Abstract
Cytokine release syndrome (CRS) is a systemic inflammatory disease caused by a variety of factors, including infections and certain drugs. A 70‐year‐old man who was diagnosed with a postoperative recurrence of lung adenocarcinoma received nivolumab, ipilimumab, pemetrexed and carboplatin every 3 weeks for two cycles followed by nivolumab and ipilimumab, which resulted in a partial response. Four days after the dose of nivolumab, the patient returned with diarrhea and fever. The patient was diagnosed with COVID‐19 infection accompanied by severe colitis. Although intensive care was performed, the patient suddenly went into cardiopulmonary arrest. Examination revealed an abnormally high interleukin‐6 level, suggesting CRS. This is the first report of a patient with CRS accompanied with COVID‐19 infection during treatment with ICIs. Cytokine release syndrome (CRS) is a systemic inflammatory disease caused by a variety of factors, including infections and certain drugs. Here, we report a case of non‐small cell lung cancer with CRS caused by COVID‐19 infection during treatment with nivolumab and ipilimumab. Fever is a common event in cancer patients, especially in COVID‐19‐infected patients, but when fever develops during cancer immunotherapy, CRS should always be kept in mind.
Keywords: cancer immunotherapy, cytokine release syndrome, immune‐related adverse events, SARS‐CoV‐2
Cytokine release syndrome (CRS) is a systemic inflammatory disease caused by a variety of factors, including infections and certain drugs. Here, we report a case of non‐small cell lung cancer with CRS caused by COVID‐19 infection during treatment with nivolumab and ipilimumab. Fever is a common event in cancer patients, especially in COVID‐19‐infected patients, but when fever develops during cancer immunotherapy, CRS should always be kept in mind.
INTRODUCTION
Cytokine release syndrome (CRS) is a systemic inflammatory disease characterized by a massive release of cytokines, and triggered by a variety of factors such as infections and certain drugs. The clinical picture of CRS is a systemic inflammatory disease that begins with fever, and severe cases manifests hypotension, hypoxia, organ dysfunction requiring urgent treatment. Interleukin‐6 (IL‐6) is known to play an important role in the pathogenesis of CRS, and although immunosuppressive drugs such as corticosteroids have been used, therapies that suppress IL‐6 are now being applied clinically. 1 , 2 , 3 , 4 , 5
COVID‐19 is now a global pandemic. COVID‐19 patients are known to have elevated IL‐6 levels. Up to 20% of COVID‐19 patients develop acute respiratory distress syndrome, suggesting CRS. 1 , 2 , 6
Immune checkpoint inhibitors (ICIs) are the latest breakthrough in the treatment of patients with advanced tumors. Immune‐related adverse events (irAEs) can develop with ICIs, and ICI‐related CRS has been recognized as an irAE. 7 , 8 Herein, we report a case of CRS acompanied with COVID‐19 infection during treatment with ICIs.
CASE REPORT
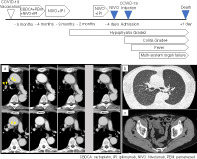
A 70‐year‐old Japanese man visited our hospital. He had undergone thoracoscopic surgery 2 years prior to admission and had been diagnosed with left upper lobe lung adenocarcinoma pT3N0M0, stage IIB. Five months prior to admission, he had received the second dose of COVID‐19 vaccine with no adverse reactions. Around the same time, the lung adenocarcinoma recurred in the right upper lobe with a PD‐L1 expression level of 30%. The tumor was negative for driver genes, and the patient was started on nivolumab plus ipilimumab combined with two cycles of carboplatin and pemetrexed. After two and four cycles of therapy, chest contrast‐enhanced computed tomography (CT) showed that the tumor had decreased in size (Figure 1). Three months prior to admission, the patient developed grade 2 hypophysitis (isolated adrenocorticotropic hormone deficiency), an irAE. Immunotherapy was held for 1 month and the patient was treated with corticosteroids. One and a half months prior to admission, immunotherapy was resumed. After confirmation of tumor regression and no evidence of pneumonitis and colitis on the contrast‐enhanced CT, the patient was administered nivolumab (Figure 1). Three days after administration, the patient had diarrhea more than 10 times a day. The next day, the patient presented with diarrhea and fever. Although the patient had no respiratory symptoms, he underwent polymerase chain reaction (PCR) testing for severe acute respiratory syndrome coronavirus 2 and was diagnosed with COVID‐19. CT showed no evidence of pneumonia, but there was bowel edema (Figure 1). The COVID‐19 infection was considered mild because the patient had no respiratory symptoms and no pneumonitis on CT. The patient was diagnosed with COVID‐19 infection accompanied by severe colitis and admitted to the intensive care unit. On admission, his body temperature was 39°C, blood pressure was 120/40 mmHg, and pulse was 110/min. He did not require treatment with oxygen or a vasopressor. Although intensive care was performed, including hydration, high dose corticosteroids, and antibiotics, the patient suddenly went into cardiopulmonary arrest 3 h after admission. Remdesivir was planned but had not yet been administered. A total of 27 min of cardiopulmonary resuscitation and one electrical defibrillation were performed, and the patient was returned to spontaneous circulation. His laboratory tests submitted before the sudden change showed increased C‐reactive protein and suggested multisystem organ failure (Table 1). An additional examination revealed an abnormally high IL‐6 level of 69 586 pg/ml. Accordingly, we diagnosed CRS due to systemic symptoms with inflammation and elevated IL‐6. Despite all efforts, he died the day after admission. Blood cultures were later confirmed negative.
FIGURE 1.

Clinical course of lung adenocarcinoma. Lung adenocarcinoma recurred 5 months prior to admission, and nivolumab plus ipilimumab was started combined with two cycles of carboplatin and pemetrexed. After two and four cycles of therapy, contrast‐enhanced computed tomography (CT) showed that the tumor had decreased in size. Three months prior to admission, the patient developed grade 2 hypophysitis (isolated adrenocorticotropic hormone deficiency) as an immune‐related adverse event. Immunotherapy was stopped, then restarted a month and a half later. CT showed no tumor regrowth just 4 days prior to admission. The final dose of nivolumab was administered on this day. Four days after resuming treatment with nivolumab, the patient returned with diarrhea and high fever. The patient was diagnosed with COVID‐19 infection and suggested cytokine release syndrome. Findings of thin‐section computed tomography. (a) Transition of CT findings over time. The lung adenocarcinoma shrank after the start of treatment and did not regrow. (b) Chest CT on admission. There was no evidence of pneumonia or acute respiratory distress syndrome which would indicate COVID‐19 infection in the lung fields. (c) Abdominal CT on admission. Bowel edema suspicious of colitis was present
TABLE 1.
Results of laboratory tests
| Four days before admission | Upon admission | |
|---|---|---|
| WBC (/μl) | 6.0 × 103 | 9.1 × 103 |
| Neutrophils (%) | 57.0 | 83.3 |
| Lymphocytes (%) | 25.5 | 9.9 |
| Monocytes (%) | 9.6 | 6.6 |
| Basophils (%) | 0.7 | 0.2 |
| Eosinophils (%) | 7.2 | 0.0 |
| RBC (/μl) | 4.21 × 106 | 4.76 × 106 |
| Hemoglobin (g/dl) | 12.4 | 13.9 |
| Platelets (/μl) | 2.0 × 105 | 1.3 × 105 |
| AST (U/l) | 22 | 904 |
| ALT (U/l) | 11 | 179 |
| LDH (U/l) | 188 | 1616 |
| γ‐GTP(U/l) | 31 | 35 |
| Total protein (g/dl) | 7.4 | 7.2 |
| Albumin (g/dl) | 4.1 | 3.8 |
| Total bilirubin (mg/dl) | 0.8 | 0.7 |
| BUN (mg/dl) | 24 | 52 |
| Creatinine (mg/dl) | 1.22 | 4.73 |
| CK (U/l) | 76 | 57 864 |
| CK‐MB (U/l) | ‐ | 101 |
| Sodium (mmol/l) | 141 | 134 |
| Potassium (mmol/l) | 4.8 | 5.7 |
| Chloride (mmol/l) | 105 | 101 |
| D‐dimer (μg/ml) | ‐ | 46.4 |
| Procalcitonin (ng/ml) | ‐ | 29.05 |
| Lactate (mmol/l) | ‐ | 4.97 |
| Glucose (mg/dl) | 103 | 87 |
| CRP (mg/dl) | 0.25 | 10.29 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CK, creatine kinase; CK‐MB, creatine kinase‐myocardial band; CRP, C‐reactive protein; γ‐GTP, gamma‐glutamyl transpeptidase; LDH, lactate dehydrogenase; RBC, red blood cells; WBC, white blood cells.
DISCUSSION
This is the first report of a patient with CRS acompanied with COVID‐19 infection during treatment with ICIs. It is not clear whether COVID‐19 infection or ICIs were the cause of CRS, but it was determined that CRS was triggered by COVID‐19 infection because it coincided with the timing of COVID‐19 infection. IrAEs have been suggested to reflect the favorable therapeutic efficacy of ICI treatment, but can sometimes be life‐threatening. 7 , 9 , 10 , 11 In addition, irAEs can be triggered by infections. 12 , 13 In the present case, the patient developed an irAE and showed a favorable response to immunotherapy. The CRS in this case may have been an irAE triggered by infection. Especially in cases of favorable tumor response, it is unfortunate that complications can lead to a poor prognosis.
ICIs are known as a cause of CRS in various types of cancer, and patients who received ICIs have been reported to develop CRS at a ratio of 0.06%–0.14%. 8 Several ICIs are linked to CRS, including anti‐programmed cell death protein‐1/ligand‐1 antibodies, and anticytotoxic T lymphocyte antigen‐4 antibodies. Because of the progressively increasing use of ICIs, CRS induced by ICIs is becoming more easily recognized and diagnosed. 8 , 14 , 15 , 16
Previously reported CRS cases induced by nivolumab plus ipilimumab are summarized in Table 2. IL‐6 values varied from case to case, but the patient described herein had the highest value. In all previous cases, immunosuppressive therapy had been used with favorable outcomes. The addition of immunosuppressive therapy such as an IL‐6 blocker or IL‐6 receptor antagonist may improve the prognosis of patients with CRS.
TABLE 2.
Previously reported CRS cases induced by nivolumab plus ipilimumab
| Authors | Age/sex | Primary cancer | IL‐6 level (pg/ml) | Immunosuppressive therapy | Outcome |
|---|---|---|---|---|---|
| Urasaki et al. 13 | 46/F | Renal cell carcinoma | No date |
Tocilizumab MMF IVIg |
Recovered |
| Ohira et al. 14 | 70/M | Renal cell carcinoma | 467 |
mPSL/PSL MMF IVIg |
Recovered |
| Kunimasa et al. 15 | 64/F | Lung cancer | 25 100 |
Tocilizumab Infliximab mPSL/PSL MMF |
Recovered |
| Present study | 70/M | Lung cancer | 69 586 | Hydrocortisone 100 mg | Death |
Abbreviations: IL‐6, interleukin‐6; IVIg, intravenous immunoglobulin; MMF, mycophenolate mofetil; mPSL, methylprednisolone; PSL, prednisolone.
Fever is a common event in cancer patients, especially in COVID‐19‐infected patients, but when fever develops during cancer immunotherapy, CRS should always be kept in mind.
CONFLICT OF INTEREST
KA reports receiving personal fees from AstraZeneca, MSD, Bristol Myers Squibb, Ono Pharmaceutical, Takeda Pharmaceutical, Pfizer and Chugai Pharmaceutical. TT reports receiving personal fees from AstraZeneca, Bristol Myers Squibb, MSD, Novartis and Chugai Pharmaceutical. The remaining authors have no conflicts of interest to disclose.
Murata D, Azuma K, Tokisawa S, Tokito T, Hoshino T. A case of cytokine release syndrome accompanied with COVID‐19 infection during treatment with immune checkpoint inhibitors for non‐small cell lung cancer. Thorac Cancer. 2022;13(20):2911–2914. 10.1111/1759-7714.14632
REFERENCES
- 1. Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96. 10.1038/s41577-021-00547-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368(6490):473–4. 10.1126/science.abb8925 [DOI] [PubMed] [Google Scholar]
- 3. Gödel P, Shimabukuro‐Vornhagen A, von Bergwelt‐Baildon M. Understanding cytokine release syndrome. Intensive Care Med. 2018;44(3):371–3. 10.1007/s00134-017-4943-5 [DOI] [PubMed] [Google Scholar]
- 4. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–95. 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimabukuro‐Vornhagen A, Gödel P, Subklewe M, Stemmler H, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. 10.1186/s40425-018-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune‐checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–80. 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 8. Ceschi A, Noseda R, Palin K, Verhamme K. Immune checkpoint inhibitor‐related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. 2020;11:557. 10.3389/fphar.2020.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park R, Lopes L, Saeed A. Anti‐PD‐1/L1‐associated immune‐related adverse events as harbinger of favorable clinical outcome: systematic review and meta‐analysis. Clin Transl Oncol. 2021;23:100–9. 10.1007/s12094-020-02397-5 [DOI] [PubMed] [Google Scholar]
- 10. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, et al. Association between immune‐related side effects and efficacy and benefit of immune checkpoint inhibitors: a systematic review and meta‐analysis. Cancer Treat Rev. 2021;92:102134. 10.1016/j.ctrv.2020.102134 [DOI] [PubMed] [Google Scholar]
- 11. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta‐analysis. JAMA Oncol. 2018;4:1721–8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makunts T, Burkhart K, Abagyan R, Lee P. Retrospective analysis of clinical trial safety data for pembrolizumab reveals the effect of co‐occurring infections on immune‐related adverse events. PLoS One. 2022;17(2):e0263402. 10.1371/journal.pone.0263402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takemura M, Motegi M, Kuroiwa Y, Itai M, Taguchi K, Umetsu K, et al. Immune‐related adverse events caused by treatment with pembrolizumab in a patient with lung cancer who infected influenza virus. Respir Med Case Rep. 2021;32:101361. 10.1016/j.rmcr.2021.101361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Urasaki T, Ono M, Mochizuki T, Takeda K, Nishizawa A, Fukagawa E, et al. Case report: a case of trimethoprim/sulfamethoxazole‐triggered hypotensive shock: cytokine release syndrome related to immune checkpoint inhibitors and drug‐induced hypersensitivity syndrome. Front Oncol. 2021;11:681997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohira J, Kawamoto M, Sugino Y, Kohara N. A case report of fulminant cytokine release syndrome complicated by dermatomyositis after the combination therapy with immune checkpoint inhibitors. Medicine. 2020;99:15. 10.1097/MD.0000000000019741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kunimasa K, Inoue T, Matsueda T, et al. Cytokine release syndrome and immune‐related pneumonitis associated with tumor progression in a pulmonary pleomorphic carcinoma treated with Nivolumab plus Ipilimumab treatment: a case report. JTO Clin Res Rep. 2021;3(2):100272. 10.1016/j.jtocrr.2021.100272 [DOI] [PMC free article] [PubMed] [Google Scholar]


