Abstract
Objective
Oblique lumbar interbody fusion (OLIF) involves inserting large cages into the interbody disc space. This expands the spinal canal and neural foramen by stretching the ligament flavum and releasing the facet joint, resulting in indirect neural decompression. Our objective was to investigate the changes in the spinal canal and ligament flavum over time after OLIF.
Methods
This was a prospective observational study involving 30 patients who underwent OLIF L4–5 between 2015 and 2018. In total, 27 of the 30 patients underwent preoperative, early follow-up (< 5 days), and late follow-up (10–14 months) magnetic resonance imaging to measure the area of the spinal canal and ligament flavum. Based on the results, the patients were divided into subsidence and nonsubsidence groups for further analysis.
Results
After OLIF, the spinal canal area gradually increased during the preoperative, early postoperative, and late postoperative periods (p<0.001). The thickness and area of the ligament flavum decreased gradually over the same periods (p<0.001). Low-grade subsidence (2–4.4 mm) did not influence the effects on the spinal canal and ligament.
Conclusion
After OLIF, the spinal canal and ligament flavum gradually change, which is effective for indirect neural decompression. In addition, the effects of low-grade subsidence on the remodeling of the spinal canal and ligament flavum are insignificant.
Keywords: Oblique lateral interbody fusion, Indirect decompression, Spinal canal, Ligament flavum, Subsidence
INTRODUCTION
Oblique lumbar interbody fusion (OLIF) reduces the risk of injury to the lumbar plexus and psoas muscle compared with direct lumbar interbody fusion (DLIF). It is increasingly used as an alternative to conventional anterior or posterior procedures [1,2]. OLIF is useful for sagittal and coronal balance correction, allowing large cages to be inserted into the disc space while preserving the anterior and posterior longitudinal ligaments [3,4].
In OLIF, the disc height is increased by inserting a large cage into the interbody space. This results in indirect neural decompression by correcting spondylolisthesis, stretching the ligament flavum, and releasing the facet joints. Oliveira et al. [5] investigated indirect neural decompression using stand-alone extreme lateral interbody fusion (XLIF). They reported an increase of 41.9% in disc height, 24.7% in the foraminal area, and 33.1% in the central canal diameter. However, if postoperative subsidence occurred, it reduced the effect of indirect neural decompression.
Many studies have reported that DLIF, XLIF, or OLIF provide efficient indirect decompression by releasing the spinal canal and foraminal areas [3,6-9]. However, the study used computed tomography (CT) and magnetic resonance imaging (MRI) examinations shortly after surgery. There is no research on the long-term effects of indirect decompression.
In our prospective study, we examined the effect of indirect decompression using MRI in the preoperative, early postoperative, and late postoperative periods. In addition, since subsidence frequently occurs after OLIF, we investigated whether subsidence could affect the spinal canal and ligament flavum remodeling.
MATERIALS AND METHODS
1. Patients and Methods
We enrolled 27 patients who underwent OLIF L 4–5 between 2015 and 2018 in this prospective observational study.
The study included 27 patients diagnosed with degenerative spondylolisthesis on L4–5 for back pain and radiating pain after more than 6 months of conservative treatment. We included only patients who underwent L4–5 level OLIF to enable consistent measurements. We excluded patients with spinal tumors, infections, vertebral fracture, and revision surgery from the study. The age, sex, and degree of osteoporosis of the patients were examined.
Twenty-seven patients underwent MRI before surgery, within 5 days of surgery (early follow-up), and 10–14 months after surgery (late follow-up). The postoperative results were evaluated by the Oswestry Disability Index (ODI) and visual analog scale (VAS). Plain radiography was done to evaluate spondylolisthesis and the intervertebral disc height (IVH) preoperatively, 3 days, 3 months, 6 months, and 12 months postoperatively (Fig. 1A). IVH was defined as the distance between the superior and inferior endplate at the midpoint of the anteroposterior diameter of the inferior vertebral body.
Fig. 1.
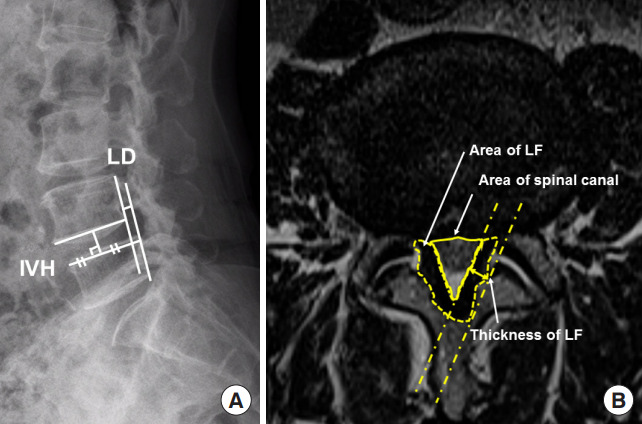
(A) Measurement of intervertebral disc height (IVH) and spondylolisthesis distance (LD) on plain radiograph. (B) The area of the spinal canal and the thickness and area of the ligament flavum (LF) on middisc level T2-weighted magnetic resonance imaging.
Subsidence was defined as a decrease in the IVH of more than 2 mm immediately and 12 months after surgery [10]. Patients were divided into subsidence (+) and subsidence (–) groups according to the degree of subsidence.
The fusion rate was graded with the Bridwell fusion grading system (Table 1) [11]. Based on this classification system, grade 1–2 was a successful fusion. We evaluated fusion using plain radiography 1 year after surgery.
Table 1.
Fusion grading system
| Grade | Fusion status |
|---|---|
| I | Completely remodeled with trabeculae across the disc space |
| II | Graft intact with no lucent lines seen between the graft and adjacent endplates |
| III | Graft intact, but a radiolucent line is seen between the graft and an adjacent endplate |
| IV | Lucency along an entire border of the graft or around a pedicle screw or subsidence of the graft |
We obtained informed consent from all the patients. The study was carried out in accordance with relevant guidelines and regulations. The study was approved by the Institutional Review Board of Kyungpook National University Hospital (No. 2014-10-034-001).
2. Surgical Technique
OLIF involves minimally invasive lateral interbody fusion using a left-sided retroperitoneal approach and percutaneous pedicle screw fixation.
The intervertebral disc was exposed through an open corridor between the psoas muscle and aorta. The sympathetic chain and ureter were mobilized anteriorly. The procedure was performed using an OLIF system (Medtronic, Memphis, TN, USA), fusion material (Grafton, Medtronic, Memphis, TN, USA), and a percutaneous pedicle screw fixation system (Longitude system, Medtronic, Memphis, TN, USA). We determined the height of the cage to be inserted by using the presurgery CT scan. If the intervertebral disc space was < 6 mm, a 10-mm cage was inserted. If the intervertebral disc space was ≥ 6 mm, a cage 4 mm greater than the disc height was inserted. The indirect decompression effect on the constant disc height was evaluated. We did not perform posterior decompression on any of the patients.
3. Measurement of the Spinal Canal and Ligament Flavum
The MRI scans were acquired on a 1.5-T EXCITE whole-body imaging system (General Electric, Milwaukee, WI, USA). An axial localizing sequence was then done to identify the lumbar L4–5 disc-space intervals. Four slices per level were obtained, with 4.0-mm thickness at 1.0 mm intervals. T2-weighted images were then obtained using the following imaging sequence: repetition time, 3,800 msec; echo times, 102 msec; matrix, 416 × 224; excitations, 4; and field of view, 20 cm. The images were displayed and analyzed using PiView (INFINITT, Seoul, Korea) digital image viewing software. The cross-sectional areas of the spinal canal and ligament flavum area were outlined using a graphic cursor. The thickness of the ligament flavum was averaged by measuring the greatest thickness of the ligament on both sides (Fig. 1B). The areas of the spinal canal and thickness and area of the ligament flavum were measured by MRI at the L4–5 middisc level preoperatively and at < 5 days (early follow-up) and 10–14 months (late follow-up) postoperatively. The radiography measurements and evaluations were performed by 2 other surgeons who reviewed and confirmed the results. The interobserver reliability for measurements was excellent (intraclass correlation coefficient, 0.91; 95% confidence interval, 0.80–0.96).
4. Statistical Analysis
The parametric and nonparametric variables preoperatively and at 3 days, 6 months, and 12 months postoperatively were compared using repeated-measures analysis of variance. A post hoc comparison was also performed using the paired t-test. The comparison of variables between the subsidence (+) and subsidence (–) groups preoperatively and at 3 days, 3 months, 6 months, and 12 months postoperatively was made using the t-test and Mann-Whitney U-test. A p-value of < 0.05 was considered statistically significant. Statistical analyses were done using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA).
RESULTS
1. Patient Demographics
Of the 27 patients who underwent OLIF at L4–5, 3 (11.1%) were male, and 24 (88.9%) were female. Subsidence occurred in 7 patients (25.9%). The subsidence was 1.5±1.8 mm on average, and it occurred within 5 mm in all patients. The Bridwell fusion grades were grade 1 in 12 patients, grade 2 in 13, and grade 3 in 2. Therefore, the fusion rate was 92.6%, with grades 1–2 regarded as successful fusion (Table 2).
Table 2.
Patient demographic data
| Variable | Value | p-value† |
|---|---|---|
| Age (yr) | 66.0 ± 9.1 | |
| Sex | ||
| Male | 3 (11.1) | |
| Female | 24 (88.9) | |
| Osteoporosis | 6 (22.2) | |
| Subsidence > 2 mm | 7 (25.9) | |
| Fusion rate | 25 (92.6) | |
| Spondylolisthesis (mm) | ||
| Preoperative | 5.3 ± 2.3 | |
| 3 Days after surgery | 2.5 ± 1.6 | < 0.001 |
| 3 Months after surgery | 2.5 ± 1.8 | < 0.001 |
| 6 Months after surgery | 2.4 ± 1.7 | < 0.001 |
| 12 Months after surgery | 2.5 ± 1.8 | < 0.001 |
| Intervertebral height (mm) | ||
| Preoperative | 8.2 ± 2.4 | |
| 3 Days after surgery | 13.6 ± 1.5 | < 0.001 |
| 3 Months after surgery | 13.4 ± 1.5 | < 0.001 |
| 6 Months after surgery | 12.5 ± 1.6 | < 0.001 |
| 12 Months after surgery | 12.2 ± 1.6 | < 0.001 |
Values are presented as mean±standard deviation or number (%).
Compared to preoperative.
2. Changes in Spondylolisthesis and IVH
After OLIF, the correction of spondylolisthesis was from 5.3±2.3 mm before surgery, to 2.5±1.6 mm at 3 days, 2.5±1.8 mm at 3 months, 2.4±1.7 mm at 6 months, and 2.5±1.8 mm at 12 months postoperatively (p<0.001). After OLIF, the IVH changed from 8.2 ±2.4 mm preoperatively to 13.6 ±1.5 mm at 3 days, 13.4±1.5 mm at 3 months, 12.5±1.6 mm at 6 months, and 12.2 ±1.6 mm at 12 months postoperatively (p<0.001) (Fig. 2).
Fig. 2.
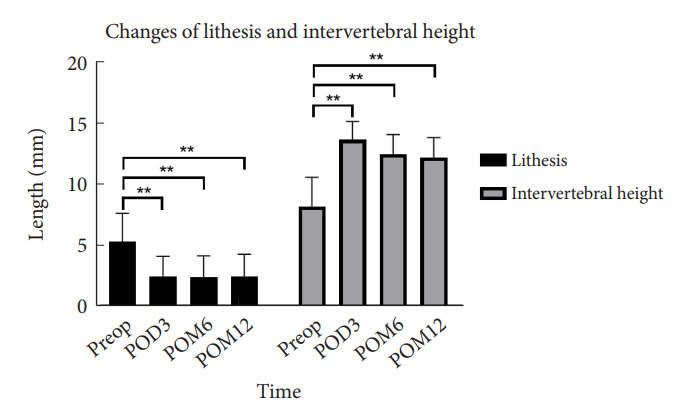
Changes of spondylolisthesis and intervertebral height after surgery. Preop, preoperative; POD, postoperative day; POM, postopertative month. **p<0.05.
3. Changes in the Spinal Canal
After OLIF, the spinal canal area gradually increased from 78.5 ±42.8 mm2 preoperatively, 112.5 ±47.8 mm2 at early follow-up, and 151.8±62.4 mm2 at late follow-up postoperatively (p<0.001) (Fig. 3).
Fig. 3.
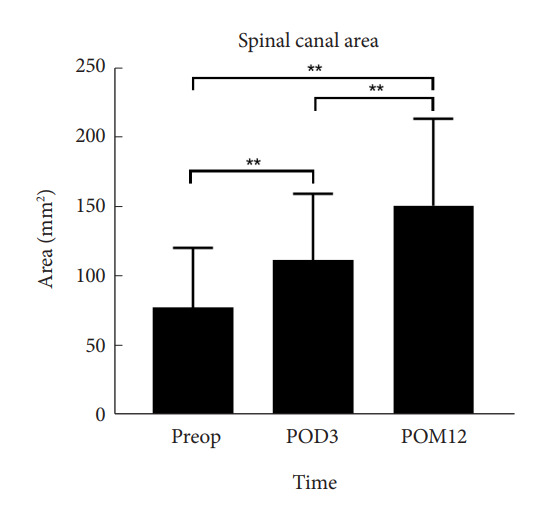
Changes in the spinal canal area after surgery. Preop, preoperative; POD, postoperative day; POM, postopertative month. **p<0.05.
4. Changes of Ligament Flavum
After OLIF, the ligament flavum thickness gradually decreased from 4.2±1.1 mm preoperatively to 3.7±0.9 mm at early follow-up and 2.6±0.74 mm at late follow-up postoperatively (p<0.001). In addition, the ligament flavum area gradually decreased from 123.5 ±37.1 mm2 before surgery to 102.2 ±28.3 mm2 at early follow-up and 70.1±26.1 mm2 at late follow-up (p<0.001) (Fig. 4).
Fig. 4.
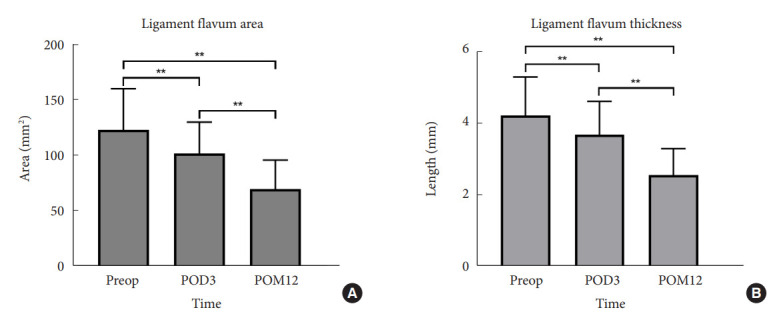
Changes in the ligament flavum area (A) and thickness (B) after surgery. Preop, preoperative; POD, postoperative day; POM, postopertative month. **p<0.05.
5. Changes of the Spinal Canal and Ligament Flavum According to Subsidence (>2 mm)
The spinal canal area of the subsidence (+) and subsidence (−) groups were compared between the preoperative and follow-up periods. The spinal canal area was 81.7±48.8 mm2 in subsidence (+), 77.4±42.9 mm2 in subsidence (−) at preoperative. At early postoperative follow-up, the spinal canal area was 110.8 ±48.9 mm2 in subsidence (+) and 113.2 ±49.8 mm2 in subsidence (−). At the late postoperative follow-up, the spinal canal area of subsidence (+) was 121.4±52.7 mm2 and subsidence (−) was 162.5±64.8 mm2 (p> 0.05).
The ligament flavum thickness and area of the subsidence (+) and subsidence (−) groups were compared between the preoperative and follow-up periods. The ligament flavum thickness was 4.2±0.6 mm in subsidence (+) and 4.2±1.2 mm in subsidence (−). And at early postoperative follow-up, it was 3.6±0.7 mm in subsidence (+) and 3.7±1.0 mm in subsidence (−). There was no statistical difference in subsidence (+) of 2.6±0.6 mm and subsidence (−) of 2.6±0.8 mm at late follow-up after surgery (p> 0.05). The ligament flavum area was 111.9±34.5 mm2 in subsidence (+) and 127.6±38.9 mm2 in subsidence (−) preoperatively. It was 92.5±20.3 mm2 in subsidence (+) and 105.6 ±31.0 mm2 in subsidence (−) at early follow-up. There was no statistical difference in ligament flavum area at late follow-up after surgery: 72.4±20.2 mm2 in subsidence (+) and 69.3±29.0 mm in subsidence (−) (p> 0.05) (Table 3).
Table 3.
The changes in the spinal canal and ligament flavum after surgery according to subsidence (>2 mm)
| Variable | Preoperative |
p-value | Early follow-up (<5 days) |
p-value | Late follow-up (10–14 months) |
p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Subsidence (–) | Subsidence (+) | Subsidence (–) | Subsidence (+) | Subsidence (–) | Subsidence (+) | ||||
| Spinal canal area (mm2) | 77.4 ± 42.9 | 81.7 ± 48.8 | 0.740 | 113.2 ± 49.8 | 110.8 ± 48.9 | 1.000 | 162.5 ± 64.8 | 121.4 ± 52.7 | 0.184 |
| Ligament flavum thickness (mm) | 4.2 ± 1.2 | 4.2 ± 0.6 | 0.618 | 3.7 ± 1.0 | 3.6 ± 0.7 | 0.599 | 2.5 ± 0.8 | 2.6 ± 0.6 | 0.719 |
| Ligament flavum area (mm2) | 127.6 ± 38.9 | 111.9 ± 34.5 | 0.293 | 105.6 ± 31.0 | 92.5 ± 20.3 | 0.319 | 69.3 ± 29.0 | 72.4 ± 20.2 | 0.825 |
Values are presented as mean±standard deviation.
6. Clinical Outcomes
The ODI was 27.6±4.7 preoperatively, 13.5±4.8 at 3 months, 13.4±5.2 at 6 months, and 11.1±4.5 at 12 months postoperatively. ODI improved significantly at 3, 6, and 12 months postoperatively (p<0.001). The VAS-leg was 7.2±1.1 preoperatively, 1.8±1.2 at 3 months, 2.0±1.5 at 6 months, and 1.5±1.3 at 12 months postoperatively. The VAS-back was 4.2±1.5 preoperatively, 2.7±1.4 at 3 months, 2.8±1.5 at 6 months, and 2.4±1.3 at 12 months postoperatively. VAS-leg and -back improved significantly at 3, 6, and 12 months postoperatively (p<0.001) (Table 4).
Table 4.
Clinical outcomes
| Variable | Mean ± SD | p-value† |
|---|---|---|
| ODI | ||
| Preoperative | 27.6 ± 4.7 | |
| 3 Months after surgery | 13.5 ± 4.8 | < 0.001 |
| 6 Months after surgery | 13.4 ± 5.2 | < 0.001 |
| 12 Months after surgery | 11.1 ± 4.5 | < 0.001 |
| VAS-leg | ||
| Preoperative | 7.2 ± 1.1 | |
| 3 Months after surgery | 1.8 ± 1.2 | < 0.001 |
| 6 Months after surgery | 2.0 ± 1.5 | < 0.001 |
| 12 Months after surgery | 1.5 ± 1.3 | < 0.001 |
| VAS-back | ||
| Preoperative | 4.2 ± 1.5 | |
| 3 Months after surgery | 2.7 ± 1.4 | < 0.001 |
| 6 Months after surgery | 2.6 ± 1.5 | < 0.001 |
| 12 Months after surgery | 2.4 ± 1.3 | < 0.001 |
SD, standard deviation; ODI, Oswestry Disability Index; VAS, visual analog scale.
Compared to preoperative.
DISCUSSION
There has been much research into indirect neural decompression in lateral lumbar interbody fusion (XLIF, DLIF, and OLIF) [4,5,12]. These procedures allow the insertion of large cages at intervertebral disc space. As a result, the height of the intervertebral disc can be increased. The indirect neural decompression is effective because the spinal canal and the neural foramen are expanded. In this study, we performed an MRI series to investigate the changes and remodeling that result from indirect neural decompression.
OLIF, a lateral lumbar interbody fusion procedure, is effective in treating lumbar foraminal stenosis. One study reported that the foraminal area increased from 110.3 mm2 preoperatively to 142.6 mm2 postoperatively, an increase of 36.4%. The foraminal height also increased from 16.0 mm to 20.3 mm, an increase of 29.5% [9]. In a previous study, we also found that the foraminal area of the cage insertion side (left) after DLIF increased from 99.5±31.1 mm2 preoperatively to 159.2±44.8 mm2 postoperatively, while it increased from 102.9±32.9 mm2 to 151.2±39.1 mm2 on the contralateral side (right) [3].
OLIF is also effective in expanding the spinal canal. After lateral lumbar interbody fusion, the spinal canal area showed an expansion of 14%–54% [5-8]. A systematic review found that the central canal area increased by 25.4%, and the central canal diameter increased by 33.1% [9]. In our study, the spinal canal area increased by 43.3% at early follow-up and 93.4% at late follow-up. This change in the spinal canal area is caused by a reduction of the disc bulge, stretching of a buckled ligament flavum, atrophy of ligament flavum due to spinal stability, facet joint release, and spondylolisthesis correction. These changes occur early or late. Early effects come from stretching the disc annulus and ligament flavum, correction of the spondylolisthesis, and release of the facet joints. The late effects are caused by atrophy of the disc annulus and ligament flavum.
In our study, we found that ligament flavum was remodeled by stretching and atrophy after OLIF. Ohtori et al. [13] reported a ligament flavum changes from 150 mm2 to 78 mm2 after anterior lumbar interbody fusion. This study was conducted with an inconsistent periods MRI scan within 10 years of surgery. In contrast, our study demonstrated the stretching effect of the ligament flavum in the early follow-up (< 5 days) MRI and the atrophic changes in the ligament flavum resulting from spinal stability through the late follow-up (10–14 months) MRI. The results showed the thickness and area of ligament flavum decreased by 11.9% and 17.2% from stretching (early effect) and decreased by 29.7% and 29.5% because of atrophic change (late effect) after OLIF (Fig. 5).
Fig. 5.
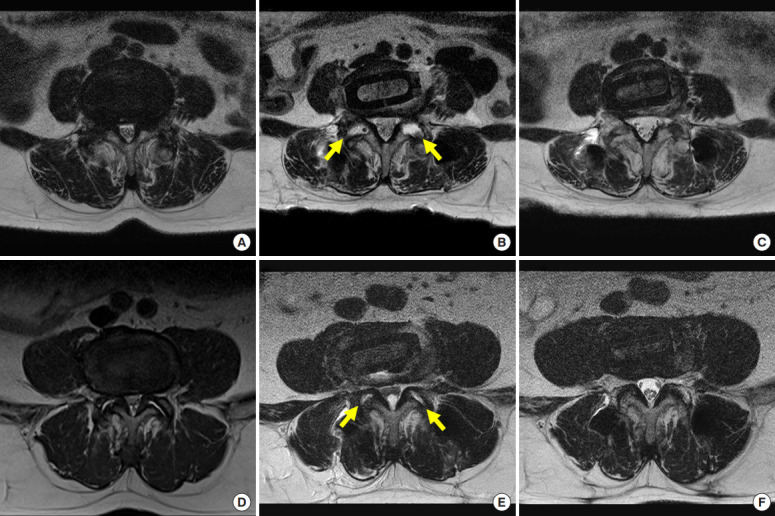
(A, D) Two cases of L4–5 oblique lumbar interbody fusion. Panels A–C are the same patients, panels D–F are the same patient. Central spinal stenosis in preoperative axial magnetic resonance images (B, E), early (postoperative 2 days), late (postoperative 12 months) axial images (C, F). In panels B and E, the area of the foramen and spinal canal were enlarged by facet joint release (arrows) and ligament flavum and disc annulus stretching (early effects). In panels C and F, the area of the foramen and spinal canal were widened by atrophy of the ligament flavum and disc annulus (late effects).
Hypertrophy and buckling of the ligament flavum are the leading causes of spinal stenosis. Hypertrophy of the ligament flavum leads to fibrosis due to a decrease in elastic fibers and an increase in collagen fibers [14,15]. Altinkaya et al. [15] suggest that buckling of the ligament flavum influences the ligament flavum thickness in spinal stenosis. In our study, as the buckling of the ligament flavum was released early follow-up, the area of the spinal canal increased, and the thickness and area of the ligament flavum decreased. Mechanical stress affects hypertrophy of the ligament flavum [16]. Since the surgical site was stabilized by fusion and fixation, the decrease of mechanical stress on the ligament flavum led to its late atrophy (Fig. 6). However, the effect of indirect decompression of OLIF on spinal stenosis caused by calcified discs and disc fragments has not yet been clearly elucidated.
Fig. 6.
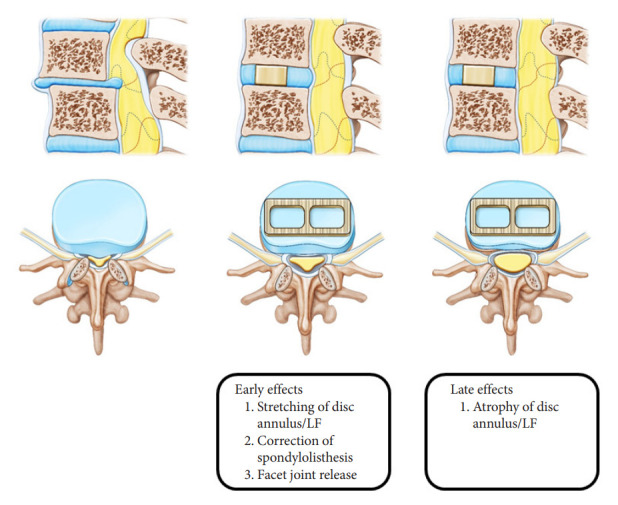
Early and late effects of remodeling of the spinal canal and ligament flavum (LF) after oblique lumbar interbody fusion in degenerative spondylolisthesis.
A previous study reported an incidence of subsidence after lateral lumbar interbody fusion of 13.8% [3]; however, the incidence of subsidence gradually decreased because of the use of wider cages and the improvement in surgical techniques [17-19]. However, subsidence still occurred at 25.9% in this study. We investigated whether subsidence after OLIF caused deterioration of the indirect neural decompression. The subsidence causes a decrease in the stretching effect on the ligament flavum and a reduction in spondylolisthesis correction. In our study, subsidence ranged from 2.0 to 4.4 mm. Applying the evaluation of cage subsidence described by Marchi et al. [20], subsidence was classified using the following scale: grade 0 (0%–24%), grade I (25%–49%), grade II (50%–74%), and grade III (75%–100%) loss of postoperative disc height. All patients in our study had grade 0 or I (low-grade subsidence). Thus the occurrence of low-grade subsidence did not affect the spinal canal area. It also did not affect the thickness and area of the ligament flavum. However, high-grade subsidence (> 5 mm) might affect the indirect neural decompression effect by reducing disc height and thus the neural foramen.
Also, Lang et al. [19] found that the use of 26 mm wide cages in OLIF significantly reduced the incidence of subsidence compared to 18- and 22-mm cages. The use of larger cages may preserve the indirect neural decompression effects by a reduction in the incidence of subsidence.
Our study showed similar results to others for the release of the spinal canal. However, we confirmed the remodeling of the spinal canal and ligament flavum by examining serial MRIs. In addition, the remodeling effect of the spinal canal and ligament flavum was not affected even if low-grade subsidence occurred (2–5 mm).
The main limitation of our study is its small sample size. However, only patients who underwent a single level of surgery, L4–5, were selected to enable a direct comparison of the effects of indirect neural decompression. Longer follow-up observations may be necessary, but a 1-year follow-up has yielded adequate results. In addition, as the disc height increases for each patient, the area of the spinal canal increases, and the ligament flavum stretching effect may be different. However, we tailored the height of the cage to the patient, using CT, before surgery.
CONCLUSION
After OLIF, the spinal canal and ligament flavum undergo remodeling over time. A decrease in the ligament area and thickness were induced by ligament flavum stretching and atrophy. In addition, changes in the spinal canal area were caused by increased disc height, spondylolisthesis correction, and changes in the ligament flavum. In addition, low-grade subsidence after OLIF did not affect the changes in the spinal canal and ligament flavum.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported by a Biomedical Research Institute grant, Kyungpook National University Hospital (2014).
Author Contribution
Conceptualization: KK; Data curation: YL, DL, HK, KK; Formal analysis: YL, CHK; Funding acquisition: KK; Methodology: YL; Project administration: DL, DC, IH, CHK, HK; Visualization: YL, DL; Writing - original draft: YL; Writing - review & editing: DC, IH, CHK, HK, KK.
REFERENCES
- 1.Pham MH, Diaz-Aguilar LD, Shah V, et al. Simultaneous robotic single position oblique lumbar interbody fusion with bilateral sacropelvic fixation in lateral decubitus. Neurospine. 2021;18:406–12. doi: 10.14245/ns.2040774.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xi Z, Chou D, Mummaneni PV, et al. The navigated oblique lumbar interbody fusion: accuracy rate, effect on surgical time, and complications. Neurospine. 2020;17:260–7. doi: 10.14245/ns.1938358.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YS, Park SW, Kim YB. Direct lateral lumbar interbody fusion: clinical and radiological outcomes. J Korean Neurosurg Soc. 2014;55:248–54. doi: 10.3340/jkns.2014.55.5.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976) 2015;40:E175–82. doi: 10.1097/BRS.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 2010;35:S331–7. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 6.Elowitz EH, Yanni DS, Chwajol M, et al. Evaluation of indirect decompression of the lumbar spinal canal following minimally invasive lateral transpsoas interbody fusion: radiographic and outcome analysis. Minim Invasive Neurosurg. 2011;54:201–6. doi: 10.1055/s-0031-1286334. [DOI] [PubMed] [Google Scholar]
- 7.Khajavi K, Shen AY. Two-year radiographic and clinical outcomes of a minimally invasive, lateral, transpsoas approach for anterior lumbar interbody fusion in the treatment of adult degenerative scoliosis. Eur Spine J. 2014;23:1215–23. doi: 10.1007/s00586-014-3246-6. [DOI] [PubMed] [Google Scholar]
- 8.Khajavi K, Shen A, Hutchison A. Substantial clinical benefit of minimally invasive lateral interbody fusion for degenerative spondylolisthesis. Eur Spine J. 2015;24 Suppl 3:314–21. doi: 10.1007/s00586-015-3841-1. [DOI] [PubMed] [Google Scholar]
- 9.Lang G, Perrech M, Navarro-Ramirez R, et al. Potential and limitations of neural decompression in extreme lateral interbody fusion-a systematic review. World Neurosurg. 2017;101:99–113. doi: 10.1016/j.wneu.2017.01.080. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Yuan C, Liu C, et al. Hounsfield unit value on CT as a predictor of cage subsidence following stand-alone oblique lumbar interbody fusion for the treatment of degenerative lumbar diseases. BMC Musculoskelet Disord. 2021;22:960. doi: 10.1186/s12891-021-04833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410–8. [PubMed] [Google Scholar]
- 12.Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J. 2017;26:671–8. doi: 10.1007/s00586-015-4170-0. [DOI] [PubMed] [Google Scholar]
- 13.Ohtori S, Orita S, Yamauchi K, et al. Change of lumbar ligamentum flavum after indirect decompression using anterior lumbar interbody fusion. Asian Spine J. 2017;11:105–12. doi: 10.4184/asj.2017.11.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong ZM, Zha DS, Xiao WD, et al. Hypertrophy of ligamentum flavum in lumbar spine stenosis associated with the increased expression of connective tissue growth factor. J Orthop Res. 2011;29:1592–7. doi: 10.1002/jor.21431. [DOI] [PubMed] [Google Scholar]
- 15.Altinkaya N, Yildirim T, Demir S, et al. Factors associated with the thickness of the ligamentum flavum: is ligamentum flavum thickening due to hypertrophy or buckling? Spine (Phila Pa 1976) 2011;36:E1093–7. doi: 10.1097/BRS.0b013e318203e2b5. [DOI] [PubMed] [Google Scholar]
- 16.Nakatani T, Marui T, Hitora T, et al. Mechanical stretching force promotes collagen synthesis by cultured cells from human ligamentum flavum via transforming growth factorbeta1. J Orthop Res. 2002;20:1380–6. doi: 10.1016/S0736-0266(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 17.Park MK, Kim KT, Bang WS, et al. Risk factors for cage migration and cage retropulsion following transforaminal lumbar interbody fusion. Spine J. 2019;19:437–47. doi: 10.1016/j.spinee.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Lee YS, Kim YB, et al. Clinical and radiological outcomes of a new cage for direct lateral lumbar interbody fusion. Korean J Spine. 2014;11:145–51. doi: 10.14245/kjs.2014.11.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang G, Navarro-Ramirez R, Gandevia L, et al. Elimination of subsidence with 26-mm-wide cages in extreme lateral interbody fusion. World Neurosurg. 2017;104:644–52. doi: 10.1016/j.wneu.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19:110–8. doi: 10.3171/2013.4.SPINE12319. [DOI] [PubMed] [Google Scholar]


