Abstract
Jaboticaba is a Brazilian berry, which is rich in fibers and bioactive compounds and shows high antioxidant and antiproliferative activities. Prostate cancer (PCa) is the second most common type of cancer among men and its progression is influenced by androgens and inflammation. Previous studies reported the ability of the jaboticaba to modulate pathways involved in prostate diseases. The main objective of this study was to provide significant data about molecular targets of the jaboticaba peel extract (JPE) and its mechanisms of action in PCa cell lines with different androgenic status (LNCaP and PC-3). The results showed that JPE was able to decrease cell viability in both cell lines. LNCaP showed more sensitivity to JPE exposure, indicating the efficacy of the JPE treatment in terms of androgen responsiveness. JPE showed a distinct hormone dependent effect on the NF-κB signaling, with reduced NF-κB levels for LNCaP and increased NF-κB levels in PC-3 cells. Mechanisms related to cell death by apoptosis were stimulated after the JPE treatment, modulating B-cell lymphoma 2 and BAX for LNCaP and PC-3. Particularly for PC-3, the JPE treatment resulted in cytokine-cytokine receptor interaction activation mostly by up regulating pro-inflammatory, pro-angiogenic, immunostimulatory and immunosuppressive genes. Also, a set of genes related to angiogenesis and metastasis were down-regulated by JPE. In conclusion, JPE exerted an antitumor effect on PCa for both cell lines which can be enhanced if androgenic reliance is considered.
Keywords: Biological products, Jaboticaba, Polyphenols, Prostate cancer, Inflammation
INTRODUCTION
Jaboticaba (Myrciaria cauliflora) is a Brazilian berry presenting a purple peel, and can be found in different parts of Brazil [1]. According to Leite-Legatti et al. [2], the jaboticaba peel is rich in fibers and bioactive compounds, such as anthocyanins, delphinidin and cyanidin 3-glucoside, which show high antioxidant capacity and antiproliferative action against leukemia and prostate cancer cells. Our group demonstrated that jaboticaba peel extract (JPE) was able to prevent hepatic damages and metabolic disorders, particularly, associated with overweight and aging [3]. In addition to these findings, for the first time, we identified that JPE administration in senile mice also prevented prostate damages caused by aging and high-fat diet consumption, mostly by anti-inflammatory, antioxidant and anti-angiogenic mechanisms and recovery of hormonal balance [4,5]. However, the action mechanisms related to JPE in the prostatic microenvironment and its targets in the cell are still unclear.
Prostate cancer (PCa) is the second most common type of malignancy between men, and 65,840 new cases were estimated in Brazil in 2020 [6], representing 29.2% of all male tumor incidences. According to Global Cancer Statistics 2020, almost 1.4 million new cases of PCa were estimated around the world, making it the fifth cause of death due to cancer among men in 2020 [7]. Different studies, using bioactive compounds from food, have shown positive effects against PCa, in both in vitro and in vivo [8-11]. It is well known that both the prostatic morphology and physiology are regulated by androgen recepter (AR) and have an important role in prostatic pathogenesis [12]. AR signaling is fundamental to PCa development, as can be seen from the fact that androgen deprivation, by either or surgical castration, is the first-line treatment for PCa [13]. De Amacis et al. [14] identified resveratrol effects on steroid receptor signaling, showing the potential of this type of compound in the treatment of hormone-dependent cancers. This study also indicated that bioactive compounds interfere in steroid receptors and therefore they are useful in combined therapies in the hormone-dependent cancer patients.
Another important point for prostatic disease is inflammation. The association between inflammation, innate immunity and cancer is widely studied, but molecular and cellular mechanisms underlying theirs association, are still unclear [15]. Among the most commonly reported features in the literature about polyphenol effects include the antioxidant action; the modulating effects on NF-κB pathway, chromatin structure, antioxidant system and, consequently on the regulation of inflammatory genes in immune cells and in different tissues [16,17].
The jaboticaba has shown great biological potential and the use of its by-products, such as the extract used in the present study, can be an opportunity to improve circular economy not only by the application of sustainable technologies but also by giving support to the efficient employment of natural resources [18]. Over the last few years, our research group has been committed to understanding the different roles of the jaboticaba by-products especially in prostate diseases. Giving continuity to these studies, the main objective herein was to provide significant data about the effects of JPE on the inflammatory and immune responses and potential gene targets in PCa.
MATERIALS AND METHODS
JPE
JPE is a patented formulation made from freeze-dried jaboticaba peel, which was solubilized in ethanol and then submitted to solvent removal [3]. Lamas et al. [3] identified 121 mg GAE/g total phenolic, 1,381 mg cyd 3-glu 100 g–1 monomeric anthocyanins, 24.5 mg CAT g–1 flavonoids and specific bioactive compounds such as ellagic acid, rutin, gallic acid and ascorbic acid. In the present study, prostate cancer cells were exposed to different JPE doses (125, 250 and 500 µg/mL) according to studies that used rich-polyphenols extracts [2,19]. JPE was diluted in RPMI-1640 medium (Vitrocell Embriolife, Campinas, Brazil).
Cell lines
The PC-3 (androgen-independent human prostate cancer cell) line was purchased from the American Type Culture Collection (Manassas, VA, USA), and LNCaP (androgen-dependent human prostate cancer cell) from Rio de Janeiro Cell Bank (Rio de Janeiro, Brazil). Both cell lines were maintained in RPMI-1640 medium supplemented with 10% of FBS and 1% of penicillin-streptomycin antibiotic solution. The cells were maintained in 25cm2 or 75 cm2 culture flasks at 37°C in a 90% humidified atmosphere of 5% CO2.
Reagents
RPMI-1640 medium, PBS, penicillin-streptomycin antibiotic solution, and trypsin were purchased from Vitrocell Embriolife (Campinas, Brazil). Anti-β-actin (sc-47778), anti-COX-2 (sc376861), and anti-Toll-like receptor 4 (TLR-4) (sc16240) were purchased from Santa Cruz Biotechnology (Irvine, CA, USA); anti-NF-κB (ab13594), anti-IκBα (ab32518), anti-IκBβ (ab7547), anti-TNF-α (ab8348), and anti-BAX (ab32503) were acquired from Abcam (Cambridge, UK) and anti-B-cell lymphoma 2 (BCL-2) was purchased from Cell Signaling (Danvers, MA, US). RNeasy® Micro kit (74004), RT2 First Strand kit (330401) and RT2 Profiler PCR array (PAHS 181ZA-Human Cancer Inflammation and Immunity Crosstalk) were obtained from Qiagen (Hilden, Germany).
Cell viability
The cells (LNCaP, 3.5 × 104; PC-3, 3 × 104) were plated in 12-well plates in triplicate and allowed to attach by overnight incubation. In brief, the cells were with or without JPE (125, 250 and 500 µg/mL). The plates were incubated for 24, 48 or 72 hours at 37°C. Then, the cells were collected by trypsinization and suspended in 0.1% trypan blue solution, and live cells were counted using a hemocytometer.
Western blotting
The cells (LNCaP, 4.5 × 105; PC-3, 4 × 105) were seeded in 60-mm dishes, allowed to attach overnight, and then treated with JPE. Based on cell viability results, LNCaP cells were exposed to 125 and 250 µg/mL and PC-3 cells to 250 and 500 µg/mL during 24 hours. The cell lysis, protein quantification and electrophoresis were performed as described in our previous study [10]. The following antibodies were used in immunoblotting: anti-TLR-4 (1:250), anti-NF-κB (1:500), anti-IκBα (1:500), anti-IκBβ (1:500), anti-BAX (1:250), anti-BCL-2 (1:250), anti-COX-2 (1:500), and anti-TNF-α (1:500). The bands were visualized by enhanced SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL, USA) followed by image acquisition (Syngene GeneGnome XRQ, Cambridge, UK). The software UN-SCAN-IT5.1 (Silk Scientific Orem, UT, USA) was used to quantify the total pixel from bands and the protein level changes relative to endogen control.
RT2 Profiler PCR array
PC-3 cells were seeded at 6 × 105 cells in 100 mm dishes and allowed to attach overnight. The cells were treated with the highest dose of JPE (500 µg/mL). Following 24-hour treatment, cells were released by trypsinization and 5 × 105 viable cell were collected into denaturing lysis buffer and the RNA isolated with RNeasy® Micro kit (Qiagen) following the manufacturer’s instructions. The concentration of RNA and quality of the samples were measured in a nanophotometer considering the ratios A260/280 and A260/230. cDNA synthesis was achieved using RT2 First Strand kit (Qiagen) and 0.5 μg of total RNA were used from each sample. Then, diluted cDNA samples were added to RT2 SYBR® green master mix (Qiagen) and aliquoted into RT2 Profiler PCR Arrays (Qiagen). The samples were analyzed with the Human Cancer Inflammation and Immunity Crosstalk array (#PAHS-181ZA; Qiagen). RNA quality control analysis was performed using a control plate RT2 RNA QC PCR Array (Qiagen) to assure that all samples used in the experiment were free of genomic contamination and to guarantee reverse transcription and polymerase chain reaction efficiency. RT-PCR was performed on Stratagene® model Mx3005P® (Agilent, Santa Clara, CA, USA), and the results were obtained with MxPro software ver 4.10 (Agilent, Santa Clara, CA, USA). The experiment conditions followed manufacturer’s instructions: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds, 60°C for 1 minute, followed by a melt curve. The PCR array data analysis was performed through Qiagen online platform GeneGlobe (https://geneglobe.qiagen.com) and the results were confirmed manually using 2–ΔΔCt method. Data are presented as biologic triplicate.
Protein-protein interaction network (PPI)
Protein-protein interaction networks are often used to identify and visualize interactions among proteins of a given dataset. Here we used PC-3 cells from RT-PCR array data, to create a PPI network. To do so, we used the STRING protein query on the Cytoscape software platform (ver. 3.8.2; https://cytoscape.org/). We used log2FCs from all plate genes, except for the housekeeping genes (actin beta [ACTB], beta-2-microglobulin [B2M], glyceraldehyde-3-phosphate dehydrogenase [GAPDH], hypoxanthine phosphoribosyltransferase 1 [HPRT1], ribosomal protein lateral stalk subunit P0 [RPLP0]) and the ones whose analysis met suggested exclusion criteria (activation induced cytidine deaminase, CC chemokine ligand [CCL] 18, interleukin 2) as input. The confidence score was set at 0.8 and 10 additional interactions were allowed and singletons proteins with no interactions within the dataset) were not shown.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (ver. 7.02; GraphPad, San Diego, CA, USA). ANOVA followed by Dunnet’s or Bonferroni test was carried out for statistical comparisons with the level of significance set at 5%. The results were expressed as the mean ± standard deviation.
RESULTS
JPE treatment decreased the cell viability of human PCa cell lines
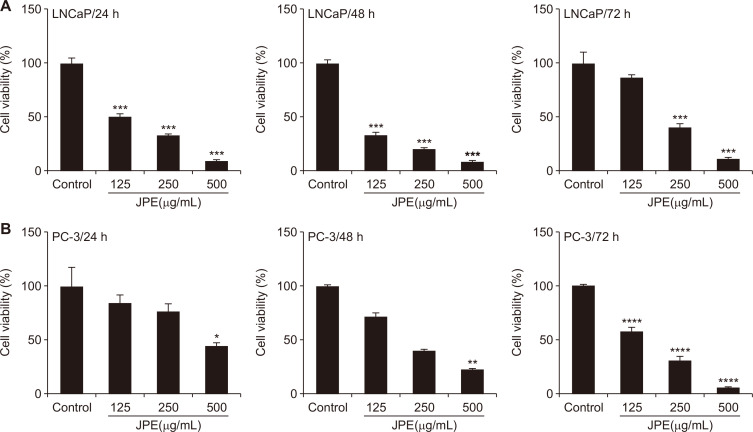
LNCaP and PC-3 cells were exposed to various concentrations of JPE for different time periods, in order to determine the most optimal experimental conditions, which are able to decrease the viability of both lines in dose- and time-dependent manners. LNCaP cells were more sensitive to JPE exposure, showing a significant reduction in their viability even at the lowest dose after 24 hour of treatment (IC50 = 118.3 µg/mL) (Fig. 1A). The maximum effect of JPE on LNCaP viability was verified after 48 hours, (IC50 = 70.0 µg/mL), whereas after 72 hours, only the highest dose was able to reduce cell viability (IC50 = 203.5 µg/ mL) (Fig. 1A). The PC-3 viability was significantly inhibited by JPE after 24 hours and 48 hours at the 500 µg/mL concentration (IC50 = 219.9 and 154.6 μg/mL, respectively) (Fig. 1B). After 72-hour exposure, all doses were able to significantly reduce the PC-3 cell viability (IC50 = 144.3 µg/mL) (Fig. 1B). These results suggest that the efficacy of JPE treatment could be related to the androgen responsive status of human prostate cancer cells, as shown by the early response of LNCaP at lower doses of JPE, and the less sensitive late response of PC-3 cells.
Figure 1. Effect of JPE on cell viability of LNCaP and PC-3 cells.
(A) LNCaP and (B) PC-3 cells were exposed to different doses of JPE during 24, 48, and 72 hours. The results were expressed as mean ± SD (n = 3). Statistical significance indicated as *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, when compared with control group. JPE, jaboticaba peel extract; SD, standard deviation.
JPE shows a distinct hormone dependent effect on the NF-κB signaling in human prostate cancer cells
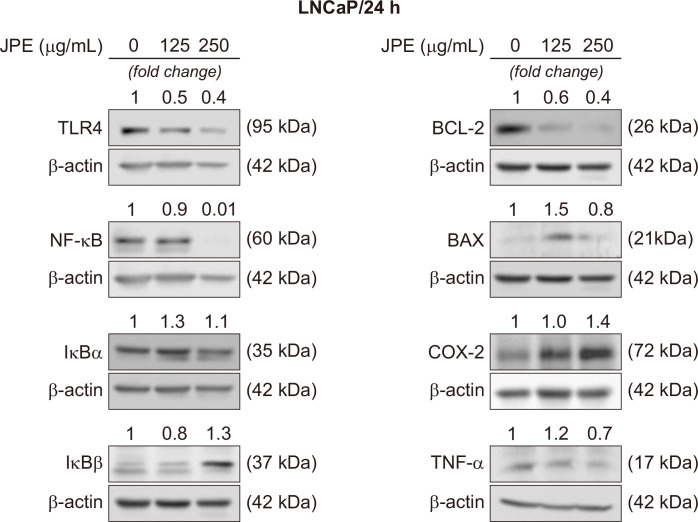
In order to determine the anti-inflammatory potential of JPE in human prostate cancer cells, the protein levels of different molecules related to the NF-κB pathway were quantified (Fig. 2 and 3). NF-κB and TLR-4 were markedly reduced after 24 hour of exposure to JPE in LNCaP cells (Fig. 2). Increased IκBα levels were observed after exposure to JPE (125 and 250 µg/mL), as well as an increase in the β form (250 µg/mL), demonstrating the ability of JPE to modulate NF-κB inhibitors (Fig. 2). TNF-α levels decreased only after exposure to the JPE highest dose (250 µg/mL) (Fig. 2). Contrary to what was expected, COX-2 levels were similar to the control levels or were increased in LNCaP cells after exposure to different JPE doses (125 and 250 µg/mL) (Fig. 2).
Figure 2. JPE effect on the proteins expression related to the inflammatory process and cell death.
Representative membranes demonstrating the effect of different JPE doses (125 and 250 µg/mL) on LNCaP cells. The numbers on the bands indicate a fold-change of the protein level when compared to the control group. β-actin was used as endogenous control. The experiments were repeated at least three times with consistent results. JPE, jaboticaba peel extract; TLR-4, Toll-like receptor 4; BCL-2, B-cell lymphoma 2.
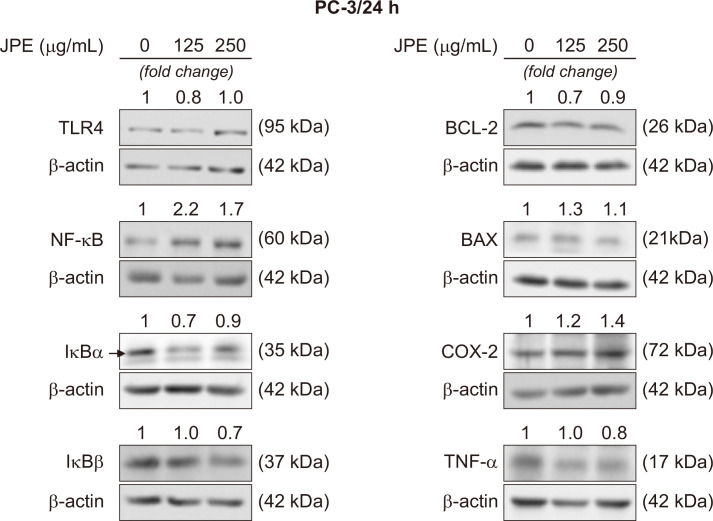
Figure 3. JPE effect on the proteins expression related to the inflammatory process and cell death.
Representative membranes demonstrating the effect of different JPE doses (250 and 500 µg/mL) on PC-3 cells. The numbers on the bands indicate a fold-change of the protein level when compared to the control group. β-actin was used as endogenous control. The experiments were repeated at least three times with consistent results. JPE, jaboticaba peel extract; TLR-4, Toll-like receptor 4; BCL-2, B-cell lymphoma 2.
The PC-3 cell exposure to JPE induced an increase in NF-κB levels at both doses tested (250 µg/mL and 500 µg/mL). Furthermore, both NF-κB inhibitors (IκBα and IκBβ) decreased, when exposed to the highest JPE dose (500 µg/mL); the same occurred with TNF-α. Similar to what was seen in LNCaP cells, JPE induced an increase in COX-2 levels (Fig. 2). Regarding TLR-4, only the 250 µg/mL dose reduced the levels of this receptor.
The present results indicate that the JPE effects on the NF-κB pathway were different in the PC-3 cell line, when compared to the effects seen in the LNCaP cells, pointing to a greater resistance of the androgen-independent cell phenotype.
JPE treatment can stimulate mechanisms related to cell death by apoptosis
Western blotting results demonstrated that JPE treatment may stimulate cell death via apoptosis. In both cell lines, the presence of JPE reduced the BCL-2 anti-apoptotic protein levels (Fig. 2 and 3). Furthermore, the 250 µg/mL dose stimulated the increase of the BAX pro-apoptotic protein in both LNCaP and PC-3 cells. Taken together, these results suggest that JPE can act as a pro-apoptotic agent in human prostate cancer cells, but more data are needed to confirm this possible action mechanism.
JPE treatment modulates the expression of cancer immunology-related genes
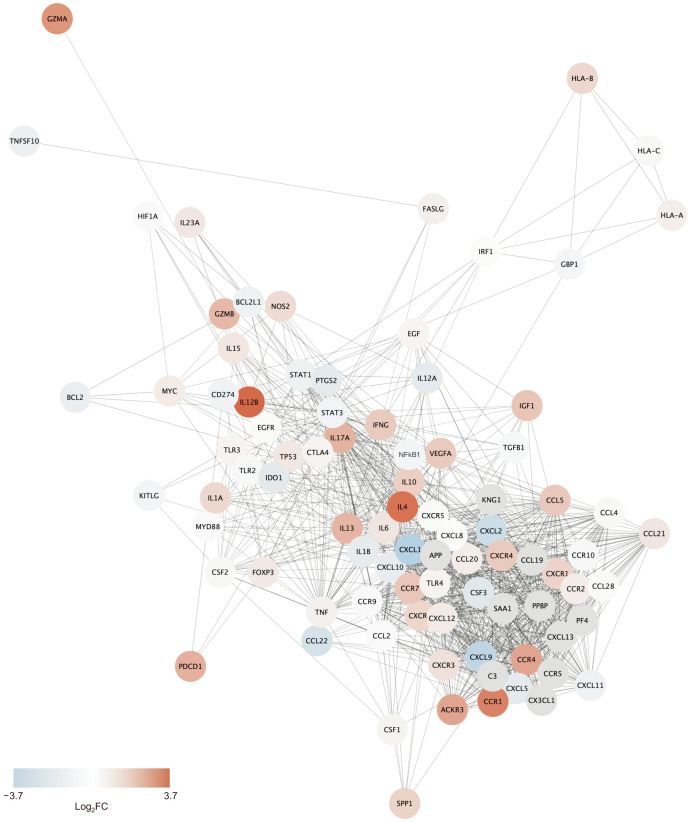
PCR array of PC-3 cells revealed the effect of JPE treatment on the expression of genes related to cancer immunology. Out of the 84 analyzed genes (Table 1), 25 showed increased expression (fold change [FC] > 2) after JPE treatment, while only 4 showed decreased expression (FC < 0.5) following JPE treatment. Such results indicate that JPE treatment affected the expression of ~35% of the plate’s selected genes, revealing its potential to affect the referred processes. From PCR array data we created a PPI network, which allowed us to visualize links between the analyzed genes and possible additional interactions (Fig. 4). The annotated network reveals a strong clustering of genes related to cytokine-cytokine receptor interaction, which is the most enriched KEGG pathway among our differentially expressed genes (Supplementary Table 1).
Table 1.
Gene panel–based on human cancer inflammation and immunity from PCR–array data
| Gene | Fold change | log2FC | Gene | Fold change | log2FC | Gene | Fold change | log2FC |
|---|---|---|---|---|---|---|---|---|
| ACKR3 | 5.76 | 2.53 | CXCL9 | 0.24 | –2.06 | IL6 | 1.83 | 0.87 |
| BCL2 | 0.65 | –0.62 | CXCR1 | 2.76 | 1.46 | CXCL8 | 0.97 | –0.04 |
| BCL2L1 | 0.73 | –0.45 | CXCR2 | 2.57 | 1.36 | IRF1 | 1.02 | 0.03 |
| CXCL11 | 0.73 | –0.45 | CXCR3 | 2.05 | 1.04 | KITLG | 0.80 | –0.32 |
| CCL2 | 0.95 | –0.07 | CXCR4 | 3.21 | 1.68 | MICA | 2.10 | 1.07 |
| CCL20 | 1.53 | 0.61 | CXCR5 | 1.08 | 0.11 | MICB | 1.36 | 0.44 |
| CCL21 | 1.84 | 0.88 | EGF | 1.18 | 0.24 | MIF | 1.69 | 0.76 |
| CCL22 | 0.38 | –1.40 | EGFR | 1.03 | 0.04 | MYD88 | 1.05 | 0.07 |
| CCL28 | 1.06 | 0.08 | FASLG | 1.40 | 0.49 | NFkB1 | 0.79 | –0.34 |
| CCL4 | 1.13 | 0.18 | FOXP3 | 1.62 | 0.70 | NOS2 | 2.32 | 1.21 |
| CSF2 | 1.13 | 0.18 | GBP1 | 0.81 | –0.30 | PDCD1 | 4.87 | 2.28 |
| CCL5 | 3.27 | 1.71 | GZMA | 7.21 | 2.85 | SPP1 | 2.78 | 1.48 |
| CCR1 | 9.21 | 3.20 | GZMB | 4.32 | 2.11 | STAT1 | 0.72 | –0.47 |
| CCR10 | 0.88 | –0.18 | HIF1A | 0.96 | –0.06 | STAT3 | 0.76 | –0.40 |
| TLR2 | 0.88 | –0.18 | HLA–A | 1.46 | 0.55 | TGFB1 | 0.86 | –0.22 |
| CCR2 | 1.52 | 0.60 | HLA–B | 2.55 | 1.35 | TLR3 | 1.26 | 0.33 |
| CCR4 | 5.78 | 2.53 | HLA–C | 1.15 | 0.20 | TLR4 | 1.26 | 0.33 |
| CCR7 | 3.49 | 1.80 | IDO1 | 0.53 | –0.92 | TNF | 1.38 | 0.46 |
| CCR9 | 0.94 | –0.09 | IFNG | 3.23 | 1.69 | TNFSF10 | 0.67 | –0.58 |
| CD274 | 0.74 | –0.43 | IGF1 | 3.50 | 1.81 | TP53 | 1.74 | 0.80 |
| CSF1 | 1.22 | 0.29 | IL10 | 2.95 | 1.56 | VEGFA | 3.26 | 1.70 |
| CSF3 | 0.50 | –1.00 | IL12A | 0.54 | –0.89 | |||
| CTLA4 | 1.25 | 0.32 | IL12B | 12.98 | 3.70 | |||
| CXCL1 | 0.21 | –2.25 | IL13 | 4.54 | 2.18 | |||
| CXCL10 | 0.59 | –0.76 | IL15 | 1.77 | 0.82 | |||
| CXCL12 | 1.59 | 0.67 | IL17A | 4.67 | 2.22 | |||
| MYC | 1.59 | 0.67 | IL1A | 2.42 | 1.28 | |||
| CXCL2 | 0.31 | –1.69 | IL1B | 0.57 | –0.81 | |||
| CXCL5 | 0.56 | –0.84 | IL23A | 1.73 | 0.79 | |||
| PTGS2 | 0.56 | –0.84 | IL4 | 11.30 | 3.50 |
Figure 4. Jaboticaba peel extract treatment effects on the expression of cancer immunology related genes.
Network annotates protein-protein interactions predicted on STRING database for the PCR array analyzed genes. Nodes represent proteins and edges the predicted interactions. Color of nodes represent gene’s log2FCs and gray nodes represent additional interactions. Confidence score = 0.8, additional interactions = 10.
DISCUSSION
The results presented herein revealed that JPE inhibited the growth of androgen dependent and independent prostate cancer cells, being the androgen-dependent cell line more sensitive to the jaboticaba extract effects. It is noteworthy that JPE exposure induced a pro-apoptotic response in prostate cancer cells. The gene expression data from PCR-array showed that the androgen independent phenotype can be an obstacle to JPE action, since different gene sets, involving pro-inflammatory response and angiogenesis were upregulated. However, another subgroup of genes, involved in metastasis inhibition mechanisms, was also regulated by JPE, suggesting specific chemokine-related targets to be investigated.
Bioactive compounds act on the carcinogenesis process mainly through the induction of cellular defense systems, which include detoxifying and antioxidant enzymatic systems, as well as the inhibition of inflammatory and proliferative signaling pathways, which leads to cell cycle delay or programmed cell death [20]. Hence, the natural phytochemicals present in the diet can exert their antitumor effects through mechanisms at different cellular levels that vary from direct modulation of gene expression to epigenetic and post-transcriptional alterations [21].
In the present study, we evaluated two different cell lines, showing distinct growth rates and molecular characteristics, specially related to AR reliance. Whereas LNCaP has a mutation for AR (T877A), PC-3 cells are AR null and exhibit an aggressive phenotype when compared to LNCaP. Our results showed that JPE exerted an early and sustained anti-proliferative effect on LNCaP cell growth associated with a potent anti-inflammatory action. On the other hand, an opposite trend was observed in PC-3 cells, which showed a late JPE response in cell growth, probably due to the metabolizing process of bioactive compounds, as well as an upregulation of pro-inflammatory markers after 24 hours. A previous study from our group also verified an opposite response in LNCaP cells exposed to piceatannol, a bioactive compound in passion-fruit, when compared to androgen-independent ones [10]. Piceatannol inhibited LNCaP growth at 24 hours of exposure even with the lowest dose, whereas PC-3 and 22Rv1 cells needed to be exposed for 72 hours to achieve the same response of LNCaP [10]. The same authors reported that cell cycle arrest induced by piceatannol extract was probably due to direct interaction with p53 in LNCaP cells, but not in 22Rv1 [10]. Similarly, an anthocyanins-rich extract from potato inhibited LNCaP and PC-3 proliferation and differentially induced cell death in both cell lines, showing that bioactive compounds can achieve similar results by different pathways [22]. Accordingly, combined phytochemicals can show different effects when compared to purified ones. Eskra et al. [23] showed that black raspberry extract itself and its isolated phytochemicals (anthocyanin cyanidin-3-rutinoside and protocatechuic acid) did not affect prostate cancer cell growth (LAPC-4, LNCaP, C4-2, 22Rv1, VCaP, and PC-3). However their metabolites (ellagic acid and urolithin A) did so at high concentrations. The same authors emphasized that instability and low access of bioactive compounds and metabolites hinder tissue exposure and may not be enough to prevent cancer [23]. The results herein confirmed anti-proliferative JPE effect in PCa, but this is probably cell-context dependent.
The interaction between the bioactive compounds and the prostate could be due to the fact that its responsiveness might be dependent on the AR status [24]. Polyphenols are commonly reported as anti-androgenic due to their ability to interact with AR and its transactivators [24]. Therefore, finding an anti-cancer compound able to modulate the cell response in an androgen-dependent condition is a fundamental aspect to the development of new chemopreventive drugs. The strong anti-inflammatory response observed in LNCaP cells, after JPE exposure, lead us to suggest that androgen reliance could be a key factor that makes this cell line more susceptible to bioactive compound action. The literature already reported that NF-κB is a target of jaboticaba compounds, both those found in the whole fruit extracts or in its by-products [25]. Here, we identified that JPE decreased the protein levels of TLR4, NF-κB, NF-κB inhibitors and TNF-α in LNCaP cells. It is known that the stimulation of TLR4 in the prostate epithelial cells leads to upregulation of NF-κB, TGF-β1, and VEGF, creating an inflammatory and pro-tumoral environment [26,27]. The inihibition of TLR4 and NF-κB-mediated signaling pathways by quercetin, one of the main JPE constituents, inhibited invasiveness capacity of human colon cancer Caco-2 cells through matrix metalloproteinase (MMP)-2 and MMP-9 decrease [28]. Similar effects were also observed in human lung cancer cells [29]. Interestingly, different authors have pointed out that phenolic compounds such as anthocyanins and flavonoids are also considered to be anti-androgenic due to their ability to downregulate AR expression, by inhibiting the receptor activity and decreasing the tumor biomarkers linked to the androgenic disease status such as prostate-specific antigen and human kallikrein 2 [30,31]. Taken together, our results suggest that the combined phytochemicals present in JPE were able to affect or disrupt the signaling between the NF-κB mediators in the LNCaP cells, indicating that the androgen dependent feature, in prostate cancer models, is crucial for the anti-cancer role of the polyphenolic compounds.
The beneficial effects of bioactive compounds on human health have been largely attributed to their anti-oxidant and anti-inflammatory role [32] and, contrary to what was expected, JPE intensified the PC-3 cell inflammatory response. Considering these conflicting facts, we decided to analyze the expression of a panel of genes related to cancer, inflammation and immunity. To the best of our knowledge, this is a pioneer study that uses real time PCR array to access possible jaboticaba targets in PCa. JPE treatment resulted in gene modulation related to cytokine-cytokine receptor interaction, together with the upregulation of pro-inflammatory, pro-angiogenic, immunostimulatory and immunosuppressive genes in PC-3 cells. Also, a set of genes related to angiogenesis and metastasis (CCL22, C-X-C motif chemokine ligand [CXCL]1, CXCL2 and CXCL9) were down-regulated by JPE. Although the molecular mechanisms are unknown, these findings suggest that JPE can induce a protumor immunity activity in PC-3 cells, enhancing proinflammatory and proangiogenic genes in PCa. One possible explanation for these results could be associated with the estrogenic activity of polyphenols via estrogen receptor alpha (ER-α) and beta already reported in different studies [33-35]. The ER-α expression is upregulated in castration-resistant prostate cancer (CRPC) and contributes to tumor progression by increasing inflammation and metastasis dissemination [36]. PC-3 is a CRPC cell line and expresses ER-α in the membrane and the nucleus [37], which could allow JPE constituents to interact with this receptor and promote a pro-inflammatory reponse, however, our findings are not focused on this specific aspect. The present results suggest a potential adverse interaction between JPE, thus more studies are necessary to identify possible relationships between polyphenols and estrogen receptors in advanced PCa.
Based on western blotting results, JPE interfered in the protein balance related to cell death, especially in PC-3 cells, probably by lowering apoptosis inhibition, as seen in the BCL-2 decrease and BAX increase. Until the present moment, only two studies have reported the pro-apoptotic role of the jaboticaba extracts on cancer cell lines [19,38]. Wang et al. [38] observed a decreased survivin expression, an anti-apoptotic protein, associated with activating caspase 3-mediated Bit cleavage in oral carcinoma cells (HSC-3) after exposure to the jaboticaba seed extract. High doses (500 and 1,000 µg/mL) of jaboticaba peel extract also induced cell death by apoptosis in colon cancer cells (HT-29), when compared to other anthocyanin-rich extracts from the myrtaceae family species [19]. Furthermore, early and late apoptotic cells significantly increased after the treatment with jaboticaba peel extract; however, this study did not investigate the mechanisms and proteins involved in this process [19]. Taking into consideration, purified bioactive compounds, such as anthocyanins, they can stimulate cell death by the regulation of BCL-2, BAX and p53, as a consequence of AR inhibition in prostate cancer cells [39]. Reddivari et al. [22] also demonstrated that rich-anthocyanin potato extract induced caspase 3-dependent apoptosis in LNCaP cells by PARP cleavage cells, whereas in PC-3 a caspase-independent pathway seems to be activated. Apoptosis is a complex mechanism that in PCa is intrinsically related to both hormone dependence and many other factors such as NF-κB and TNF-α modulation [40]. Our data showed pro-apoptotic potential of JPE in PCa cells with a distinct genetic background. However more studies are necessary to determine the specific pathway that promoted cell death or if this action was a direct or indirect consequence of the inflammatory regulation.
In conclusion, the results herein confirmed that JPE is a NF-κB pathway inhibitor, especially in androgen-dependent cells. Also, the JPE effects on PCa prevention seem involve different action mechanisms that are still unclear, mostly if the immune response and cell death are considered, which need further validation. The present data certainly pave the way for new studies involving jaboticaba by-products, but also warn the indiscriminate use of natural products during cancer progression, especially in CRPC. Finally, we also show that the antitumor effect can be enhanced in an androgen-dependent end of period. This was reflected, to a greater of lesser extent, in both the suppression of cell viability and anti-inflammatory response.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.15430/JCP.2022.27.3.182
ACKNOWLEDGMENTS
VHAC acknowledges Prof. Dr. Alexandre Leite Rodrigues de Oliveira for his assistance providing his laboratory to PCR array analysis.
Footnotes
FUNDING
This study was financed by São Paulo Research Foundation – FAPESP (grants: 2015/50333-1; 2018/04579-7; 2018/11069-5; 2015/13320-9, 2019/13465-8) and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -Finance Code 001; CNPq 403976/2021-9; 301496/2019-6). MRMJ acknowledges Red Iberomericana de Alimentos Autoctonos Subutilizados (ALSUB-CYTED, 118RT0543).
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Batista ÂG, Lenquiste SA, Cazarin CBB, da Silva JK, Luiz-Ferreira A, Bogusz S, et al. Intake of jaboticaba peel attenuates oxidative stress in tissues and reduces circulating saturated lipids of rats with high-fat diet-induced obesity. J Funct Foods. 2014;6:450–61. doi: 10.1016/j.jff.2013.11.011. [DOI] [Google Scholar]
- 2.Leite-Legatti AV, Batista ÂG, Dragano NRV, Marques AC, Malta LG, Riccio MF, et al. Jaboticaba peel: antioxidant compounds, antiproliferative and antimutagenic activities. Food Res Int. 2012;49:596–603. doi: 10.1016/j.foodres.2012.07.044. [DOI] [Google Scholar]
- 3.Lamas CA, Lenquiste SA, Baseggio AM, Cuquetto-Leite L, Kido LA, Aguiar AC, et al. Jaboticaba extract prevents prediabetes and liver steatosis in high-fat-fed aging mice. J Funct Foods. 2018;47:434–46. doi: 10.1016/j.jff.2018.06.005. [DOI] [Google Scholar]
- 4.Lamas CA, Kido LA, Hermes TA, Nogueira-Lima E, Minatel E, Collares-Buzato CB, et al. Brazilian berry extract (Myrciaria jaboticaba): a promising therapy to minimize prostatic inflammation and oxidative stress. Prostate. 2020;80:859–71. doi: 10.1002/pros.24017. [DOI] [PubMed] [Google Scholar]
- 5.Lamas CA, Kido LA, Montico F, Collares-Buzato CB, Maróstica MR, Junior, Cagnon VHA. A jaboticaba extract prevents prostatic damage associated with aging and high-fat diet intake. Food Funct. 2020;11:1547–59. doi: 10.1039/C9FO02621E. [DOI] [PubMed] [Google Scholar]
- 6.INCA, author. Estimate/2020 - cancer incidence in Brazil. National Cancer Institute José Alencar Gomes da Silva; Rio de Janeiro: 2019. pp. 1–122. [DOI] [Google Scholar]
- 7.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Baseggio AM, Kido LA, Viganó J, Carneiro MJ, Lamas CA, Martínez J, et al. Systemic antioxidant and anti-inflammatory effects of yellow passion fruit bagasse extract during prostate cancer progression. J Food Biochem. 2022;46:e13885. doi: 10.1111/jfbc.13885. [DOI] [PubMed] [Google Scholar]
- 9.Venkateswaran V, Fleshner NE, Sugar LM, Klotz LH. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004;64:5891–6. doi: 10.1158/0008-5472.CAN-04-0690. [DOI] [PubMed] [Google Scholar]
- 10.Kido LA, Hahm ER, Kim SH, Baseggio AM, Cagnon VHA, Singh SV, et al. Prevention of prostate cancer in Transgenic Adenocarcinoma of the Mouse Prostate mice by yellow passion fruit extract and antiproliferative effects of its bioactive compound piceatannol. J Cancer Prev. 2020;25:87–99. doi: 10.15430/JCP.2020.25.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahm ER, Singh KB, Kim SH, Powolny AA, Singh SV. The role of lysosome-associated membrane protein 2 in prostate cancer chemopreventive mechanisms of sulforaphane. Cancer Prev Res (Phila) 2020;13:661–72. doi: 10.1158/1940-6207.CAPR-20-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tindall DJ, Rittmaster RS. The rationale for inhibiting 5alpha-reductase isoenzymes in the prevention and treatment of prostate cancer. J Urol. 2008;179:1235–42. doi: 10.1016/j.juro.2007.11.033. Erratum in: J Urol 2008;179:2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gucalp A, Traina TA. The androgen receptor: is it a promising target? Ann Surg Oncol. 2017;24:2876–80. doi: 10.1245/s10434-017-5961-9. [DOI] [PubMed] [Google Scholar]
- 14.De Amicis F, Chimento A, Montalto FI, Casaburi I, Sirianni R, Pezzi V. Steroid receptor signallings as targets for resveratrol actions in breast and prostate cancer. Int J Mol Sci. 2019;20:1087. doi: 10.3390/ijms20051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biesalski HK. Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care. 2007;10:724–8. doi: 10.1097/MCO.0b013e3282f0cef2. [DOI] [PubMed] [Google Scholar]
- 17.Santangelo C, Varì R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43:394–405. [PubMed] [Google Scholar]
- 18.Gabriel da Rosa R, Sganzerla WG, Barroso TLCT, Buller LS, Berni MD, Forster-Carneiro T. Sustainable production of bioactive compounds from jabuticaba (Myrciaria cauliflora): a bibliometric analysis of scientific research over the last 21 years. Sustain Chem Pharm. 2022;27:100656. doi: 10.1016/j.scp.2022.100656. [DOI] [Google Scholar]
- 19.Simas Frauches N, Montenegro J, Amaral T, Abreu JP, Laiber G, Junior J, et al. Antiproliferative activity on human colon adenocarcinoma cells and in vitro antioxidant effect of anthocyanin-rich extracts from peels of species of the Myrtaceae family. Molecules. 2021;26:564. doi: 10.3390/molecules26030564.d8ae183d430643409979fc08999740e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briguglio G, Costa C, Pollicino M, Giambò F, Catania S, Fenga C. Polyphenols in cancer prevention: new insights (Review) Int J Funct Nutr. 2020;1:9. doi: 10.3892/ijfn.2020.9. [DOI] [Google Scholar]
- 21.Izzo S, Naponelli V, Bettuzzi S. Flavonoids as epigenetic modulators for prostate cancer prevention. Nutrients. 2020;12:1010. doi: 10.3390/nu12041010.df9b2a463cd5457584ec90bde45e31be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reddivari L, Vanamala J, Chintharlapalli S, Safe SH, Miller JC., Jr Anthocyanin fraction from potato extracts is cytotoxic to prostate cancer cells through activation of caspase-dependent and caspase-independent pathways. Carcinogenesis. 2007;28:2227–35. doi: 10.1093/carcin/bgm117. [DOI] [PubMed] [Google Scholar]
- 23.Eskra JN, Dodge A, Schlicht MJ, Bosland MC. Effects of black raspberries and their constituents on rat prostate carcinogenesis and human prostate cancer cell growth in vitro. Nutr Cancer. 2020;72:672–85. doi: 10.1080/01635581.2019.1650943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costea T, Nagy P, Ganea C, Szöllősi J, Mocanu MM. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int J Mol Sci. 2019;20:1062. doi: 10.3390/ijms20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inada KOP, Silva TBR, Lobo LA, Domingues RMCP, Perrone D, Monteiro M. Bioaccessibility of phenolic compounds of jaboticaba (Plinia jaboticaba) peel and seed after simulated gastrointestinal digestion and gut microbiota fermentation. J Funct Foods. 2020;67:103851. doi: 10.1016/j.jff.2020.103851. [DOI] [Google Scholar]
- 26.Gatti G, Rivero V, Motrich RD, Maccioni M. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol. 2006;79:989–98. doi: 10.1189/jlb.1005597. [DOI] [PubMed] [Google Scholar]
- 27.González-Reyes S, Fernández JM, González LO, Aguirre A, Suárez A, González JM, et al. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol Immunother. 2011;60:217–26. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han M, Song Y, Zhang X. Quercetin suppresses the migration and invasion in human colon cancer Caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn Mag. 2016;12(Suppl 2):S237–44. doi: 10.4103/0973-1296.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu TC, Chan ST, Chang CN, Yu PS, Chuang CH, Yeh SL. Quercetin and chrysin inhibit nickel-induced invasion and migration by downregulation of TLR4/NF-κB signaling in A549 cells. Chem Biol Interact. 2018;292:101–9. doi: 10.1016/j.cbi.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Ghafouri-Fard S, Shabestari FA, Vaezi S, Abak A, Shoorei H, Karimi A, et al. Emerging impact of quercetin in the treatment of prostate cancer. Biomed Pharmacother. 2021;138:111548. doi: 10.1016/j.biopha.2021.111548. [DOI] [PubMed] [Google Scholar]
- 31.Singh AN, Baruah MM, Sharma N. Structure based docking studies towards exploring potential anti-androgen activity of selected phytochemicals against Prostate Cancer. Sci Rep. 2017;7:1955. doi: 10.1038/s41598-017-02023-5.121f8ace6fc94522aa3dbf154fa90106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila) 2009;2:187–94. doi: 10.1158/1940-6207.CAPR-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanashima N, Horie K, Maeda H. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules. 2017;23:74. doi: 10.3390/molecules23010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papoutsi Z, Kassi E, Tsiapara A, Fokialakis N, Chrousos GP, Moutsatsou P. Evaluation of estrogenic/antiestrogenic activity of ellagic acid via the estrogen receptor subtypes ERalpha and ERbeta. J Agric Food Chem. 2005;53:7715–20. doi: 10.1021/jf0510539. [DOI] [PubMed] [Google Scholar]
- 35.Eskra JN, Schlicht MJ, Bosland MC. Effects of black raspberries and their ellagic acid and anthocyanin constituents on taxane chemotherapy of castration-resistant prostate cancer cells. Sci Rep. 2019;9:4367. doi: 10.1038/s41598-019-39589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semenas J, Wang T, Sajid Syed Khaja A, Firoj Mahmud A, Simoulis A, Grundström T, et al. Targeted inhibition of ERα signaling and PIP5K1α/Akt pathways in castration-resistant prostate cancer. Mol Oncol. 2021;15:968–86. doi: 10.1002/1878-0261.12873.76e6e359dd224187a7177058d52aa91f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pisolato R, Lombardi AP, Vicente CM, Lucas TF, Lazari MF, Porto CS. Expression and regulation of the estrogen receptors in PC-3 human prostate cancer cells. Steroids. 2016;107:74–86. doi: 10.1016/j.steroids.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 38.Wang WH, Tyan YC, Chen ZS, Lin CG, Yang MH, Yuan SS, et al. Evaluation of the antioxidant activity and antiproliferative effect of the jaboticaba (Myrciaria cauliflora) seed extracts in oral carcinoma cells. Biomed Res Int. 2014;2014:185946. doi: 10.1155/2014/185946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha US, Bae WJ, Kim SJ, Yoon BI, Hong SH, Lee JY, et al. Anthocyanin induces apoptosis of DU-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med J. 2015;56:16–23. doi: 10.3349/ymj.2015.56.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catz SD, Johnson JL. BCL-2 in prostate cancer: a minireview. Apoptosis. 2003;8:29–37. doi: 10.1023/A:1021692801278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.