaling in DU145 cells, as also reported in our previous finding [11]. Of note, the radiosensitizing effect of silibinin in EGFR-knockdown DU145 cells is mediated by targeting DNA-PK and Rad51 proteins, but not the Ku proteins.
The activation of p53 protein in response to IR-induced DNA damage increases its transcriptional activity for various genes involved in DNA repair, cell cycle arrest and apoptosis [34-36]. However, p53 mutations in the DNA binding domain in DU145 cells render p53 transcriptionally inactive [37] Thus, silibinin as well as IR caused an increase in the phosphorylation of p53 (ser15) irrespective of EGFR status of the cells which was further increased in the combination treatment.
Notably, there was a corresponding decrease in the total p53 protein level which is indicative of its reduced expression or enhanced degradation. These observations suggested that p53 activation is independent of EGFR status of PCa cells and corresponded to the level of DNA damage as well as cell death.
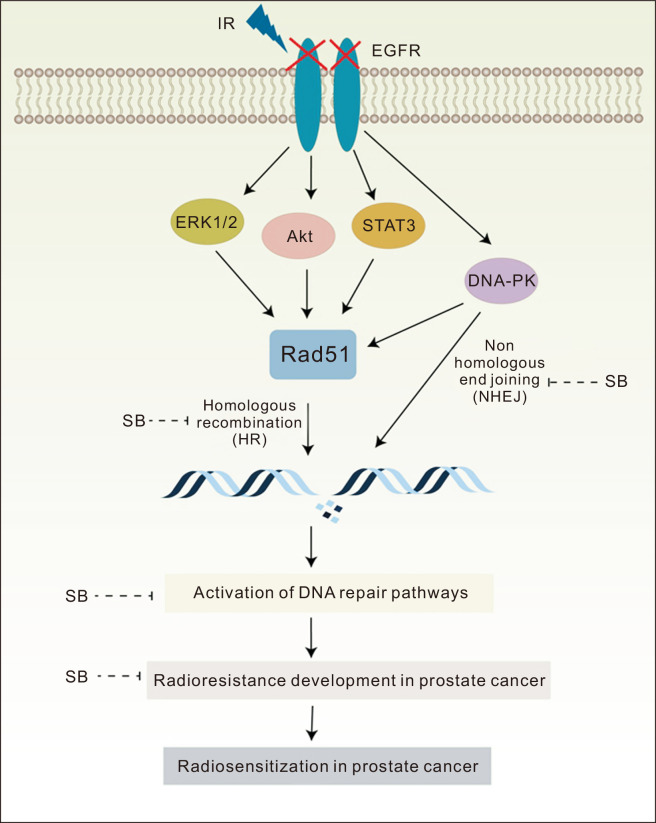
Notably, radiotherapy also activates the pro-survival signaling pathways in tumor cells which play an important role in emergence of radioresistance. The activation of ERK1/2, Akt and STAT3 signaling pathways in response to radiation therapy has been reporeted [38-41]. In the present study, EGFR-knockdown decreased the activation of Akt and ERK1/2 but not the STAT3, suggesting an EGFR-independent regulation of STAT3 in PCa cells. Further, silibinin combination with IR suppressed the activation of STAT3 proteins only in EGFR-deficient cells suggesting that in the absence of EGFR, silibinin may sensitize the PCa cells exposed to IR through down-regulation of STAT3. The silibinin also sensitized the PCa cells exposed to IR through inhibition of ERK1/2 and Akt signaling in EGFR-deficient cells suggesting a further downregulation of pro-survival signaling. Moreover, activated Akt and STAT3 are known to upregulate Rad51 thus suggesting their role in DNA repair which was inhibited by silibinin and IR combination (Fig. 6).
Figure 6. Schematic representation of radiosensitizing effects of a low non-toxic dose of SB in radioresistant DU145 PCa cells.
SB-induced radiosensitization of PCa cells via down-regulating DSBs DNA repair pathways (HR and NHEJ) proteins, Rad51 and DNA-PK, and this effect was further increased in EGFR-deficient cells. Further, SB inhibited pro-survival signaling molecules, ERK1/2, Akt and STAT3 in EGFR-deficient PCa cells exposed to radiation. SB, silibinin; IR, ionizing radiation; PCa, prostate cancer; DSB, double strand break; HR, homologous recombination; NHEJ, non-homologous end joining; DNA-PK, dependent protein kinase; ERK1/2, extracellular signal-regulated kinases1/2; EGFR, EGF receptor.
In conclusion, we report the novel molecular alterations associated with a low dose silibinin-mediated radiosensitizing effects in PCa cells by targeting HR and NHEJ pathways. Silibinin mitigated the IR-induced DNA repair through Rad51 and DNA-PK and sensitized the cells to death which was more effective in EGFR-deficient cells. This radiosensitizing effect of silibinin was further supported by the downregulation of pro-survival signaling pathways, ERK1/2, Akt and STAT3, in PCa cells. Together, these findings suggested that the combination approach of targeting EGFR signaling along with the application of silibinin in radioresistant PCa cells could be a better strategy to enhance the radiotherapeutic index in clinical settings.
ACKNOWLEDGMENTS
We are thankful for the technical assistance for flow cytometry, fluorescence microscopy and Gamma irradiation chamber from the Central Instrumentation Facility (CIF) at School of Life Sciences, Jawaharlal Nehru University, India.
Footnotes
FUNDING
Mohit Rajput, Deepali Mishra and Kunal Kumar were supported by Fellowships from University Grant Commission (UGC), India. The work was supported in part by DST, UGC-DRS and RNW, UPE-2 and DST-PURSE, DST-DPRP, India are gratefully acknowledged.
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–99. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, current, and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus. 2018;4:121–7. doi: 10.1016/j.euf.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace TJ, Torre T, Grob M, Yu J, Avital I, Brücher B, et al. Current approaches, challenges and future directions for monitoring treatment response in prostate cancer. J Cancer. 2014;5:3–24. doi: 10.7150/jca.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gay HA, Michalski JM. Radiation therapy for prostate cancer. Mo Med. 2018;115:146–50. doi: 10.5772/53180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khullar K, Parikh RR. The role of radiotherapy in metastatic prostate cancer. Am J Clin Exp Urol. 2019;7:92–7.83449ebb8d3d4828ba3d539745f5891b [PMC free article] [PubMed] [Google Scholar]
- 6.Bolla M, Henry A, Mason M, Wiegel T. The role of radiotherapy in localised and locally advanced prostate cancer. Asian J Urol. 2019;6:153–61. doi: 10.1016/j.ajur.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F, et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. 2018;37:87. doi: 10.1186/s13046-018-0758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5:60. doi: 10.1038/s41392-020-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava M, Raghavan SC. DNA double-strand break repair inhibitors as cancer therapeutics. Chem Biol. 2015;22:17–29. doi: 10.1016/j.chembiol.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Nickoloff JA, Taylor L, Sharma N, Kato TA. Exploiting DNA repair pathways for tumor sensitization, mitigation of resistance, and normal tissue protection in radiotherapy. Cancer Drug Resist. 2021;4:244–63. doi: 10.20517/cdr.2020.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajput M, Singh R, Singh N, Singh RP. EGFR-mediated Rad51 expression potentiates intrinsic resistance in prostate cancer via EMT and DNA repair pathways. Life Sci. 2021;286:120031. doi: 10.1016/j.lfs.2021.120031. [DOI] [PubMed] [Google Scholar]
- 12.Hazra B, Ghosh S, Kumar A, Pandey BN. The prospective role of plant products in radiotherapy of cancer: a current overview. Front Pharmacol. 2012;2:94. doi: 10.3389/fphar.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagunas-Rangel FA, Bermúdez-Cruz RM. Natural compounds that target DNA repair pathways and their therapeutic potential to counteract cancer cells. Front Oncol. 2020;10:598174. doi: 10.3389/fonc.2020.598174.a91b00dab6e34032bd745096d0ae44d7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nambiar D, Rajamani P, Singh RP. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res. 2011;728:139–57. doi: 10.1016/j.mrrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Nambiar DK, Rajamani P, Deep G, Jain AK, Agarwal R, Singh RP. Silibinin preferentially radiosensitizes prostate cancer by inhibiting DNA repair signaling. Mol Cancer Ther. 2015;14:2722–34. doi: 10.1158/1535-7163.MCT-15-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar M, Jaiswal RK, Prasad R, Yadav SS, Kumar A, Yadava PK, et al. PARP-1 induces EMT in non-small cell lung carcinoma cells via modulating the transcription factors Smad4, p65 and ZEB1. Life Sci. 2021;269:118994. doi: 10.1016/j.lfs.2020.118994. [DOI] [PubMed] [Google Scholar]
- 17.Rajput M, Kujur PK, Mishra A, Singh RP. Flavonoids inhibit chronically exposed arsenic-induced proliferation and malignant transformation of HaCaT cells. Photodermatol Photoimmunol Photomed. 2018;34:91–101. doi: 10.1111/phpp.12357. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Ye HL, Zhang G, Yao WM, Chen XZ, Zhang FC, et al. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PLoS One. 2014;9:e85771. doi: 10.1371/journal.pone.0085771.7ebcdbde417f456ca28e16f294079916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soica C, Oprean C, Borcan F, Danciu C, Trandafirescu C, Coricovac D, et al. The synergistic biologic activity of oleanolic and ursolic acids in complex with hydroxypropyl-γ-cyclodextrin. Molecules. 2014;19:4924–40. doi: 10.3390/molecules19044924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Punia R, Raina K, Agarwal R, Singh RP. Acacetin enhances the therapeutic efficacy of doxorubicin in non-small-cell lung carcinoma cells. PLoS One. 2017;12:e0182870. doi: 10.1371/journal.pone.0182870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar K, Mishra JPN, Singh RP. Usnic acid induces apoptosis in human gastric cancer cells through ROS generation and DNA damage and causes up-regulation of DNA-PKcs and γ-H2A.X phosphorylation. Chem Biol Interact. 2020;315:108898. doi: 10.1016/j.cbi.2019.108898. [DOI] [PubMed] [Google Scholar]
- 22.Sabarwal A, Agarwal R, Singh RP. Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol Carcinog. 2017;56:499–514. doi: 10.1002/mc.22512. [DOI] [PubMed] [Google Scholar]
- 23.Jayakumar S, Kunwar A, Sandur SK, Pandey BN, Chaubey RC. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim Biophys Acta. 2014;1840:485–94. doi: 10.1016/j.bbagen.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Biau J, Chautard E, Verrelle P, Dutreix M. Altering DNA repair to improve radiation therapy: specific and multiple pathway targeting. Front Oncol. 2019;9:1009. doi: 10.3389/fonc.2019.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim YC, Roberts TL, Day BW, Harding A, Kozlov S, Kijas AW, et al. A role for homologous recombination and abnormal cell-cycle progression in radioresistance of glioma-initiating cells. Mol Cancer Ther. 2012;11:1863–72. doi: 10.1158/1535-7163.MCT-11-1044. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Zuo W, Zeng Q, Li Y, Lu T, Bu Y, et al. The homologous recombination repair pathway is associated with resistance to radiotherapy in nasopharyngeal carcinoma. Int J Biol Sci. 2020;16:408–19. doi: 10.7150/ijbs.37302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balbous A, Cortes U, Guilloteau K, Rivet P, Pinel B, Duchesne M, et al. A radiosensitizing effect of RAD51 inhibition in glioblastoma stem-like cells. BMC Cancer. 2016;16:604. doi: 10.1186/s12885-016-2647-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King HO, Brend T, Payne HL, Wright A, Ward TA, Patel K, et al. RAD51 is a selective DNA repair target to radiosensitize glioma stem cells. Stem Cell Reports. 2017;8:125–39. doi: 10.1016/j.stemcr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gachechiladze M, Škarda J, Soltermann A, Joerger M. RAD51 as a potential surrogate marker for DNA repair capacity in solid malignancies. Int J Cancer. 2017;141:1286–94. doi: 10.1002/ijc.30764. [DOI] [PubMed] [Google Scholar]
- 30.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–42. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–8. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781–91. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 33.Krajewska M, Fehrmann RS, de Vries EG, van Vugt MA. Regulators of homologous recombination repair as novel targets for cancer treatment. Front Genet. 2015;6:96. doi: 10.3389/fgene.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakin ND, Jackson SP. Regulation of p53 in response to DNA damage. Oncogene. 1999;18:7644–55. doi: 10.1038/sj.onc.1203015. [DOI] [PubMed] [Google Scholar]
- 35.Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280:20375–83. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 36.Williams AB, Schumacher B. p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med. 2016;6:a026070. doi: 10.1101/cshperspect.a026070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chappell WH, Lehmann BD, Terrian DM, Abrams SL, Steelman LS, McCubrey JA. p53 expression controls prostate cancer sensitivity to chemotherapy and the MDM2 inhibitor Nutlin-3. Cell Cycle. 2012;11:4579–88. doi: 10.4161/cc.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol. 2009;4:43. doi: 10.1186/1748-717X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hein AL, Ouellette MM, Yan Y. Radiation-induced signaling pathways that promote cancer cell survival (review) Int J Oncol. 2014;45:1813–9. doi: 10.3892/ijo.2014.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marampon F, Ciccarelli C, Zani BM. Biological rationale for targeting MEK/ERK pathways in anti-cancer therapy and to potentiate tumour responses to radiation. Int J Mol Sci. 2019;20:2530. doi: 10.3390/ijms20102530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zhang X, Qiu C, Yang N. STAT3 contributes to radioresistance in cancer. Front Oncol. 2020;10:1120. doi: 10.3389/fonc.2020.01120.b27bb3f2965e49b8a5b5858f022d52b0 [DOI] [PMC free article] [PubMed] [Google Scholar]