Abstract
Objective
This study is an investigator-initiated, prospective, randomized, controlled study to evaluate the efficacy and safety of the combined use of recombinant human BMP-2 (rhBMP-2) and a hydroxyapatite (HA) carrier in multilevel fusion in patients with adult spinal deformity (ASD).
Methods
Thirty patients underwent posterolateral fusion for lumbar spinal deformities at 3 to 5 segments between L1 and S1. The patients received rhBMP-2+HA or HA on the left or right side of the transverse processes. They were followed up regularly at 1, 3, 6, and 12 months postoperatively. Fusion was defined according to the bone bridging on computed tomography scans. The fusion rate per segment was subanalyzed. Function and quality of life as well as pain in the lower back and lower extremities were evaluated.
Results
The union rate for the rhBMP-2+HA group was 100% at 6 and 12 months. The union rate for the HA group was 77.8% (21 of 27) at 6 months and 88.0% (22 of 25) at 12 months (p = 0.014 at 6 months; not significant at 12 months). All segments were fused at 6 and 12 months in the rhBMP-2+HA group (p < 0.001). In the HA group, 108 of 115 segments (93.5%) were fused at 6 months and 105 of 109 segments (96.3%) at 12 months. Other clinical parameters (visual analogue scale, 36-item Short Form Health Survey, and Scoliosis Research Society-22 scores) improved compared to baseline.
Conclusion
Combining rhBMP-2 and an HA carrier is a safe and effective method to achieve multilevel fusion in patients with ASD.
Keywords: Adult spinal deformity, Bone graft, Bone morphogenetic protein-2, Hydroxyapatite, Posterolateral fusion, Lumbar fusion
INTRODUCTION
Posterolateral lumbar fusion (PLF) is performed to correct adult spinal deformity (ASD) [1]. Autologous bone grafts for PLF are usually harvested from the iliac crest, requiring additional surgery; moreover, it may not yield sufficient bone. It can also be associated with donor site complications (hematoma, fracture, wound healing problems, persistent pain, pelvic deformity, and neurovascular injuries) [2-5]. Many ASD cases require multilevel fusion, requiring more bone grafts than typical single-level fusion. Thus, multilevel fusion decreases the union rate and increases surgical blood loss, operative time, length of hospital stay, and recovery time [6].
Various materials have been explored as autogenous bone substitutes. However, noncalcium phosphate-based bone substitutes demonstrate poor mechanical properties, low biocompatibility, and poor tissue adhesion. Therefore, calcium phosphate-based ceramics, such as hydroxyapatite (HA), have been developed to address these issues. HA is a naturally-occurring form of calcium phosphate and the most abundant inorganic constituent of human bones [7]. Unlike allografts, HA shows no risk of virus transmission. Moreover, it is nonallergenic with excellent osseointegration. Therefore, HA is preferred as a bone graft or extender in orthopedics [8-11].
Bone morphogenetic proteins (BMPs) have been considered for bone graft enhancement. Among the various BMPs, factors with osteogenic activity are limited [12]. Recombinant human BMP-2 (rhBMP-2) is commercially available and approved by the U.S. Food and Drug Administration for anterior lumbar interbody fusion (ALIF) [13]. Treatment with rhBMP-2 results in a higher union rate than autologous bone and significantly improves several clinical parameters in a single-level fusion through ALIF and PLF [14-16]. Mulconrey et al. [17] demonstrated rhBMP-2 efficacy in multilevel fusion in 98 patients with ASD. Application of rhBMP-2 at an average of 2.6 levels per patient resulted in a 95% union rate. However, these studies have not identified any interpatient factors with statistically significant effects on the bone union. Therefore, this investigator-initiated exploratory study on multilevel fusion compared the outcomes of the combined use of rhBMP-2 and HA to HA alone in the same patients to exclude interpatient factors.
MATERIALS AND METHODS
1. Study Design
This study was a investigator-initiated, prospective, single-center, randomized, controlled, exploratory clinical trial. Patients were enrolled with institutional review board approval between October 2016 and December 2019. This study was registered with the Clinical Research Information Service (CRIS No. KCT-0006545) and conducted according to the principles of the Declaration of Helsinki and the guidelines of Good Clinical Practice.
Patients who visited to the clinic and met the following inclusion criteria were consecutively enrolled: (1) age, 19–80 years, (2) pain associated with ASD at 3–5 segments between L1 and S1 necessitating intertransverse process lateral fusion, (3) voluntary participation in this clinical trial with written consent. The exclusion criteria were as follows: (1) participation in another clinical trial within 1 month before enrollment, (2) history of fusion surgery on the same site, (3) osteoporosis; average T-score ≤ -3.0 at lumbar, (4) immunosuppression or autoimmune disease, (5) rhBMP-2 hypersensitivity, (6) history of malignant tumors, (7) fractures, acute infections, hemorrhagic diseases, active systemic infections, osteodystrophy, or infections at the surgical site, (8) serious diseases that could affect surgery, (9) use of contraindicated concomitant drugs, (10) alcohol or drug addiction, or mental illness, (11) pregnancy, lactation. Detailed criteria for ASD are scoliosis Cobb angle of 20° or more, sagittal vertical axis of 5 cm or more, pelvic tilt of 25° or more, and/or pelvic incidence–lumbar lordosis of 10° or more. All patients enrolled in this study received the same posterior column osteotomy, and none underwent 3-column osteotomy that would have been a confounder to the study. Since this is an investigator-initiated exploratory trial, the sample size was not statistically calculated. In addition, the clinically significant effect sizes, limits, and standard deviations required to calculate the number of subjects could not be obtained due to the absence of adequate previous studies. The number of subjects was determined based on clinical experience (the number of patients visiting the hospital, the enrollment rate, etc.) and the dropout rate of 20%. Eligible subjects were assigned numbers according to pregenerated randomization of the order of participation. A block randomization was conducted through a web-based simple randomization service (https://www.sealedenvelope.com/simple-randomiser/v1/lists) provided by Sealed Envelope. Control (HA) or test sites (rhBMP-2+HA) were randomly assigned to the left or right side of one patient's transverse processes in a 1:1 ratio. The surgeon was blinded until the day of surgery and was unable to preidentify the randomization code for the patient. Patients received follow-ups at 1, 3, 6, and 12 months after surgery with physical examination and static radiography at every follow-up visit, dynamic radiography at 3, 6, and 12 months after surgery, and computed tomography (CT) scans at 6 and 12 months following surgery. For clinical outcomes, we evaluated visual analogue scale (VAS) and Oswestry Disability Index (ODI) scores for back and leg pain at baseline and at 6 and 12 months after surgery. 36-item Short Form Health Survey (SF-36) and Scoliosis Research Society-22 (SRS-22) questionnaires were administered at baseline and 12 months after surgery. Samples for rhBMP-2 antibody testing were collected and analyzed at baseline and at 3 and 12 months after surgery.
2. Intervention
After general anesthesia, we used a midline skin incision and a posterior approach [18,19] to expose the origin of the transverse processes on both sides, with bilateral retraction. The affected nerve root was decompressed by laminectomy. Pedicle screws were inserted into the vertebral body with an appropriate medical angulation. The anterior cortical bone of the vertebra was fixed to sufficient depth without damage using a rod. The lateral surface of the vertebral joint, the recess at the origin of the transverse process, and the bone surface of the transverse process were removed and irrigated with saline. Bone grafts were placed on the assigned side according to randomization results. Instrumentation was performed at all fused segments. The test site (rhBMP-2+HA) received 3.0 g of a porous HA carrier (Bongros-HA, CG Bio Co., Ltd., Seoul, Korea) adsorbed with 3.0 mg of E. coli-derived rhBMP-2 (Novosis, CG Bio Co., Ltd., Seoul, Korea) per level for posterolateral bone fusion. The average amount of rhBMP-2 applied per patient was 12.78 ± 2.71 mg (9–15 mg rhBMP-2 for 3–5 level fusion). The dose setting of 3.0 mg of rhBMP-2 per level was based on previous studies [16]. For the control site (HA), 3.0 g of HA carrier, loaded with saline, was mixed with 2.5 g of HA carrier only and transplanted.
3. Evaluation
The primary efficacy endpoint was the CT-based union rate at 12 months, and the secondary efficacy endpoint was the CT-based union rate at 6 months. The union was judged as grade I–IV according to the bone bridging pattern on the CT scan as follows [20]: grade I, complete fusion; grade II, partial fusion; grade III, unipolar pseudarthrosis; grade IV, bipolar pseudarthrosis. Grades I and II were considered “bone union.” Two spinal neurosurgeons who did not participate in this study evaluated the results as independent evaluators. Where there were conflicting opinions between the 2 evaluators, the result was nonunion. In this study, “union” was judged only when all segments were fused and “nonunion” when even one segment was not fused. The evaluation was subject and operator-blinded, and the sites for assessment (test and control site) were also blinded to minimize bias. Changes in VAS, ODI, SF-36, and SRS-22 scores were considered secondary endpoints. We counted subjects with adverse events (AEs) during the clinical trial to evaluate safety. The proportion of subjects experiencing AEs was analyzed separately for treatment-emergent AEs (TEAEs) and adverse device events (ADEs), with an additional analysis based on severity.
4. rhBMP-2 Antibody Analysis
We modified the study plan for analyzing increased rhBMP-2 antibodies during the clinical trial. An enzyme-linked immunosorbent assay-based rhBMP-2 antibody test was performed on 11 patients enrolled after plan modification, and serum samples were collected at baseline and at 3 and 12 months after surgery. A difference in absorbance before and after surgery of more than 3 times the standard deviation of baseline samples was considered positive for antibody elevation (following antibody test guidelines).
5. Statistical Analysis
Demographic data were descriptively analyzed. The union rate was presented as a number (n) and a ratio (%), and the difference between the groups was analyzed using McNemar test. Interobserver reliability for the reading of fusion status on CT scans was evaluated using Cohen kappa (Definition [21]: 0.01‒0.20 slight agreement, 0.21‒0.40 fair agreement, 0.41‒0.60 moderate agreement, 0.61‒0.80 substantial agreement, 0.81‒1.00 almost perfect or perfect agreement). Changes in VAS, ODI, SF-36, and SRS-22 scores from baseline were analyzed using the paired t-test or Wilcoxon signed-rank test. Two-sample t-test or Mann-Whitney U-test was used to compare demographic factors in union and non-union cases. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The threshold for statistical significance was set at p< 0.05.
RESULTS
1. Patients
Thirty subjects were enrolled after screening. We included 27 subjects for analysis at 6 months and 25 subjects at 12 months. The number of subjects enrolled, assigned, lost at follow-up, and analyzed are summarized in the flow diagram (Fig. 1). Data on patient baseline characteristics and operated segments are presented in Table 1.
Fig. 1.
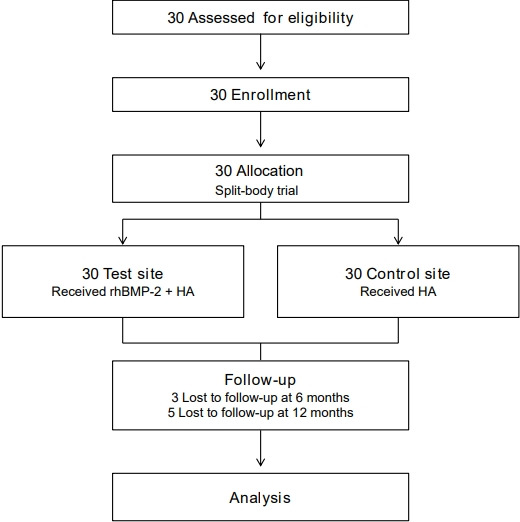
Flow diagram showing study enrollment, allocation, follow-up, and analysis. rhBMP-2, recombinant human bone morphogenetic protein-2; HA, hydroxyapatite.
Table 1.
Patient demographics (n=27)
| Characteristic | Value |
|---|---|
| Age (yr) | 71.07 ± 5.78 |
| Sex, n (%) | |
| Male | 7 (25.9) |
| Female | 20 (74.1) |
| Height (cm) | 153.61 ± 9.13 |
| Weight (kg) | 59.01 ± 11.31 |
| BMD (T-score) | 0.08 ± 1.65 |
| Smoking | 3 (11.1) |
| Drinking | 5 (18.5) |
| No. of fused levels | |
| 3 Levels | 8 (29.6) |
| 4 Levels | 4 (14.8) |
| 5 Levels | 15 (55.6) |
| Fusion levels | |
| L1‒4 | 2 (7.4) |
| L2‒5 | 2 (7.4) |
| L3‒S1 | 4 (14.8) |
| L1‒5 | 1 (3.7) |
| L2‒S1 | 3 (11.1) |
| L1‒S1 | 15 (55.6) |
| Fused levels per patient (level) | 4.26 |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
BMD, bone mineral densitometry.
2. Union Rate
We evaluated the union rates separately for control and test sites (Table 2). At 6 months, the union rate was 100% (27 of 27) at the test site and 77.8% (21 of 27) at the control site, indicating a statistically significantly higher union rate at the test site (p= 0.014). At 12 months, the union rate was 100% (25 of 25) at the test site and 88.0% (22 of 25) at the control site (p= 0.083). Based on Cohen kappa (0.86), interobserver reliability between evaluators (98.1%) for fusion evaluation indicated almost perfect agreement. The union rate per segment was subanalyzed (Table 3). In the subanalysis, a total of 115 segments (in 27 patients) were included at 6 months and 109 segments (in 25 patients) at 12 months. The union rate at the control site was 93.9% (108 of 115) at 6 months and 96.3% (105 of 109) at 12 months. At 6 months, 6 of 7 nonunion segments were L5–S1, and one was L3–4. At 12 months, 3 of 4 nonunion segments were L5–S1 and one L3–4. In the test group, the union rates at 6 and 12 months were both 100%, and the differences between the groups were significant at both time points (p< 0.001). Fusion characteristics on CT scans were slightly different between the 2 groups. Although both sites showed fusion mass, continuity of the fused mass was more prominent and uniformly observed at the test site than at the control site (Fig. 2).
Table 2.
Fusion rate on computed tomography scan
| Timepoints | Test site (rhBMP-2+HA) | Control site (HA) | p-value† |
|---|---|---|---|
| 6 Months (n = 27) | 27 (100) | 21 (77.8) | 0.014* |
| 12 Months (n = 25) | 25 (100) | 22 (88.0) | 0.083 |
Values are presented as number (%).
rhBMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; HA, hydroxyapatite.
p<0.05, statistically significant differences.
McNemar test was used to compare the fusion rates between the test and control sites.
Table 3.
Subanalysis of fusion rate according to the segment on computed tomography
| Timepoints | Test site (rhBMP-2+HA) | Control site (HA) | p-value† |
|---|---|---|---|
| 6 Months (n = 115) | 115 (100) | 108 (93.9) | < 0.001* |
| 12 Months (n = 109) | 109 (100) | 105 (96.3) | < 0.001* |
Values are presented as number (%).
rhBMP-2, Escherichia coli-derived recombinant human bone morphogenetic protein-2; HA, hydroxyapatite.
p<0.05, statistically significant differences.
McNemar test was used to compare the fusion rates between the test and control sites.
Fig. 2.
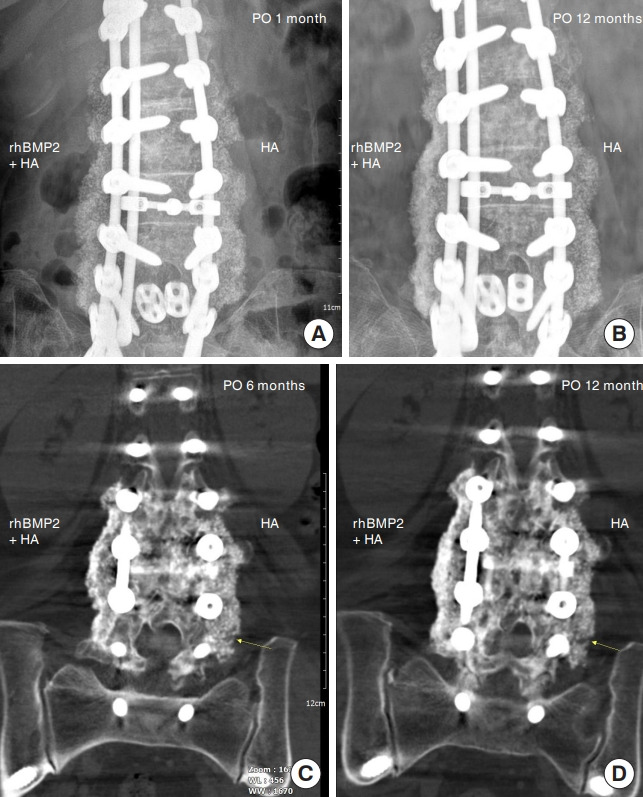
Characteristics of fusion mass in the postoperative period. (A) Fusion mass on a 1-month postoperative plain radiograph. (B) A 12-month postoperative plain radiograph showed a more prominent fusion mass on the rhBMP-2 site. (C) Fusion mass on a 6-month postoperative CT scan. Although fusion mass was detected at both sites, patients with the HA carrier showed suspicious nonunion lesion of fusion mass (arrow). (D) Fusion mass on a 12-month postoperative CT scan. Continuity of fused mass is more prominent and uniformly observed at the rhBMP-2 than at the control site. Defect of fusion mass at the control site is still noted (arrow). CT, computed tomography; rhBMP-2, recombinant human bone morphogenetic protein-2; HA, hydroxyapatite.
3. Clinical Outcomes
ODI and VAS scores for back and leg pain significantly decreased compared to baseline at all time points (all p < 0.01) (Table 4). The Physical Component Summary and Mental Component Summary scores in the SF-36 questionnaire (to evaluate the quality of daily life) had significantly improved 12 months after surgery compared to baseline (p < 0.001) (Table 5). The SRS-22 score significantly improved compared to baseline values (p< 0.001) (Table 5).
Table 4.
Changes from baseline to 6 and 12 months in the Oswestry Disability Index (ODI) and visual analogue scale (VAS) scores
| Variable | Baseline (n = 27) | 6 Months (n = 27) | p-value† | 12 Months (n = 27) | p-value† |
|---|---|---|---|---|---|
| ODI | 25.59 ± 8.51 | 19.11 ± 5.90 | 0.004* | 18.85 ± 8.37 | 0.004* |
| VAS | |||||
| Back pain | 6.70 ± 2.15 | 2.89 ± 1.95 | < 0.001* | 2.62 ± 2.06 | < 0.001* |
| Left leg | 5.78 ± 3.04 | 2.78 ± 2.39 | < 0.001* | 3.04 ± 2.78 | < 0.001* |
| Right leg | 5.22 ± 3.85 | 2.48 ± 2.83 | 0.001* | 2.41 ± 2.85 | 0.004* |
Values are presented as mean±standard deviation.
p<0.05, statistically significant differences.
Paired t-test was used.
Table 5.
Changes from baseline to 12 months in 36-item Short Form Health Survey (SF-36) and Scoliosis Research Society-22 (SRS-22) scores
| Variable | Baseline (n = 25) | 12 Months (n = 25) | p-value† |
|---|---|---|---|
| SF-36 | |||
| Physical functioning | 14.80 ± 15.38 | 35.80 ± 24.82 | 0.001* |
| Role-physical | 24.90 ± 21.41 | 48.63 ± 18.95 | < 0.001* |
| Bodily pain | 21.78 ± 15.89 | 53.64 ± 23.15 | < 0.001* |
| General health | 33.40 ± 20.55 | 45.60 ± 15.16 | 0.006* |
| Vitality | 35.14 ± 19.96 | 48.06 ± 19.45 | 0.023* |
| Social functioning | 33.44 ± 22.42 | 51.30 ± 19.78 | 0.004* |
| Role-emotional | 20.60 ± 15.67 | 58.80 ± 21.69 | < 0.001* |
| Mental health | 44.20 ± 21.92 | 61.00 ± 18.98 | 0.008* |
| Physical component summary | 23.64 ± 13.08 | 45.71 ± 17.66 | < 0.001* |
| Mental component summary | 33.22 ± 15.08 | 54.53 ± 16.80 | < 0.001* |
| SRS-22 | 2.22 ± 0.60 | 3.20 ± 0.65 | < 0.001* |
Values are presented as mean±standard deviation.
p<0.05, statistically significant differences.
Paired t-test was used.
4. Safety
We analyzed safety in 30 enrolled subjects. Eleven subjects had TEAEs (36.67%, 23 cases). Among TEAEs, fluid collection at the surgical site developed in 2 patients, but symptoms (buttock pain) improved after percutaneous catheter drainage, and bacterial cultures were negative.
Three patients (10.0%) had 4 serious TEAEs, 1 mild (adjacent disc herniation at T8‒9 at 9‒12 months), and 3 moderate (acute kidney injury between baseline and 1 month, thoracic vertebral compression fracture at T11 at 1‒3 months, gait disturbance after 12 months). Most serious TEAEs (3 patients) resolved with conservative drug treatment and rehabilitation. However, one patient with a fracture recovered after 8 weeks of fusion extension surgery. No subject developed ADEs or serious ADEs. Laboratory and electrocardiogram tests revealed no abnormal findings.
We analyzed the antibody response according to the high-dose rhBMP-2. One patient was temporarily positive at 3 months but negative at 12 months. This patient achieved radiographic union at both control and test sites at all time points, with no rhBMP2-related complications.
DISCUSSION
This study had a split-body trial design with a control or test site assigned to the left/right side of the transverse process of one subject. An average of 4.26 segments were fused. The union rate at the test site was 100% at 6 and 12 months. Despite using multilevel fusion, our results were similar to previously reported union rates achieved with rhBMP-2 with single-level fusion. Cho et al. [16] applied 3.0 mg of rhBMP-2 along with an HA carrier to a single level PLF. The union rate achieved in the rhBMP-2+HA group at 3 and 6 months was 100%, superior to that of the ICBG group (3 months, 90.2%; 6 months, 94.1%). Therefore, the present study demonstrated that 3.0 mg of rhBMP-2 per level was sufficient to achieve bone fusion, even at multiple fusion levels (3–5 levels).
In the HA group, we applied a high-purity synthetic HA ceramic with a structure in which 300‒500 μm pores are 3-dimensionally interconnected by mimicking human cancellous bone. HA is a low-biodegradable osteoconductive material that can serve as a long-term carrier due to high rhBMP-2 affinity. Because HA effectively adsorbs rhBMP-2 to the surface due to its high affinity and porosity, it can slowly and continuously release rhBMP-2 through adjacent bodily fluid streams [22-24]. This study showed a 77.8% union rate for the HA group at 6 months and 88.0% at 12 months, higher than the union rates achieved in previous PLF studies on a single level [25]. Nam and Yi [25] applied HA or demineralized bone matrix (DBM) as a bone graft extender for PLF, but the union rate at 12 months in the HA group was 58%, not significantly different from the 73% achieved in the DBM group. Our study used only the HA carrier at the control site for multilevel fusion. The high union rate achieved at the control site could be due to earlier fusion at the contralateral test site that received rhBMP-2 with HA. Early union of the test site would have a positive effect on the union rate by providing stability to the control site. Subanalysis of the union rate per segment showed that the HA group exhibited a 93.9% (108 of 115) union rate at 6 months and 96.3% (105 of 109) at 12 months. There were differences between nonunion and union cases in female ratio, BMD, smoking, drinking, number of fusion levels, and fusion sites, but only smoking showed a statistically significant difference (p= 0.001). Smoking may be a major confounder of spinal fusion. The demographic factors of the nonunion case were compared with that of the union case (Table 6). As a result of analyzing the union rate at the control site excluding 3 smokers, the union rate at 6 months was 79.2% (19 of 24), and the union rate at 12 months was 87.0% (20 of 23). The union rate per segment at 6 months was 94.2% (98 of 104), and the union rate per segment at 12 months was 96.0% (97 of 101).
Table 6.
Analysis of the demographic factors of nonunion cases
| Characteristic | Nonunion (n = 6) | Union (n = 21) | p-value† |
|---|---|---|---|
| Age (yr) | 69.83 ± 2.23 | 71.43 ± 6.45 | 0.562 |
| Sex | |||
| Male | 2 (33.3) | 8 (23.8) | 1.000 |
| Female | 4 (66.7) | 16 (76.2) | |
| Height (cm) | 155.93 ± 16.31 | 152.95 ± 6.31 | 0.492 |
| Weight (kg) | 59.38 ± 11.28 | 58.90 ± 11.60 | 0.929 |
| BMD (T-score) | 0.27 ± 2.20 | 0.02 ± 1.53 | 0.758 |
| Smoking | 5 (83.3) | 2 (9.5) | 0.001* |
| Drinking | 3 (50.0) | 2 (9.5) | 0.056 |
| No. of fused levels | |||
| 3 Levels | 0 (0) | 8 (38.1) | 0.171 |
| 4 Levels | 1 (16.7) | 3 (14.3) | |
| 5 Levels | 5 (83.3) | 10 (47.6) | |
| Fusion levels | |||
| L1‒4 | 0 (0) | 2 (9.5) | 0.757 |
| L2‒5 | 0 (0) | 2 (9.5) | |
| L3‒S1 | 0 (0) | 4 (19.1) | |
| L1‒5 | 0 (0) | 1 (4.8) | |
| L2‒S1 | 1 (16.7) | 2 (9.5) | |
| L1‒S1 | 5 (83.3) | 10 (47.6) | |
| Fused levels per patient (level) | 4.83 | 4.10 | - |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
BMD, bone mineral densitometry.
p<0.05, statistically significant differences.
Two-sample t-test or Mann-Whitney U-test was used.
The segment with the highest nonunion rate was L5–S1 (at 6 months; 85.7%; 12 months, 75.0%). The L5–S1 region exhibits a high nonunion rate due to poor sacral bone quality, complex sacral anatomy, and high biomechanical forces exerted on the lumbosacral junction [26-29]. Nevertheless, the rhBMP-2+HA group showed union at all segments and time points. Therefore, the application of rhBMP-2 with HA can increase the union rate in long-segment fusion, including the lumbosacral junction (L5–S1), where nonunions are frequent.
The rhBMP-2 is a growth factor with osteoinductive ability. Consequently, there have been concerns about side effects with high doses. This study used 3.0 mg per segment and 9–15 mg rhBMP-2 per patient. Assuming that the average weight of patients was 60 kg, the dose per patient was 0.15‒0.25 mg/kg, which was lower than the dose at which no adverse effects of rhBMP-2 were observed in rats (0.5 mg/kg) [30] and markedly lower than the lethal dose in rats (7.0 mg/kg) [31].
Some side effects associated with rhBMP-2 in the lumbar region are postoperative radiculitis, postoperative nerve root injury, ectopic bone formation, vertebral osteolysis/edema, and retrograde ejaculation [32]. However, no severe ADEs due to rhBMP-2 occurred in this study. Furthermore, this clinical trial evaluated the antibody response according to rhBMP-2 application in some subjects at 3 and 12 months. Only one subject temporarily tested positive at 3 months but negative at 12 months, a finding consistent with the results of a prospective, longitudinal cohort study. Burkus et al. [33] analyzed antibody production and clinical symptoms in patients receiving rhBMP-2 in the lumbar region. Antibody formation against rhBMP-2 peaked at 3 months and decreased to baseline at 12 months. The overall antibody elevation rate was 0.8%‒6.4%, but all were non-neutralizing antibodies [33]. These nonneutralizing antibodies generated due to an immune response to the therapeutic protein can bind to the protein without affecting its activity. Therefore, even if an antibody is present, it is important to determine whether it is a neutralizing antibody and exhibits actual clinical effects [33]. In this study, one subject with antibody formation showed bone union at both control and test sites, with no other clinical AEs.
This study had several limitations. The number of patients was too small to sufficiently detect differences in effects between groups. The follow-up period was short (1 year). Therefore, it is necessary to verify long-term clinical results with a larger cohort in the future. We applied 2 interventions simultaneously in each subject that could have mutually influenced the outcomes of each treatment group. The split-body trial has an advantage that the confounding factors between groups can be removed because the subjects of the test group are the same. However, since half of the human body is not completely independent, the difference in effects between groups may be underestimated, and systemic side effects may be masked [34]. Therefore, this study was able to directly compare the difference in effects according to BMP-2 without confounding factors between individuals, but the degree of difference may have been underestimated. In addition, safety could not be evaluated separately between groups.
In conclusion, this study demonstrated the clinical efficacy and safety of combined rhBMP-2 and HA in multilevel PLF fusion for ASD correction. Complete fusion was achieved at 6 months with 3 mg of rhBMP-2 per level for multilevel fusion (3‒5 levels) without causing antibody production that resulted in clinical symptoms.
CONCLUSION
This prospective, randomized, controlled trial investigated the efficacy and safety of the combined use of rhBMP-2 and a HA carrier in multilevel fusion in patients with ASD. The union rate for the rhBMP-2+HA group was 100% at 6 and 12 months. The union rate for the HA group was 77.8% (21 of 27) at 6 months and 88.0% (22 of 25) at 12 months. (p= 0.014 at 6 months; not significant at 12 months). In subgroup analysis per segment, 108 (93.9%) of a total of 115 segments at the control site and all segments at the test site were fused at 6 months (p< 0.001). At 12 months, 105 (96.3%) of a total of 109 segments at the control site and all segments at the test site were fused (p< 0.001). Clinical- and functional parameters (VAS, SF-36, and SRS-22 scores) improved significantly compared to baseline.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
This work was supported by the New Faculty Startup Fund from Seoul National University, Seongnam, Republic of Korea (grant numbers; 800-20210285) and a research grant for clinical studies from CG Bio Co., Ltd., Seoul, Republic of Korea.
Author Contribution
Conceptualization: SH, MYR; Data curation: SH; Formal analysis: CHL; Funding acquisition: SH; Methodology: HYC; Project administration: SH; Writing - original draft: SH; Writing - review & editing: SH, CHL, JHY, KK.
REFERENCES
- 1.Daniels AH, DePasse JM, Eberson CP, et al. Adult spinal deformity: contemporary treatment and patient outcomes. R I Med J (2013) 2015;98:32–41. [PubMed] [Google Scholar]
- 2.Frymoyer JW, Howe J, Kuhlmann D. The long-term effects of spinal fusion on the sacroiliac joints and ilium. Clin Orthop Relat Res. 1978:196–201. [PubMed] [Google Scholar]
- 3.Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin Orthop Relat Res. 1994:208–13. [PubMed] [Google Scholar]
- 4.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine (Phila Pa 1976) 1989;14:1324–31. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Good CR, Auerbach JD, O'Leary PT, et al. Adult spine deformity. Curr Rev Musculoskelet Med. 2011;4:159–67. doi: 10.1007/s12178-011-9101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshikawa H, Myoui A. Bone tissue engineering with porous hydroxyapatite ceramics. J Artif Organs. 2005;8:131–6. doi: 10.1007/s10047-005-0292-1. [DOI] [PubMed] [Google Scholar]
- 8.Bucholz RW, Carlton A, Holmes RE. Hydroxyapatite and tricalcium phosphate bone graft substitutes. Orthop Clin North Am. 1987;18:323–34. [PubMed] [Google Scholar]
- 9.Daculsi G, Passuti N, Martin S, et al. Macroporous calcium phosphate ceramic for long bone surgery in humans and dogs. Clinical and histological study. J Biomed Mater Res. 1990;24:379–96. doi: 10.1002/jbm.820240309. [DOI] [PubMed] [Google Scholar]
- 10.Korovessis P, Koureas G, Zacharatos S, et al. Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur Spine J. 2005;14:630–8. doi: 10.1007/s00586-004-0855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acharya NK, Kumar RJ, Varma HK, et al. Hydroxyapatite-bioactive glass ceramic composite as stand-alone graft substitute for posterolateral fusion of lumbar spine: a prospective, matched, and controlled study. J Spinal Disord Tech. 2008;21:106–11. doi: 10.1097/BSD.0b013e31805fea1f. [DOI] [PubMed] [Google Scholar]
- 12.Cheng H, Jiang W, Phillips FM, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85:1544–52. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Fu R, Selph S, McDonagh M, et al. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013;158:890–902. doi: 10.7326/0003-4819-158-12-201306180-00006. [DOI] [PubMed] [Google Scholar]
- 14.Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–49. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Dimar JR, Glassman SD, Burkus KJ, et al. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976) 2006;31:2534–9. doi: 10.1097/01.brs.0000240715.78657.81. [DOI] [PubMed] [Google Scholar]
- 16.Cho JH, Lee JH, Yeom JS, et al. Efficacy of Escherichia coliderived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: an open, active-controlled, randomized, multicenter trial. Spine J. 2017;17:1866–74. doi: 10.1016/j.spinee.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Mulconrey DS, Bridwell KH, Flynn J, et al. Bone morphogenetic protein (RhBMP-2) as a substitute for iliac crest bone graft in multilevel adult spinal deformity surgery: minimum two-year evaluation of fusion. Spine (Phila Pa 1976) 2008;33:2153–9. doi: 10.1097/BRS.0b013e31817bd91e. [DOI] [PubMed] [Google Scholar]
- 18.Park BJ, Hyun SJ, Wui SH, et al. Surgical outcomes and complications following all posterior approach for spinal deformity associated with neurofibromatosis type-1. J Korean Neurosurg Soc. 2020;63:738–46. doi: 10.3340/jkns.2019.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyun SJ, Lenke LG, Kim Y, et al. The incidence of addingon or distal junctional kyphosis in adolescent idiopathic scoliosis treated by anterior spinal fusion to L3 was significantly higher than by posterior spinal fusion to L3. Neurospine. 2021;18:457–63. doi: 10.14245/ns.2142182.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyun SJ, Lenke LG, Kim YC, et al. Comparison of standard 2-rod constructs to multiple-rod constructs for fixation across 3-column spinal osteotomies. Spine (Phila Pa 1976) 2014;39:1899–904. doi: 10.1097/BRS.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 22.Lee JH, Hwang CJ, Song BW, et al. A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A. 2009;90:804–10. doi: 10.1002/jbm.a.32113. [DOI] [PubMed] [Google Scholar]
- 23.Rohanizadeh R, Chung K. Hydroxyapatite as a carrier for bone morphogenetic protein. J Oral Implantol. 2011;37:659–72. doi: 10.1563/AAID-JOI-D-10-00005. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W, Fu H, Rahaman MN, et al. Hollow hydroxyapatite microspheres: a novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater. 2013;9:8374–83. doi: 10.1016/j.actbio.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam WD, Yi J. Bone union rate following instrumented posterolateral lumbar fusion: comparison between demineralized bone matrix versus hydroxyapatite. Asian Spine J. 2016;10:1149–56. doi: 10.4184/asj.2016.10.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuklo TR, Bridwell KH, Lewis SJ, et al. Minimum 2-year analysis of sacropelvic fixation and L5-S1 fusion using S1 and iliac screws. Spine (Phila Pa 1976) 2001;26:1976–83. doi: 10.1097/00007632-200109150-00007. [DOI] [PubMed] [Google Scholar]
- 27.Lebwohl NH, Cunningham BW, Dmitriev A, et al. Biomechanical comparison of lumbosacral fixation techniques in a calf spine model. Spine (Phila Pa 1976) 2002;27:2312–20. doi: 10.1097/00007632-200211010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Kim DH, Hyun SJ, Kim KJ. Selection of fusion level for adolescent idiopathic scoliosis surgery: selective fusion versus postoperative decompensation. J Korean Neurosurg Soc. 2021;64:473–85. doi: 10.3340/jkns.2020.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim CW, Hyun SJ, Kim KJ. Surgical impact on global sagittal alignment and health-related quality of life following cervical kyphosis correction surgery: systematic review. Neurospine. 2020;17:497–504. doi: 10.14245/ns.2040476.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JH, Lee EN, Nam SH. The short-term effects of repetitive E. coli-derived rhBMP-2 administration through intravenous injection in rats. Drug Chem Toxicol. 2014;37:40–7. doi: 10.3109/01480545.2013.806530. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Lee EN, Chang BS, et al. Acute intravenous injection toxicity study of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in rat. Asian Spine J. 2014;8:113–8. doi: 10.4184/asj.2014.8.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14:552–9. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- 33.Burkus JK, Gornet MF, Glassman SD, et al. Blood serum antibody analysis and long-term follow-up of patients treated with recombinant human bone morphogenetic protein-2 in the lumbar spine. Spine (Phila Pa 1976) 2011;36:2158–67. doi: 10.1097/BRS.0b013e3182059a8c. [DOI] [PubMed] [Google Scholar]
- 34.Nair B. Clinical trial designs. Indian Dermatol Online J. 2019;10:193–201. doi: 10.4103/idoj.IDOJ_475_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


