Abstract
There is overwhelming evidence to suggest that male gender is at a higher risk of developing more severe Covid‐19 disease and thus having poorer clinical outcomes. However, the relationship between testosterone (T) and Covid‐19 remains unclear with both protective and deleterious effects on different aspects of the disease suggested. Here, we review the current epidemiological and biological evidence on the role of testosterone in the process of SARS‐CoV‐2 infection and in mediating Covid‐19 severity, its potential to serve as a biomarker for risk stratification and discuss the possibility of T supplementation as a treatment or preventative therapy for Covid‐19.
Keywords: prostate, sexual medicine, testosterone
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2, also known as Corona Virus Disease 2019 or Covid‐19) exhibits differences in morbidity and mortality between sexes, with a male bias towards severe disease. 1 , 2 , 3 , 4 A meta‐analysis of over 3 million cases globally (accumulated from 92 data sets that contained information on the total number of infections by sex, and the severity of disease as measured by admission to intensive therapy unit (ITU) and death) has shown that whilst there are no sex‐based differences in the proportion of Covid‐19 cases, male patients have higher odds for requiring Intensive treatment (odds ratio OR = 2.84) and death (OR = 1.39) compared to females, despite this analysis not controlling for lifestyle, comorbidities, testing method and case type (hospital vs. community). 5 This sex difference is apparent across different countries when comparing Covid‐19 fatality (Figure 1). 6 Epidemiological data from the previous coronavirus epidemic of SARS‐CoV (2002) also show preponderancy of poorer outcomes for males. 7 , 8 , 9 One study reported the age–adjusted mortality risk ratio for males of 1.62 (95% CI = 1.21–2.16) again without either study controlling for comorbidities and lifestyle factors, 7 and an OR for ITU admission or death was reported to be 3.10 (95% CI = 1.64–5.87) for males by a separate study when using gender as a univariate prognostic factor. 10 A similar trend of higher case fatality rate for males (52%) compared to females (23%) was also observed for Middle Eastern respiratory syndrome coronavirus (MERS, 2012). 8
FIGURE 1.
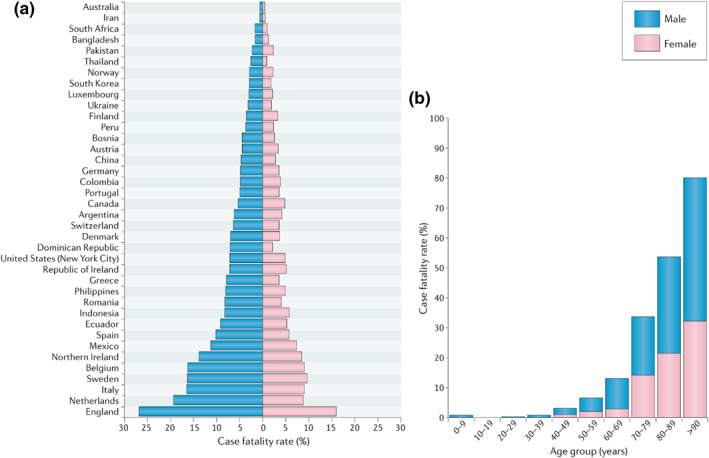
Covid‐19 case fatality rates (CFRs) for males and females across 38 countries or regions reporting sex‐disaggregated data on Covid‐19 cases and deaths. (a) Male CFR is significantly higher than the female CFR in 37 of the 38 regions investigated, with an average male CFR 1.7 times greater than the average female CFR. (b) Covid‐19 CFR increases for both sexes with advancing age, but males have a significantly higher CFR than females at all ages from 30 years in 12 countries currently reporting sex‐ and age‐disaggregated data on Covid‐19 cases and deaths (Australia, Columbia, Denmark, Italy, Mexico, Norway, Pakistan, Philippines, Portugal, Spain, Switzerland and England). Reprinted by permission from Springer Nature© in Scully et al 6
Evolutionary and biological basis for these differences have been suggested, and numerous reports have attempted to understand the sex discordance as a result of lifestyle and cultural disparity as well as innate physiological differences. 11 Indeed, gender based socio‐cultural and behavioural differences between the sexes are also thought to contribute to severity in Covid‐19. For instance males are more likely to smoke than females, and several studies have reported increased Covid‐19 severity in patients who smoke. 12 , 13 Cultural and regional gender differences in a healthcare‐seeking behaviours, 14 and access to healthcare may also lead to the observed bias in disease severity. 15 , 16
Biological reasons such as higher prevalence of comorbidities like chronic obstructive pulmonary disease, diabetes, cerebrovascular disease, cancer and hypertension in males have been associated with poorer Covid‐19 clinical outcomes. 17 , 18 Sex‐based differences in both innate and adaptive immunity is a well characterised evolutionarily conserved phenomena that could confer improved outcomes for females. 11 , 19 The influence of gonadal hormones facilitates the differential biological milieu between the sexes, and it has been postulated that they may affect pathogenicity and immune response to SARS‐CoV‐2 and thus contribute to the sex bias. In fact, many of the risk factors for Covid‐19 are influenced by sex hormones. Some studies investigating oestrogen supplementation in post‐menopausal women have observed a protective effect of oestrogen in Covid‐19. 20 , 21 A similar observation was reported which showed that post‐menopausal women were at a greater risk of hospitalisation, and that oestrogen levels had a protective effect against disease severity. 22 In men, hypogonadism and low testosterone levels are associated with increased comorbidities such as type 2 diabetes, obesity and cardiovascular diseases, 23 which are risk factors for severe Covid‐19. 24 , 25 , 26 In contrast, there is evidence to suggest that the receptor for SARS‐CoV‐2, angiotensin converting enzyme‐2 (ACE2), and the major viral fusogenic protease transmembrane protease serine 2 (TMPRSS2) are under transcriptional regular by androgens, 27 and high T levels can thus facilitate infection and disease progression in men.
The relationship between T and Covid‐19's sex‐bias therefore remains unclear, and its improved understanding can give an insight into the disease mechanisms, predict patient outcomes and eventually help treat severe disease. In this review, we discuss the evidence to date on the effect of testosterone on the severity and mortality associated with Covid‐19.
2. TESTOSTERONE AND SARS‐COV‐2 INFECTIONS
SARS‐CoV‐2 viral attachment is mediated by binding of the S‐glycoprotein to ACE2 receptor on target cells, and entry is facilitated by the protease activity of TMPRSS2 that allows fusion of the viral and cellular membranes (Figure 2). 28 This indicates that high expression of ACE2 receptor and or TMPRSS2, particularly in the lung alveolar epithelial cells but also enterocytes of the small intestine, may be a primary factor in SARS‐CoV‐2 infections. A recent study that conducted a high‐throughput screen of nearly 1500 FDA approved drugs to identify molecules that reduce ACE2 levels in human cells found androgen signalling as a key modulator of ACE2 expression. 29 The study also showed that anti‐androgenic drugs reduced ACE2 expression in human embryonic stem cells and was protective against SARS‐CoV‐2 infections. 29 A decrease in ACE2 expression was found in older men as a consequence of age‐related androgen level decline 30 and testosterone significantly upregulates ACE2 expression in primary isolated human airway smooth muscle cells from healthy males. 31 TMPRSS2 transcription is regulated by androgen receptor (AR) and may partially mediate the sex discordance observed in Covid‐19. 32 , 33
FIGURE 2.
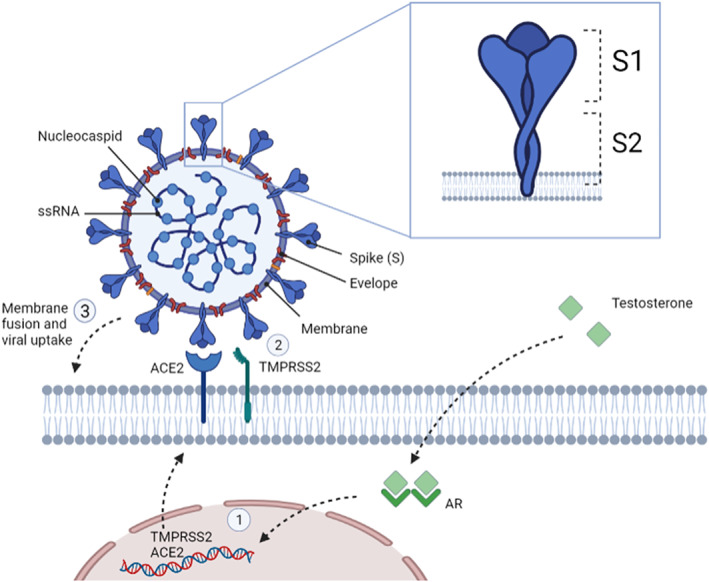
SARS‐CoV‐2 structure, viral entry into host cells and the influence of testosterone. SARS‐CoV‐2 is made up of four structural proteins, including the spike (S), membrane, envelop and nucleocapsid proteins. The S protein has 2 functional domains known as S1 and S2. S1 is recognized and binds to angiotensin‐converting enzyme 2 (ACE2). Following ACE2 binding, cleavage of the viral spike protein (S) by proteases including transmembrane protease serine 2 (TMPRSS2) a crucial step to allow host cell membrane fusion and viral uptake. TMPRSS2 interacts with and primes ACE2 in this process and is considered crucial for host cell infection. TMPRSS2 transcription is exclusively regulated by the androgen receptor (AR) and ACE2 expression is increased in an androgen dependent manner (1). Mechanistically it is considered that androgen‐regulated TMPRSS2 promotes SARS‐CoV‐2 entry by two separate processes: ACE2 interaction/cleavage (2), which might promote viral uptake, and SARS‐2‐S cleavage, which enhances membrane fusion (3) via activation of the S protein. Created with BioRender.com
As SARS‐CoV‐2 relies on ACE2 and TMPRSS2 expression for cellular entry, and androgen dependent prostate cancer cells have shown high expression of TMPRSS2 in response to androgens, 34 androgen deprivation therapy (ADT) was thought to be protective in this patient group. Indeed, a large‐scale study involving 4532 prostate cancer patients showed that patients receiving ADT had a lower risk of SARS‐CoV‐2 infection (OR of 4.05; 95% CI 1.55–10.59). 35 This supports a partial role of androgens in the pathogenesis of Covid‐19. However, the study did not describe the clinical and biological characteristics of the patient populations and the analysis wasn't corrected for multiple variables which are likely confounders. Furthermore, androgen deprivation in mice by castration, or anti‐androgen treatment in vitro, led to a reduction in TMPRSS2 and ACE2 transcript and protein levels. 36 Mechanistically it is considered that androgen‐regulated TMPRSS2 promotes SARS‐CoV‐2 entry by two separate processes: ACE2 interaction/cleavage, which might promote viral uptake, and SARS‐2‐S cleavage, which enhances membrane fusion via activation of the S protein (Figure 2). 28 , 37 Since this collective evidence, multiple studies have advocated ADT and anti‐androgens, commonly used for prostate cancer treatment, as potential therapeutic options in Covid‐19 providing protection against SARS‐CoV‐2 infection or reduce viral amplification. 35 , 38 , 39 , 40 , 41
Conversely, in a prospective registry cohort study, Klein et al. (2021) reported that 5.6% of prostate cancer patients on ADT were infected with SARS‐CoV‐2 compared to 5.8% of patients not on ADT, and thus concluded that ADT therapy is not protective against or a risk for infection. 42 This observation was also confirmed by an epidemiological study consisting of 7894 prostate cancer patients from the Swedish National registers with long‐term androgen inhibition which showed no beneficial or detrimental effects on Covid‐19 infection rate or severity. 43 In fact, as per the Global Health 50/50 data, there is no evidence that males are more likely to be infected than females. This was also observed in data from over 700,000 cases collated by WHO which showed that there were almost equal number of cases of males and females (1.03:1). Taken together, the evidence suggests that male sex or T may not mediate SARS‐CoV‐2 infections per se, but through cellular mechanisms involving ACE2 and TMPRSS2 has the potential to play a role in the observed sex disparity in Covid‐19 severity and mortality outcomes, 11 , 27 despite some conflicting evidence. 43
3. TESTOSTERONE AND COVID‐19 SEVERITY
The sex bias in Covid‐19 severity and mortality has led to several studies exploring the role of T in disease progression. In a case‐control study, significantly lower total T (TT) levels were observed in men with Covid‐19 at hospital admission compared to healthy controls (2.5 nmol/L vs. 10.4 nmol/L; p < 0.0001). 44 Furthermore, 89.8% of Covid‐19 patients compared to 14.9% of healthy individuals were observed to be hypogonadal (TT < 9.2 nmol/L). 44 A meta‐analysis of 11 studies that evaluated male hormonal parameters in over 2000 patients also reported that SARS‐CoV‐2 patients were characterised by reduced TT levels, with no alterations in luteinizing hormone and follicle stimulating hormone (FSH) (Figure 3). 45 A prospective cohort study of 358 patients diagnosed with Covid‐19 and 92 non‐Covid‐19 patients reported lower serum TT levels in the Covid‐19 patients (median, 140 ng/dl vs. 322 ng/dl). 46 On stratifying Covid‐19 patients on disease severity, lower TT levels were observed in patients with severe disease compared to those with mild‐moderate symptoms (85.1 ng/dl vs. 315 ng/dl), and in patients needing intensive care versus those who did not need it (64 ng/dl vs. 286 ng/dl). 46 This association was also demonstrated by an independent study which reported a steep increase in both ICU transfers and mortality risk in men with low levels of TT and calculated free T (cFT). 47
FIGURE 3.
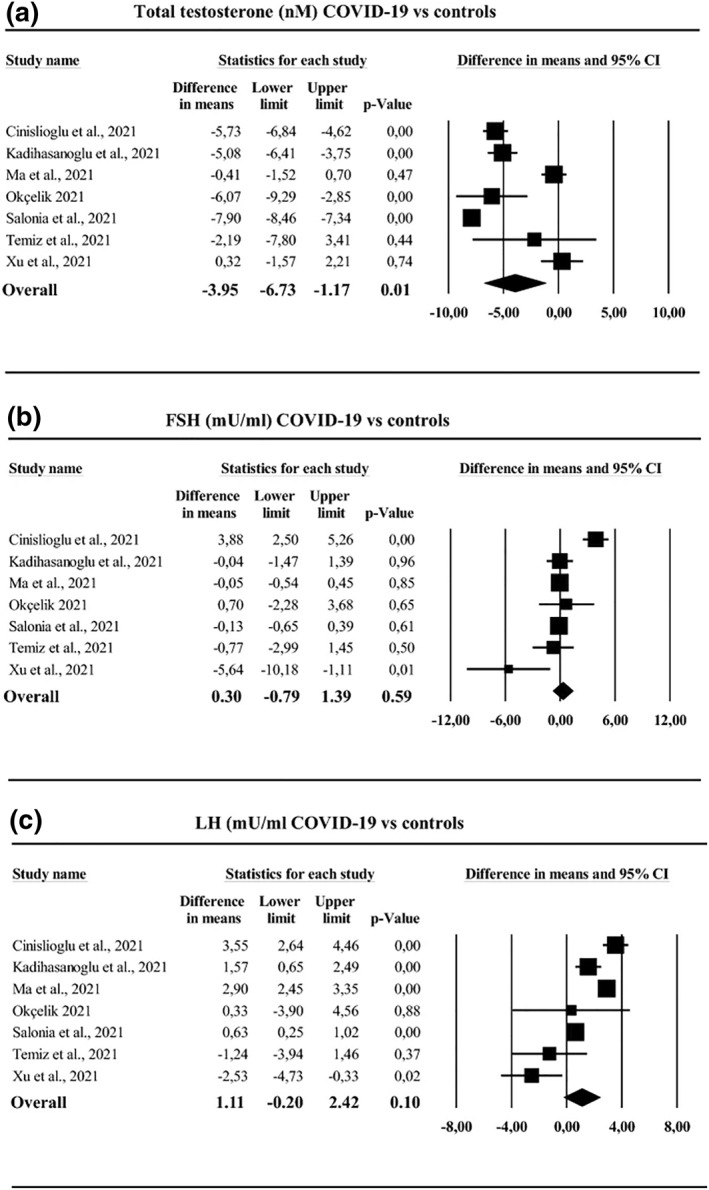
Hormone analysis in Covid‐19 subjects versus non‐infected controls. SARS‐Cov‐2‐infected patients were characterised by reduced total testosterone levels (a), whereas no difference in either follicular stimulating hormone (FSH) levels (b) or luteinizing hormone (LH) levels (c) was observed. Figure reproduced with permission from Corona et al 45
A larger single centre cohort study involving 90 men observed that patients who had severe Covid‐19 at presentation or developed it following admission, had lower levels of serum T compared to patients with milder disease (53 ng/dl vs. 151 ng/dl; p = 0.01). 48 Whilst lower T levels were associated with increased disease severity in this cohort, levels of T in patients with mild Covid‐19 were also observed to be lower than the normal reference range. 48 The requirement for continuous positive airway pressure therapy, a measure of adverse clinical outcome, was associated with lower levels of TT and cFT at time of hospitalisation in a multicenter observational study of 35 men. 49 Lower TT and cFT also correlated with longer hospital stays (rho = −0.51, p < 0.01 and rho = −0.55, p < 0.01, respectively), and TT levels were an independent predictive factor for the length of hospitalisation. 49 A similar observation was reported by Camici et al. (2021) who observed that low TT and low cFT were associated with longer hospital stay following Covid‐19 infection (p = 0.052 and p = 0.041 respectively) and the increased occurrence of hyperinflammatory syndrome determined by lymphocyte count, and levels of ferritin, lactate dehydrogenase, d‐dimer and c‐reactive proteins (p = 0.018 and p = 0.048 respectively). This has significant implications on mortality as the first cause of death in patients with Covid‐19 was acute respiratory failure syndrome (ARDS), 50 and the second reason has been found to be hyperinflammatory syndrome. 51 , 52 ARDS in critically injured adults has been shown to be associated with women, and men with low testosterone levels. 53 In the context of Covid‐19, Pagano et al. (2021) demonstrated that lower plasma T levels in and lymphocyte counts were associated with more severe ARDS at hospital admission in males, and with deterioration of the condition post‐admission. 54 Low levels of serum TT and higher oestrogen to T ratio (often considered a marker of systemic inflammation) 55 was associated with increased risk of in‐hospital mortality in a separate retrospective study in symptomatic men. 56
Whilst several studies have described an associated of lower T levels to poorer clinical outcomes, it still remains unclear whether reduced T is a marker or a mediator of Covid‐19 severity. Critical illness can cause transient hypogonadism thereby causing a rapid decrease in serum T. 57 , 58 Whilst T deficiency is associated with increased pro‐inflammatory cytokines, 59 inflammatory mediators have also been shown to lower T levels during acute illness via increased metabolic clearance and suppression of gonadal axis. 60 , 61 In a study comparing Covid‐19 patients with non‐Covid‐19 patients with respiratory tract infections and age‐matched healthy controls, TT levels were decreased in both Covid‐19 and non Covid‐19 patients suggesting that hormonal imbalance might be a consequence of critical illness in general. 62 A multi‐organ proteomic analyses from organs of 19 Covid‐19 patients showed evidence of testicular injuries specifically to T producing Leydig cells was observed. 63 SAR‐CoV‐2 spike protein has been observed in the nuclei and cytoplasm of Leydig cells along with increased inflammation, lymph and macrophage infiltration in postmortem testes after SARS‐CoV‐2 sepsis. 64 Therefore, it is possible that the association of lower T to disease progression might partly be due to the suppression of T production as a consequence of viral infection. Supporting this in part, Salonia et al. showed in a follow‐up cohort study that testosterone levels increased during the recovery phase at 7‐month. 65 Despite this finding, 50% of patients remained hypogonadal at follow‐up and testosterone levels even further decreased in as many as 10% of cases.
In order to understand the biological basis of hypogonadism and its correlation to Covid‐19 severity, Baldassarri et al. (2021) focussed on the role of AR polymorphisms. 66 The X‐linked AR gene is characterised by a highly polymorphic poly glutamine (polyQ) tract at its N‐terminus, which may have 9 to 36 glutamine coding CAG repeats, with 20 repeats having the highest frequency in humans. 67 Whilst AR is expressed in males and females, the bioavailability of its ligands is much higher in men. The length of this polyQ tract, which varies with ethnicity, is inversely correlated with the activation of AR 67 and may offer some biological basis of the differences in Covid‐19 severity between ethnic populations. In a nested case‐control study of 638 male and female Covid‐19 patients, Baldassarri et al. (2021) observed that shorter polyQ tracts (<22) which would have higher AR functionality was protective of worse clinical outcomes in individuals of <60 years. 66 The authors also show that the longer polyQ tracts (>23) are associated with higher serum T levels, possibly due to a dysregulation in hypothalamo‐pituitary negative feedback, and an increased pro‐inflammatory status. 66 Taken together, their study suggests that the risk of disease severity can be predicted by the ‘androgenicity’ of the individual which is a function of polyQ status and their serum T levels.
Although there is consensus that low T levels are associated with poorer outcomes, the changes in T levels longitudinally following Covid‐19 is less explored. This was addressed in an elegant study where Toscano‐Guerra et al. (2022) analyzed the longitudinal relationship between serum T levels and immune cell populations with disease outcomes in Covid‐19 patients. 68 They reported that the positive trajectories of serum T (p = 0.0038) and lymphocyte counts (p = 0.01), and negative trajectory for neutrophil counts (p = 0.0023) were significantly different between the disease severity groups and were remarkably accurate predictors of patient outcome. 68 The authors concluded that a potential functional role for T in Covid‐19 recovery is possible based on the association of its reinstatement with improved Covid‐19 outcomes and reduced signs of inflammation. 68
However, there is a paucity of studies that describe the role of circulating T levels prior to SARS‐CoV‐2 infection on Covid‐19 outcomes. A study by Manning and Fink (2020) aimed to understand if the prenatal sex‐steroid levels, as measured by digit ratio (2D:4D) as a surrogate marker, associated with increased severity of disease by comparing data from 41 countries. 69 They observed that low prenatal testosterone (indicated by a high 2D:4D ratio), led to increased Covid‐19 severity in men. 69 Conversely, in community‐dwelling men aged 40–69 years, serum total testosterone measured years prior to any exposure to SARS‐CoV‐2 a U‐shaped association of total testosterone with risk of death from Covid‐19 was apparent. 70 Interestingly, when the model was adjusted for additional covariates including multiple lifestyle and demographic variables, blood cholesterol, and prevalent medical conditions, Testosterone levels were positively correlated with risk of dying from Covid‐19. 70 This suggests that potential beneficial effects of testosterone may occur only via influence on the presence of medical comorbidities including obesity and type 2 diabetes which are known to increase Covid‐19 severity. However, the significance of this relationship is diminished by the testosterone assessment taking place years prior to Covid‐19 infection, and therefore may not reflect the man's T level at time of pandemic.
4. TESTOSTERONE THERAPY AND COVID‐19
In one of the few studies investigating 32 men hypogonadal men receiving testosterone therapy (TTh) at time of Covid‐19 infection, the authors concluded that TTh was not associated with a worse clinical outcome in men diagnosed with Covid‐19 as no differences were observed in clinical outcomes when compared to 63 age, race, body mass index, and socioeconomic status matched controls diagnosed with Covid‐19 and not on TTh. 71 Despite a lack of statistical significance, patients on TTh had lower rates of ICU admission (12.5% vs. 25.4%) and mechanical ventilator utilization (9.3% vs. 19.0%) than patients not on TTh, but none were. 71 This study did not, however, compare clinical outcomes with patient who were hypogonadal prior to Covid‐19 infection and not receiving TTh as an indication of testosterone's potential protective effects in men with low T. Interestingly, men in the TTh group had increased incidence of baseline comorbidities, including cardiovascular disease, diabetes, hypertension and immunosuppression allowing the possibility that no increase in clinical outcomes in this group may in fact indicate a protective effect of T. Of clinical relevance, evidence indicating post Covid‐19 preferential testosterone level recovery over time in men with a lower burden of comorbid conditions at presentation, 65 is suggestive that TTh may aid Covid‐19 recovery via its reported beneficial effects on cardiometabolic comorbidities. 72 , 73 , 74
A large‐scale nested case‐control study that investigated if TTh was associated with reduced disease progression, male patients over the age of 50 who were hospitalised in 30 days after Covid‐19 diagnosis (n = 33,380), or those who required ITU admission or mechanical ventilation post hospitalisation (n = 10,273) were matched 1:1 to controls. 75 The study observed that the patients who had received TTh prescription for 120 days or less were not associated with decreased odds of hospitalisation or ITU/mechanical ventilation. 75 The study however did not have access to serum T levels either prior to or during Covid‐19 diagnosis and thus could not assess the if hypogonadism was effectively treated in the patient population. There is a possibility that undertreated hypogonadism may have moderated the protective effective of TTh. Moreover, the study relied on electronic health records and therefore data on several potential confounders affecting patient health were unavailable. 75
5. LIMITATIONS AND FUTURE PROSPECTS
The evidence so far on the role of T on Covid‐19 severity is based on correlation of T measurements at diagnosis or hospitalisation and therefore make interpretations of causality difficult. Prospective studies that have longitudinal hormonal measurements, and pre‐infection hormonal milieu assessment, could provide a deeper insight into the role of T in SAR‐CoV‐2 infection and severity risk. Furthermore, pre‐clinical animal models can help robustly demonstrate or validate mechanistic relationships between T status (i.e. deprivation or replacement) and SARS‐CoV‐2 infection outcomes. Indeed, the seemingly conflicting roles of testosterone in the pathogenesis of Covid‐19 requires further investigation. Testosterone may facilitate cell infection with the SARS‐CoV‐2 but also be protective of worse clinical outcomes during active infections. Future studies investigating testosterone levels of men prior to Covid‐19 infection (baseline) and sequential timepoints throughout disease pathology and recovery may help further delineate this relationship. Similarly, studies comparing hypogonadal men at baseline either receiving TTh or not, for key Covid‐19 clinical outcomes may uncover any therapeutic potential of testosterone in Covid‐19 protection. Finally, most studies do not assess the effects of free or bioavailable T concentrations, which are a better predictor of T status, on disease outcomes.
6. CONCLUSION
Taken together, the evidence so far suggests that the role of testosterone in Covid‐19 is a double‐edged sword, with some studies suggesting that a low testosterone state is protective in men in certain situations, yet increasing amounts of evidence suggests lower T in male patients is associated with increased Covid‐19 severity and mortality. However, lack of evidence on the effect of pre‐infection circulating T, contradictory reports on the effects of ADT on SAR‐CoV‐2 infection, and incomplete understanding of the underlying biological mechanisms makes it difficult to conclude if T is a marker or mediator of Covid‐19 severity. Indeed, the mechanisms are likely to be multidimensional and influenced by a wide range of interacting factors that can be specific to the individual patient. Furthermore, the potential of TTh in men with Covid‐19 to aid recovery and the effect of testosterone on long Covid are still to be extensively investigated. Therefore, both longitudinal studies and prospective placebo‐controlled trials are necessary to further understand the long‐term impact of testosterone in Covid‐19 severity, recovery, and mortality.
AUTHOR CONTRIBUTIONS
Aksam Yassin: Supervision, Concept, Manuscript Writing, Manuscript Grammatically Revision, Critical Revision, Final Revision (Editor and Reviewer Comments). Ridwan Shabsigh: Concept, Manuscript Grammatically Revision. Raed M. Al‐Zoubi: Literature Search, Critical Revision. Omar M. Aboumarzouk: Literature Search, Critical Revision. Mustafa Alwani: Literature Search, Manuscript Grammatically Revision. Joanne Nettleship: Manuscript Grammatically Revision, Critical Revision, Final Revision (Editor and Reviewer Comments). Daniel Kelly: Concept, Supervision, Literature Search, Manuscript Writing, Manuscript Grammatically Revision, Critical Revision, Final Revision (Editor and Reviewer Comments).
CONFLICT OF INTEREST
Aksam Yassin has received partial compensation for research, honoraria and occasionally travel grants from Bayer AG. Member of Advisory Board for Testosterone, Besin Health Care, Pharma. DMK has previously received honoraria, research funding and occasionally travel grants from Bayer AG.
ACKNOWLEDGEMENT
Editorial support for this manuscript was provided by Astra‐Health, www.Astra‐Health.com. The publication of this article was funded by the Qatar National Library.
Yassin A, Sabsigh R, Al‐Zoubi RM, et al. Testosterone and Covid‐19: an update. Rev Med Virol. 2022;e2395. 10.1002/rmv.2395
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;8:152. 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. JAMA. 2020;323(20):2052‐2059. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kragholm K, Andersen MP, Gerds TA, et al. Association between male sex and outcomes of Coronavirus Disease 2019 (COVID‐19)—a Danish nationwide, register‐based study. Clin Infect Dis. 2021;73(11):e4025‐e4030. 10.1093/cid/ciaa924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen NT, Chinn J, De Ferrante M, Kirby KA, Hohmann SF, Amin A. Male gender is a predictor of higher mortality in hospitalized adults with COVID‐19. PLoS One. 2021;16(7):e0254066. 10.1371/journal.pone.0254066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID‐19 outcomes. Nat Rev Immunol. 2020;20(7):442‐447. 10.1038/s41577-020-0348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229‐231. 10.1093/aje/kwh056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alghamdi IG, Hussain I, Almalki SS, Alghamdi MS, Alghamdi MM, El‐Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417‐423. 10.2147/IJGM.S67061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jansen A, Chiew M, Konings F, Lee CK, Ailan L. On behalf the World Health Organization regional office for the western pacific MEMT. Sex matters—a preliminary analysis of Middle East respiratory syndrome in the Republic of Korea. Western Pac Surveill Response J. 2015;6(3):68‐71. 10.5365/WPSAR.2015.6.3.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leong HN, Earnest A, Lim HH, et al. SARS in Singapore—predictors of disease severity. Ann Acad Med Singapore. 2006;35(5):326‐331. [PubMed] [Google Scholar]
- 11. Alwani M, Yassin A, Al‐Zoubi RM, et al. Sex‐based differences in severity and mortality in COVID‐19. Rev Med Virol. 2021;31(6):e2223. 10.1002/rmv.2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clift AK, von Ende A, Tan PS, et al. Smoking and COVID‐19 outcomes: an observational and Mendelian randomisation study using the UK Biobank cohort. Thorax. 2022;77(1):65‐73. 10.1136/thoraxjnl-2021-217080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai H. Sex difference and smoking predisposition in patients with COVID‐19. Lancet Respir Med. 2020;8(4):e20. 10.1016/S2213-2600(20)30117-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Team WHOER, Agua‐Agum J, Ariyarajah A, et al. Ebola Virus disease among male and female persons in west Africa. N Engl J Med. 2016;374(1):96‐98. 10.1056/NEJMc1510305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Regitz‐Zagrosek V. Sex and gender differences in health. Science & society series on sex and science. EMBO Rep. 2012;13(7):596‐603. 10.1038/embor.2012.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bischof E, Oertelt‐Prigione S, Morgan R, Klein S. Towards precision medicine: inclusion of sex and gender aspects in COVID‐19 clinical studies‐acting now before it is too late—a joint call for action. Int J Environ Res Publ Health. 2020;17(10):3715. 10.3390/ijerph17103715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789‐1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID‐19 and intensive care unit admission: a systematic review and meta‐analysis. Int J Public Health. 2020;65(5):533‐546. 10.1007/s00038-020-01390-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626‐638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 20. Costeira R, Lee KA, Murray B, et al. Estrogen and COVID‐19 symptoms: associations in women from the COVID symptom study. PLoS One. 2021;16(9):e0257051. 10.1371/journal.pone.0257051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seeland U, Coluzzi F, Simmaco M, et al. Evidence for treatment with estradiol for women with SARS‐CoV‐2 infection. BMC Med. 2020;18(1):369. 10.1186/s12916-020-01851-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding T, Zhang J, Wang T, et al. Potential influence of menstrual status and sex hormones on female severe acute respiratory syndrome coronavirus 2 infection: a cross‐sectional multicenter study in Wuhan, China. Clin Infect Dis. 2021;72(9):e240‐e248. 10.1093/cid/ciaa1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeo S, Holl K, Penaherrera N, Wissinger U, Anstee K, Wyn R. Burden of male hypogonadism and major comorbidities, and the clinical, economic, and humanistic benefits of testosterone therapy: a narrative review. Clinicoecon Outcomes Res. 2021;13:31‐38. 10.2147/CEOR.S285434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bae S, Kim SR, Kim MN, Shim WJ, Park SM. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID‐19 according to age: a systematic review and meta‐analysis. Heart. 2021;107(5):373‐380. 10.1136/heartjnl-2020-317901 [DOI] [PubMed] [Google Scholar]
- 25. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;31(7):e3319. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID‐19: a systematic review and meta‐analysis. Metabolism. 2020;113:154378. 10.1016/j.metabol.2020.154378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baratchian M, McManus JM, Berk MP, et al. Androgen regulation of pulmonary AR, TMPRSS2 and ACE2 with implications for sex‐discordant COVID‐19 outcomes. Sci Rep. 2021;11(1):11130. 10.1038/s41598-021-90491-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samuel RM, Majd H, Richter MN, et al. Androgen signaling regulates SARS‐CoV‐2 receptor levels and is associated with severe COVID‐19 symptoms in men. Cell Stem Cell. 2020;27(6):876‐889. 10.1016/j.stem.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS‐CoV‐2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19(7). 10.1111/acel.13168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalidhindi RSR, Borkar NA, Ambhore NS, Pabelick CM, Prakash YS, Sathish V. Sex steroids skew ACE2 expression in human airway: a contributing factor to sex differences in COVID‐19? Am J Physiol Lung Cell Mol Physiol. 2020;319(5):L843‐L847. 10.1152/ajplung.00391.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leach DA, Mohr A, Giotis ES, et al. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS‐CoV‐2 in human lung cells. Nat Commun. 2021;12(1):4068. 10.1038/s41467-021-24342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clinckemalie L, Spans L, Dubois V, et al. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol Endocrinol. 2013;27(12):2028‐2040. 10.1210/me.2013-1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin B, Ferguson C, White JT, et al. Prostate‐localized and androgen‐regulated expression of the membrane‐bound serine protease TMPRSS2. Cancer Res. 1999;59(17):4180‐4184. [PubMed] [Google Scholar]
- 35. Montopoli M, Zumerle S, Vettor R, et al. Androgen‐deprivation therapies for prostate cancer and risk of infection by SARS‐CoV‐2: a population‐based study (N = 4532). Ann Oncol. 2020;31(8):1040‐1045. 10.1016/j.annonc.2020.04.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng Q, Rasool RU, Russell RM, Natesan R, Asangani IA. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID‐19. iScience. 2021;24(3):102254. 10.1016/j.isci.2021.102254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffmann M, Hofmann‐Winkler H, Smith JC, et al. Camostat mesylate inhibits SARS‐CoV‐2 activation by TMPRSS2‐related proteases and its metabolite GBPA exerts antiviral activity. bioRxiv. 2020. 10.1101/2020.08.05.237651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhowmick NA, Oft J, Dorff T, et al. COVID‐19 and androgen‐targeted therapy for prostate cancer patients. Endocr Relat Cancer. 2020;27(9):R281‐R292. 10.1530/ERC-20-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mjaess G, Karam A, Aoun F, Albisinni S, Roumeguere T. COVID‐19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol. 2020;30(10):484‐487. 10.1016/j.purol.2020.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patel VG, Zhong X, Liaw B, et al. Does androgen deprivation therapy protect against severe complications from COVID‐19? Ann Oncol. 2020;31(10):1419‐1420. 10.1016/j.annonc.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID‐19: serendipity or opportunity for intervention? Cancer Discov. 2020;10(6):779‐782. 10.1158/2159-8290.CD-20-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klein EA, Li J, Milinovich A, et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS‐CoV‐2. J Urol. 2021;205(2):441‐443. 10.1097/JU.0000000000001338 [DOI] [PubMed] [Google Scholar]
- 43. Welen K, Rosendal E, Gisslen M, et al. A phase 2 trial of the effect of antiandrogen therapy on COVID‐19 outcome: No evidence of benefit, supported by epidemiology and in vitro data. Eur Urol. 2022;81(3):285‐293. 10.1016/j.eururo.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salonia A, Pontillo M, Capogrosso P, et al. Severely low testosterone in males with COVID‐19: a case‐control study. Andrology. 2021;9(4):1043‐1052. 10.1111/andr.12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corona G, Vena W, Pizzocaro A, et al. Andrological effects of SARS‐Cov‐2 infection: a systematic review and meta‐analysis. J Endocrinol Invest. 2022. 10.1007/s40618-022-01801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cinislioglu AE, Cinislioglu N, Demirdogen SO, et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID‐19 disease in male patients: a prospective study. Andrology. 2022;10(1):24‐33. 10.1111/andr.13081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS‐CoV‐2 pneumonia patients. Andrology. 2021;9(1):88‐98. 10.1111/andr.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dhindsa S, Zhang N, McPhaul MJ, et al. Association of circulating sex hormones with inflammation and disease severity in patients with COVID‐19. JAMA Netw Open. 2021;4(5):e2111398. 10.1001/jamanetworkopen.2021.11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marinelli L, Beccuti G, Zavattaro M, et al. Testosterone as a biomarker of adverse clinical outcomes in SARS‐CoV‐2 pneumonia. Biomedicines. 2022;31(4):10. 10.3390/biomedicines10040820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. 10.1016/j.jaut.2020.102452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heffernan DS, Dossett LA, Lightfoot MA, et al. Gender and acute respiratory distress syndrome in critically injured adults: a prospective study. J Trauma. 2011;71(4):878‐883. discussion 883‐5. 10.1097/TA.0b013e31822c0d31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pagano MT, Peruzzu D, Busani L, et al. Predicting respiratory failure in patients infected by SARS‐CoV‐2 by admission sex‐specific biomarkers. Biol Sex Differ. 2021;12(1):63. 10.1186/s13293-021-00407-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Koeverden ID, de Bakker M, Haitjema S, et al. Testosterone to oestradiol ratio reflects systemic and plaque inflammation and predicts future cardiovascular events in men with severe atherosclerosis. Cardiovasc Res. 2019;115(2):453‐462. 10.1093/cvr/cvy188 [DOI] [PubMed] [Google Scholar]
- 56. Infante M, Pieri M, Lupisella S, et al. Low testosterone levels and high estradiol to testosterone ratio are associated with hyperinflammatory state and mortality in hospitalized men with COVID‐19. Eur Rev Med Pharmacol Sci. 2021;25(19):5889‐5903. 10.26355/eurrev_202110_26865 [DOI] [PubMed] [Google Scholar]
- 57. Lazzerini PE, Cantara S, Bertolozzi I, et al. Transient hypogonadism is associated with heart rate‐corrected QT prolongation and torsades de Pointes risk during active systemic inflammation in men. J Am Heart Assoc. 2022;11(1):e023371. 10.1161/JAHA.121.023371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Woolf PD, Hamill RW, McDonald JV, Lee LA, Kelly M. Transient hypogonadotropic hypogonadism caused by critical illness. J Clin Endocrinol Metab. 1985;60(3):444‐450. 10.1210/jcem-60-3-444 [DOI] [PubMed] [Google Scholar]
- 59. Mohamad NV, Wong SK, Wan Hasan WN, et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129‐140. 10.1080/13685538.2018.1482487 [DOI] [PubMed] [Google Scholar]
- 60. Tremellen K, McPhee N, Pearce K, Benson S, Schedlowski M, Engler H. Endotoxin‐initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. 2018;314(3):E206‐E213. 10.1152/ajpendo.00279.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spratt DI, Bigos ST, Beitins I, Cox P, Longcope C, Orav J. Both hyper‐ and hypogonadotropic hypogonadism occur transiently in acute illness: bio‐ and immunoactive gonadotropins. J Clin Endocrinol Metab. 1992;75(6):1562‐1570. 10.1210/jcem.75.6.1464665 [DOI] [PubMed] [Google Scholar]
- 62. Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS‐CoV‐2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J Sex Med. 2021;18(2):256‐264. 10.1016/j.jsxm.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nie X, Qian L, Sun R, et al. Multi‐organ proteomic landscape of COVID‐19 autopsies. Cell. 2021;184(3):775‐791. 10.1016/j.cell.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Enikeev D, Taratkin M, Morozov A, et al. Prospective two‐arm study of the testicular function in patients with COVID‐19. Andrology. 2022;10:1047‐1056. 10.1111/andr.13159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salonia A, Pontillo M, Capogrosso P, et al. Testosterone in males with COVID‐19: a 7‐month cohort study. Andrology. 2022;10(1):34‐41. 10.1111/andr.13097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baldassarri M, Picchiotti N, Fava F, et al. Shorter androgen receptor polyQ alleles protect against life‐threatening COVID‐19 disease in European males. EBioMedicine. 2021;65:103246. 10.1016/j.ebiom.2021.103246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Callewaert L, Christiaens V, Haelens A, Verrijdt G, Verhoeven G, Claessens F. Implications of a polyglutamine tract in the function of the human androgen receptor. Biochem Biophys Res Commun. 2003;306(1):46‐52. 10.1016/s0006-291x(03)00902-1 [DOI] [PubMed] [Google Scholar]
- 68. Toscano‐Guerra E, Martinez‐Gallo M, Arrese‐Munoz I, et al. Recovery of serum testosterone levels is an accurate predictor of survival from COVID‐19 in male patients. BMC Med. 2022;20(1):129. 10.1186/s12916-022-02345-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Manning JT, Fink B. Understanding COVID‐19: digit ratio (2D:4D) and sex differences in national case fatality rates. Early Hum Dev. 2020;146:105074. 10.1016/j.earlhumdev.2020.105074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yeap BB, Marriott RJ, Manning L, et al. Higher premorbid serum testosterone predicts COVID‐19‐related mortality risk in men. Eur J Endocrinol. 2022;187(1):159‐170. 10.1530/EJE-22-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rambhatla A, Bronkema CJ, Corsi N, et al. COVID‐19 infection in men on testosterone replacement therapy. J Sex Med. 2021;18(1):215‐218. 10.1016/j.jsxm.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47‐R71. 10.1530/JOE-12-0582 [DOI] [PubMed] [Google Scholar]
- 73. Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013;217(3):R25‐R45. 10.1530/JOE-12-0455 [DOI] [PubMed] [Google Scholar]
- 74. Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16(7):581‐606. 10.1111/obr.12282 [DOI] [PubMed] [Google Scholar]
- 75. Baillargeon J, Kuo YF, Westra J, et al. Association of testosterone therapy with disease progression in older males with COVID‐19. Andrology. 2022;10(6):1057‐1066. 10.1111/andr.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


