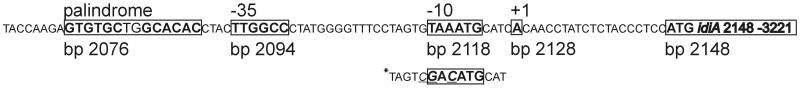Abstract
Expression of a thylakoid membrane-associated protein called IdiA (iron-deficiency-induced protein A) is highly elevated and tightly regulated by iron limitation in Synechococcus elongatus PCC 6301 and PCC 7942. Although this protein is not essential for photosystem II (PSII) activity, it plays an important role in protecting the acceptor side of PSII against oxidative damage, especially under iron-limiting growth conditions, by an unknown mechanism. We defined the iron-responsive idiA promoter by using insertional inactivation mutagenesis and reporter gene assays. A 67-bp DNA region was sufficient for full iron deficiency-inducible idiA promoter activity. Within this fragment is a palindromic sequence 4 bp upstream of a putative −35 promoter element, which resembles the binding site of FNR/CAP-type helix-turn-helix transcription factors. The absence of this palindromic sequence or a 3-bp mutation in a putative −10 region eliminated promoter activity completely. A previously identified candidate for a positively acting transcription factor is the IdiB protein, whose gene lies immediately downstream of idiA. IdiB shows strong similarity to helix-turn-helix transcription factors of the FNR/CAP family. A His6x-tagged IdiB that was overexpressed in Escherichia coli bound to a 59-bp fragment of the idiA regulatory region that included the palindrome. Although the idiA promoter lacks a consensus binding site for the iron-sensing regulator Fur, we attempted to inactivate fur in order to investigate the potential role of this factor. The resulting merodiploid mutants showed constitutive partial derepression of IdiA expression under iron-sufficient growth conditions. We concluded that IdiB is a specific iron-responsive regulator of idiA and that Fur has an indirect role in influencing idiA expression.
Numerous cyanobacterial genes that are tightly regulated in response to iron availability have been identified in Synechococcus species and other cyanobacteria. These genes include irpA, isiA, isiB, mapA, cpcG, slr0374, nblA, and idiA (21–23, 29, 32, 35, 38). The fact that upstream DNA regions of isiAB, irpA, and mapA contain operator sequences resembling Fur (ferric iron uptake repressor) boxes of gram-negative bacteria led to the assumption that expression of these genes under iron-limiting growth conditions is mediated via the Fur system (36). Fur was first discovered in the gram-negative bacteria Salmonella and Escherichia coli (5, 13), in which it is responsible for specific regulation of many genes involved in iron metabolism. The E. coli Fur protein is a dimeric DNA binding protein related to the CAP family (18). Each 17-kDa monomer contains an N-terminal DNA binding motif and a C-terminal metal binding domain. Fur can act as a transcription repressor only in the presence of its corepressor, Fe2+. Under iron-sufficient growth conditions it binds to specific DNA sequences, known as Fur boxes, and inhibits transcription of virtually all genes and operons that are repressed by iron (14). When iron becomes a limiting resource, the dimeric Fur complex releases its bound Fe2+, can no longer bind to its repressor site, and, therefore, allows transcription of iron-regulated genes. Fur boxes consist of a 19-bp sequence with dyad symmetry (two 9-bp inverted repeats), but they can also be interpreted as three 5-bp direct repeats (6).
Only one of the cyanobacterial genes mentioned above, isiA, has been shown to exhibit iron-regulated expression facilitated by a cyanobacterial Fur homolog (10). This cyanobacterial Fur homolog exhibits only moderate sequence similarity to the E. coli protein. A typical Fe2+ binding site shared with other gram-negative Fur proteins (HHXHXXCXXC) justifies classification of this protein as a Fur homolog and not a DtxR homolog, the major iron-dependent regulation system of gram-positive organisms (10, 15). However, some features of the cyanobacterial Fur are unusual, and the Synechococcus elongatus gene cannot complement an E. coli fur mutant (10). Additionally, insertional inactivation of fur in S. elongatus results in merodiploid mutants, whereas in E. coli mutants there is complete segregation of the mutant allele. Despite the fact that no fully fur-inactivated strain has been obtained, the merodiploid cyanobacterial mutant does exhibit derepression of several iron-regulated genes and proteins, probably due to a significantly lower number of intracellular Fur molecules (10).
Another prominent iron-regulated protein of S. elongatus PCC 7942 and PCC 6301 is IdiA (iron-deficiency-induced protein A) (29). The amount of this 35-kDa protein, which is attached to the cytoplasmic side of thylakoid membranes, is elevated under iron-deficient growth conditions and to some extent under manganese-deficient growth conditions (26). Although biochemical assays revealed that IdiA is most likely involved in protection of photosystem II against oxidative stress, especially under iron-limiting growth conditions, its precise function is unknown (7). Expression analyses using Western and Northern blot techniques in the presence of protein and RNA biosynthesis inhibitors, respectively, have indicated that idiA is monocistronically transcribed and that expression of IdiA is most likely regulated at the transcriptional level (27). A 5′ transcript end for idiA has been mapped to 193 bp (bp 1929 in the idiA EMBL database accession no. Z48754 entry) upstream of the first of at least three potential translational start codons (bp 2121, 2148, and 2160). Biochemically purified IdiA is processed at a procaryotic AA cleavage site (RRRAEAAEGEV), which is consistent with the idiA mRNA size determined from Northern blots. A putative Fur box was identified upstream of the mapped 5′ end of the idiA mRNA. However, the 19-bp sequence only poorly resembles the Fur box consensus sequence from E. coli (11 of 19 possible matches), and it lacks the typical dyad symmetry. Additional sequencing of the DNA regions flanking the idiA gene and interposon mutagenesis led to identification of two downstream genes, idiB and dpsA, which encode a putative helix-turn-helix transcriptional activator of the FNR/CAP family and a Dps-PexB homolog, respectively. Loss of either IdiB or DpsA by gene inactivation led to a drastic decrease in IdiA content and loss of the ability to increase expression under inducing conditions (27). These data suggest that idiA is inducible by iron deficiency rather than repressed under iron-sufficient conditions via Fur.
Because idiA is one of the most prominent cyanobacterial genes regulated by iron availability (7, 35) and/or oxidative stress and because available data suggested regulatory involvement of DNA binding proteins other than Fur, we performed a detailed analysis of the idiA promoter in S. elongatus PCC 7942. Functional analysis of the idiA promoter was required because although idiA transcription is tightly regulated in response to manganese and iron limitation, no typical promoter structures were evident upstream of the previously mapped 5′ end of the idiA transcript.
We determined that the mapped mRNA 5′ end lies upstream of the iron-regulated promoter of idiA and, therefore, could not represent the transcription start site. The functional idiA promoter is located in a 67-bp region which includes the first of three potential start codons and requires a 14-bp region of dyad symmetry, which was bound by the IdiB protein in mobility shift assays. Despite the absence of a consensus Fur box in the idiA regulatory region, expression of idiA is partially derepressed in merodiploid Δfur mutants.
MATERIALS AND METHODS
Culture conditions.
S. elongatus wild-type strain PCC 7942 (formerly Anacystis nidulans R2 or Synechococcus sp. strain PCC 7942) (17) and mutant strains were grown in BG-11M medium (4) in 100-ml cultures in shaking flasks with 1% CO2-enriched air at a light intensity (photosynthetic photon flux density) of 150 microeinsteins m−2 s−1 (standard fluorescent light bulbs). For reporter gene experiments starter cultures that had optical densities at 750 nm (OD750) of 0.9 to 1.2 were used to inoculate 40-ml tubes at an OD750 of 0.25. The cultures were placed in an aquarium, incubated at 30°C with a light intensity of 250 microeinsteins m−2 s−1, bubbled with 1% CO2 in air, and sampled after 24, 48, and 72 h. For growth under iron-deficient conditions, cells from starter cultures were harvested, washed twice with distilled water, and then transferred to medium from which iron was omitted. All cyanobacterial strains were grown on BG-11M agar plates containing 1.5% agar (Difco Bacto Agar). As indicated below, antibiotics were added at the following concentrations: ampicillin, 10 mg liter−1; chloramphenicol, 10 mg liter−1; gentamicin, 2 mg liter−1; kanamycin, 25 mg liter−1; and spectinomycin, 20 mg liter−1.
E. coli
E. coli DH10B and JM107 were hosts for all plasmids. Cells were cultivated in liquid Luria-Bertani medium or on Luria-Bertani medium containing 1.5% agar. As indicated below, antibiotics were added at the following concentrations: ampicillin, 200 mg liter−1; chloramphenicol, 50 mg liter−1; gentamicin, 10 mg liter−1; kanamycin, 50 mg liter−1; and spectinomycin, 50 mg liter−1.
Construction of mutant strains and DNA manipulations.
Plasmid clone analysis, cleavage with restriction endonucleases, agarose electrophoresis, ligation, Southern blotting, and transformation of E. coli strains were performed by using standard procedures (33). The plasmids, strains, and oligonucleotides used for PCR amplification of genomic template DNA with Pwo polymerase (Roche Molecular Biochemicals, Indianapolis, Ind.) are listed in Table 1. All plasmids used in β-galactosidase assays were derivatives of neutral site I (NS1) (GenBank accession no. U30252) targeting vector pAM990 (25). Promoter fragments of idiA generated by PCR were cloned into a unique SmaI site of pAM990 to produce out-of-frame transcriptional fusions with a promoterless lacZ gene. The constructs used to create strains for bioluminescence assays were derivatives of neutral site II (NS2) (GenBank accession no. U44761) targeting vector pAM1580 (1). PCR-derived idiA promoter fragments were cloned into StuI- or SmaI-SalI-digested pAM1580 to create out-of-frame transcriptional fusions to promoterless luxAB genes. All fragments made with primers AMO524 (EMBL database accession no. Z48754 entry starting at bp 2299) and AMO536 (EMBL database accession no. Z48754 entry starting at bp 2129) were cut with SalI prior to cloning into pAM1580. Transformation of the S. elongatus wild-type strain with pAM990 derivatives and transformation of strain AMC395 (psbAI::luxCDE background in NS1) with pAM1580 derivatives occurred through homologous recombination with the chromosome (11). This method was also used to generate the interposon mutants in the upstream idiA region and the sigma factor genes.
TABLE 1.
S. elongatus strains and plasmids
| Strain | Parent strain | Plasmida | Characteristics | Primer combination | Source or reference(s) |
|---|---|---|---|---|---|
| PCC 7942 | Wild type | Laboratory collection | |||
| AMC520 | AMC411 | pAM1997 | psbAII::luxAB in NS2, psbAI::luxCDE in NS1, Cmr Spr | Laboratory collection | |
| AMC539 | Wild type | pAM1518b | conII::luxAB in NS2, psbAI::luxCDE in NS1, Cmr Spr | 19 | |
| AMC777 | Wild type | pAM2338 | −54 to +43 psbAI::luxAB in NS2, psbA1::luxCDE in NS1, Cmr Spr | 30 | |
| pAM1518 | |||||
| AMC395 | Wild type | pAM1518 | psbAI::luxCDE in NS1, Spr | Laboratory collection | |
| Interposon mutagenesisc | |||||
| AMC838 | Wild type | pKPM226 | Kmr in NruI site at bp 582d | 27 | |
| AMC839 | Wild type | pKPM228 | Spr in deletion of NaeI sites at bp 1586 to 1777d | This study | |
| AMC840 | Wild type | pKPM227 | Spr in KpnI site at bp 1397d | 27 | |
| AMC843 | Wild type | pKPM231 | Spr in AvaI site at bp 1901d | This study | |
| AMC844 | Wild type | pKPM234 | Spr in ScaI site at bp 1829d | This study | |
| AMC808 | Wild type | pAM2389 | Spr in NsiI site at bp 2121d | This study | |
| AMC809 | Wild type | pAM2390 | Spr in ApoI site at bp 1974d | This study | |
| AMC810 | Wild type | pAM2392 | Spr in SfoI site at bp 2060d | This study | |
| luxABCDE reporter strains | |||||
| AMC811 | AMC395 | pAM1580 | Promoterless luxAB, Cmr Spr | Laboratory collection | |
| AMC812 | AMC395 | pAM2393 | 1,419-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO537-AMO536e | This study |
| AMC814 | AMC395 | pAM2395 | 1,589-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO537-AMO524e | This study |
| AMC815 | AMC395 | pAM2396 | 902-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO538-AMO524e | This study |
| AMC816 | AMC395 | pAM2397 | 747-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO539-AMO524e | This study |
| AMC817 | AMC395 | pAM2398 | 480-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO540-AMO524e | This study |
| AMC818 | AMC395 | pAM2399 | 357-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO541-AMO524e | This study |
| AMC819 | AMC395 | pAM2400 | 623-bp PCR fragment in StuI site in pAM1580, Cmr Spr | AMO539-AMO525 | This study |
| AMC820 | AMC395 | pAM2401 | 611-bp PCR fragment in StuI site in pAM1580, Cmr Spr | AMO539-AMO526 | This study |
| AMC821 | AMC395 | pAM2402 | 357-bp PCR fragment in StuI site in pAM1580, Cmr Spr | AMO540-AMO525 | This study |
| AMC822 | AMC395 | pAM2403 | 345-bp PCR fragment in StuI site in pAM1580, Cmr Spr | AMO540-AMO526 | This study |
| AMC823 | AMC395 | pAM2404 | 259-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO529-AMO524e | This study |
| AMC824 | AMC395 | pAM2405 | 222-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO530-AMO525 | This study |
| AMC899 | AMC395 | pAM2534 | 192-bp PCR fragment in SmaI-SalI sites in pAM1580, Cmr Spr | AMO531-AMO524e | This study |
| AMC911 | AMC395 | pAM2559 | 211-bp PCR fragment in StuI site in pAM1580, Cmr Spr | AMO541-AMO528 | This study |
| AMC912 | AMC395 | pAM2558 | 201-bp PCR fragment in StuI site in pAM1580, Cmr Spr | AMO541-AMO527 | This study |
| lacZ reporter strains | |||||
| AMC801 | Wild type | pAM990 | Promoterless lacZ in NS1 Spr | Laboratory collection | |
| AMC802 | Wild type | pAM2406 | 1,422-bp PCR fragment in SmaI site in pAM990, Spr | AMO537-AMO536e | This study |
| AMC805 | Wild type | pAM2409 | 761-bp PCR fragment in SmaI site in pAM990, Spr | AMO539-AMO524e | This study |
| AMC807 | Wild type | pAM2411 | 371-bp PCR fragment in SmaI site in pAM990, Spr | AMO541-AMO524e | This study |
| Sigma factor mutant strains | |||||
| AMC851 | Wild type | pAM1344 | Insertional inactivation of rpoD2, Kmr | 30; this study | |
| AMC852 | Wild type | pAM2414 | Insertional inactivation of rpoD3, Kmr | 30; this study | |
| AMC853 | Wild type | pAM2413 | Insertional inactivation of rpoD4, Kmr | 30; this study | |
| AMC854 | Wild type | pAM2331 | Insertional inactivation of sigC, Gmr | 30; this study | |
| PCC 7942 ΔFur strains | |||||
| AMC915 | Wild type | pAM2571 | 864-bp Fur fragment in pUC19, Kmr in PpuMI site | This study | |
| AMC930 | Wild type | pAM2571 | 864-bp Fur fragment in pUC19, Kmr in PpuMI site | This study | |
| AMC916 | Wild type | pAM2572 | 864-bp Fur fragment in pUC19, Cmr in PpuMI site | This study |
Homologous recombination substrate for integration of genes into the chromosome.
conII::luxAB reporter plasmid not archived.
Mutagenesis by insertion of antibiotic resistance genes into the chromosome was mediated by homologous recombination.
EMBL database accession no. Z48754 entry.
AMO536 and AMO524 create a SalI site that is not present in the EMBL database accession no. Z48754 entry. All pAM990 derivatives carrying a PCR-amplified fragment obtained with AMO536 have three mutated bases near the right end of the PCR fragment, and all fragments used for luxAB reporter experiments were cut with SalI prior to cloning.
Insertional inactivation of the fur locus in S. elongatus PCC 7942.
Primers AMO534 (5′-AAGTTTTGAGGCTCCGACTGCTG-3′; database accession no. L41065 entry starting at bp 1) and AMO535 (5′-GATCGCCTCGAACAGCTCTATCA-3′; database accession no. L41065 entry starting at bp 864) were used to PCR amplify an 864-bp fragment encoding the entire fur gene and flanking DNA regions (10). This fragment was cloned into SacI-cut and T4 DNA polymerase-blunted pUC19. To interrupt the fur gene, a Kmr cassette and a Cmr cassette were individually cloned into a unique PpuMI site of fur, leaving flanking regions of the same size on both sides to allow efficient homologous recombination with the S. elongatus chromosome.
In vivo luciferase measurements.
For in vivo bioluminescence measurements whole cells of lux reporter strains grown under iron-sufficient, -deficient, or -replete growth conditions were taken from a liquid culture and diluted in the corresponding medium to an OD750 of 0.2. Aliquots (10 μl) were transferred to a scintillation vial and dark adapted for 3 min to allow decay of chlorophyll fluorescence. Luciferase expression was measured as light production in counts per minute with a Beckman LS5301 scintillation counter with coincidence disabled. In vivo bioluminescence measurements were also obtained for cyanobacterial cultures streaked onto BG-11M agar pads in 96-well microtiter plates by using a modified Packard Instrument Co. TopCount luminometer as previously described (19). Prior to these measurements a 12-h dark pulse was administered to all cultures to reset the circadian clock.
Protein determination and β-galactosidase assays.
The methods used for quantification of soluble proteins by a modified Lowry technique and for determination of specific β-galactosidase activity (in nanomoles per minute per milligram of protein) by a colorimetric assay with the substrate o-nitrophenyl-β-d-galactopyranoside have been described previously (34).
Preparation of French press extracts, SDS-PAGE, and immunoblotting.
To prepare soluble protein extracts for Western blot experiments, 35-ml cultures of S. elongatus cells were harvested by centrifugation, washed once with 10 mM sodium phosphate buffer (pH 7), and then resuspended in the same buffer to a final volume of 4 ml. Each cell suspension was passed twice through a prechilled French press at 137.9 MPa (20,000 lb/in2). Unbroken cells were removed by centrifugation at 4,000 × g for 5 min at 4°C. Protein samples from the supernatant fraction were denatured for 30 min at 65°C by using a dithiothreitol-containing buffer. E. coli cells were lysed and extracts were denatured by heating them at 100°C for 5 min in a β-mercaptoethanol-containing denaturing buffer system. Protein samples (20 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (20) and transferred to a nitrocellulose membrane (BA85S; Schleicher & Schuell, Keene, N.H.) as previously described (28). Proteins were immunostained with an anti-IdiA antiserum (28) at the dilutions indicated in the figure legends and were detected with an ECL detection kit (Amersham Pharmacia Biotech, Piscataway, N.J.).
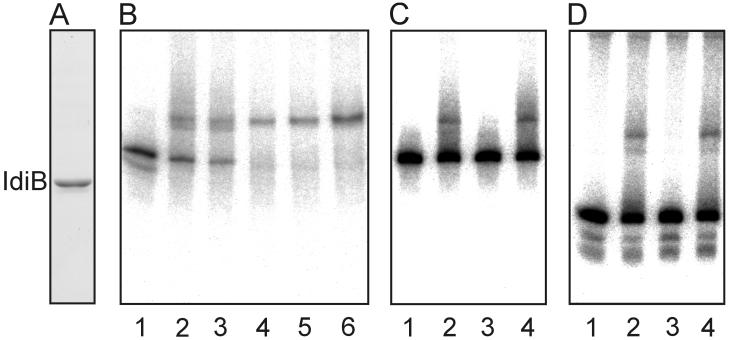
Overexpression of IdiB and electrophoretic mobility shift assays with partially purified IdiB protein.
IdiB was overexpressed by using the Qiaexpress overexpression system (Qiagen, Valencia, Calif.) for synthesis of N-terminal His-tagged proteins (His6). A PCR-derived 629-bp fragment obtained by using primers AMO532 (5′-PATGATTGCCAGTCACGTAACC-3′; EMBL database accession no. Z48754 entry starting at bp 4090) and AMO533 (5′-PGCTTAGGCATCTATGGCATC-3′; EMBL database accession no. Z48754 entry starting at bp 3463), encoding the entire idiB gene, was cloned into BamHI-digested and T4 DNA polymerase-blunted overexpression vector pQE32. After induction with 50 μM isopropyl-β-d-thiogalactopyranoside (IPTG) at an OD600 of 0.5 and growth for an additional 1 h at 25°C to minimize the formation of insoluble protein, cells were harvested and passed twice through a prechilled French press at 137.9 MPa. The extract, containing at least 50% soluble IdiB, was centrifuged for 30 min at 12,000 × g to prepare a cleared lysate for affinity purification on an Ni-nitrilotriacetic acid (NTA) matrix (Qiagen). The partially purified IdiB was used for electrophoretic mobility shift assays as previously described (24). The 222-, 163-, and 59-bp idiA promoter fragments used for binding and competition assays were released from pAM2405 by digestion with HindIII, XhoI, and NsiI, gel purified, and end labeled as described previously (24), except that the binding buffer did not contain KCl. After electrophoresis (5% polyacrylamide) at 4°C, the gels were dried and images were captured with a Fujix BAS 2000 phosphor imaging system.
RESULTS
Interposon mutagenesis of upstream idiA gene regions located the idiA promoter downstream of the previously mapped 5′ end.
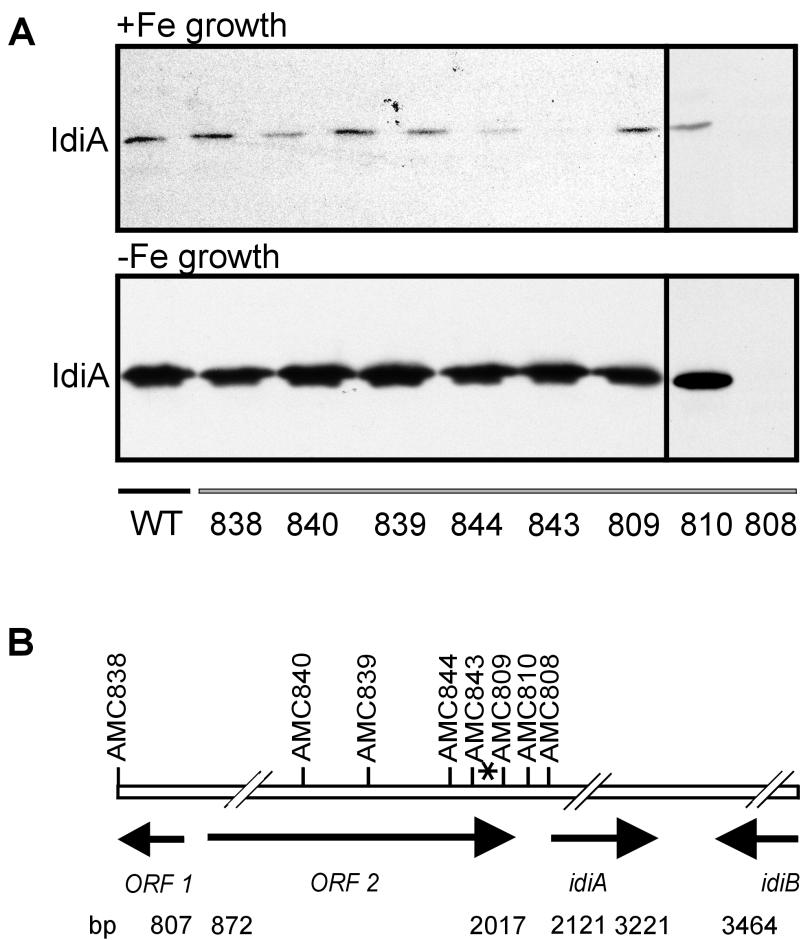
Interposon cassettes bearing spectinomycin or kanamycin resistance genes and Ω terminators (8) were introduced at eight different locations on the S. elongatus chromosome, approaching the putative start codon of idiA at bp 2121 (Fig. 1B). All recombination events were verified by Southern analysis (data not shown). Only one of the mutant strains, AMC808, was affected in terms of IdiA expression compared to the wild type under iron-sufficient or iron-deficient growth conditions. Strain AMC808, carrying a Spr cassette in the putative start codon of idiA at bp 2121, did not show any detectable IdiA expression under either type of growth conditions. These results show that neither ORF1 nor ORF2 (both of which are upstream of idiA) is necessary for idiA expression and that idiA is transcribed monocistronically, as previously concluded from Northern analysis (27). Furthermore, there is no autonomously functional promoter activity downstream of bp 2121. Surprisingly, neither AMC809 (Spr in ApoI at bp 1974) nor AMC810 (Spr in SfoI at bp 2060) showed modified expression of IdiA under iron-sufficient or -deficient growth conditions, although the interposons in those strains are between the only detectable 5′ end of idiA mRNA at bp 1929 and the idiA gene (Fig. 1B). Thus, the functional promoter and the actual transcription start site of idiA are downstream of bp 1929.
FIG. 1.
(A) Comparative immunoblot with French press extracts from S. elongatus wild-type (WT) and mutant strains carrying antibiotic resistance cassettes in the chromosomal DNA region upstream of idiA. Cells were grown for 3 days under iron-sufficient conditions (+Fe growth) or iron-deficient conditions (-Fe growth). After preparation of French press extracts, samples containing 25 μg of protein were subjected to SDS-PAGE, transferred to nitrocellulose, and immunostained with an anti-IdiA antiserum (dilution, 1:20,000). (B) Location of antibiotic resistance cassettes inserted near the idiA locus. The AMC strains are mutants that carry insertions at different positions. The hash marks indicate breaks in the scale required to include several loci on the map. The numbers are positions in the EMBL database accession no. Z48754 entry. The asterisk indicates the position of the previously mapped 5′ transcript end.
Initial mapping of the idiA promoter by idiA::lacZ reporter assays.
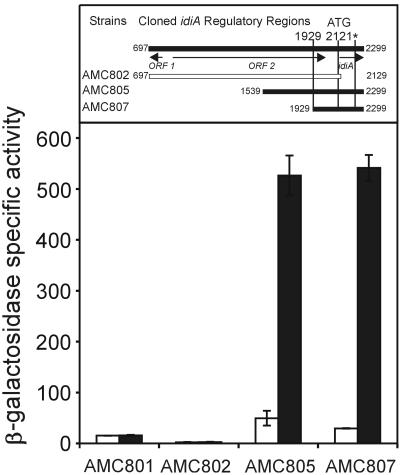
In order to define promoter elements of the idiA gene, we constructed transcriptional fusions between different idiA upstream DNA fragments and a promoterless E. coli lacZ gene in a recombinational vector that targets the reporter gene to a neutral site (NS1) in the S. elongatus genome. Three PCR-derived fragments of the upstream DNA region of idiA, with two different downstream ends and three different upstream ends, were cloned into pAM990 to create reporter strains AMC802 (bp 687 to 2129), AMC805 (bp 1539 to 2299), and AMC807 (bp 1929 to 2299). S. elongatus reporter strains were tested for β-galactosidase activity after growth for 2 days under iron-sufficient and -deficient growth conditions. Strain AMC801, carrying a promoterless lacZ gene, served as a negative control. Fragments with a right endpoint (orientated as shown in Fig. 1B) at bp 2299, located in the coding region of idiA and downstream of a protein cleavage site of IdiA at bp 2255, showed promoter activity, whereas strain AMC802, which carried a fragment with a downstream end at bp 2129, showed no activity (Fig. 2). Strains AMC805 and AMC807 exhibited up to 20-fold increases in β-galactosidase activity after 2 days of growth in iron-deficient medium compared to the values obtained for iron-sufficient cultures. The fact that strain AMC807 showed inducible reporter activity indicates that the previously mapped 5′ end of the idiA mRNA is not the transcription start site. The uninduced level of idiA::lacZ expression in inducible strains AMC805 and AMC807 was only twice the level in strain AMC801, which carried a promoterless lacZ gene, indicating that the idiA promoter is nearly shut off under regular growth conditions. The fact that AMC802 exhibited levels of activity that are about 10% of the AMC801 background level is rather surprising, because the fragment tested comprised bp 697 to 2129 and, therefore, included the previously suggested start codon at bp 2121. However, the right endpoint of this fragment carried a 3-bp mutation, which did not include the ATG potential start codon. The sequence change was introduced to create a SalI site for directional cloning into pAM1580 for finer mapping of the idiA promoter with luxAB-based reporter assays.
FIG. 2.
β-Galactosidase activities from idiA::lacZ fusions in S. elongatus reporter strains after 48 h of growth under iron-sufficient (open bars) and iron-deficient (solid bars) growth conditions. At least three experiments were carried out to calculate the average activity and the standard error of the means. Strain AMC801, which carries a promoterless lacZ gene, was used as a negative control. The numbers given for cloned idiA regulatory regions are the positions in the EMBL database accession no. Z48754 entry.
Fine mapping of the idiA promoter and identification of distinctive regulatory elements with idiA::luxAB-based reporter gene assays.
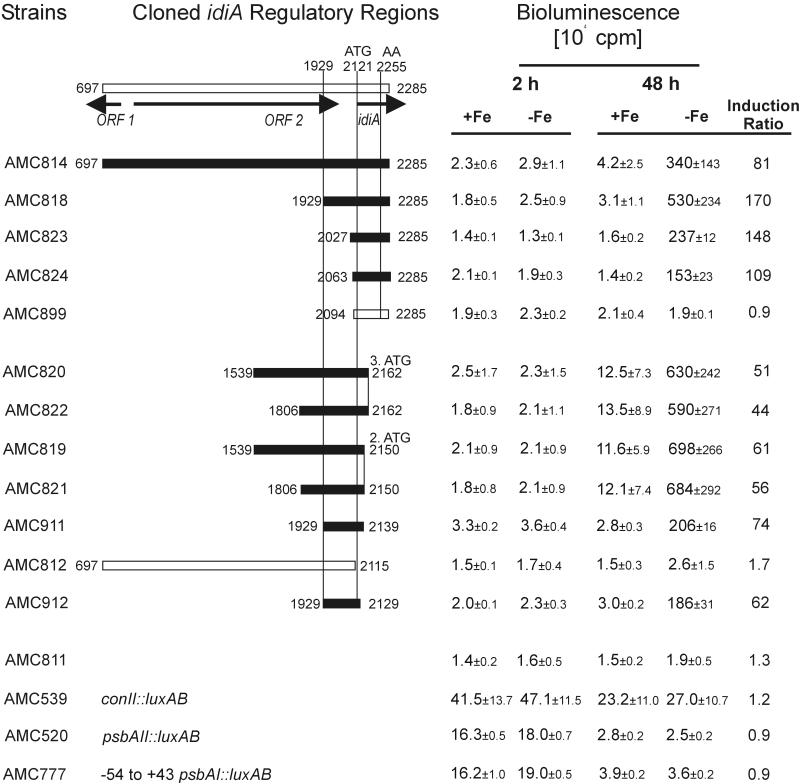
To narrow down the idiA regulatory region to a minimal functional promoter and to identify distinctive structural elements, we constructed transcriptional fusions between different idiA upstream fragments and promoterless luxAB genes from Vibrio harveyi in recombinational vector pAM1580, which targets the reporter gene to a specific locus (NS2) in the S. elongatus genome. Altogether, we created 15 PCR-derived fragments of the idiA DNA region with different left and right endpoints (between bp 697 and 2285). Each fragment was cloned into pAM1580 and then used to transform S. elongatus AMC395 (with psbAI::luxCDE genes providing aldehyde as a substrate for LuxAB bioluminescence) (Table 1). After the ability to express native IdiA in immunoblots with the anti-IdiA antiserum (data not shown) was verified, reporter strains were tested for bioluminescence under iron-sufficient and -deficient growth conditions at two time points (Fig. 3). Strain AMC811, carrying promoterless luxAB genes, served as a negative control, whereas AMC539 (E. coli consensus promoter, conII::luxAB in NS2, psbAI::luxCDE in NS1), AMC520 (psbAII::luxAB in NS2, psbAI::luxCDE in NS1), and AMC777 (−54 to +43 psbAI::luxAB in NS2, psbAI::luxCDE in NS1) were used to define the transcriptional characteristics of known S. elongatus promoters under iron-deficient conditions and to measure idiA promoter strength. As our data illustrate, none of the control strains exhibited an increase in bioluminescence with time under either iron-sufficient or iron-deficient growth conditions. Whereas AMC811 exhibited a constant very low level of bioluminescence, strain AMC539 had a 50% decrease in the initial level of bioluminescence and strains AMC520 and AMC777 showed more than 75% decreases in the initial levels of bioluminescence after 48 h of growth under both types of conditions, which may have reflected physiological changes in the cultures as light penetration decreased with increasing cell density. To track down the idiA promoter region from the left end, we constructed reporter strains AMC814, AMC818, AMC823, and AMC824 by using a right-end primer in the coding region of idiA that worked in β-galactosidase assays, except that the fragment was 14 bp shorter due to SalI digestion for cloning into pAM1580. All strains exhibited low initial levels of bioluminescence similar to that of AMC811 under both iron-sufficient and iron-deficient conditions 2 h after inoculation of cultures (t2 h), verifying results obtained with the lacZ reporter strains and showing that transcription of idiA under regular growth conditions is nearly shut off independent of the growth phase. Whereas the bioluminescence at t48 h for all test strains grown in iron-sufficient medium remained constant or increased slightly (up to 2.5-fold), the levels of bioluminescence of these strains grown in iron-deficient medium increased tremendously. With strain AMC818 (bp 1929 to 2285) we detected a 170-fold increase in bioluminescence (when t48 h values obtained in the presence and in the absence of Fe were compared). The levels of induction in the inducible strains varied between 81- and 170-fold.
FIG. 3.
Expression of idiA::luxAB fusions in S. elongatus reporter strains, as determined by measuring bioluminescence after growth for 2 and 48 h under iron-sufficient and -deficient conditions. The numbers above and to the left and right of bars are the positions of the ends of cloned PCR fragments with respect to the positions in the EMBL database accession no. Z48754 entry. The map at the top shows the positions of relevant open reading frames. AA indicates the position of the IdiA protein cleavage site. Strains AMC520, AMC539, and AMC777 were used as well-characterized controls for general transcriptional and translational activity. The average values and standard deviations were calculated on the basis of at least three independent growth experiments; 2 and 48 h refer to the duration of growth under iron-sufficient or -deficient conditions prior to sampling. The induction ratios are the ratios of the values obtained with iron-deficient samples to the values obtained with iron-sufficient samples after 48 h of growth.
To narrow down the location of the idiA promoter from the right end, we constructed reporter strains AMC812, AMC819, AMC820, AMC821, AMC822, and AMC911 by using left-end primers that were previously shown to be sufficient for promoter activity. All strains showed a low initial rate of bioluminescence 2 h after inoculation under both iron-sufficient and iron-deficient conditions. The level of bioluminescence at t48 h in all strains grown in regular medium was the same as the t2 h value or was up to 7.5-fold greater than the t2 h value. After 48 h of growth under iron-deficient conditions the bioluminescence of all strains increased dramatically (when t48 h values obtained in the presence and in the absence of Fe were compared); the increases ranged from 44-fold for AMC822 to 74-fold for AMC911. The only exception was AMC812, which exhibited only background (promoterless) levels of bioluminescence. These findings reveal that a DNA region between bp 2115 and 2139 is essential for idiA promoter activity. Thus, a minimal, fully iron deficiency-inducible idiA promoter comprises bp 2063 (e.g., AMC824) to bp 2139 (e.g., AMC911). A closer examination of this DNA region revealed a 14-bp palindromic sequence (GTGTGCTGGCACAC) at bp 2076 that resembles binding sites of helix-turn-helix transcriptional activators, followed by a 6-bp sequence (TTGGCC) at bp 2094 that is similar to the E. coli −35 consensus sequence (Fig. 4). A region at bp 2118, TAAATG, may represent a −10 box. This segment carries a 3-bp mutation in the reporter fusion in AMC802, which exhibited very low noninducible β-galactosidase activity (Fig. 2). To test whether a wild-type promoter fragment with a right end at bp 2129 (as in AMC802, but without mutations) would create a fully functional idiA promoter fragment and to test the influence of the palindromic sequence on transcriptional activity, we created reporter strains AMC912 (bp 1929 to 2129) and AMC899 (bp 2094 to 2285, without palindrome). As strain AMC912 exhibited all of the features of inducible idiA::luxAB transcriptional activity, the mutations in AMC802 at bp 2020, 2018, and 2017 eliminated inducible promoter activity. In contrast, promoter activity was barely detectable in AMC899. Therefore, the absence of the palindromic sequences eliminated promoter activity even though the putative −35 region was still present and intact. These data are consistent with the presence of a promoter having the −35 and −10 elements shown in Fig. 4 and requiring the palindromic sequence for induction.
FIG. 4.
Structural elements of the idiA promoter. Proposed functional elements are enclosed in boxes and labeled. The proposed start codon, the −35 and −10 sites, and the palindrome are indicated by boldface type. The bp numbers are the positions in the EMBL database accession no. Z48754 entry. Mutated bases in the AMC802 sequence compared to the wild-type sequence are italicized and underlined.
His-tagged IdiB binds to the upstream region of idiA.
A gene designated idiB (bp 4090 to 3464), which lies downstream of idiA and is transcribed in the opposite direction (Fig. 1B) (27), encodes a putative helix-turn-helix DNA binding protein that is similar to another S. elongatus transcriptional activator, CysR, which is involved in sulfur metabolism (31). Both IdiB and CysR belong to the FNR/CAP family of transcriptional activator proteins (37). Because of the predicted function of IdiB and the limited ability of an IdiB-free mutant to express IdiA (27), we overexpressed and partially purified a His6x-tagged IdiB molecule to test its DNA binding capacities in gel mobility shift experiments with idiA probes. After the IPTG concentration was reduced to 50 μM and the growth temperature (25°C) and time of induction (1.25 h) were reduced to prevent the formation of insoluble protein, 50% of the induced His6x-tagged IdiB remained soluble in the cleared lysate and was affinity purified to homogeneity (Fig. 5A). Three DNA probes corresponding to the following regions of the idiA gene were prepared: (i) a 222-bp probe from bp 2063 to 2285 (including the minimal promoter and parts of the translated region), (ii) a 163-bp probe from bp 2121 to 2285 (lacking the minimal promoter but with parts of the translated region), and (iii) a 59-bp probe from bp 2063 to 2121 (including the minimal promoter fragment only). Greater amounts of IdiB bound greater amounts of the 222-bp probe (Fig. 5B). IdiB also bound to the 59-bp probe containing the minimal promoter (Fig. 5D, lane 2). Unlabeled 222-bp (Fig. 5D, lane 3) and 59-bp (Fig. 5C, lane 3) probes competed for binding, but the unlabeled 163-bp probe did not compete (Fig. 5C and D, lanes 4). This shows that binding of IdiB to the idiA promoter region is specific. Moreover, IdiB never bound to the radiolabeled 163-bp probe (data not shown).
FIG. 5.
Electrophoretic mobility shift assay with idiA-specific probes and purified His6x-tagged IdiB protein. (A) Coomassie blue-stained SDS-PAGE gel of IdiB representing 15 μl of eluate from Ni-NTA affinity matrix. (B) Different amounts of IdiB eluate (0.1, 0.5, 1, 2, and 5 μl in lanes 2 to 6, respectively) were incubated with 10 to 20 pg of the radiolabeled 222-bp fragment (bp 2063 to 2285 of the accession no. Z48754 sequence). Lane 1 contained only radiolabeled probe. (C) Radiolabeled 222-bp probe was incubated with 0.5 μl of IdiB eluate (lane 2) and in the presence of unlabeled 59-bp (bp 2063 to 2121) (lane 3) and 163-bp (bp 2122 to 2285) (lane 4) competitor fragments. (D) Radiolabeled 59-bp probe was incubated with 0.5 μl of purified IdiB eluate (lane 2) and in the presence of unlabeled 222-bp (lane 3) and 163-bp (lane 4) competitor fragments. In panels B to D lane 1 contained the probe fragment with no IdiB extract added. Competitor fragments were added at a 50-fold molar excess to binding assay mixtures. All assay mixtures contained 0.5 μg of poly(dI-dC) as a nonspecific competitor.
Comparative time courses of idiA::luxAB gene expression under iron-sufficient, -depleted, and -replete conditions.
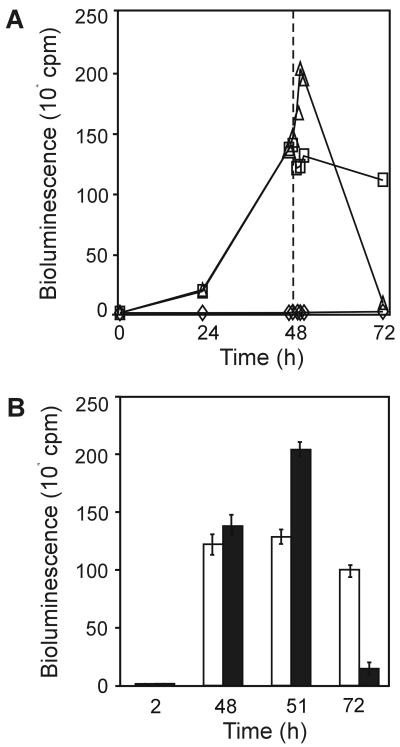
We predicted that if idiA transcription were regulated via the Fur system, idiA::luxAB transcription would stop as soon as iron entered the cells and formed stable Fe2+-Fur transcription repressor complexes. To test whether idiA is regulated by iron in this way, we investigated the kinetic characteristics of idiA expression by monitoring bioluminescence over a 72-h period under standard growth conditions, under iron-depleted conditions, and after iron-replete conditions were restored (Fig. 6). As stated above, transcription of idiA::luxAB in AMC824 (bp 2063 to 2285), measured as bioluminescence, seemed to stop under iron-sufficient growth conditions. In two cultures of AMC824 grown in parallel in iron-deficient medium, the bioluminescence values increased 13- to 14-fold by 24 h and 93-fold by 47 h (compared to t2 h values). A linear increase in the levels of bioluminescence in the iron-depleted strains up to 48 h was verified with additional time points by growing the reporter strains on solid BG-11M medium lacking iron and measuring activity every 1.61 h with a cycling luminometer (TopCount; Packard Instrument Co.) (data not shown). After bioluminescence was measured at t48 h, we added 30 μM ferric ammonium citrate (the regular iron content of BG-11M medium) to one of the iron-depleted parallel cultures. While the strain which remained under iron-deficient growth conditions bioluminesced at a nearly constant level for at least another 24 h, the strain grown under iron-replete conditions exhibited a burst of bioluminescence that seemed to peak at t51 h (the value was 137-fold higher than the t2 h value). This represented a 48% increase in bioluminescence within 3 h after repletion of iron. This increase cannot be attributed to a growth phenomenon because all values were normalized to cell number and the increase was consistently observed in multiple experiments (Fig. 6B). Despite the initial induction after iron addition, by t72 h bioluminescence had decreased to almost noninduced levels. These results are inconsistent with direct regulation via the Fur system.
FIG. 6.
Time course of idiA::luxAB gene expression in AMC824 under iron-sufficient, -depleted, and -replete conditions. Strains were grown under iron-sufficient or -deficient conditions. (A) Single time course assay with AMC824. The dashed line indicates when the regular amount of iron components in BG-11M medium (30 μM ferric ammonium citrate) was added to the iron-replete culture. Symbols: ◊, iron-sufficient conditions; □, iron-deficient conditions; ▵, iron-deficient and -replete conditions. (B) Average values (with error bars) for the key time points in three experiments like those whose results are shown in panel A. Open bars, iron-deficient growth conditions; solid bars, iron-deficient and -replete growth conditions.
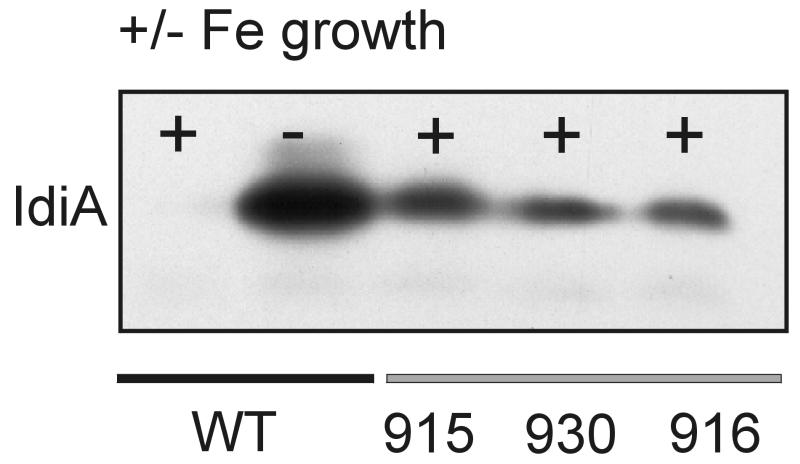
Insertional inactivation of fur in S. elongatus PCC 7942 creates a merodiploid mutant that shows partial derepression of idiA expression under iron-sufficient growth conditions.
Because Fur is known to act as a repressor, we would have predicted that if idiA were under Fur control, the promoter analysis of idiA would have identified a derepressed promoter that lacked the Fur binding site. However, the only two classes of promoter fragments were the fragments that were iron inducible and the fragments that did not activate reporter expression at all. The kinetics of reporter gene expression also did not fit criteria for Fur regulation. However, because Fur is known to be involved in iron-responsive expression of at least one gene in cyanobacterial strain PCC 7942, we tested the possibility that Fur affects idiA expression. Attempts to construct insertionally inactivated S. elongatus mutants with plasmids pAM2571 (Kmr Apr) and pAM2572 (Cmr Apr) resulted in an unusually high frequency of single-crossover mutants (11). Although double-crossover transformants (Aps) were obtained with both donor plasmids, Southern blot analysis and colony PCR revealed a merodiploid phenotype (unsegregated mutant and wild-type chromosomes). This result is consistent with previously published data showing that fur is an essential gene in S. elongatus PCC 7942 (10). While fur mutant strains AMC915 (Kmr), AMC930 (Kmr), and AMC916 (Cmr) grew slowly on solid BG-11M agar plates, the growth rates in liquid cultures were comparable to wild-type growth rates (approximately ≥80%). Immunoblot analysis performed with an anti-IdiA antiserum and French press extracts from wild-type and mutant strains revealed that the Fur mutants have higher IdiA contents under iron-sufficient growth conditions than wild-type strains have (Fig. 7). However, the amount of detectable IdiA was only 25% of the amount in wild-type cells grown under iron-deficient conditions.
FIG. 7.
Analysis of IdiA expression in S. elongatus PCC 7942 and three merodiploid Δfur mutants. Wild-type (WT) or fur mutant cultures (AMC915, AMC930, AMC916) were grown for 3 days in iron-sufficient (+) or iron-deficient (−) medium. After preparation of French press extracts, samples containing 25 μg of protein were subjected to SDS-PAGE, transferred to nitrocellulose, and immunostained with an anti-IdiA antiserum (dilution, 1:15,000).
Insertional inactivation of four group II sigma factor genes (rpoD2, rpoD3, rpoD4, and sigC) in S. elongatus PCC 7942 has no effect on IdiA expression.
We tested the hypothesis that one of four known class 2 sigma factors in S. elongatus PCC 7942 (12, 30) is responsible for, or at least affects, regulation of idiA transcription, as is true for FecI regulation of genes involved in uptake of ferric iron citrate in E. coli (2). We insertionally inactivated each of the group 2 sigma factor genes (GenBank accession no. AB006910, AB024709, AB024710, and AF288784) by using previously described constructs (30) and tested the corresponding mutants, AMC851 (ΔrpoD2), AMC852 (ΔrpoD3), AMC853 (ΔrpoD4), and AMC854(ΔsigC), for the ability to express IdiA under iron-sufficient and -deficient growth conditions. Immunoblot analysis showed that none of the mutants was affected in terms of its ability to express IdiA or to significantly enhance its expression under iron-limiting conditions (data not shown). We concluded that either the idiA promoter is redundantly recognized by sigma factors or expression of this promoter specifically requires a sigma factor that has not been tested yet.
DISCUSSION
Functional analysis of the idiA regulatory region revealed promoter elements that were not predicted by in vitro transcript mapping. Despite the consistent data obtained with four primers, a previous primer extension analysis revealed an mRNA end that cannot represent the idiA transcription start site; polar insertions downstream of the mapped site still allowed normal idiA expression (Fig. 1), and the DNA fragment that drives reporter gene expression with the characteristic properties of idiA regulation is located much closer to the idiA open reading frame (Fig. 2). Deletion analysis also indicated that unlike iron-regulated genes of the Fur regulon in many gram-negative bacteria, idiA is positively regulated. No deletions were capable of derepressing expression, and regulation by iron was not separable from promoter activity per se. If the −35 and −10 elements shown in Fig. 4 are the actual RNA polymerase promoter recognition sites, they seem to require the upstream palindrome for initiation of transcription. In the presence of iron or in the absence of either the palindrome or idiB function, idiA promoter activity is negligible. The data are consistent with the hypothesis that IdiB plays a role in binding to the palindrome to induce expression under iron-limiting conditions.
The start codon for idiA is ambiguous, as three in-frame ATG triplets precede the sequence that gives rise to the mature, processed N terminus of IdiA in vivo. Our data indicate that the first possible ATG is unlikely to be the start codon but rather is part of the −10 element of the promoter. The results obtained with reporter strains AMC802 and AMC812 showed that a fragment with a right endpoint just beyond the first ATG can drive luxAB expression in a transcriptional fusion when the sequence is the wild-type sequence but not when base substitutions are incorporated that would alter the proposed −10 element immediately adjacent to the ATG in question. It is unlikely that these nucleotide alterations would completely abolish transcription by modifying the context of the translation signals. Moreover, the left endpoint deletions of the idiA promoter leave few other options for placement of a promoter element upstream of the idiA open reading frame, unless the transcript has no untranslated leader, which has not been demonstrated previously in cyanobacteria. We propose that either the second or third ATG, at bp 2148 or bp 2160, is the genuine start codon for the open reading frame. However, neither of these potential start codons is preceded by a recognizable ribosome binding site.
The position of the idiA palindrome (GTGTGCTGGCACAC), 4 bp in front of a −35 box, and its position relative to the −10 box meet the expectations for the binding site for a transcriptional activator that positively interacts with the C-terminal domain of the α subunit of RNA polymerase (3, 9, 16, 39). IdiB, a putative helix-turn-helix transcription activator of the FNR/CAP family, is encoded next to idiA and bound specifically to the minimal idiA promoter comprising the palindromic sequence. Binding experiments with IdiB and a 30-bp minimal probe derived from overlapping oligonucleotides (bp 2068 to 2097) that included the palindromic sequence and a minimal flanking DNA region failed, perhaps merely because of poor stability of binding with such a small fragment of DNA (24). We believe that IdiB is the transcriptional activator of idiA, a hypothesis supported by the inability of an IdiB-free mutant to express IdiA at wild-type levels (27).
A search for potential IdiB binding sites of other iron-regulated genes in S. elongatus identified a sequence nearly identical to the idiA palindrome, GTGTGATGCCACAC, upstream of the irpA gene, which encodes a protein that is localized at the cytoplasmic membrane and might be involved in iron acquisition (32). Located 4 bp downstream of this palindrome is a putative −35 box, TTGCCC, which is very similar in terms of sequence and spacing to the proposed −35 element of idiA (Fig. 4). However, neither the transcriptional start of irpA nor the functional promoter of irpA has been mapped. Binding of IdiB as an activator of irpA contrasts with the previous model, which suggested that irpA is regulated via the Fur system (32, 36). The proposed Fur box in front of irpA, however, is very unusual as it comprises 28 bp instead of the 19-bp consensus sequence of E. coli. Another idiA type of palindromic sequence is found upstream of mapA (38) but is more degenerate (GTGN9CAC), and previous work placed it in the 5′ untranslated region (UTR) of the mapA transcript (38). However, we note that physical mapping would have placed the IdiB binding palindrome in the 5′ UTR before functional mapping revised the position of the promoter. Examination of the idiA homologs slr1295, slr0513, and sll0237 in the Synechocystis sp. strain PCC 6803 genome did not reveal any idiA type of palindromic sequences, suggesting that IdiB is not a universal cyanobacterial iron-responsive regulatory factor.
Despite arguments against Fur regulation of idiA, the known role of Fur as a key iron response regulator and evidence for involvement of Fur in regulation of isiA led us to examine idiA expression in a Fur mutant. As previously shown in the isiA study, an attempt to insertionally inactivate fur in S. elongatus resulted in merodiploid mutants (10). Neither repeated selection on antibiotic-containing medium nor the use of different antibiotics allowed total segregation of the mutant allele. Therefore, the cyanobacterial Fur protein plays a role in metabolism different from that of the E. coli protein, which is not essential (13). However, even without complete segregation, fur merodiploid mutants showed derepression of IdiA expression under iron-sufficient growth conditions. We concluded that the number of wild-type alleles and thus the number of Fur molecules per cell are reduced in the merodiploids. The effect of Fur reduction on idiA was surprising, especially since neither IdiA nor a protein of its size was among the proteins previously identified as derepressed in a merodiploid S. elongatus Fur mutant (10).
The tight regulation of reporter genes by iron suggests that the idiA promoter has great potential as a tool for conditional expression of heterologous genes in S. elongatus. When the promoter is induced by transfer to iron-deficient medium, in which S. elongatus cells grow robustly, the promoter strength is in the range of the promoter strength of the psbAI promoter, the strongest promoter characterized so far for this organism (1, 24, 30). Reporter expression remained high for at least 48 h after induction. Because lacI-based repression in S. elongatus is leaky, idiA may prove to be superior for conditional expression when low constitutive expression is harmful or compromises experimental design.
ACKNOWLEDGMENT
A research fellowship awarded to Klaus-Peter Michel by the Deutsche Forschungsgemeinschaft is gratefully acknowledged.
REFERENCES
- 1.Andersson C R, Tsinoremas N F, Shelton J, Lebedeva N V, Yarrow J, Min H, Golden S S. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 2000;305:527–542. doi: 10.1016/s0076-6879(00)05511-7. [DOI] [PubMed] [Google Scholar]
- 2.Angerer A, Enz S, Ochs M, Braun V. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. Fecl belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol. 1995;18:163–174. doi: 10.1111/j.1365-2958.1995.mmi_18010163.x. [DOI] [PubMed] [Google Scholar]
- 3.Berg O G, von Hippel P H. Selection of DNA binding sites by regulatory proteins. I. The binding specificity of cyclic AMP receptor protein to recognition sites. J Mol Biol. 1988;200:709–723. doi: 10.1016/0022-2836(88)90482-2. [DOI] [PubMed] [Google Scholar]
- 4.Bustos S A, Golden S S. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J Bacteriol. 1991;173:7525–7533. doi: 10.1128/jb.173.23.7525-7533.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst J F, Bennett R L, Rothfield L I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135:928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escolar L, Perez-Martin J, de Lorenzo V. Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol. 1999;181:6223–6229. doi: 10.1128/jb.181.20.6223-6229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Exss-Sonne P, Toelle J, Bader K P, Pistorius E K, Michel K P. The IdiA protein of Synechococcus sp. PCC 7942 functions in protecting photosystem II under oxidative stress. Photosynth Res. 2000;63:145–157. doi: 10.1023/A:1006322925324. [DOI] [PubMed] [Google Scholar]
- 8.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Dominguez M, Reyes J C, Florencio F J. NtcA represses transcription of gifA and gifB, genes that encode inhibitors of glutamine synthetase type I, from Synechocystis sp. PCC 6803. Mol Microbiol. 2000;35:1192–1201. doi: 10.1046/j.1365-2958.2000.01789.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghassemian M, Straus N A. Fur regulates the expression of iron-stress genes in the cyanobacterium Synechococcus sp. strain PCC 7942. Microbiology. 1996;142:1469–1476. doi: 10.1099/13500872-142-6-1469. [DOI] [PubMed] [Google Scholar]
- 11.Golden S S, Brusslan J, Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- 12.Goto-Seki A, Shirokane M, Masuda S, Tanaka K, Takahashi H. Specificity crosstalk among group 1 and group 2 sigma factors in the cyanobacterium Synechococcus sp. PCC7942: in vitro specificity and a phylogenetic analysis. Mol Microbiol. 1999;34:473–484. doi: 10.1046/j.1365-2958.1999.01608.x. [DOI] [PubMed] [Google Scholar]
- 13.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 14.Hantke K, Braun V. Control of bacterial iron transport by regulatory proteins. In: Silver S, Walden W, editors. Metal ions in gene regulation. New York, N.Y: International Thomson Publishing; 1998. pp. 11–45. [Google Scholar]
- 15.Helman J D. Metal cation regulation in Gram-positive bacteria. In: Silver S, Walden W, editors. Metal ions in gene regulation. New York, N.Y: International Thomson Publishing; 1988. pp. 45–76. [Google Scholar]
- 16.Hennecke H. Regulation of bacterial gene expression by metal-protein complexes. Mol Microbiol. 1990;4:1621–1628. doi: 10.1111/j.1365-2958.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 17.Herdman M, Castenholz R W, Iteman I, Waterbury J B, Rippka R. Subsection I: order “Chroococcales” Wettstein 1924, emend. Rippka, Deruelles, Waterbury, Herdman and Stanier 1979. In: Boone D R, Castenholz R W, Garrity G M, editors. Bergey's manual of systematic bacteriology. 2nd ed. 1. The archaea, cyanobacteria and deeply branching bacteria. New York, N.Y: Springer-Verlag; 2001. p. 776. [Google Scholar]
- 18.Holm L, Sander C, Ruterjans H, Schnarr M, Fogh R, Boelens R, Kaptein R. LexA repressor and iron uptake regulator from Escherichia coli: new members of the CAP-like DNA binding domain superfamily. Protein Eng. 1994;7:1449–1453. doi: 10.1093/protein/7.12.1449. [DOI] [PubMed] [Google Scholar]
- 19.Katayama M, Tsinoremas N F, Kondo T, Golden S S. cpmA, a gene involved in an output pathway of the cyanobacterial circadian system. J Bacteriol. 1999;181:3516–3524. doi: 10.1128/jb.181.11.3516-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Laudenbach D E, Straus N A. Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J Bacteriol. 1988;170:5018–5026. doi: 10.1128/jb.170.11.5018-5026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonhardt K, Straus N A. An iron stress operon involved in photosynthetic electron transport in the marine cyanobacterium Synechococcus sp. PCC 7002. J Gen Microbiol. 1992;138:1613–1621. doi: 10.1099/00221287-138-8-1613. [DOI] [PubMed] [Google Scholar]
- 23.Leonhardt K, Straus N A. Photosystem II genes isiA, psbDI and psbC in Anabaena sp. PCC 7120: cloning, sequencing and the transcriptional regulation in iron-stressed and iron-repleted cells. Plant Mol Biol. 1994;24:63–73. doi: 10.1007/BF00040574. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Dickerson N S, Mueller U W, Golden S S. Specific binding of Synechococcus sp. strain PCC 7942 proteins to the enhancer element of psbAII required for high-light-induced expression. J Bacteriol. 1995;177:508–516. doi: 10.1128/jb.177.3.508-516.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li R, Golden S S. Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc Natl Acad Sci USA. 1993;90:11678–11682. doi: 10.1073/pnas.90.24.11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michel K P, Exss-Sonne P, Scholten-Beck G, Kahmann U, Ruppel H G, Pistorius E K. Immunocytochemical localization of IdiA, a protein expressed under iron or manganese limitation in the mesophilic cyanobacterium Synechococcus PCC 6301 and the thermophilic cyanobacterium Synechococcus elongatus. Planta. 1998;205:73–81. doi: 10.1007/s004250050298. [DOI] [PubMed] [Google Scholar]
- 27.Michel K P, Kruger F, Puhler A, Pistorius E K. Molecular characterization of idiA and adjacent genes in the cyanobacteria Synechococcus sp. strains PCC 6301 and PCC 7942. Microbiology. 1999;145:1473–1484. doi: 10.1099/13500872-145-6-1473. [DOI] [PubMed] [Google Scholar]
- 28.Michel K P, Pistorius E K. Isolation of a photosystem II associated 36 kDa polypeptide and an iron stress 34 kDa polypeptide from thylakoid membranes of the cyanobacterium Synechococcus PCC 6301 grown under mild iron deficiency. Z Naturforsch Teil C Biochem Biophys Biol Virol. 1992;47:867–874. [Google Scholar]
- 29.Michel K P, Thole H H, Pistorius E K. IdiA, a 34 kDa protein in the cyanobacteria Synechococcus sp. strains PCC 6301 and PCC 7942, is required for growth under iron and manganese limitations. Microbiology. 1996;142:2635–2645. doi: 10.1099/00221287-142-9-2635. [DOI] [PubMed] [Google Scholar]
- 30.Nair U, Thomas C, Golden S S. Functional elements of the strong psbAI promoter of Synechococcus elongatus PCC 7942. J Bacteriol. 2001;183:1740–1747. doi: 10.1128/JB.183.5.1740-1747.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson M L, Laudenbach D E. Genes encoded on a cyanobacterial plasmid are transcriptionally regulated by sulfur availability and CysR. J Bacteriol. 1995;177:2143–2150. doi: 10.1128/jb.177.8.2143-2150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy K J, Bullerjahn G S, Sherman D M, Sherman L A. Cloning, nucleotide sequence, and mutagenesis of a gene (irpA) involved in iron-deficient growth of the cyanobacterium Synechococcus sp. strain PCC7942. J Bacteriol. 1988;170:4466–4476. doi: 10.1128/jb.170.10.4466-4476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 34.Schaefer M R, Golden S S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989;171:3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A K, Sherman L A. Identification of iron-responsive, differential gene expression in the cyanobacterium Synechocystis sp. strain PCC 6803 with a customized amplification library. J Bacteriol. 2000;182:3536–3543. doi: 10.1128/jb.182.12.3536-3543.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straus N A. Iron deprivation: physiology and gene regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 731–750. [Google Scholar]
- 37.Unden G, Guest J R. Isolation and characterization of the Fnr protein, the transcriptional regulator of anaerobic electron transport in Escherichia coli. Eur J Biochem. 1985;146:193–199. doi: 10.1111/j.1432-1033.1985.tb08638.x. [DOI] [PubMed] [Google Scholar]
- 38.Webb R, Troyan T, Sherman D, Sherman L A. MapA, an iron-regulated, cytoplasmic membrane protein in the cyanobacterium Synechococcus sp. strain PCC7942. J Bacteriol. 1994;176:4906–4913. doi: 10.1128/jb.176.16.4906-4913.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wing H J, Williams S M, Busby S J. Spacing requirements for transcription activation by Escherichia coli FNR protein. J Bacteriol. 1995;177:6704–6710. doi: 10.1128/jb.177.23.6704-6710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]