Abstract
Objectives
We describe clinical characteristics, pregnancy, and infant outcomes in pregnant people with laboratory‐confirmed SARS‐CoV‐2 infection by trimester of infection.
Study Design
We analyzed data from the Surveillance for Emerging Threats to Mothers and Babies Network and included people with infection in 2020, with known timing of infection and pregnancy outcome. Outcomes are described by trimester of infection. Pregnancy outcomes included live birth and pregnancy loss (<20 weeks and ≥20 weeks gestation). Infant outcomes included preterm birth (<37 weeks gestation), small for gestational age, birth defects, and neonatal intensive care unit admission. Adjusted prevalence ratios (aPR) were calculated for pregnancy and selected infant outcomes by trimester of infection, controlling for demographics.
Results
Of 35,200 people included in this analysis, 50.8% of pregnant people had infection in the third trimester, 30.8% in the second, and 18.3% in the first. Third trimester infection was associated with a higher frequency of preterm birth compared to first or second trimester infection combined (17.8% vs. 11.8%; aPR 1.44 95% CI: 1.35–1.54). Prevalence of birth defects was 553.4/10,000 live births, with no difference by trimester of infection.
Conclusions
There were no signals for increased birth defects among infants in this population relative to national baseline estimates, regardless of timing of infection. However, the prevalence of preterm birth in people with SARS‐CoV‐2 infection in pregnancy in our analysis was higher relative to national baseline data (10.0–10.2%), particularly among people with third trimester infection. Consequences of COVID‐19 during pregnancy support recommended COVID‐19 prevention strategies, including vaccination.
Keywords: COVID‐19, pregnancy, SARS‐CoV‐2
Abbreviations
- aPR
adjusted prevalence ratio
- CDC
centers for disease control and prevention
- COVID‐19
coronavirus disease 2019
- EDD
expected delivery date
- ICD‐10
international classification of diseases 10th revision
- ICU
intensive care unit
- IQR
inter‐quartile range
- LMP
last menstrual period
- MRA
medical record abstraction
- NICU
neonatal intensive care unit
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SET‐NET
surveillance for emerging threats to mothers and babies network
- SGA
small for gestational age
1. INTRODUCTION
Pregnant people are at increased risk for severe coronavirus disease 2019 (COVID‐19) compared to those who are not pregnant (Allotey, Stallings, Bonet, et al., 2020; Zambrano, Ellington, Strid, et al., 2020). In addition, people with COVID‐19 during pregnancy have an increased risk of adverse pregnancy outcomes, such as preterm delivery and stillbirth, compared to pregnant people without COVID‐19 (Allotey et al., 2020; DeSisto, Wallace, Simeone, et al., 2021). Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) infection during pregnancy may result in stillbirth and has been associated with negative effects in the placenta and conditions such as chronic histiocytic intervillositis, perivillous fibrin deposition, and trophoblast necrosis (Di Girolamo, Khalil, Alameddine, et al., 2021; Watkins, Torous, & Roberts, 2021). While rare, in utero transmission of SARS‐CoV‐2 infection to fetuses has been described, and multiple reports of perinatal or postnatal transmission in neonates have been published (Allotey, Chatterjee, Kew, et al., 2022). The full effects of SARS‐CoV‐2 infection during pregnancy and the biologic mechanisms associated with negative outcomes are still being elucidated.
Most of what is known to date about COVID‐19 in pregnancy comes from surveillance or research cohorts composed primarily of people with infections late in pregnancy (Allotey et al., 2020). Few studies have assessed infection in early pregnancy when disease might impact critical periods of fetal development and growth. For example, fever early in pregnancy is associated with neural tube defects, (Kerr, Parker, Mitchell, Tinker, & Werler, 2017), and congenital infections (e.g., toxoplasmosis, cytomegalovirus, and Zika virus) acquired in the first trimester are more likely to lead to serious birth defects than those acquired later in pregnancy (Megli & Coyne, 2021). Risk of other adverse pregnancy outcomes may also be associated with timing of infection (e.g., risk of hydrops fetalis and pregnancy loss in parvovirus infection(Lamont et al., 2011; March of Dimes, 2006)). Additionally, the timing of infection, as well as the pathogen, can result in varying degrees and patterns of fetal growth restriction (MacDonald & Mary, 2015).
Using data from the Surveillance for Emerging Threats to Mothers and Babies Network (SET‐NET), a population‐based linked longitudinal surveillance cohort, we describe pregnancy and infant outcomes, including birth defects, among pregnant people with laboratory‐confirmed SARS‐CoV‐2 infection by trimester of infection. Additionally, we examined report of COVID‐19 specific treatment among pregnant people with more severe illness.
2. MATERIALS AND METHODS
We report on people with SARS‐CoV‐2 infections during pregnancy from January 25, 2020, to December 31, 2020, and reported by December 3, 2021, from 22 state, local, and territorial health departments participating in SET‐NET. Laboratory confirmed (by polymerase chain reaction [PCR] testing) SARS‐CoV‐2 infections in pregnant people were ascertained through reporting of pregnancy status in COVID‐19 surveillance or through linkages with local data systems (e.g., vital statistics, prenatal screening, administrative data) to determine pregnancy status by jurisdictions (Woodworth et al., 2021). Additional data elements were obtained by jurisdictions through COVID‐19 case report forms, vital statistics, and/or administrative datasets.
Some data elements submitted by the jurisdictions to SET‐NET are obtained through medical record abstraction (MRA). For this analysis, data on maternal disease severity, and maternal treatment were only available for pregnancies with completed MRA. Of the 22 jurisdictions included in this analysis, staff from 12 participating jurisdictions systematically collected medical record data for all pregnancies meeting inclusion criteria, while staff from six jurisdictions used a random sampling approach to identify a subset of pregnancies for MRA. Cases from the 12 jurisdictions are in varying stages of MRA completeness, and all cases from the six sampling jurisdictions have MRA completed. As of December 2021, four jurisdictions were not conducting MRA for any pregnancies and were excluded from the maternal treatment analysis.
Data on birth defects was obtained through MRA or by linking to jurisdiction‐level birth defect surveillance systems; seven jurisdictions did not submit data on birth defects and are excluded from the birth defect analysis. For the analysis of birth defects and maternal treatment, we applied MRA sampling weights to account for selection probability and nonresponse, such that the sampling weights totaled the number of pregnancies meeting the case definition. Additional information on SET‐NET methodology is published elsewhere (Centers for Disease Control and Prevention, 2021a; Woodworth et al., 2021).
Trimester of infection was determined using date of first positive PCR test and calculated date of last menstrual period (LMP). LMP was derived using information submitted by the jurisdiction or calculated from the jurisdiction submitted expected delivery date (EDD) or gestational age at delivery, if LMP was missing. For discrepancies in gestational dating (for gestational age at delivery and gestational age at first PCR positive result that occurred during pregnancy), the following hierarchy based on recommendations from the National Center for Health Statistics and the American College of Obstetricians and Gynecologists(American College of Obstetricians and Gynecologists, 2017; Martin, Osterman, Kirmeyer, & Gregory, 2015) was applied to calculate gestational age: EDD, LMP, or estimate of gestational age from the birth certificate as reported by the jurisdiction. Trimester of infection was based on completed weeks of gestation and defined as: first (0–13 weeks), second (14–27 weeks), or third (28–42 weeks). For selected statistical analyses, stillbirth, preterm birth, and NICU admissions had similar prevalence among pregnancies with first and second trimester infections, so these groups were combined for comparison to third trimester; detailed information by trimester of infection is provided in Supplemental Table S1.
Outcomes were defined as live birth and pregnancy loss (<20 weeks gestation and ≥ 20 weeks gestation [stillbirth]); and among live births, preterm birth (<37 weeks of gestation at delivery), small for gestational age (SGA) (weight < 10% by sex and age per INTERGROWTH‐21st) (Papageorghiou et al., 2018), neonatal intensive care unit (NICU) admission, and birth defects based on ICD‐10 codes from birth hospitalization records for liveborn infants. Stillbirth rate per 100,000 live births and stillbirths were reported for the entire cohort, and a secondary analysis was performed using a subset of five jurisdictions that linked their data to fetal death certificates. In the full cohort and among these five jurisdictions, median gestational age and duration between date of infection and date of stillbirth were examined. Frequency of preterm births was examined after excluding those not at risk for preterm delivery if they had infection ≥37 weeks gestation. Frequencies and weighted prevalence rates per 10,000 live births of selected birth defects overall and by trimester of infection are reported along with relevant population comparators from the literature where available.
COVID‐19 specific treatments (i.e., remdesivir, azithromycin with hydroxychloroquine, hydroxychloroquine, convalescent plasma, monoclonal antibodies, and dexamethasone) (National Institutes of Health, 2021a) administered to pregnant people were described among a subset of pregnant people with moderate‐to‐critical illness. Maternal disease severity was categorized using adapted National Institutes of Health criteria (Galang, Newton, Woodworth, et al., 2021). Illness severity categories were asymptomatic infection, mild, moderate‐to‐severe, critical, and insufficient information. Briefly, criteria were applied to classify severity using available data including symptoms, intensive care unit (ICU) admission, invasive ventilation, use of COVID‐19 therapies, complications associated with COVID‐19, and death. For this analysis, moderate‐to‐severe and critical illness severity categories were combined into a single category, moderate‐to‐critical illness. Treatment analyses incorporated sampling weights to account for jurisdictional MRA approaches as described above.
The analysis included pregnancies with known trimester of primary infection and pregnancy outcome. Since the study population included pregnancies with infection in 2020 prior to COVID‐19 vaccination being available in the U.S., the impact of vaccination was not assessed in this analysis. Using Poisson regression in SAS v 9.4, we calculated adjusted prevalence ratios (aPR) to examine differences in pregnancy and infant outcomes by trimester of infection comparing third trimester infections with first and second trimester infections combined, controlling for maternal demographics (age, race/ethnicity, and health insurance at delivery).
Data were submitted to the Centers for Disease Control and Prevention (CDC) and uploaded into REDCap version 11.1 (Research Electronic Data Capture, Nashville, TN, USA). Analyses were conducted in SAS version 9.4; 95% Confidence Intervals (CI) were used to determine statistical significance. This activity was reviewed by CDC and conducted consistent with applicable federal law and policy (45 C.F.R. part 46, 2018).
3. RESULTS
A total of 44,914 pregnancies with SARS‐CoV‐2 infection were reported as of December 3, 2021, and 35,200 (78.3%) had information on timing of infection and pregnancy outcome included in this analysis (Figure 1). The median age of pregnant people was 29.1 years (interquartile range [IQR]: 23.0–33.2), and 32.2% of pregnant people were Hispanic or Latina and 42.7% were White, non‐Hispanic (Table 1). Half (50.8%) of pregnant people had infection in the third trimester, 30.8% in the second trimester, and 18.3% in the first trimester.
FIGURE 1.
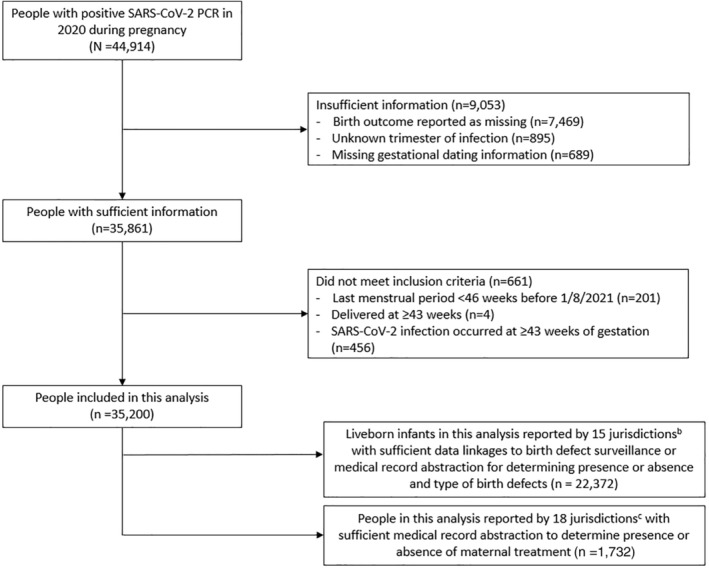
Flow‐chart of pregnant people with SARS‐CoV‐2 infection included in this analysis, SET‐NET, 22 Jurisdictionsa, January 25, 2020–December 31, 2020, reported as of December 3, 2021. (a) 22 jurisdictions included in this analysis are Arkansas, City of Chicago, Georgia, City of Houston, Iowa, Illinois (excluding Chicago), Massachusetts, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Hampshire, New Jersey, New York (excluding New York City), Pennsylvania (excluding Philadelphia), Philadelphia County, Puerto Rico, South Carolina, Tennessee, U.S. Virgin Islands, and Washington. (b) 15 jurisdictions included for birth defects subanalysis are Arkansas, City of Chicago, City of Houston, Iowa, Illinois (excluding Chicago), Minnesota, Missouri, Nebraska, New Jersey, New York State (excluding New York City), Pennsylvania, Philadelphia, Puerto Rico, South Carolina, and Washington. (c) 18 jurisdictions included for COVID‐19 treatment subanalysis are Arkansas, City of Chicago, City of Houston, Illinois (excluding Chicago), Massachusetts, Michigan, Minnesota, Missouri, Nebraska, New Hampshire, New Jersey, New York (excluding New York City), Pennsylvania, Puerto Rico, South Carolina, Tennessee, U.S. Virgin Islands, and Washington.
TABLE 1.
Demographic and pregnancy characteristics, underlying medical conditions, and SARS‐CoV‐2 infection characteristics of pregnant people with a known pregnancy outcome, by trimester of infection, SET‐NET, 22 Jurisdictions, January 25, 2020–December 31, 2020
| Total n (%) | First trimester (<14 weeks) n (%) a | Second trimester (14–27 weeks) n (%) b | Third trimester (28–42 weeks) n (%) | |
|---|---|---|---|---|
| Total | 35,200 | 6,458 (18.3) | 10,848 (30.8) | 17,894 (50.8) |
| Age at infection, years c | 35,193 | 6,457 | 10,846 | 17,890 |
| Median (IQR Q1‐Q3) | 29.1 (23.0–33.2) | 28.8 (25.0–32.6) | 29.4 (25.4–33.2) | 28.9 (24.8–33.3) |
| <20 | 1821 (5.2) | 304 (4.7) | 497 (4.6) | 1,020 (5.7) |
| 20–24 | 6,923 (19.7) | 1,306 (20.2) | 1948 (18.0) | 3,669 (20.5) |
| 25–29 | 10,891 (30.9) | 2,119 (32.8) | 3,411 (31.4) | 5,361 (30.0) |
| 30–34 | 9,715 (27.6) | 1819 (28.2) | 3,162 (29.2) | 4,734 (26.5) |
| 35–39 | 4,742 (13.5) | 747 (11.6) | 1,506 (13.9) | 2,489 (13.9) |
| 40+ | 1,101 (3.1) | 162 (2.5) | 322 (3.0) | 617 (3.4) |
| Unknown | 7 (0.0) | 1 (0.0) | 2 (0.0) | 4 (0.0) |
| Race/ethnicity c | 34,541 | 6,356 | 10,650 | 17,535 |
| Hispanic or Latina | 11,127 (32.2) | 1763 (27.7) | 3,151 (29.6) | 6,213 (35.4) |
| Asian, non‐Hispanic | 1,310 (3.8) | 225 (3.5) | 402 (3.8) | 683 (3.9) |
| Black, non‐Hispanic | 6,143 (17.8) | 1,057 (16.6) | 1829 (17.2) | 3,257 (18.6) |
| White, non‐Hispanic | 14,758 (42.7) | 3,132 (49.3) | 4,906 (46.1) | 6,720 (38.3) |
| Multiple or other d race, non‐Hispanic | 1,203 (3.5) | 179 (2.8) | 362 (3.4) | 662 (3.8) |
| Unknown | 659 (1.9) | 102 (1.6) | 198 (1.8) | 359 (2.0) |
| Health insurance c | 30,306 | 5,482 | 9,232 | 15,592 |
| Private | 13,935 (46.0) | 2,815 (51.3) | 4,621 (50.1) | 6,499 (41.7) |
| Medicaid | 14,787 (48.8) | 2,426 (44.3) | 4,195 (45.4) | 8,166 (52.4) |
| Other e | 917 (3.0) | 173 (3.2) | 278 (3.0) | 466 (3.0) |
| Self‐pay/none | 667 (2.2) | 68 (1.2) | 138 (1.5) | 461 (3.0) |
| Unknown | 4,894 (13.9) | 976 (15.1) | 1,616 (14.9) | 2,302 (12.9) |
| Underlying medical conditions c | 34,256 | 6,360 | 10,527 | 17,369 |
| Any underlying condition | 14,597 (42.6) | 2,499 (39.3) | 4,315 (41.0) | 7,783 (44.8) |
| Obesity (pre‐pregnancy BMI ≥30 kg/m2) | 11,281 (32.9) | 2,152 (33.8) | 3,715 (35.3) | 5,414 (31.2) |
| Chronic lung disease | 1,159 (3.4) | 187 (2.9) | 379 (3.6) | 593 (3.4) |
| Diabetes mellitus | 670 (2.0) | 109 (1.7) | 238 (2.3) | 323 (1.9) |
| Chronic hypertension | 1,621 (4.7) | 327 (5.1) | 587 (5.6) | 707 (4.1) |
| Cardiovascular disease | 401 (1.2) | 51 (0.8) | 114 (1.1) | 236 (1.4) |
| Immunosuppression | 175 (0.5) | 31 (0.5) | 63 (0.6) | 81 (0.5) |
| Other f | 1,312 (3.8) | 200 (3.1) | 378 (3.6) | 734 (4.2) |
| Pregnancy complications c | 6,062 | 1,119 | 1995 | 2,948 |
| Gestational hypertension | 3,025 (9.1) | 578 (9.4) | 975 (9.5) | 1,472 (8.8) |
| Gestational diabetes mellitus | 3,503 (10.6) | 630 (10.2) | 1,172 (11.4) | 1701 (10.2) |
| Prenatal care c | 33,796 | 6,326 | 10,474 | 16,996 |
| Yes (trimester initiated) g | 33,221 (98.3) | 6,241 (98.7) | 10,350 (98.8) | 16,630 (97.8) |
| First trimester | 22,471 (67.6) | 4,151 (66.5) | 7,404 (71.5) | 10,916 (65.6) |
| Second trimester | 5,073 (15.3) | 848 (13.6) | 1,355 (13.1) | 2,870 (17.3) |
| Third trimester | 913 (2.7) | 111 (1.8) | 210 (2.0) | 592 (3.6) |
| Unknown trimester | 4,764 (14.3) | 1,131 (18.1) | 1,381 (13.3) | 2,252 (13.5) |
| No | 575 (1.7) | 85 (1.4) | 124 (1.2) | 366 (2.2) |
| Unknown | 1,404 (4.0) | 132 (2.0) | 374 (3.4) | 898 (5.0) |
Trimester of SARS‐CoV‐2 infection is based on calculated date of last menstrual period and date of first positive COVID‐19 laboratory result.
Participating jurisdictions included Arkansas, City of Chicago, Georgia, City of Houston, Iowa, Illinois (excluding Chicago), Massachusetts, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Hampshire, New Jersey, New York (excluding New York City), Pennsylvania (excluding Philadelphia), Philadelphia County, Puerto Rico, South Carolina, Tennessee, U.S. Virgin Islands, and Washington.
Totals for each of the demographic and pregnancy characteristics do not include “Unknown.”
Other race includes Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, and self‐reported as other.
Other insurance includes Indian Health Service, CHAMPUS or TRICARE; other government (federal, state, or local), or charity.
Other underlying medical conditions includes chronic renal disease, chronic liver disease, psychological/psychiatric condition, disability, and autoimmune condition.
Trimester of prenatal care initiation was derived from the date of first prenatal care visit and calculated last menstrual period.
Among 35,767 (35,191 singleton and 576 multiple gestation) pregnancy outcomes, there were 35,574 (99.5%) liveborn infants and 193 pregnancy losses (0.1% <20 weeks gestation and 0.4% ≥20 weeks gestation) (Table 2). For the full cohort, 0.4% (395 per 100,000 live births and stillbirths) of pregnancies ended in stillbirth (i.e., pregnancy loss that occurred ≥20 weeks of gestation), with no significant difference by trimester of infection (aPR 0.59, 95% CI: 0.34–1.03). The median gestational age at stillbirth was 31 weeks (IQR 23.6–35.8), and the median duration between infection and stillbirth was 18 days (IQR 1–79). In the secondary analysis restricted to five jurisdictions that linked their data with fetal death certificates, findings were similar; 0.5% (57/12,081; 472 per 100,000 live births and stillbirths) of pregnancies ended in stillbirth, with no differences by trimester of infection. Additionally, the median gestational age at stillbirth was 31 weeks (IQR 20.0–36.0), and the median duration between infection and stillbirth was 42 days (IQR 1–105) (data not shown). The prevalence of stillbirths in the general U.S. population was 578 fetal deaths per 100,000 fetal deaths and live births in 2017(Hoyert & Gregory, 2020).
TABLE 2.
Pregnancy, birth, and infant outcomes among pregnancies with SARS‐CoV‐2 infection during by trimester of infection, SET‐NET, 22 Jurisdictions, January 25, 2020–December 31, 2020
| Total n (%) a | First or second trimester infection n (%) b | Third trimester infection n (%) a | Adjusted prevalence ratio c (95% CI) d | |
|---|---|---|---|---|
| Total | 35,767 | 17,615 (49.2) | 18,152 (50.8) | |
| Days from first positive PCR test to pregnancy outcome (median, IQR Q1‐Q3) | 71 (12–147) | 149 (108–199) | 13 (1–43) | |
| Pregnancy outcome e | 35,767 | 17,615 | 18,152 | |
| Live birth | 35,574 (99.5) | 17,470 (99.2) | 18,104 (99.7) | |
| Pregnancy loss | 193 (0.5) | 145 (0.8) | 48 (0.3) | |
| <20 weeks gestation | 52 (0.1) | 52 (0.3) | n/a | |
| ≥20 weeks gestation (stillbirth) | 141 (0.4) | 93 (0.5) | 48 (0.3) | 0.59 (0.34–1.03) |
| NICU admission f , g | 32,319 | 16,200 | 16,119 | |
| Yes | 3,529 (10.9) | 1,655 (10.2) | 1874 (11.6) | 1.13 (1.06–1.21) d |
| Term (≥37 weeks) | 1821 (5.6) | 735 (4.5) | 1,086 (6.7) | 1.29 (1.16–1.36) c |
| Preterm (<37 weeks) | 1708 (5.3) | 920 (5.7) | 788 (4.9) | |
| No | 28,790 (89.1) | 14,545 (89.8) | 14,245 (88.4) | |
| Small for gestational age f | 34,522 | 17,101 | 17,421 | |
| Yes | 1844 (5.3) | 827 (4.8) | 1,017 (5.8) | 1.16 (1.06–1.27) d |
| No | 32,678 (94.7) | 16,274 (95.2) | 16,404 (94.2) | |
| Gestational age h | 27,430 | 17,459 | 9,971 | |
| Term (≥37 weeks) | 23,598 (86.0) | 15,398 (88.2) | 8,200 (82.2) | |
| Preterm (<37 weeks) | 3,832 (14.0) | 2061 (11.8) | 1771 (17.8) | 1.44 (1.35–1.54) d |
| Late preterm (34–37 weeks) | 2,825 (10.3) | 1,410 (8.1) | 1,415 (14.2) | |
| Moderately preterm (28 to <34 weeks) | 831 (3.0) | 475 (2.7) | 356 (3.6) | |
| Very preterm (<28 weeks) | 176 (0.6) | 176 (1.0) | n/a | |
| Unknown | 11 (0.0) | 11 (0.1) | 0 (0.0) |
Abbreviations: CI, confidence interval; IQR, interquartile range.
Participating jurisdictions included Arkansas, City of Chicago, Georgia, City of Houston, Iowa, Illinois (excluding Chicago), Massachusetts, Maryland, Michigan, Minnesota, Missouri, Nebraska, New Hampshire, New Jersey, New York (excluding New York City), Pennsylvania (excluding Philadelphia), Philadelphia County, Puerto Rico, South Carolina, Tennessee, U.S. Virgin Islands, and Washington.
Trimester of SARS‐CoV‐2 infection is based on calculated date of last menstrual period and date of first positive COVID‐19 laboratory result.
Covariates include maternal age, race/ethnicity, and health insurance. For trimester of infection analyses, the reference group is first or second trimester infection.
95% confidence interval excluded 1.0 indicating an association that is statistically significant at the 5% level.
Other pregnancy outcomes (e.g., terminations and nonlive births) were reclassified as pregnancy losses based on gestational age of outcome provided. Pregnancy outcomes include multiple gestations.
Among Live births.
Reason for admission may be for infection control and prevention purposes.
Among those with infection occurring <37 weeks gestation (i.e., could have had a preterm birth), restricted to live births with known gestational age.
Among term infants (≥37 weeks), 5.6% were admitted to the NICU; this was highest among infants born to people with third trimester infection (6.7%, aPR: 1.29, 95% CI; 1.16–1.36). The frequency of SGA was 4.8% of liveborn infants born to persons with first and second trimester infections compared to 5.8% of infants born to persons with third trimester infections (aPR: 1.16, 95% CI; 1.06–1.27).
When restricting to those with infection occurring <37 weeks gestation (i.e., could have had a preterm birth), 14.0% of liveborn infants with known gestational age were born preterm, with the highest frequency among those born to pregnant people with third trimester infection (17.8%) compared to those with first or second trimester infections (11.8%, aPR: 1.44, 95% CI: 1.35–1.54). The overall prevalence of preterm birth in the United States was 10.0% in 2018, 10.2% in 2019 and 10.1% in 2020 (Centers for Disease Control and Prevention, 2022a).
Data regarding birth defects for liveborn infants were reported by 15 jurisdictions with sufficient linkages to birth defect surveillance data or MRA for determining presence of birth defects. Of 22,372 liveborn infants, the overall weighted prevalence of birth defects was 553.4 per 10,000 live births (95% CI: 510.3–596.5). For each specific birth defect with population prevalence estimates available, the weighted prevalence per 10,000 live births observed in this analysis were consistent with the reported ranges in the literature (Table 3). Additionally, no differences in weighted prevalence by trimester of infection were observed for specific defects.
TABLE 3.
Birth defects reported among liveborn infants born to people with SARS‐CoV‐2 infection in pregnancy, by trimester of infection and in comparison, with prevalence estimates from published literature, when available, SET‐NET, 15 Jurisdictions, January 25, 2020–December 31, 2020 (unweighted N = 22,372)
| Trimester of infection a unweighted n (weighted prevalence per 10,000 live births [95% CI]) | Prevalence per 10,000 live births in literature | |||||
|---|---|---|---|---|---|---|
| Total unweighted n b | Total prevalence per 10,000 live births c weighted prevalence (95% CI) | First n = 3,119 | Second n = 5,696 | Third n = 10,651 | ||
| Total | 825 | 553.4 (510.3–596.5) |
88 (434.5 [325.4–543.7]) |
258 (623.0 [531.7–714.3]) |
479 (550.0 [496.3–603.7]) |
— |
| Central nervous system | 31 | 20.9 (11.9–30.0) |
3 (25.8 [0–56.6]) |
14 (27.1 [9.9–44.3]) |
14 (15.8 [5.8–25.9]) |
— |
| Eye | 4 | 2.1 (0–4.3) | 0 | 1 |
3 (3.2 [0–7.1]) |
— |
| Ear | 40 | 26.5 (16.6–36.3) |
6 (30.9 [0.1–61.7]) |
9 [16.4 (5.1–27.8)] |
25 (31.1 [16.4–45.8]) |
— |
| Cardiovascular | 240 | 136.3 (115.6–157.0) |
29 (100.9 [57.2–144.5]) |
93 (191.4 [142.8–239.9]) |
118 (115.3 [91.8–138.7]) |
— |
| Critical congenital heart disease (CCHD) d , e | 26 | 13.5 (7.4–19.6) |
5 (12.8 [1.6–24.1]) |
8 (16.3 [1.5–31.1]) |
13 (12.1 [5.2–19.0]) |
15.6 (95% CI 10.8–15.3) (Reller, Strickland, Riehle‐Colarusso, Mahle, & Correa, 2008) |
| Atrial septal defect | 109 | 58.3 (45.5–71.2) |
10 (43.8 [10.3–77.3]) |
41 (64.5 [42.1–86.9]) |
58 (59.3 [41.7–77.0]) |
64.7 (95% CI 0.0–171.7) (Mai et al., 2015) |
| Pulmonary valve atresia and stenosis | 7 | 3.4 (0.8–6.1) | 0 |
5 (6.9 [0.9–12.9]) |
2 |
9.65 (95% CI 9.38, 9.92) (Mai, Isenburg, Canfield, et al., 2019) |
| Tetralogy of fallot | 6 | 4.2 (0–8.6) | 1 | 1 |
4 (3.2 [0.1–6.4]) |
4.60 (95% CI 4.42, 4.79) (Mai et al., 2019) |
| Ventricular septal defect | 74 | 43.1 (30.8–55.3) |
10 (35.5 [7.0–63.9]) |
23 (49.7 [23.8–75.6]) |
41 (41.6 [26.3–56.8]) |
43.4 (95% CI 10.1–76.6) (Mai et al., 2015) |
| Orofacial | 25 | 15.0 (7.8–22.3) |
4 (12.8 [0–26.2]) |
5 (12.1 [0–26.2]) |
16 (17.5 [7.1–27.8]) |
— |
| Cleft lip with or without cleft palate | 21 | 11.7 (5.8–17.6) |
3 (10.3 [0–22.6]) |
4 (5.5 [0.1–10.9]) |
14 (15.8 [5.8–25.9]) |
10.25 (95% CI 9.97, 10.54) (Mai et al., 2019) |
| Gastrointestinal | 92 | 60.0 (45.7–74.5) |
6 (20.5 [3.1–38.0]) |
31 (76.9 [43.2–110.7]) |
55 (62.7 [44.5–80.7]) |
— |
| Genitourinary | 156 | 86.1 (70.9–101.3) |
16 (58.6 [24.2–93.0]) |
41 (73.7 [46.8–100.5]) |
99 (102.0 [80.3–123.8]) |
|
| Hypospadius f | 29 | 25.9 (16.2–35.5) |
5 (25.6 [3.2–48.1]) |
5 (16.3 [1.3–31.4]) |
19 (31.4 [17.0–45.8]) |
64.7 (95% CI 23.0–106.3) (Mai et al., 2015) |
| Musculoskeletal | 117 | 70.6 (55.1–86.2) |
13 (58.7 [17.7–99.6]) |
39 (93.3 [56.3–130.3]) |
65 (61.2 [45.6–76.7]) |
— |
| Gastroschisis | 4 | 1.7 (0–3.4) | 1 | 1 | 2 | 5.39 (95% CI 5.19, 5.59) (Mai et al., 2019) |
| Polydactyly | 20 | 13.3 (6.2–20.5) | 2 |
7 (21.1 [1.8–40.5]) |
11 (11.3 [4.2–18.4]) |
14.22 g (Kucik, Alverson, Gilboa, & Correa, 2012) |
| Talipes equinovarus (clubfoot) | 27 | 15.1 (8.0–22.3) | 1 |
10 (24.3 [4.4–44.1]) |
16 (13.7 [6.8–20.5]) |
17.07 (95% CI 16.67, 17.48) (Mai et al., 2019) |
| Chromosomal | 24 | 15.3 (7.6–23.1) |
5 (34.1 [0–68.2]) |
7 (14.9 [0.3–29.4]) |
12 (9.7 [4.2–15.1]) |
— |
| Trisomy 21 | 16 | 10.3 (4.0–16.6) |
4 (31.5 [0–65.3]) |
2 |
10 (8.0 [3.1–13.0]) |
14.85 (95% CI 14.52, 15.19) (Mai et al., 2019) |
Trimester of SARS‐CoV‐2 infection is based on calculated date of last menstrual period and date of first positive COVID‐19 laboratory result.
Fifteen jurisdictions with sufficient data linkages to birth defects surveillance or medical record abstraction for determining presence or absence and type of birth defects are Arkansas, City of Chicago, City of Houston, Iowa, Illinois (excluding Chicago), Minnesota, Missouri, Nebraska, New Jersey, New York (excluding New York City), Pennsylvania, Philadelphia, Puerto Rico, South Carolina, and Washington.
Prevalence was calculated when total unweighted case count for a defect was ≥3.
Subcategories may not add to total for the system level categories because there may be additional infrequent defects not individually described.
CCHD included single ventricle, tricuspid atresia, Ebstein anomaly, hypoplastic left heart, hypoplastic right heart, common truncus, transposition, atrioventricular septal defects, tetralogy of Fallot, aortic valve atresia/stenosis, coarctation, total anomalous pulmonary venous return, anomalous coronary artery.
Denominator for calculation of prevalence included only male infants.
Metropolitan Atlanta Congenital Defects Program (MACDP) does not report 95% confidence intervals.
Of 1,732 pregnant people with moderate‐to‐critical illness and sufficient MRA to determine presence or absence of maternal treatment, approximately a quarter (422 cases, 24.4%) were reported to have received treatment, with 265 (15.2%) receiving a COVID‐19 specific treatment (Table 4). The most common COVID‐19 specific treatments for this 2020 cohort were remdesivir (57.0%), dexamethasone (45.8%), and azithromycin with hydroxychloroquine (15.4%). Median age of pregnant people receiving COVID‐19 specific treatments was 31.9 years (IQR: 27.2–35.3), 39.3% were Hispanic or Latina, and 68.7% had an underlying medical condition.
TABLE 4.
Demographic and pregnancy characteristics, underlying medical conditions, and SARS‐CoV‐2 infection characteristics of people with moderate‐to‐critical COVID‐19 illness in pregnancy by treatment status, SET‐NET, 18 Jurisdictions, January 25, 2020–December 31, 2020 (unweighted N = 1,732)
| COVID‐19 specific treatment b (unweighted n = 265) | Non‐COVID‐19 specific treatment (unweighted n = 157) a | No treatment reported c (unweighted n = 1,310) | |
|---|---|---|---|
| Unweighted n weighted % (95% CI) | |||
| Total a | 15.2% (12.9%–17.4%) | 7.7% (6.3%–9.1%) | 77.1% (74.6%–79.6%) |
| Age at infection, years | 265 | 157 | 1,310 |
| Median (IQR Q1‐Q3) | 31.9 (27.2‐35.3) | 30.7 (26.4‐34.8) | 29.6 (25.4‐33.6) |
| <20 | 1.8 (0.6–3.1) | 6.6 (1.3–11.9) | 3.8 (2.5–5.1) |
| 20–24 | 15.5 (7.9–22.9) | 16.2 (8.1–24.3) | 18.5 (15.6–21.4) |
| 25–29 | 21.8 (15.8–27.8) | 28.6 (20.4–36.8) | 30.5 (27.0–34.0) |
| 30–34 | 30.2 (23.2–37.2) | 24.9 (17.5–32.4) | 28.6 (25.4–31.8) |
| 35–39 | 26.1 (18.6–33.6) | 19.6 (12.3–26.8) | 14.8 (12.0–17.6) |
| 40+ | 4.7 (2.1–7.2) | 4.0 (1.5–6.6) | 3.8 (2.6–5.1) |
| Unknown | 0 | 0 | 0 |
| Race/ethnicity | 256 | 151 | 1,282 |
| Hispanic or Latina | 39.3 (31.8–46.8) | 45.6 (36.2–55.0) | 35.7 (32.3–39.0) |
| Asian, non‐Hispanic | 8.4 (4.3–12.3) | 8.1 (2.3–13.9) | 3.7 (2.0–5.4) |
| Black, non‐Hispanic | 18.7 (12.4–24.9) | 17.4 (10.7–24.1) | 14.2 (11.8–16.7) |
| White, non‐Hispanic | 30.4 (21.4–39.5) | 21.3 (13.4–29.1) | 42.7 (39.0–46.4) |
| Multiple or other d race, non‐Hispanic | 3.3 (0.7–5.8) | 7.6 (2.2–13.1) | 3.7 (2.0–5.3) |
| Unknown | 4.1 (1.4–6.8) | 4.0 (0.6–7.4) | 2.0 (1.2–2.8) |
| Health insurance | 251 | 153 | 1,273 |
| Private | 42.3 (34.0–50.7) | 42.9 (33.3–52.5) | 41.6 (37.9–45.3) |
| Medicaid | 53.6 (45.2–62.0) | 50.8 (41.4–60.2) | 54.3 (50.6–58.1) |
| Other e | 2.1 (0.8–3.5) | 5.3 (0.7–10.0) | 2.2 (1.1–3.3) |
| Self‐pay/none | 1.9 (0.7–3.2) | 0.9 (0.0–2.1) | 1.9 (1.0–2.8) |
| Unknown | 4.5 (2.0–7.1) | 1.8 (0.1–3.5) | 2.4 (1.5–3.3) |
| Underlying medical condition f | 68.7 (61.8–75.6) | 59.9 (50.5–69.2) | 57.1 (53.5–60.8) |
| Death | 2.4 (0.7–4.1) | 1.0 (0.0–2.3) | 0.5 (0.1–0.9) |
| Trimester of infection | 265 | 157 | 1,310 |
| First | 5.9 (0.0–11.8) | 8.4 (1.5–15.3) | 12.7 (10.4–15.0) |
| Second | 22.2 (15.5–28.9) | 35.0 (25.9–44.1) | 36.0 (32.4–39.6) |
| Third | 71.9 (64.0–79.8) | 56.6 (47.1–66.1) | 51.3 (47.7–55.0) |
| Timing of infection | 265 | 157 | 1,310 |
| January–June 2020 | 37.4 (30.4–44.5) | 45.0 (35.9–54.0) | 45.0 (41.5–48.5) |
| July–December 2020 | 62.6 (55.5–69.6) | 55.0 (46.0–64.1) | 55.0 (51.5–58.5) |
| Timing of treatment (days after first positive PCR test) | 238 | 133 | — |
| 0–5 | 66.8 (58.8–74.9) | 72.7 (63.2–82.3) | — |
| 6–10 | 30.4 (22.5–38.2) | 12.0 (5.5–18.4) | — |
| ≥11 | 2.8 (0.1–5.6) | 15.3 (6.7–23.8) | — |
| COVID‐19 specific treatments g | [265] | — | — |
| Remdesivir | 57.0 (48.7–65.4) | — | — |
| Dexamethasone | 45.8 (38.2–53.3) | — | — |
| Azithromycin with hydroxychloroquine | 15.4 (11.3–19.5) | — | — |
| Convalescent plasma | 12.4 (7.5–17.3) | — | — |
| Hydroxychloroquine alone | 7.3 (4.6–10.0) | — | — |
| Immunosuppressants | 1.2 (0.0–2.5) | — | — |
| Monoclonal antibodies | 1.1 (0.0–2.6) |
Participating jurisdictions included Arkansas, City of Chicago, City of Houston, Illinois (excluding Chicago), Massachusetts, Michigan, Minnesota, Missouri, Nebraska, New Hampshire, New Jersey, New York (excluding New York City), Pennsylvania (excluding Philadelphia), Puerto Rico, South Carolina, Tennessee, U.S. Virgin Islands, and Washington.
Included remdesivir, dexamethasone, azithromycin with hydroxychloroquine, convalescent plasma, hydroxychloroquine alone, immunosuppressants, and monoclonal antibodies.
Reported as not receiving treatment, missing not included in the analysis.
Other race includes Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, and self‐reported as other.
Other insurance includes Indian Health Service, CHAMPUS or TRICARE; other government (federal, state, or local), or charity.
Underlying medical conditions included obesity, chronic lung disease, diabetes mellitus, chronic hypertension, cardiovascular disease, immunosuppression, renal disease, liver disease, psychological/psychiatric condition, disability, or autoimmune condition. For people with SARS‐CoV‐2 infection in the third trimester, hypertensive disorders of pregnancy and gestational diabetes were also included as underlying medical conditions.
Not mutually exclusive.
Among those receiving COVID‐19 specific treatments, 5.9% were reported to have received treatment in the first trimester, compared to 22.2% and 71.9% among those with second and third trimester infection, respectively. Among those with no treatment reported, 12.7% were infected in the first, 36.0% in the second, and 51.3% in the third trimester (Table 4). Those with first trimester infection were treated a median of 2 days after first positive PCR (IQR 0.0–5.0), while persons with second trimester and third trimester infection were treated a median of 3 days after (IQR 0.0–17.2 and 1.0–6.0 respectively) (data not shown). Two‐thirds (66.8%) of those who had moderate‐to‐critical illness and received COVID‐19 specific treatment were treated within 5 days of a positive PCR test.
4. COMMENTS
4.1. Principal findings
In this population‐based, linked‐longitudinal COVID‐19 surveillance analysis, we did not detect signals for increases in adverse outcomes of stillbirth, small‐for‐gestational age, or birth defects among infants born to people with SARS‐CoV‐2 infection in pregnancy relative to national baseline estimates; however, we observed an increase in preterm birth, NICU admissions, and SGA among infants born to people with infection in the third trimester relative to earlier infection in the first or second trimester. While other reports have described an increased risk of stillbirth associated with COVID‐19, (Allotey et al., 2020; DeSisto et al., 2021) the frequency of stillbirth in this surveillance cohort was not higher than the baseline estimate in the general U.S. population (578 fetal deaths per 100,000 fetal deaths and live births in 2017) (Hoyert & Gregory, 2020) overall or when examined by trimester of infection. This might be due to differences in case ascertainment, inclusion criteria, or circulating SARS‐CoV‐2 variants. Using a comparison population of pregnant persons without COVID‐19 and a longer study period, DeSisto et al. described an increased risk for stillbirth among pregnant people with COVID‐19 documented at delivery during the period of Delta variant predominance during July–September 2021(DeSisto et al., 2021), which was beyond the study period of the current analysis.
While fevers in early pregnancy are associated with certain birth defects, the overall frequency of birth defects in this cohort was not higher than published population estimates of 2–6% (CDC, 2008; Feldkamp, Carey, Byrne, Krikov, & Botto, 2017; March of Dimes, 2006). Direct comparisons to national estimates are challenging because of differences in surveillance methodologies used for birth defects; however, among individual birth defects with a frequency of >3 cases within our cohort, none were reported at a prevalence greater than estimates from the literature. Placental changes have been reported in the context of COVID‐19, including evidence of maternal vascular malperfusion (Shanes et al., 2020) and placentitis, (Watkins et al., 2021) which could impact fetal growth. However, frequency of SGA was not greater than expected by INTERGROWTH‐21st standards (10%), and there were no differences in frequency of SGA by trimester of infection. These findings should be corroborated in other surveillance systems with comprehensive birth defects ascertainment among infants with exposure to COVID‐19 in utero.
In this cohort, 14.0% of pregnancies with infection at <37 weeks resulted in preterm birth, higher than may be expected based on preterm birth rates in the United States in 2018 (10.0%), 2019 (10.2%) and 2020 (10.1%) (Centers for Disease Control and Prevention, 2022a). This was consistent with prior SET‐NET reports and a living systematic review and meta‐analysis findings describing higher rates of preterm birth among pregnant people with COVID‐19 compared to those without COVID‐19 or national estimates (Allotey et al., 2020). A previous analysis of SET‐NET data indicated that, among people with SARS‐CoV‐2 infection during pregnancy, critical COVID‐19 illness was associated with an increased risk of preterm birth compared to those with mild‐to‐severe COVID‐19 illness (Newton, Reeves, Olsen, et al., n.d.). Differences in characteristics between pregnant people with and without SARS‐COV‐2 infection may explain the higher preterm rates in this cohort. (Martin, Osterman, & Valenzuela, 2021)
COVID‐19 in pregnancy is associated with adverse fetal and infant outcomes in addition to risk of severe disease for the pregnant person themselves, necessitating access to safe and effective vaccines, as well as treatment. The National Institutes of Health (NIH) provides COVID‐19 treatment guidelines for adults. The Society for Maternal‐Fetal Medicine and American College of Obstetricians and Gynecologists support the NIH COVID‐19 treatment guidelines and recommends that antiviral and steroid treatment with remdesivir and dexamethasone be offered to pregnant patients with COVID‐19 who require oxygen or mechanical ventilation (National Institutes of Health, 2021b). In this cohort of pregnant people with SARS‐CoV‐2 infection in 2020 (prior to availability of COVID‐19 vaccines), of those with moderate‐to‐critical illness and available treatment data, only 15.2% received a COVID‐19 specific treatment. This is lower than reports in the general population for 2020, (Best et al., 2021; Wiltz, Feehan, Molinari, et al., 2022) and consistent with at least one other study of COVID‐19 treatment among pregnant people. Sekkarie et al. assessed hospitalized COVID‐19 cases and determined that the pregnant patients were less likely to receive remdesivir and systemic steroids when compared to similar hospitalized nonpregnant women (Sekkarie, Whitaker, et al., 2022). It is unclear if lower prevalence of treatment may have been because of limited availability of effective and established treatments, or hesitancy to treat these individuals due to their pregnant status. In addition, fewer pregnant people with first trimester infection were reported to have received treatment compared to those with later infection, and it is unclear if this may represent hesitancy to treat pregnant people due to reproductive health or birth defect concerns. An international observational cohort also described lower frequency of treatment among pregnant people with first trimester infection relative to other trimesters, as well as wide variation in treatment patterns by country (Westhoff, Smith, Wyszynski, & Hernandez‐Diaz, 2022). Pregnant people have historically been excluded from pharmaceutical trials leading to a lack of evidence‐based treatment recommendations. More information is needed regarding the treatment patterns of pregnant people with SARS‐CoV‐2 infection, potential impact on adverse pregnancy outcomes, as well as safety and efficacy in this population; however, this requires large collaborative efforts to pool surveillance cohorts to assess for rare outcomes while appropriately adjusting for disease severity and underlying risk factors.
4.2. Public health implications
COVID‐19 vaccination is recommended for people who are pregnant, breastfeeding, trying to become pregnant now, or might become pregnant in the future, and treatment for COVID‐19, if indicated, should not be withheld from pregnant people (Centers for Disease Control and Prevention, 2021b; National Institutes of Health, 2021b). Data on treatments are evolving, including efficacy and safety in pregnancy. Vaccination rates among pregnant people remain lower than among nonpregnant people as of February 2022 (Centers for Disease Control and Prevention, 2022b) despite accumulating data on the safety and effectiveness of COVID‐19 vaccination during pregnancy (Centers for Disease Control and Prevention, 2021b). Understanding the impact of COVID‐19 throughout pregnancy may help pregnant people or those who may become pregnant, as well as families and communities, to make informed decisions regarding recommended COVID‐19 personal protective practices and vaccination. More research is needed to understand potential impacts of SARS‐CoV‐2 infection in pregnancy on longer term infant outcomes.
4.3. Strengths and limitations
This analysis used a large population‐based cohort from 22 U.S. jurisdictions. In contrast to much of the existing literature, this cohort includes pregnant people with infection early in pregnancy and includes those with asymptomatic infections or mild illness severity. Despite these strengths, the analysis has several limitations. First, pregnancy losses <20 weeks are under ascertained; therefore, our ability to understand the association between infection and early pregnancy loss is limited. Second, stillbirths are also likely under ascertained, and although the analysis restricted to a subset of jurisdictions that linked cases to fetal death certificates yielded results similar to those of the full cohort, there may be differences in reporting of stillbirths by area, which can underestimate the true frequency. Third, some pregnancies in this surveillance cohort are still undergoing medical record abstraction, which affects the completion of clinical variables including birth defects, treatment, and disease severity. Presently, SET‐NET captures ICD‐10 codes and verbatim text of birth defects documented during the birth hospitalization. More robust birth defects surveillance will need to include detailed case finding often up to 1 year of age. Fourth, SET‐NET does not capture indication for treatments, which may lead to misclassification of whether use of specific treatments was for COVID‐19 or some other reason. For example, dexamethasone is a COVID‐19 treatment but is also used for management of preterm birth. As this analysis included pregnant people with moderate‐critical illness, this misclassification is likely low. Additionally, some of the treatments used early in the pandemic and reflected in this study are no longer recommended (e.g., hydroxychloroquine and azithromycin). Fifth, the reason for NICU admission was not reported and might have included admission for isolation and infection prevention and control purposes only, potentially explaining the higher frequency of NICU admission for infants born to pregnant people with third trimester infection. Finally, this analysis included pregnant people with SARS‐CoV‐2 infection confirmed by PCR testing in 2020. Additional surveillance is needed to determine if outcomes differ in subsequent years.
5. CONCLUSIONS
Findings from this large surveillance cohort may help to inform pregnant people and their communities about the risk of COVID‐19 to their pregnancies by timing of infection and inform risk–benefit discussions regarding recommended COVID‐19 prevention strategies, including vaccination. Longitudinal surveillance of infants up to 6 months of age is ongoing to describe the impacts of SARS‐CoV‐2 infection more completely in pregnancy.
AUTHOR CONTRIBUTION
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING INFORMATION
This study was performed as regular work of the Centers for Disease Control and Prevention. This work is supported by the Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement (CK19‐1904) and by contractual mechanisms. University of Iowa reported support by the National Center for Advancing Translational Services of the National Institutes of Health under Award Number UL1TR002537; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
All authors have no conflicts of interest to disclose.
Supporting information
Table S1 Pregnancy, birth, and infant outcomes among pregnancies with SARS‐CoV‐2 infection by trimester of infectiona, SET‐NET, 22 Jurisdictions, January 25, 2020–December 31, 2020
ACKNOWLEDGEMENTS
We thank the staff supporting SET‐NET, including the health departments for data collection and reporting. We thank the following persons for their contributions to this project: Theo Calloway, William Greenfield, Lucille IM, Mallory Jayroe, Rachel Lee Jones, Wendy Nembhard, Alice Rogers‐Johnson, Tabatha M. Scarborough, Arkansas Department of Health; Erika Fuchs, CDC, Nebraska Department of Health and Human Services; Elizabeth Harvey, CDC, Tennessee Department of Health; Beatriz Cabello, Lynn Pistorio, Hillary Spencer, Chicago Department of Health; Cosme Harrison, Jasmine A. McClam, Amber Palmer, Chickasaw Health Consulting; Sashawn Lawrence, Cristina Meza, Victoria Sanon, Anam Syed, Bill Williamson, Georgia Department of Public Health; Dieula Casimyr, Ikram Cheref, Elizabeth Herrera, Syed F. Imam, Salma Khuwaja, Kirstin Short, Houston Health Department; Kimberly Noble Piper, Iowa Department of Public Health; Jane Fornoff, Illinois Department of Public Health; Ademilola Badejo, Aliya Marroquin, Kate Schneider, Maryland Department of Health; Eirini Nestoridi, Hanna Shephard, Mahsa Yazdy, Massachusetts Department of Public Health; Alexandra Gold, Michigan Department of Health and Human Services; Shannon Baack, Kathryn Como‐Sabetti, Ona Loper, Minnesota Department of Health; Tyler Faulkner, Nebraska Department of Health and Human Services; Carolyn Fredette, Katrina Hansen, New Hampshire Department of Health and Human Services; Jamie N. Sommer, Nadia Thomas, Eva M. Williford, New York State Department of Health; Paula Dzimira, Allison Longenberger, Lauren Orkis, Sharon Watkins, Pennsylvania Department of Health; Katie Karen Anderson, My‐Phoung Huynh, Philadelphia Department of Public Health; Nadjarie Aviles Ramos, Mariam Marcano Huertas, Leishla Nieves‐Ferrer, Glorimar Melendez Rosario, Miguel Valencia‐Prado, Puerto Rico Department of Health; Andrew T. Broadway, Brian K. Humphries, Jane M. Kelly, Vinita Leedom, Minna M. Miller, Claire Youngblood, South Carolina Department of Health & Environmental Control; Lindsey Sizemore, Tennessee Department of Health; Leah de Wilde, U.S. Virgin Islands Department of Health; Carrie J. Fall, Soman Puzhankara, University of Iowa College of Public Health; Tiffany Chen, Washington State Department of Health.
Finally, we thank the COVID‐19 Pregnancy and Infant Linked Outcomes Team, the Epidemiology and Surveillance Task Force.
Neelam, V. , Reeves, E. L. , Woodworth, K. R. , O'Malley Olsen, E. , Reynolds, M. R. , Rende, J. , Wingate, H. , Manning, S. E. , Romitti, P. , Ojo, K. D. , Silcox, K. , Barton, J. , Mobley, E. , Longcore, N. D. , Sokale, A. , Lush, M. , Delgado‐Lopez, C. , Diedhiou, A. , Mbotha, D. , … Gilboa, S. M. (2022). Pregnancy and infant outcomes by trimester of SARS‐CoV‐2 infection in pregnancy–SET‐NET, 22 jurisdictions, January 25, 2020–December 31, 2020. Birth Defects Research, 1–15. 10.1002/bdr2.2081
Funding information Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases Cooperative Agreement, Grant/Award Number: CK19‐1904
REFERENCES
- 45 C.F.R. part 46 , 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq, 2018.
- Allotey, J. , Chatterjee, S. , Kew, T. , Gaetano, A., Stallings, E., Fernandez‐Garcia, S., … Thangaratinam, S. (2022). SARS‐CoV‐2 positivity in offspring and timing of mother‐to‐child transmission: Living systematic review and meta‐analysis. BMJ, 376, e067696. 10.1136/bmj-2021-067696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allotey, J. , Stallings, E. , Bonet, M. , Yap, M., Chatterjee, S., Kew, T., … Thangaratinam, S. (2020). Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta‐analysis. BMJ, 370, m3320. 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists . (2017). Committee opinion no 700: Methods for estimating the due date. Obstetrics and Gynecology, 129(5), e150–e154. 10.1097/aog.0000000000002046 [DOI] [PubMed] [Google Scholar]
- Best, J. H. , Kong, A. M. , Kaplan‐Lewis, E. , Brawley, O. W. , Baden, R. , Zazzali, J. L. , … Mohan, S. V. (2021). Treatment patterns in US patients hospitalized with COVID‐19 and pulmonary involvement. Journal of Medical Virology, 93(9), 5367–5375. 10.1002/jmv.27049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Surveillance for Emerging Threats to Mothers and Babies Network, Sampling and Weighting Methods For COVID‐19 End of Pregnancy Medical Record Abstraction, 2021a. https://www.cdc.gov/ncbddd/set-net/documents/set-net-pregnancy-sampling-white-paper-508.pdf
- Centers for Disease Control and Prevention . COVID‐19 Vaccines While Pregnant or Breastfeeding. 2021b. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- Centers for Disease Control and Prevention . National Center for Health Statistics, Birth Data. 2022a. https://www.cdc.gov/nchs/nvss/births.htm
- Centers for Disease Control and Prevention . COVID‐19 Vaccination Coverage and Vaccine Confidence Among Adults. 2022b. https://www.cdc.gov/vaccines/imz‐managers/coverage/covidvaxview/interactive/adults.html
- Centers for Disease Control and Prevention (CDC) . (2008). Update on overall prevalence of major birth defects–Atlanta, Georgia, 1978–2005. Morbidity and Mortality Weekly Report, 57(1), 1–5. [PubMed] [Google Scholar]
- DeSisto, C. L. , Wallace, B. , Simeone, R. M. , Polen, K., Ko, J. Y., Meaney‐Delman, D., & Ellington, S. R. (2021). Risk for stillbirth among women with and without COVID‐19 at delivery hospitalization ‐ United States, march 2020–September 2021. Morbidity and Mortality Weekly Report, 70(47), 1640–1645. 10.15585/mmwr.mm7047e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Girolamo, R. , Khalil, A. , Alameddine, S. , D'Angelo, E., Galliani, E., Matarrelli, B., … D'Antonio, F. (2021). Placental histopathology after SARS‐CoV‐2 infection in pregnancy: A systematic review and meta‐analysis. American Journal of Obstetrics & Gynecology MFM, 6(3), 100468. 10.1016/j.ajogmf.2021.100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp, M. L. , Carey, J. C. , Byrne, J. L. B. , Krikov, S. , & Botto, L. D. (2017). Etiology and clinical presentation of birth defects: Population based study. BMJ, 357, j2249. 10.1136/bmj.j2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galang, R. R. , Newton, S. M. , Woodworth, K. R. , Griffin, I., Oduyebo, T., Sancken, C. L., … Gilboa, S. M. (2021). Risk factors for illness severity among pregnant women with confirmed severe acute respiratory syndrome coronavirus 2 infection‐surveillance for emerging threats to mothers and babies network, 22 state, local, and territorial health departments. Clinical Infectious Diseases, 73(1), S17–s23. 10.1093/cid/ciab432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyert, D. L. , & Gregory, E. C. W. (2020). Cause‐of‐death data from the fetal death file, 2015‐2017. National Vital Statistics Reports, 69(4), 1–20. [PubMed] [Google Scholar]
- Kerr, S. M. , Parker, S. E. , Mitchell, A. A. , Tinker, S. C. , & Werler, M. M. (2017). Periconceptional maternal fever, folic acid intake, and the risk for neural tube defects. Annals of Epidemiology., 27(12), 777–782.e1. 10.1016/j.annepidem.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik, J. E. , Alverson, C. J. , Gilboa, S. M. , & Correa, A. (2012). Racial/ethnic variations in the prevalence of selected major birth defects, metropolitan Atlanta, 1994‐2005. Public Health Reports, 127(1), 52–61. 10.1177/003335491212700106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont, R. F. , Sobel, J. D. , Vaisbuch, E. , Kusanovic, J. P. , Mazaki‐Tovi, S. , Kim, S. K. , … Romero, R. (2011). Parvovirus B19 infection in human pregnancy. BJOG: An International Journal of Obstetrics and Gynaecology, 118(2), 175–186. 10.1111/j.1471-0528.2010.02749.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, M. G. S. , & Mary, M. (2015). Neonatology: Pathophysiology and Management of the Newborn (7th ed.). Philadelphia, PA: Wolters Kluwer Health. [Google Scholar]
- Mai, C. T. , Isenburg, J. , Langlois, P. H. , Alverson, C. J. , Gilboa, S. M. , Rickard, R. , … for the National Birth Defects Prevention Network . (2015). Population‐based birth defects data in the United States, 2008 to 2012: Presentation of state‐specific data and descriptive brief on variability of prevalence. Birth Defects Research. Part A, Clinical and Molecular Teratology, 103(11), 972–993. 10.1002/bdra.23461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai, C. T. , Isenburg, J. L. , Canfield, M. A. , Meyer, R. E., Correa, A., Alverson, C. J., … National Birth Defects Prevention Network. (2019). National population‐based estimates for major birth defects, 2010–2014. Birth Defects Research, 111(18), 1420–1435. 10.1002/bdr2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March of Dimes . March of Dimes Global Report on Birth Defects. 2006. https://www.marchofdimes.org/materials/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabled-children-executive-summary.pdf
- Martin, J. A. , Osterman, M. J. , Kirmeyer, S. E. , & Gregory, E. C. (2015). Measuring gestational age in vital statistics data: Transitioning to the obstetric estimate. National Vital Statistics Reports, 64(5), 1–20. [PubMed] [Google Scholar]
- Martin, J. A. , Osterman, Michelle, J. K. , Valenzuela, Claudia, P. Maternal and Infant Characteristics and Outcomes Among Women with Confirmed or Presumed COVID_19 During Pregnancy: 14 States and the District of Columbia. Vital statistics rapid release. 2021. https://www.cdc.gov/nchs/data/vsrr/VSRR-17.pdf
- Megli, C. J. , & Coyne, C. B. (2021). Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nature Reviews Microbiology, 20, 67–82. 10.1038/s41579-021-00610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health . COVID‐19 Treatment Guidelines, 2021a. https://www.covid19treatmentguidelines.nih.gov/therapies/ [PubMed]
- National Institutes of Health , COVID‐19 treatment guidelines: Special considerations in pregnancy, 2021b. https://www.covid19treatmentguidelines.nih.gov/special‐populations/pregnancy/ [PubMed]
- Newton, S. M. , Reeves, E. L. , Olsen, E. O. , Woodworth, K. R., Farr, S. L., Galang, R. R., … Tong, V. T. (2022). Preterm birth among pregnant persons with severe acute respiratory syndrome coronavirus 2 infection. Journal of Perinatology. 10.1038/s41372-022-01467-6 [DOI] [PMC free article] [PubMed]
- Papageorghiou, A. T. , Kennedy, S. H. , Salomon, L. J. , Altman, D. G. , Ohuma, E. O. , Stones, W. , … International Fetal and Newborn Growth Consortium for the 21(st) Century (INTERGROWTH‐21(st)) . (2018). The INTERGROWTH‐21(st) fetal growth standards: Toward the global integration of pregnancy and pediatric care. American Journal of Obstetrics and Gynecology., 218(2s), S630–s640. 10.1016/j.ajog.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Reller, M. D. , Strickland, M. J. , Riehle‐Colarusso, T. , Mahle, W. T. , & Correa, A. (2008). Prevalence of congenital heart defects in metropolitan Atlanta, 1998‐2005. The Journal of Pediatrics, 153(6), 807–813. 10.1016/j.jpeds.2008.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekkarie, A. , Woodruff, R., Whitaker, M. , Kramer, M. R., Zapata, L. B., Ellington, S. R., … Havers, F. P. (2022). Characteristics and treatment of hospitalized pregnant women with coronavirus disease 2019. American Journal of Obstetrics & Gynecology MFM. 10.1016/j.ajogmf.2022.100715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes, E. D. , Mithal, L. B. , Otero, S. , Azad, H. A. , Miller, E. S. , & Goldstein, J. A. (2020). Placental pathology in COVID‐19. American Journal of Clinical Pathology, 154(1), 23–32. 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, J. C. , Torous, V. F. , & Roberts, D. J. (2021). Defining severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Placentitis. Archives of Pathology & Laboratory Medicine, 145(11), 1341–1349. 10.5858/arpa.2021-0246-SA [DOI] [PubMed] [Google Scholar]
- Westhoff, W. J. , Smith, L. H. , Wyszynski, D. F. , & Hernandez‐Diaz, S. (2022). COVID‐19 pharmacotherapy utilization patterns during pregnancy: International registry of coronavirus exposure in pregnancy (IRCEP). Pharmacoepidemiology and Drug Safety, 31(7), 804–809. 10.1002/pds.5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltz, J. L. , Feehan, A. K. , Molinari, N. M. , Ladva, C. N., Truman, B. I., Hall, J., … Boehmer, T. K. (2022). Racial and ethnic disparities in receipt of medications for treatment of COVID‐19 ‐ United States, march 2020–august 2021. Morbidity and Mortality Weekly Report, 71(3), 96–102. 10.15585/mmwr.mm7103e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth, K. R. , Reynolds, M. R. , Burkel, V. , Gates, C. , Eckert, V. , McDermott, C. , … Gilboa, S. M. (2021). A preparedness model for mother‐baby linked longitudinal surveillance for emerging threats. Maternal and Child Health Journal, 25(2), 198–206. 10.1007/s10995-020-03106-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano, L. D. , Ellington, S. , Strid, P. , Galang, R. R., Oduyebo, T., Tong, V. T., … Meaney‐Delman, D. (2020). Update: Characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status ‐ United States. Morbidity and Mortality Weekly Report, 69(44), 1641–1647. 10.15585/mmwr.mm6944e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Pregnancy, birth, and infant outcomes among pregnancies with SARS‐CoV‐2 infection by trimester of infectiona, SET‐NET, 22 Jurisdictions, January 25, 2020–December 31, 2020


