Abstract
This systematic review aims to present an overview of the current aerosol sampling methods (and equipment) being used to investigate the presence of SARS‐CoV‐2 in the air, along with the main parameters reported in the studies that are essential to analyze the advantages and disadvantages of each method and perspectives for future research regarding this mode of transmission. A systematic literature review was performed on PubMed/MEDLINE, Web of Science, and Scopus to assess the current air sampling methodologies being applied to SARS‐CoV‐2. Most of the studies took place in indoor environments and healthcare settings and included air and environmental sampling. The collection mechanisms used were impinger, cyclone, impactor, filters, water‐based condensation, and passive sampling. Most of the reviewed studies used RT‐PCR to test the presence of SARS‐CoV‐2 RNA in the collected samples. SARS‐CoV‐2 RNA was detected with all collection mechanisms. From the studies detecting the presence of SARS‐CoV‐2 RNA, fourteen assessed infectivity. Five studies detected viable viruses using impactor, water‐based condensation, and cyclone collection mechanisms. There is a need for a standardized protocol for sampling SARS‐CoV‐2 in air, which should also account for other influencing parameters, including air exchange ratio in the room sampled, relative humidity, temperature, and lighting conditions.
Keywords: air sampling, airborne transmission, collection media, impactor, impinge, infectivity
1. INTRODUCTION
1.1. Definition and generation of aerosol
According to the World Health Organization (WHO), airborne transmission can be defined as the spread of an infectious agent caused by the dissemination of aerosols that remain infectious when suspended in air over long distances and time. 1
Infectious aerosols are suspensions of pathogens in particles in the air, with particle size being an important determinant of aerosol behavior. 2 The Infectious Diseases Society of America has two definitions regarding aerosol particles, namely: “respirable” particles (those <10 μm that can deposit in both lower and upper airways) and “inhalable” particles (those 10–100 μm that predominantly deposit in upper airways). 3 , 4 Still, there is significant confusion over the definition and application of relevant terms, such as droplets, droplet nuclei, aerosols, and particles, primarily due to differences between professionals in defining these terms.
Any microorganism, including viruses, can become airborne under specific environmental conditions (that is, being present in aerosolised particles), representing significant health and economic risks to human and animal populations. 5 Virus‐containing aerosols can be released into the environment in two ways: (i) naturally, by sneezing, coughing, breathing, talking, or singing of an individual infected by a respiratory virus or (ii) mechanically, when air currents around contaminated surfaces disperse the viruses into the air for example. 6 The most significant aerosol source representing a risk for human health is the natural generation by other humans, 5 as these aerosols that contain respiratory viruses can be inhaled and deposited in the lower respiratory tract, resulting in disease. 6
However, mechanical generation of aerosols is also important, such as flushing a toilet containing infectious particles, resulting in significant concentrations of airborne viruses. 7 , 8 , 9 , 10 Moreover, viral aerosols can also be produced by wastewater treatment plants 11 , 12 , 13 , 14 and sewage sprinklers. 15 , 16 , 17 , 18 Although the presence of viruses in aerosols has been verified in all of these contexts, the actual risk of infection depends significantly on the stability of the viral particle in question. Viral factors such as whether it is an enveloped or non‐enveloped virus 19 play a major role in the environment's viral stability. Non‐enveloped viruses have been reported to be more stable in the environment because the capsid is more resistant to environmental factors than the envelope of enveloped viruses, 20 which is composed mainly by lipids that can be neutralized more easily by different chemical and physical agents present in the environment. 21 Moreover, viral receptors required for cell entry are usually located on the envelope, 22 which will lose its capacity to enter host cells if the envelope is impaired. In contrast, naked viruses have only the viral capsid, which is made of self‐assembled structural proteins, and are therefore more resistant to these environmental factors such as heat, moisture, pH, UV light, and etc., which suggest they might remain infectious in the environment for longer periods. 23 Other factors such as the mechanism and speed by which the droplets are ejected from the infected person, gravitational settling of respiratory droplets out of the air and onto surfaces, the concentration of viruses in respiratory secretions, the presence of particulates/organic matter, temperature and humidity (that may affect the infectivity of viruses), ventilation, heating, or air conditioning 24 , 25 , 26 also play a role on the actual risk of infection. Factors associated with the exposed person such as distance from infected person, whether exposed and infected person are wearing appropriate masks and the health status of the exposed person, and vaccination status also need to be considered.
1.2. Relevance of airborne transmission in the current SARS‐CoV‐2 pandemic
In March 2020, the WHO declared the coronavirus disease (COVID‐19) as a global pandemic, an infectious disease caused by a newly discovered coronavirus—SARS‐CoV‐2 (severe acute respiratory syndrome coronavirus 2). Since then, it has been shown that regular and thorough hand hygiene, wearing masks, and social distancing are effective ways for preventing SARS‐CoV‐2 infection. 27 , 28 , 29 , 30 However, these measures may not prevent infection by inhaling aerosols exhaled by an infected person that can travel considerable distances in the air and carry their viral content away. 31 , 32
In the beginning, it was thought that SARS‐CoV‐2 transmission occurred through direct, indirect, or close contact with infected people, mostly by droplets and fomites. 1 However, as knowledge about the transmission of SARS‐CoV‐2 is continuously evolving and new evidence accumulates, airborne transmission of SARS‐CoV‐2 started to be considered, being now recognized as a transmission mode of COVID‐19. 33 , 34 According to the WHO, SARS‐CoV‐2 spreads mainly between people who are close to each other (within 1 m), as a susceptible person can be infected when aerosols or droplets containing the virus are inhaled or come directly into contact with the eyes, nose, or mouth. This is thought to happen mainly in poorly ventilated and/or crowded indoor environments, where people tend to spend longer periods. 34
Moreover, airborne transmission seems to be the most probable explanation when considering superspreading events 35 , 36 , 37 , 38 , 39 , 40 , 41 that have occurred mainly in crowded indoor spaces with poor ventilation, 42 higher rates of infection indoors than outdoors and high rates of nosocomial infection among healthcare workers in healthcare facilities worldwide—all supporting the hypothesis that SARS‐CoV‐2's main route of transmission is airborne. 43 , 44
Some studies have detected SARS‐CoV‐2's RNA in air samples, with some of them even detecting viable viruses. 45 , 46 , 47 , 48 This highlights the need for more studies regarding SARS‐CoV‐2 airborne transmission, namely on the stability, concentration, and pathogenicity of SARS‐CoV‐2 upon being subjected to aerosolisation. Knowledge about the size distribution of virus‐laden particles and not only about total suspended particles (TSP) is also important for understanding the risk of airborne transmission 49 as it is the particle size that will determine whether or not it can be inhaled and retained in the respiratory tract, 5 health impact, residence time in ambient air, and the potential for long‐distance transport. 50
This knowledge would directly impact decisions regarding adequate control measures to be implemented for efficient prevention and mitigation of the spread of the virus. 31 , 44 Thus, it is essential to understand the different air sampling methods to collect viruses, each with its particular advantages and disadvantages, as previously reviewed by Verreault et al. 5
To the best of our knowledge, five reviews have been published regarding SARS‐CoV‐2 and/or coronaviruses air sampling. Birgand et al. 51 reported a systematic review assessing air contamination in hospital settings with twenty‐four studies and analyzed the number of studies with RNA detection and infectivity. Rahmani et al. 52 published one mini‐review about air detection methods for coronaviruses in general with eleven studies, reporting the need for more studies to investigate the method's performance to detect SARS‐CoV‐2 viruses in the air. Robotto et al. 53 reviewed the methodological approaches to SARS‐CoV‐2 air sampling and their problems and controversies. Aghalari et al. 54 published a systematic review of an evaluation of SARS‐CoV‐2 transmission through indoor air in hospitals, including 11 studies. Lastly, Dinoi et al., 55 published a systematic review of current knowledge regarding identifying and quantifying SARS‐CoV‐2 RNA in airborne samples comparing indoor and outdoor environments, including 78 articles.
However, no systematic review has been published compiling the studies that have performed air sampling for SARS‐CoV‐2 in indoor and outdoor environments with a detailed description of all methodologies used for air collection with other essential parameters, relating them to detection and infectivity results. Therefore, this systematic review aims to present a compilation of the current aerosol sampling methods (and equipment) being used to investigate the presence of SARS‐CoV‐2 in the air, along with the main parameters reported in the studies (sampling environment/microenvironment, the position of the sampler, air volume sampled, airflow, sampling duration, sampling collection medium, detection, and infectivity) that are essential to analyze the advantages and disadvantages of each method and perspectives for future research regarding this mode of SARS‐CoV‐2 transmission.
2. MATERIALS AND METHODS
This review includes studies published since the emergence of COVID‐19 56 and until December 20, 2021, in the following databases: PubMed/MEDLINE, Web of Science, and Scopus. No language restrictions were imposed during the search.
The following search terms were used “SARS‐CoV‐2,” “aerosol,” “airborne,” “airborne transmission,” “air detection,” “air sampling,” “air sampler,” and “aerosol sampler.” A total of 99 articles were found with potential interest from the initial search, and 36 additional articles were identified through the snowball method. After removing duplicates, 121 articles were screened and had their abstracts appropriately reviewed. After this, articles were selected based on the following criteria: if the study included air sampling to detect SARS‐CoV‐2, the sampling methodology and if it was written in English. Using these criteria, 45 articles were excluded, summarizing 76 articles that were reviewed in detail. Figure 1 shows the flowchart with the number of studies identified and included/excluded following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement. 57
FIGURE 1.
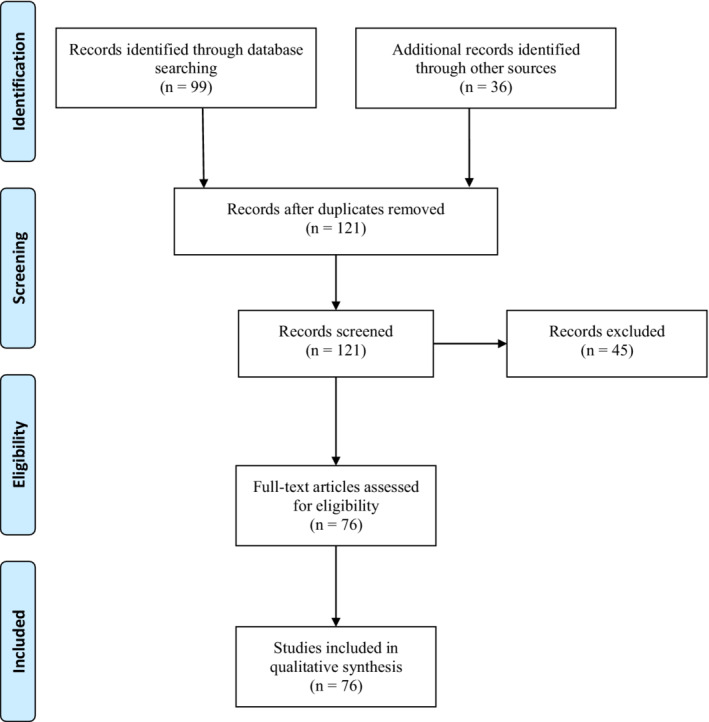
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart
All authors independently screened the databases, and relevant information was extracted. Differences in opinions about whether to include an article were solved by consensus between all the authors.
3. RESULTS
3.1. Location, environments and microenvironments
The geographical distribution of the 76 reviewed studies is represented in Figure 2. The studies were performed in 19 different countries. The majority of the studies were from Asia (China, Iran, Singapore, Kuwait, Israel, Japan, and South Korea), followed by Europe (Italy, Portugal, Germany, France, and Greece) and North America (USA, Canada, and Mexico). In South America, there were 2 studies in Brazil, and there were no studies in Africa or Oceania.
FIGURE 2.
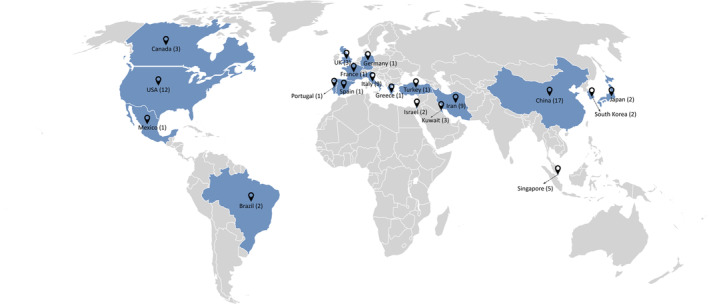
Geographical distribution of the reviewed studies
The main characteristics of the reviewed studies are summarized in Table 1. Most of the studies took place in indoor environments, mainly hospitals (58 studies), especially in COVID‐19 dedicated facilities, such as COVID‐19 wards, nursing stations, intensive care units (ICUs), emergency rooms, computational tomography rooms, staff areas, and toilets. However, other healthcare facilities were also studied, like dental clinics, long‐term healthcare facilities, and homes of infected people. Other indoor settings were also studied, such as shopping centres, post offices, banks, governmental offices, student dormitories, residential rooms, and higher education institutes. Moreover, transports (6), like buses, trains, subways, ferryboats and cruise ships, were also studied. There were also studies outdoors (7) performed in public spaces.
TABLE 1.
Summary of the main characteristics of the reviewed studies
| Reference | Sampling date | Sampling environment: microenvironment | Sampler | Collection mechanism | Volume of air sampled | Airflow rate | Duration of sampling | Sampling collection medium | RNA detected | SARS‐CoV‐2 viability |
|---|---|---|---|---|---|---|---|---|---|---|
| Chang et al. (2020) 2 | December, 2019 | Hospital: airborne infection isolation rooms | SAS Super ISO 180 model 86 834 (VWR International) with modification as previously described Cheng et al. (2019) 156 , Cheng et al. (2019) 157 | Cyclone | 1 m3 | 180 L/min | 5.5 min | Viral transport medium (VTM)a | No | NA |
| Kim et al. (2020) 115 | April, 2020 | Hospital: airborne infection isolation rooms | Airport MD8 (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Gelatin membrane filter | No | NA |
| Di Carlo et al. (2020) 101 | May, 2020 | Non‐healthcare setting: Inside a trolleybus | HE BASIC PLUS (AMS Analitica) | Filter | 18.72 m3 | 24 L/min | 6.5 h | Microbiological gelatin membrane of 80 mm diameter | No | NA |
| Li et al. (2020) 116 | February–March, 2020 | Hospital: intensive care unit (ICU) ward, general isolation wards, fever clinic, storage room for medical waste, conference rooms and the public area | BIO‐Capturer‐6 (Bioenrichment Co.) | Impinger | 2.4 m3 | 80 L/min | 30 min | Phosphate buffered saline (PBS) and magnetic beads | No | NA |
| Lane et al. (2020) 64 | Not mentioned | Hospital: patient room, bathroom, and anteroom | NIOSH BC 251 (NIOSH, CDC), connected with a 6.35‐mm Tygon tubing to an air sampling pump (PCXR‐4, 8SKC) | Cyclone | 1.26 m3 | 3.5 L/min | 6 h | VTM a | No | NA |
| Faridi et al. (2020) 106 | March, 2020 | Hospital: patient rooms | Standard midget impinger (SKC) | Impinger | 0.09 m3 | 1.5 L/min | 1 h | Dulbecco's minimal essential medium (DMEM) with 100 μg/ml streptomycin, 100 U/ml penicillin and 1% antifoam reagent (isoamyl alcohol) | No | NA |
| Ong et al. (2020) 117 | January–February, 2020 | Hospital: airborne infection isolation rooms (in the room, anteroom and outside the room) | Universal pumps with 37‐mm filter cassettes (SKC) | Filter | 1.2 m3 | 5 L/min | 4 h | 0.3‐μm polytetrafluoroethylene (PTFE) filters | No | NA |
| Airport MD8 (Sartorius) | Impactor | And 1.5 m3 respectively | 6 m3/h | 15 min | Gelatin membrane filter | No | NA | |||
| Wei et al. (2020b) 158 | April, 2020 | Hospital: patient's rooms and toilet area | FSC‐1 V (Honri Airclea Technology Co. Ltd.) | Impactor | 1.5 m3 | 100 L/min | 15 min | 0.22‐um pore‐size filter membranes | No | NA |
| Wu et al. (2020) 112 | Not mentioned | Hospital: general isolation wards, intensive care unit (ICU), fever clinic, clinical laboratory, office areas, and restrooms | Petri dishes | Natural sedimentation (Passive sampling) | NA | NA | Not mentioned | Viral transport mediuma | No | NA |
| Ahn et al. (2020) | Not mentioned | Hospital: isolation rooms | BioSampler (SKC) | Impinger | 0.25 m3 | 12.5 L/min | 20 min | Phosphate buffered saline (PBS) | No | NA |
| Swab sampler previously reported by Kim et al. (2019) 154 | Filter | 0.2 m3 | 10 L/min | 20 min | Phosphate buffered saline (PBS) | No | NA | |||
| Cheng et al. (2020) 114 | January–April, 2020 | Hospital: airborne infection isolation rooms | MD8 Airscan (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Sterile gelatin filters (80 mm in diameter and 3 μm pore size, type 17 528‐80‐ACD) | No | NA |
| Nakamura et al. (2020) | January–February, 2020 | Hospital: negative pressure bays, negative pressure room in a general ward and single negative pressure room in an isolation ward. | MD8 Airscan (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Sterile gelatin filters (80 mm diameter and 3 mm pores) | No | NA |
| Wei et al. (2020) 118 | March, 2020 | Hospital: patient rooms, toilets, and a negative pressure room | FSC‐1 V (Honri Airclea Technology Co. Ltd.) with 0.22 μm filter membranes | Impactor | 1.5 m3 | 100 L/min | 15 min | Nutrient agar (Hopebiol) | No | NA |
| Song et al. (2020) 121 | February, 2020 | Hospital: airborne infection isolation rooms | PNS 16 T‐3.1 (Derenda) | Filter | 1.5 m3 | 1.5 m3/h | 1.5 h | 46 mm membrane filter | No | NA |
| Morioka et al. (2020) 122 | Not mentioned | Hospital: negative‐pressure room and its associated bathrooms | MD8 airscan (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Sterile gelatin filters (80 mm diameter and 3‐mm pores) | No | NA |
| Masoumbeigi et al. (2020) 123 | Not mentioned | Hospital: selected wards for COVID‐19 patients, including Emergency, bedridden, ICU, CT‐SCAN and laundry wards | All‐glass impinger (AGI) | Impinger | 0.1‐1 m3 | 5 and 40 L/min | 20 and 15 min | Transmitting mediaa | No | NA |
| Declementi et al. (2020) 124 | 6 | Hospital: COVID‐19 non‐ICU of a Trauma Center | SKC Filter pumps (SKC) | Filter | 90 m3 | 15 L/min | 340 min | 47 mm filter cassettes and 0.45 μm polytetrafluoroethylene (PTFE) filters | No | NA |
| Ong et al. (2020) 117 | January–February, 2020 | Hospital: airborne infection isolation rooms (in the room, anteroom and outside the room) | Universal pumps with 37‐mm filter cassettes (SKC) | Filter | 1.2 m3 | 5 L/min | 4 h | 0.3‐μm polytetrafluoroethylene (PTFE) filters | No | NA |
| Airport MD8 (Sartorius) | Impactor | 1.5 m3 | 6 m3/h | 15 min | Gelatin membrane filter | No | NA | |||
| Lane et al. (2021) 63 | Not mentioned | Hospital: nursing stations in intensive care units (ICUs), family/visitor corridors outside of ICUs, and medical unit patient room hallways | NIOSH BC 251 (NIOSH, CDC), connected with a 6.35‐mm Tygon tubing to an air sampling pump (PCXR‐4, SKC) | Cyclone | 1.26 m3 | 3.5 L/min | 6 h | VTMa (Copan UTM) or phosphate buffered saline (PBS) | No | NA |
| Dumont‐Leblond et al. (2021) 125 | Spring, 2020 | Healthcare facilities: long‐term care facilities | IOM Sampler (SKC) attached to a portable pump Gillian Air 5 (Gillian) | Filter | 0.72 m3 | 3 L/min | 4 h | 3 μm gelatin filters (Sartorius Stedim Biotech) | No | NA |
| Conte et al. (2021) 75 | November–December, 2020 | Non‐healthcare indoor community environments: train station, supermaket, canteen, shopping centre, hair salon, pharmacy | Quartz fiber filters (47 mm diameter) in Skypost PM‐TCR (Tecora), and Zambelli Explorer Plus | Filter | 27.9 and 44.4 m3 | 38.3, 61.7, and 38.3 L/min | 12 h during the day and 12 h during the night | Quartz fiber filters (Whatman, 47 mm in diameter), pre‐fired at 400°C in a muffle furnace | No | NA |
| Vosoughi et al. (2021) 126 | Not mentioned | Hospital: COVID‐19 wards, laboratory, CT, emergency, ICU | An impinger connected to a pump | Impinger | ~1.4 m3 | 28 L/min | ~50–60 min | 15 ml of DMEM, 10 000 units/ml of penicillin, 10 000 μg/ml of streptomycin, and 25 μg/ml of fungizone (amphotericin B) | No | NA |
| Pivato et al. (2021) 69 | February–March, 2020 | Outdoor: public spaces | Quartz filters using low‐volume sampling setting according to the European standard EN 12341:2014 | Filter | 55.2 m3 | 2.3 m3/h | 24 h | Quartz fiber filters (47 mm Ø, Whatman QMA, GE Healthcare) | No | NA |
| Chui et al. (2021) 127 | February–March, 2020 | Non‐healthcare setting: poorly ventilated accommodation room | Coriolis μ (Bertin Technologies) | Cyclone | 9 m3 | 300 L/min | 30 min | VTMa | No | NA |
| Chirizzi et al. (2021) 49 | May, 2020 | Outdoor: public spaces | Skypost PM‐TCR Tecora equipped with a sequential sampler (Charlie) | Filter | 110 m3 | 38.3 L/min | 48 h each sample, total of 6 days | Quartz fiber filters | No | Not studied |
| 110 MOUDI | Impactor | 259.2 m3 | 30 L/min | 6 days | Quartz fiber filters | No | Not studied | |||
| SWAM 5a Dual Channel Monitor (FAI In‐struments) | Impactor | 328.32 m3 | 38 L/min | 6 days | Quartz fiber filters | No | Not studied | |||
| 120 MOUDI‐II™ | Impactor | 259.2 m3 | 30 L/min | 6 days | Quartz fiber filters | No | Not studied | |||
| Razzini et al. (2020) 128 | May, 2020 | Hospital: COVID‐19 ward | Airport MD8 (Sartorius) | Impactor | 2 m3 | 50 L/min | 40 min | Gelatin membrane filter | Yes | Not studied |
| Setti et al. (2020) 71 | February–March, 2020 | Outdoors: industrial area of Bergamo (Italy) | Low‐volume gravimetric air samplerb, compliant with the reference method EN12341:2014 for PM10 monitoring | Filter | 55 m3 | 38.3 L/min | 24 h | Quartz fiber filters | Yes | Not studied |
| Ge et al. (2020) 62 | Not mentioned | Hospital: hemodialysis room, general fever clinic, COVID‐19 respiratory investigation ward, ward of confirmed intensive care patients | NIOSH BC 251 (NIOSH, CDC) with air pumps (XR5000, SKC) | Cyclone | 0.105 m3 | 3.5 L/min | 30 min | Minimal essential medium (MEM) containing 1% bovine serum albumin | Yes | Not studied |
| Liu et al. (2020) 77 | February–March, 2020 |
Hospital: patient areas, medical staff areas, pharmacy, outpatient hall and outdoors. Other public and private areas: community checkpoint, residential building, supermarket |
25 mm diameter filters loaded into styrene filter cassettes (SKC) using a portable pump (APEX2, Casella) | Filter | 1.5–6.0 m3 | Not mentioned | 5–20 h, and 7 days for deposition samples | Presterilized gelatin filters with pore size 3 μm (Sartorius) | Yes | Not studied |
| Miniature cascade impactor (Sioutas Impactor, SKC) loaded with four impaction stages | Impactor | 8.9 m3 | Not mentioned | 5–20 h | Presterilized gelatin filters with pore size 3 μm (Sartorius) | Yes | Not studied | |||
| Aerosol deposition (passive sampling) | Natural sedimentation (Passive sampling) | NA | NA | 7 days | Not mentioned | Yes | Not studied | |||
| Kenarkoohi et al. (2020) 129 | May, 2020 | Hospital: ICU, ICU entrance hall, hospital entrance hall, laboratory ward, CT scan, radiology, men and woman internal ward, and emergency ward | Liquid‐phase sampler (SKC) | Impinger | 2.16 m3 | 12 L/min | 3 h | 13.38 g/L of DMEM, 1.50 g/L NaHCO3, 2 g/L bovine serum albumin with 100 μg/ml streptomycin, 100 U ml penicillin and 970 ml distilled water | Yes | Not studied |
| Chia et al. (2020) 59 | Not mentioned | Hospital: airborne infection isolation rooms | NIOSH BC 251 (NIOSH, CDC) | Cyclone | 5.04 m3 | 3.5 L/min | 4 h | Not mentioned | Yes | Not studied |
| 37 mm PTFE filter cassettes connected to universal air sampling pumps (SKC) | Filter | 1.2 m3 | 5 L/min | 4 h | 37 mm PTFE filter | No | Not studied | |||
| Lei et al. (2020) 66 | Not mentioned | Hospital: ICU and isolation wards | NIOSH BC 251 (NIOSH, CDC) | Cyclone | 0.84 m3 | 3.5 L/min | 4 h | VTMa | Yes | Not studied |
| WA‐15 (Beijing DingBlue Technology) | Cyclone | 0.42 m3 | 14 L/min | 30 min | VTMa | Yes | Not studied | |||
| Mouchtouri et al. (2020) 130 | Not mentioned | Non‐healthcare setting: Ferryboat | Airport MD8 (Sartorius) | Impactor | 0.5 m3 | 50 L/min | 10 min | Gelatin membrane filters of 80 mm diameter (Sartorius 17 528–80‐ ACD). Filters were the placed in a 50 ml conical tube filled with ¼ strength Ringer's solution | Yes | Not studied |
| Guo et al. (2020) 131 | February–March, 2020 | Hospital: intensive care unit (ICU) and a general COVID‐19 ward | SASS 2300 (Research International) | Cyclone | 9 m3 | 300 L/min | 30 min | VTM a | Yes | Not studied |
| Santarpia et al. (2021) 76 | April, 2020 | Hospital: acuity wards | NIOSH BC 251 (NIOSH, CDC) | Cyclone | 0.1 m3 | 3.5 L/min | 30 min | Gelatin filter (Sartorius, GmbH) | Yes | Yes |
| Jin et al. (2021) 132 | March, 2020 | Hospital: isolation room and staff personal protective equipment (PPE) dressing room in the intensive care unit (ICU) | WA‐400 (DingBlue Technology) | Impinger | 6 m3 | 400 L/min | 15 min | VTMa | Yes | Not studied |
| Feng et al. (2021) 61 | February–March, 2020 | Hospital: isolation wards | NIOSH BC 251 (NIOSH, CDC) | Cyclone | 0.105 m3 | 3.5 L/ min | 30 min | Not mentioned | Yes | Not studied |
| Hadei et al. (2021) 133 | June–July, 2020 | Non‐healthcare setting: Public places and public transport: bank, shopping center, post office, airport, subway station, subway train, bus | Universal PCXR4 SKC pump (SKC) with filter | Filter | From 0.2 to 0.24 m3 | 3.5 L/min | 1–1.5 h, depending on the site's limitations | Glass fiber filters (diameter = 2 cm) | Yes | Not studied |
| AV1000 sampler (China) | Filter | From 1.27 to 3.496 m3 | 40 L/min | 1–1.5 h, depending on the site's limitations | PTFE filters (diameter = 9 cm, pore size = 0.2 μm) | Yes | Not studied | |||
| Zhou et al. (2021) 105 | Not mentioned | Hospital: corridors, hospital waste storage rooms, intensive care unit (ICU) rooms, toilets, medical preparation rooms, clinical observation rooms, and general wards | WA‐15 (Dinglan Technology) | Impinger | 0.6 m3 | 15 L/min | 40 min | Virus collection liquid (Jiangsu Kangjian Medical Supply) | Yes | Not studied |
| WA‐400 (Ding Blue Technology) | Impinger | 16 m3 | 400 L/min | 40 min | Virus collection liquid (Jiangsu Kangjian Medical Supply) | Yes | Not studied | |||
| Passos et al. (2021) 78 | March, 2020 | Hospitals: COVID‐19 dedicated facilities and non‐COVID‐19 areas | Portable low flow sampler (CRIFFER) | Filter | 1.1–96 m3 | 2.5 L/min | Variable, depending on the location | 37 mm diameter filter loaded into a styrene filter cassette (SKC) | No | Not studied |
| AIR IDEAL® 3P® (bioMérieux) | Impactor | 2 m3 | Not mentioned | Variable, depending on the location | 65 mm diameter filter on an adapted perforated plate inside | Yes | Not studied | |||
| Airport MD8 (Sartorius) | Impactor | 2 m3 | Not mentioned | Variable, depending on the location | 80 mm diameter gelatin filter | No | Not studied | |||
| HANDI‐VOL (Energética) | Filter | Not mentioned | 150 L/min | Variable, depending on the location | 100 mm diameter filter | No | Not studied | |||
| 821 T (Fisatom) | Filter | 1.1–96 m3 | 18 L/min | Variable, depending on the location | 37 mm diameter filter | No | Not studied | |||
| Petri dishes | Natural sedimentation (Passive sampling) | NA | NA | ~1 week | 90 mm diameter quartz microfiber filter (ϕ = 0.3 μm, Whatman®) | Yes | Not studied | |||
| Non‐healthcare setting: sidewalks near the hospitals, outdoor outpatient hall, open car parking near hospitals and at a bus station | HVS (AGV, Energética) | Impactor | 1.1 m3–96 m3 | 1130 L/min | Variable, depending on the location | 100 mm diameter filter | No | Not studied | ||
| Ding et al. (2021) 134 | February, 2020 | Hospital: 4 isolation rooms, a nursing station, a corridor, an air‐conditioning system, and other spaces in the airborne infectious‐disease zone. | Andersen one‐stage viable impactor – QuickTake‐30 (SKC) | Impactor | 0.3 m3 | 10 L/min | 30 min | Gibco cell culture medium | No | Not studied |
| Airport MD8 (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Water‐soluble gel film | No | Not studied | |||
| ASE‐100 (Langsi Medical Technology) | Not mentioned | 10 m3 | 500 L/min | 20 min | Biological aerosol special‐collection liquid | Yes | Not studied | |||
| WA‐15 (Dinglan Technology) | Impinger | 0.42 m3 | 14 L/min | 30 min | Youkang virus‐sampling kit | No | Not studied | |||
| Amato‐Lourenço et al. (2021) 135 | September–October, 2020 | Hospital: COVID‐19 ward and ICU, non‐COVID‐19 ICU and autopsy room. | MiniVol™ TAS (Air Metrics, Innovative Air Sampling Equipment) | Filter | 2.4 m3 | 5 L/min | 8 h | Polycarbonate filters of 47‐mm diameter and 0.4‐μm pores (Millipore®) | Yes | Not studied |
| López et al. (2021) 67 | Not mentioned | Hospital: Emergency area, Internal medicine, COVID area and COVID‐19 patients care room. | Filters of 25 mm diameter with 0.22 mm pores (Millipore, AAWP02500), placed in a sterilized filter holder (Millipore, SWINNX) coupled to a vacuum system | Filter | 1.7 m3 | 9.6 L/min | 3 h | Filters of 25 mm diameter with 0.22 mm pores | Yes | Not studied |
| Habibi et al. (2021) 73 | Not mentioned | Hospital: waiting area near pharmacy, main entrance, pediatric casualty, laboratory, COVID isolation area, COVID ward, Cytology laboratory, Virology laboratory, corridor, reception area; Non healthcare setting: outdoor. | A specialized sampler developed for this purpose which utilizes a variable speed suction pump (Tisch, Environmental International) with a flow controller that allows air to pass through 3 gas wash bottles Habibi et al. 73 | Impinger | 3.6 m3 | 30 L/min | 2 h | TRIzol® | Yes | Not studied |
| Ma et al. (2021) 103 | Not mentioned | Hospital and quarantine hotel environments: corridor, hotel room, hospital CT room, ICU, toilet, Emergency room, clinical observation room, and hospital ward. | WA‐15 (Dinglan Technology) | Impinger | 0.6 m3 | 15 L/min | 40 min | 3 ml virus culture liquid (MT0301) (Yocon Biology Inc.) | Yes | Not studied |
| WA‐400 (DingBlue Technology) | Impinger | 16 m3 | 400 L/min | 40 min | 3 ml virus culture liquid (MT0301) (Yocon Biology Inc.) | No | Not studied | |||
| Stern et al. (2021) 50 , 72 | April–May, 2020 | Hospital dedicated to COVID‐19: negative and ambient‐pressure ICU, rooms of symptomatic and asympromatic patients, nurses' workstation and lockers' room, bathrooms, observation rooms and outside gates. | Custom‐designed Harvard Micro‐Environmental Cascade Impactor Demokritou et al. (2002) 155 | Impactor | 14.4 m3 | 5 L/min | 48 h | Large particles (>10.0 μm) and coarse particles (10.0–2.5 μm) are collected on polyurethane foam (PUF) impaction substrates. Fine particles (≤2.5 μm) are collected on a 37‐mm glass fiber filter | Yes | Not studied |
| Hemati et al. (2021) 136 | July–August, 2020 | Hospital: infectious wards, ICU, pediatric ward, radiology, CT scan, emergency ward, respiratory patients' clinic, laundry, toilet of COVID‐19 patients, and PPE rooms of staff. | Standard midget impinger (SKC) | Impinger | 0.48 m3 | 2 L/min | 4 h | VTM a | Yes | Not studied |
| Baboli et al. (2021) 137 | July–August, 2020 | Hospital: COVID‐19 infectious and ICU wards. | Standard Petri dishes with 8 mm diameter | Natural sedimentation (passive sampling) | NA | NA | 30 min | 5 ml of DMEM or 5 ml normal saline liquid | Yes | Not studied |
| Glass impinger (AGI) connected to SKC universal air pump | Impinger | 0.12 m3 | 4 L/min | 30 min | 5 ml of DMEM or normal saline liquid | Yes | Not studied | |||
| SKC universal air pump with 37 mm and 0.3 μm pore size PFTE filter | Filter | Not mentioned | 4 L/min | NA | 5 ml of DMEM or normal saline liquid | Yes | Not studied | |||
| Quick Take 30 pump kit (SKC) | Filter | Not mentioned | 4 L/min | NA | 5 ml of DMEM or normal saline liquid | Yes | Not studied | |||
| Moreno et al. (2021) 138 | May–July, 2020 | Non‐healthcare setting: Public transports (buses and subway trains) | 47 mm Teflon filters with PEM (Personal Environmental Monitor) | Filter | ~55.4 m3 | 10 L/min | 9–10 h | 47 mm Teflon filters | Yes | Not studied |
| Semelka et al. (2021) 139 | April–May, 2020 | Hospital: COVID‐19 patient rooms | Andersen air sampler | Impactor | Not mentioned | Not mentioned | Not mentioned | VTMa | Yes | Not studied |
| Liu et al. (2021) 74 | February–March, 2020 | Hospital: contaminated areas including ICU wards, general wards, clinical lab, radiological examination, and clean area. | WB‐15 (DINGBLUE TECH) | Based on the combination of cyclone separation and impact | 0.56 m3 | 14 L/min | 40 min | Virus protection solution a | Yes | Not studied |
| Capeyron et al. (2021) 140 | April–May, 2021 | Hospital: COVID‐19 patients' rooms | AIR IDEAL® 3P® (bioMérieux) | Impactor | 0.5 m3 | Not mentioned | 5 min | 90‐mm Muellere Hinton agar Petri dish | Yes | Not studied |
| Ang et al. (2021) 141 | Not mentioned | Hospital: COVID‐19 isolation ward and open‐cohort wards. | SASS 3100 air samplers (Research International) | Filter | 24 and 72 m3 | 50 and 150 L/min | 8 h | 44 mm diameter SASS bioaerosol filter (polyester, Research International) with two different pore sizes. | Yes | Not studied |
| Stern et al. (2021) 50 , 72 | April–May, 2020 | Hospital: outside negative‐pressure COVID‐19 wards, hospital ward not involved in COVID‐19 patient care, and emergency department | Custom‐designed Harvard Micro‐Environmental Cascade Impactor Demokritou et al. (2002) | Impactor | 14.4 m3 | 5 L/min | Not mentioned | Large particles (>10.0 μm) and coarse particles (10.0–2.5 μm) are collected on polyurethane foam (PUF) impaction substrates. Fine particles (≤2.5 μm) are collected on a 37‐mm glass fiber filter | Yes | Not studied |
| Ghaffari et al. (2021) 63 | November–December, 2020 | Hospital: four sections of ICU including the patient section, nurse station, rest room and doorway. | ESPS LVM Model (Fanpava) | Filter | 24.04 m3 | 16.7 L/min | 24 h | PTFE membrane filters with a pore size of 0.3 μm | Yes | Not studied |
| Bazzazpour et al. (2021) 142 | August–December, 2020 | Healthcare facility: dental clinic | AV1000 sampler (China) | Filter | 6.96 m3 | 30–58 L/ min | 1–2 h | PTFE, diameter = 9 cm, pore size = 0.2 μm | Yes | Not studied |
| Sousan et al. (2021) 143 | April, 2021 | Non‐healthcare setting: Student dormitories | Filter cassettes (SKC) | Filter | 7.2 m3 | 5 L/min | 24 h | 37‐mm filters | No | Not studied |
| Button sampler (SKC) | Filter | 5.76 m3 | 4 L/min | 24 h | 25‐mm filters | Yes | Not studied | |||
| BioSampler (SKC) | Impinger | 0.37–1.12 m3 | 12.5 L/min | 30–90 min | PBS solution | Yes | Not studied | |||
| AerosolSense sampler (ThermoFisher Scientific) | Filter | 288 m3 | 200 L/min | 24 h | Sample cartridge | Yes | Not studied | |||
| Di Carlo et al. (2021) 101 | April–June, 2020 | Hospital: isolation room | MD8 Airscan (Sartorius) | Impactor | 1.5 m3 | 50 L/min | 30 min | Gelatin membrane filter (80 mm diameter) | Yes | Not studied |
| Akin et al. (2021) 144 | Not mentioned | Healthcare facility: dental clinic | Glass petri dishes | Natural sedimentation (Passive sampling) | NA | NA | Not mentioned | 3 ml of VTMa | Yes | Not studied |
| Nannu Shankar et al. (2022) 145 | Not mentioned | Non‐healthcare setting: residential rooms of infected people | Airport MD8 (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Gelatin membranes (3.0 mm filtration cut‐off) | Yes | Not studied |
| Silva et al. (2022) 146 | January–February, 2020 | Hospital: COVID‐19 areas including ICU, intermediate ICU, nursing area and testing room, and non‐COVID‐19 areas including respiratory diseases observation room, waiting room, clinical decision unit, urgent care (recovery), entrance atrium and staff cafeteria. | Coriolis μ (Bertin Technologies) | Cyclone | 1 m3, 2 m3, 3 m3 | 100, 200 and 300 L/min | 10 min | 4 ml of PBS | Yes | Not studied |
| Coriolis Compact (Bertin Technologies) | Cyclone | 3 m3 | 50 L/min | 60 min | 4 ml of PBS | Yes | Not studied | |||
| Dunker et al. 60 | Not mentioned | Outdoor: public spaces | Volumetric pollen trap (Hirst‐type trap, Burkhard Manufacturing Co Ltd.) | Other: volumeric pollen trap | ~216 m3 | ~10 L/min | 15 days | 200 μl D‐PBS‐buffer (w/o calcium, w/o magnesium; Biowest) | No | Not studied |
| Cyclone trap (Burkhard Manufacturing Co Ltd.) | Cyclone | ~151.2 m3 | ~15 L/min | 7 days | 200 μl D‐PBS‐buffer (w/o calcium, w/o magnesium; Biowest) | No | Not studied | |||
| Moore et al. (2021) 147 | March–May, 2020 | Hospital: negative pressure isolation rooms, neutral pressure side rooms, ICU, high‐dependency unit (HDU) open cohorts and non‐ICU cohort bays | Coriolis μ (Bertin Technologies) | Cyclone | 3 m3 | 300 L/min | 10 min | 15 ml RNase‐free PBS | Yes | Not studied |
| Airport MD8 (Sartorius) | Impactor | 0.5 m3 | 50 L/min | 10 min | Gelatin membrane filter | No | Not studied | |||
| Santarpia et al. (2020) 48 | Not mentioned | Hospital: patient rooms and hallways of the Biocontainment Unit and Quarantine Unit | Airport MD8 (Sartorius) | Impactor | 0.75 m3 | 50 L/min | 15 min | 80 mm gelatin filters | Yes | Evidence |
| Personal Button Samplers (SKC) with Air Check pumps (SKC) | Filter | 0.06 m3 | 4 L/min | 15 min | 25 mm gelatin filters | Yes | No | |||
| Lednicky et al. (2020) 45 | Not mentioned | Hospital: room of a COVID‐19 ward | A prototype VIVAS Lednicky et al. (2020) 79 ; Lednicky et al. (2020) 45 ), and a BioSpot‐VIVAS BSS300P, which is a commercial version of the VIVAS (Aerosol Devices.) | Water‐based condensation | 1.44 m3 | 8 L/min | 3 h | 1.5 ml of 1× PBS with 0.5% (w/v) bovine albumin fraction V and a final concentration of 0.2 M sucrose | Yes | Yes |
| Lednicky et al. (2021) 47 | Not mentioned | Hospital Environment | VIVAS (Aerosol Devices) | Water‐based condensation | 0.93 m3 | 6.5 L/min | 1 h | 1.5 ml of 1x PBS with 0.5% (w/v) bovine albumin fraction V and a final concentration of 0.2 M sucrose | Yes | Yes |
| Dumont‐Leblond et al. (2020) 148 | Not mentioned | Hospital: an adapted ward dedicated to patients with non‐severe COVID‐19 | IOM Sampler (SKC), connected to the medical vacuum using a regulator (Genstar Technologies) | Filter | 2.4 m3, 3.6 m3, 10.8 m3 | 10 L/min | 4, 6, or 18 h | 3 μm gelatin filters (Sartorius), solubilized in VTM or DMEM +10% FBS | Yes | No |
| 37 mm cassette with 0.8 μm polycarbonate filters (PC; SKC), connected to the medical vacuum using a regulator (Genstar Technologies) | Filter | 2.4 m3, 3.6 m3, 10.8 m3 | 10 L/min | 4, 6, or 18 h | 2 ml or 3 ml of VTM directly introduced in the 37 mm cassettes with a transfer pipet and ejected by the pressurizing action of a 50 ml empty syringe at the opposite opening of the cassette | Yes | No | |||
| SASS 3100 (Research International) | Filter | 4.5 m3 | 300 L/min | 15 min | SASS® 3010 particle extrator with 5 ml of extraction buffer (138 mM sodium chloride, 2.7 mM potassium chloride, 0.05% Triton X‐100, <0.1% sodium azide, 10 mM sodium phosphate) | No | No | |||
| Yamagishi et al. (2020) 149 | February, 2020 | Non‐healthcare setting: cabins in a cruise ship | Airport MD8 (Sartorius) | Impactor | 1 m3 | 50 L/min | 20 min | Special gelatin filter (type 175, Sartorius; T1 phage capture rate, 99.99%; effective filtration area, 38.5 cm2) | No | No |
| Binder et al. (2020) 58 | April–May, 2020 | Hospital: COVID‐19 patients' rooms | NIOSH BC 251 (NIOSH, CDC) | Cyclone | 0.84 m3 | 3.5 L/min | 4 h | Not mentioned | Yes | No |
| Ben‐Shmuel et al. (2020) 150 | Not mentioned | Hospital: COVID‐19 isolation units | MD8 Airscan (Sartorius) | Impactor | 1 m3 | 50 L /min | 20 min | Gelatine membranes (3.0 mm filtration cut‐off) | Yes | No |
| Ong et al. (2021) 68 | Not mentioned | Hospital: airborne‐infection isolation rooms and a community isolation facility | BioSpot‐VIVAS BSS300‐P (Aerosol Devices) | Water‐based condensation | Not mentioned | 8 L/min | Not mentioned | Liquid collection mediuma | Yes | No |
| NIOSH BC 251 (NIOSH, CDC), connected to SKC AirChek TOUCH Pumps (SKC) | Cyclone | Not mentioned | Not mentioned | Not mentioned | Liquid collection mediuma | Yes | No | |||
| Zhou et al. (2021) 151 | April, 2020 | Hospital: emergency department, admissions ward, COVID‐19 cohort wards, theaters during tracheostomy procedures, ICU, a 6‐bedded bay converted into a negative pressure area for management of continuous positive airway pressure on patients with COVID‐19, and a public area of the hospital. | Coriolis μ (Bertin Technologies) | Cyclone | 1 m3 | Not mentioned | Not mentioned | 5 ml of DMEM | Yes | No |
| Barbieri et al. (2021) 152 | June, 2020 | Hospital: main corridor of COVID‐19 ward | SILENT Air Sampler (FAI Instruments S.r.l.) on quartz filters | Filter | 14.4 m3 | 10 L/min | 24 h | Quartz fiber filters (prefired 47 mm diameter Pallflex, Pall Corporation) | Yes | No |
| Mallach et al. (2021) 153 | September, 2020 to January, 2021 | Hospital rooms, long‐term care facility rooms, penitentiary cells and personal residences housing COVID‐19 residents. | Ultrasonic Personal Air Samplers (UPAS), with 10 μm selective inlet | Filter | 1.92 m3 | Not mentioned | 16 h | Sterile 37 mm gelatin filter pads (12602‐37‐ALK, Sartorius, DE) | Yes | No |
| Ultrasonic Personal Air Samplers (UPAS), with 2.5 μm selective inlet | Filter | 1.92 m3 | Not mentioned | 16 h | Sterile 37 mm gelatin filter pads (12602‐37‐ALK, Sartorius, DE) | Yes | No | |||
| Coriolis μ (Bertin Technologies) | Cyclone | 1.5 m3 | Not mentioned | 10 min | VTM (Corning 25‐500‐CM) | Yes | No | |||
| Robie et al. (2021) 70 | October 2020–January 2021 | Non‐healthcare setting: patients' houses | NIOSH BC 251 (NIOSH, CDC) | Cyclone | ~0.42 m3 | 3.5 L/min | ~2 h | Not mentioned | Yes | No |
| SKC BioSamplers (SKC) | Impinger | ~1.5 m3 | 12.5 L/min | ~2 h | 16 ml phosphate‐ buffered saline (PBS) and 0.5% bovine serum albumin (BSA) for the BioSampler | Yes | No | |||
| Lednicky et al. (2021) 46 | Not mentioned | Non‐healthcare setting: inside a car | Sioutas Personal Cascade impactor sampler (PCIS) with a Leland Legacy pump (SKC) | Impactor | Not mentioned | 9 L/min | Not mentioned | PTFE filters. PCIS filters were then immersed in 1 recovery solution (PBS with 0.5% w/v BSA fraction V and 0.2 M sucrose) | Yes | Yes |
The viral transport medium was not specified.
The sampler model was not specified.
3.2. Air sampling, duration of collection, and airflow rates
From the 76 studies included in this systematic review (Table 1), 41 included both air and surface sampling. Of those, 16 studies have assessed SARS‐CoV‐2 specifically in different PM sizes (ex: PM1.0, PM2.5, PM1.0–4.0, PM10), 50 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 with SARS‐CoV‐2 being detected in all these PM sizes and the size fraction <4 μm containing more positive samples among these studies. All other 60 studies sampled air for total suspended particles (TSP). Notably, one study used a non‐commercial sampler developed for this purpose. 73
A total of 57 different air samplers were used in the different articles included in this review. The most often used air sampling method was the filter (36), followed by impactor (30), cyclone (20), impinger (17), passive sampling (5) and water‐based condensation (3). There were also 2 studies that used different methodologies, namely one with a combination of cyclone separation and impactor (Liu et al.) 74 and another that used a volumetric pollen trap 59 . Table 2. summarizes the different sampling methods used for SARS‐CoV‐2 air collection, including a brief description of the collection mechanism, the collection media, the flow rate range, and captured particle range, as well as the main advantages and disadvantages.
TABLE 2.
Sampling methods of air samplers used for SARS‐CoV‐2
| Sampling method | Collection mechanism a | Collection media | Flow rate range | Captured particle range (d50, μm) | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|
| Passive air sampling | Sedimentation | A petri dish is opened and exposed to the air for specified periods of time to determine what microbiological particles may be present in the environment, as they may settle out of the ambient air and on the media surface of the petri dish | Filter | NA | Depending on the filter porosity | Not aggressive. Lower cost | Qualitative analysis. Collection duration is longer |
| Active air sampling | Impactor | Particles in the incoming airstream accelerate through small nozzles (in the form of holes or slits), and those with high inertia impact on the surface of collection media | Agar, slide or filter | ~1–125 L/min | <1 (single‐stage) or 0.01–10 (multi‐stage) | Collect viruses in different particle sizes. More efficient for larger particles | Wall loss. Virus deactivation upon collection. Low‐efficiency for small virus particles |
| Cyclone | Centrifugal forces deviate particles from the airflow to impact on the collection wall | Dry vial | ~10–400 L/min | >0.5 | Collect viruses in different particle sizes. More efficient for larger particles | Wall loss. Virus deactivation upon collection. Low‐efficiency for small virus particles. | |
| Filter | Particles are collected on the filter media through interception, inertial impaction, and diffusion | Filter or membrane media | ~1–1000 L/min (filter dependent) | Dependent on filter porosity (<1) | Efficient for particles from 20 nm to 10 μm or even larger. Easy to use | Inactivation of viruses due to dehydration or extraction from filters | |
| Impinger | Abrupt change in the airstream direction inside the bottle impacts particles into the liquid collection medium | Liquid | ~10–500 L/min | >1 | Maintain viability of viruses. No need to extract viruses from a surface or filter | Wall loss or inlet loss. Low efficiency for small virus particles | |
| Water‐based condensation | A laminar‐flow condensation growth tube (CGT) encapsulates airborne particles into liquid droplets and gently deposits the droplets on a liquid surface | Liquid | ~10–1000 L/min | <0.5 | Maintain viability of viruses. Efficient for particles from 8 nm to 10 μm or even larger | Bulky and complex to operate | |
Abbreviation: NA, not applicable.
Adapted from Pan et al. 6
Figure 3 presents a schematic representation of each method to better understand the working principle and key differences between each collection mechanism. Passive air sampling is the method with the lowest cost, although it only gives a qualitative analysis. Within the active methods, two are essentially dry methods (impactor and cyclone), two are wet methods (impinger and condensation‐based), and one is a gel‐based method (impactor). Among the active methods, impactors (gel), cyclones, and filters (dry) imply the inactivation of the viruses while collecting the air sample, thus not maintaining their infectivity for further infectivity analysis. 6
FIGURE 3.
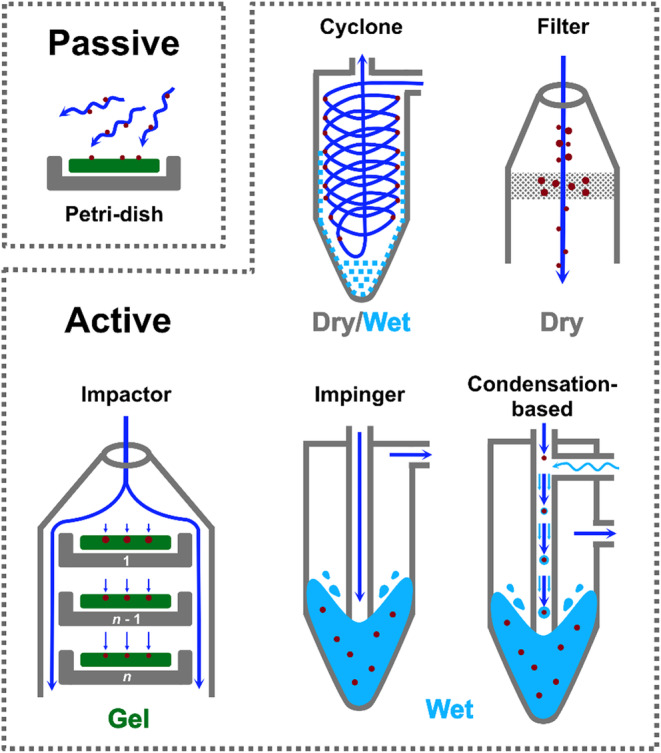
Schematic representation of each air sampling method applied for the detection of SARS‐CoV‐2
The duration of the air sampling of the studies included in this review ranged from approximately 15 min to 48 h for filter‐based samplers, 10 min to 6 days for impactor samplers, 15 min to 4 h for impinger samplers, 5.5 min to 7 days for cyclone samplers, 1 to 3 h for water‐based condensation samplers, and 30 min to 1 week for passive air sampling. Among the studies that detected viral RNA, sampling time varied between 5 min to 6 days, with many different sampling times being employed within this interval. As for the studies that could detect viral viability, sampling time varied between 30 min and 3 h. The sampling campaign was usually from one to only a few days of sampling, with a maximum of 23 days.
Collected air volumes ranged from 0.06 to 288 m3 for filter‐based samplers, 0.3 to 328.32 m3 for impactors, 0.09 to 16 m3 for impingers, 0.1 to 151.2 m3 for cyclones, and 0.93 to 1.44 m3 for water‐based condensation. Within the studies that detected viral RNA, the virus was detected in both low‐ and high‐volume samples, with the air volume ranging from 0.1 to 72 m3 in these studies. For the studies that did not detect viral RNA, the sampled air volume ranged from 0.4 to 110 m3. Among studies, airflow rates were highly variable depending on the sampler used (1.5 to 1130 L/min).
3.3. Collection media, sample processing, and SARS‐CoV‐2 detection and infectivity
Different collection media were used according to the type of air sampler employed in the study. The most common for impingers were phosphate‐buffered saline (PBS), Dulbecco's essential medium (DMEM), and magnetic beads. For cyclones, minimal essential medium (MEM) containing 1% bovine serum albumin, viral transport medium (VTM), and DMEM were used. For impactors, pre‐sterilized gelatin filters, gibco cell culture medium, virus transport medium, and 0.22‐um‐pore‐size filter membranes were used. For filter‐based method, gelatin membrane, polytetrafluoroethylene, polycarbonate, and quartz fiber filters were used. For the water‐based condensation, PBS with 0.5% (w/v) bovine albumin fraction V and a final concentration of 0.2 M sucrose was used.
The processing of the samples involved storage on ice for transportation to the laboratory facilities, followed by RNA extraction with commercially available kits according to the manufacturer's instructions. When viability was assessed, the most common media used were gelatin filters, DMEM and PBS; moreover, when only detection of viral RNA was performed, the most common media used was gelatin membrane filters, quartz fiber filters, PTFE filters, and virus transport medium.
Most of the reviewed studies used reverse transcription polymerase chain reaction (RT‐PCR) to verify the presence of SARS‐CoV‐2 viral RNA in the collected samples, with one studying using RT‐PCR and ddPCR. 73
SARS‐CoV‐2 RNA was detected with all collection mechanisms, namely in 56% of the 36 studies that used filter‐based samplers, 59% of the 17 studies that used impingers, 43% of the 30 studied that used impactors, 75% of the 20 studies that used cyclones, 100% of the 3 studies that used water‐based condensation, and 80% of the 5 studies that used passive sampling. Some of the studies detected SARS‐CoV‐2 viral RNA with more than one collection mechanism.
From the studies detecting the presence of SARS‐CoV‐2 viral RNA, 14 studies assessed infectivity. Infectivity was never assessed in the passive sampling method. As for the other methods, only three collection mechanisms were able to detect viable viruses, which were the impactor method using the Sioutas Personal Cascade Impactor (SKC, Inc) samplers (1 study out of 4 that used impactor samplers), 46 the cyclone method using the NIOSH BC251 sampler (1 study out of 5 that used cyclone samplers), 74 and the water‐based condensation method, using BioSpot‐VIVAS (Aerosol Devices Inc.) sampler (2 studies out of 3 that used water‐based condensation samplers). 45 , 47 Moreover, 1 of the 4 studies that assessed viability and used the impactor method with an Airport MD8 (Sartorius) reported evidence of viral viability in the air samples. 48
4. DISCUSSION
Positive results for SARS‐CoV‐2 RNA in air were found with all the different known methods available (filter‐based samplers, impingers, impactors, cyclones, water‐based condensation, and passive sampling). These results suggest that all used sampling methods are suitable for detecting SARS‐CoV‐2 RNA in air samples. These results support airborne transmission of SARS‐CoV‐2, but it is important to consider that except for seven studies performed outdoors ( 49 , 59 , 68 , 70 , 72 , 75 ; Liu et al.), 76 the majority of the other studies were performed in indoor healthcare settings. Thus, the virus collected may come from respiratory bioaerosols from patients or medical procedures. Also important to note is that the detection of SARS‐CoV‐2 RNA does not correlate to the infectivity of these viral particles, 76 and viral infectivity and infectivity of viruses present in air samples must be studied to fully clarify the airborne transmission of SARS‐CoV‐2. Regarding viral infectivity, two of the five studies that detected viable viruses from air samples used a water‐based condensation sampler (BioSpot‐VIVAS, Aerosol Devices Inc.), with two others using impactor samplers (Airport MD8, Sartorius and Sioutas Personal Cascade Impactor, SKC Inc) and another one using a cyclone sampler (NIOSH BC251). Interestingly, the BioSpot‐Vivas sampler mimics the human lung using a condensation‐enhanced inertial deposition method in which the collection is gently made into a liquid. A laminar‐flow condensation growth tube (CGT) collects airborne particles into liquid droplets and gently deposits the droplets onto a liquid surface. The air sample flow is set at 8 L/min, approximately the breathing rate of an average person. In this way, bioaerosols, including viable viruses, are collected with high efficiency independently of their size, shape, or hydrophobicity. 77 Notably, in a previous study by Pan et al. 78 it has been demonstrated that collecting virus aerosols by water‐based condensation is much more efficient when compared to an impinger sampler. More details regarding this sampler, how it works, and its efficiency have been previously described in the literature. 78 , 79 , 80 , 81 As for impactors, the air is drawn into the sampler and particles are deposited on a dry or coated surface, or agar. They are available as cascade impactors or slit impactors. Cascade impactors (Sioutas Personal Cascade Impactor) allow the measurement of particle size, whereas slit impactors (Airport MD8) have a rotating support stage for agar plates and allow measurement of concentration over time. 82
As for other methods listed in this article, the filter‐based sampling is typically known to desiccate the collected material as air passes through (or by) the filters, 6 which in turn can result in the inactivation of many types of viruses. Impingers are among the most common air samplers currently being used to sample SARS‐CoV‐2 and other airborne viruses and bacteria, although they are not as efficient in collecting smaller size fractions. 83 In a study by Zhu et al., 84 electron micrographs of negative‐stained SARS‐CoV‐2 particles obtained from bronchoalveolar‐lavage fluid samples were shown to have a diameter varying from about 0.06 to 0.14 μm, which in turn points to the possibility that impinger‐based samplers might not efficiently capture SARS‐CoV‐2. The cyclone, which was another frequently used method, seems to be suitable for collecting viral RNA. However, whether and how this sampler's collection mechanism might affect viral infectivity is still unknown. From the studies reviewed none detected viable viruses. And lastly, in contrast to active air samplers, passive sampling does not require active air movement from a pump and electricity. 85 The particles are collected by diffusion through membrane permeation. However, this method is not quantitative and cannot sample specific volumes of air.
Two other air sampling methodologies are described in the literature for microorganisms other than SARS‐CoV‐2, namely electrostatic precipitation and thermal precipitation. 82 Electrostatic precipitation collects air drawn over an electrostatically charged surface onto solid collecting surfaces (e.g., glass and agar). This type of sampler has a high volume sampling rate, but the equipment is complex and not practical to use in healthcare settings. 82 , 86 As for thermal precipitation, the air is drawn over a thermal gradient, particles are repelled from hot surfaces and then settle on colder surfaces such as a glass coverslip or an electron microscope grid. It can be used to determine particle size by direct observation. However, it is not frequently used because of complex adjustments and low sampling rates. 82 , 87
The performance of the virus aerosol samplers can be evaluated by their sampling efficiency, namely: (i) physical efficiency, being the ratio between the amount of collected particles and the amount of particles in the ambient environment and (ii) biological efficiency, being a measure of the fraction of biologically active virus that remains viable after collection. 6 For aerosolised viruses the same principles used for sampling bacterial and fungal aerosols are applied, 88 where particles are separated from the air through different physical mechanisms. 5 However, none of the studies included in this review evaluated the sampling performance, which is a parameter that should be included in future studies to obtain more accurate results.
Regarding viral infectivity in air, Tang et al. 41 have highlighted that air sampling technologies do not accurately replicate the actual mechanisms involved in human respiratory infection through inhalation, as natural human exhalation and inhalation flow velocities differ from the parameters involved in air sampling with current available air‐sampling techniques, making them less likely to cause shear stress damage to the viral structure. Because of that, lack of viable viruses in air samples does not necessarily correlate to the absence of viable virus in RNA positive samples, and the presence of RNA should be interpreted as the probable presence of a viable virus, especially considering that viral culture is very difficult as it requires a week or more for completion and specialized laboratory equipment and skills, therefore being much less sensitive than detection by molecular methods. 89 , 90 Nonetheless, alternative methods for assessing viral infectivity, such as sample pre‐treatment before nucleic acid extraction with propidium monoazide (PMA) should be explored and validated. 91 , 92 , 93 This method is based on the assumption that virus inactivation is associated with the loss of integrity of viral outer structures such as the envelope and capsid. 94 As PMA is a photoreactive intercalating dye with a high affinity for DNA and RNA, it forms a covalent linkage upon exposure to intense visible light. This reaction inhibits RT‐qPCR amplification of modified DNA templates by removing modified DNA during purification and inhibition of template amplification by DNA polymerases. Because the dyes cannot penetrate the intact viral capsid, when a sample containing both live and dead viruses is treated with it, only dead viruses with compromised capsids are susceptible to DNA modification, 91 which in turn allows for assessment of viable virus, expanding the applications of PCR‐based detection, and eliminating the need of a BSL‐3 laboratory facility to assess cell infectivity through culture methods.
It should also be considered that airborne virus concentrations can be low at the place and time of collection, 47 resulting in negative results that do not necessarily mean that there was no virus present at the moment of collection. Long sampling times may be needed to collect enough airborne viruses for detection by current molecular techniques; however, longer sampling time does not necessarily correlate to better recovery considering that the stability of viral RNA on sampling media is still unknown. Notably, another study by Raynor et al. 95 has also demonstrated that while high flow rate samplers may be better for detecting infectious virus and viral RNA in the air, airborne virus concentrations are measured more accurately by lower flow rate samplers, although the explanation for that is still uncertain. That study suggested that because higher flow rate samplers consolidate a sample from a large amount of air into a similar volume of liquid as the lower flow rate samplers, it would be possible that these consolidation processes damage the virus RNA in some way that reduces the measured infectious and total virus concentration. However, further studies are needed to clarify these aspects of air collection of viruses.
The collection media used for air sampling is also an important factor for successful collection of viable viruses. Sterile PBS with 0.5% bovine serum albumin fraction V has been used as collection media for influenza viruses because it helps maintain viability of the viruses. 6 , 96 As for SARS‐CoV‐2, the most common collection media reported in the literature are DMEM and PBS for liquid samplers, and gelatin membrane filters as well as PTFE filters for filter samplers. When viral viability assays are an option, DMEM is likely the most suitable collection media as it is also used in cell culture and is widely available in molecular biology laboratories, therefore the collection media can be inoculated directly onto the cell culture after collection.
Moreover, other variables could also affect the results, such as (SARS‐CoV‐2 infected) patient distance from the sampler, patient activity, coughing, and sneezing during sampling time, patient density in the sampling site, sampling conditions, storage, and transferring conditions. 52 Other environmental variables such as ultraviolet light (UV) exposure, temperature, relative humidity, wind currents, and ventilation systems can also influence the results. 97 These should, ideally, be controlled or at least measured when studying the presence of SARS‐CoV‐2 in air, as these data would give a better understanding of the dynamics and behavior of the aerosolised virus.
Moreover, it is not possible to understand the full extent of environmental contamination with aerosol samples alone. Surface sampling studies should always be conducted along with any aerosol sampling, 64 as viral particles suspended in air will, eventually, settle onto surfaces, which means that air samples negative for viral RNA do not necessarily mean that the virus is not present in the air. If negative air samples are paired with positive surface samples, these results can tell us that the virus might have been present in the ambient room previously and settled onto the surfaces before air sampling was performed.
To date, only a few studies have been published regarding cough aerosol and exhaled breath sampling from patients with COVID‐19. 60 , 98 , 99 , 100 , 101 , 102 Therefore further in vivo experiments should be performed using actual patient cough, sneeze, and breath aerosols to show the possibility of generation of the airborne size carrier aerosols and the infectivity fraction of the embedded virus in those carrier aerosols. 103 Moreover, studies on the presence and infectivity of SARS‐CoV‐2 in aerosols generated from sewage and wastewater treatment plants should also be made, as recent studies have shown the presence of SARS‐CoV‐2 RNA in wastewater 104 , 105 , 106 , 107 , 108 , 109 and exposure to SARS‐CoV‐2 in wastewater‐generated aerosols could also pose a health risk if the virus is viable. 110
There is no consensus or a defined protocol for sampling SARS‐CoV‐2 in air, with parameters such as airflow rate, the volume of air collected, the position of samplers in the sampling area, type of sampler and collection media varying greatly among all studies that detected SARS‐CoV‐2 RNA published until this date. Besides that, information regarding the number of air changes in the rooms where air collection occurred, relative humidity, temperature, and lighting conditions, which are important parameters affecting virus recovery and infectivity, are also often missing and not mentioned in the publications. This demonstrates how important and urgent is the definition of a standard method for sampling and detecting SARS‐CoV‐2 in the air, which would allow for the correct interpretation of the results of future studies regarding the behavior of this virus in the air, and also contribute to answering definitely to the question of SARS‐CoV‐2 airborne transmission.
Due to the lack of standardization for air sampling protocols, it is difficult to determine which method is more or less efficient. However, knowing the difference between each sampler and the air collection methodology used can help determine which conditions might be favorable in a specific experiment setting. The collection time, activity, and traffic of people in the environment during sampling will also influence the results, which is why these conditions should be specified for each environment.
5. CONCLUSION
The majority of the previous studies on the presence of SARS‐CoV‐2 in air samples detected viral RNA. SARS‐CoV‐2 RNA was detected in samples collected with different methods, namely filter‐based samplers, impingers, impactors, cyclones, water‐based condensation, and passive sampling. Those studies varied in terms of monitoring site (usually hospitals and other microenvironments), airflow rate, the volume of air collected, the position of samplers in the sampling area, and collection media. Nevertheless, only thirteen studies have assessed virus infectivity, and only four studies detected viable viruses from air samples using either water‐based condensation or impactor samplers.
There is a need for a standardized protocol for sampling SARS‐CoV‐2 in air, which should also account for other influencing parameters, including air exchange ratio in the room sampled, relative humidity, temperature, and lighting conditions. Air sampling should also be complemented with surface sampling.
There is still a considerable knowledge gap regarding the dynamics and behavior of the virus in aerosols and whether the viral particles suspended in air are infectious or not. Thus, further research on the airborne transmission of SARS‐CoV‐2 is urgently needed as the generated data would bring evidence that could significantly update the current infection control guidelines for dealing with COVID‐19 that, although now widely recognized as an airborne pathogen, still is not being dealt with as so in many countries.
AUTHOR CONTRIBUTIONS
PGS: Formal Analysis, Investigation, Visualization, Writing – Original Draft Preparation. PTBSB: Formal Analysis, Investigation, Validation, Writing – Review & Editing. RS: Visualization, Writing – Review & Editing. JRM: Supervision, Conceptualization, Writing – Review & Editing. SIVS: Supervision, Conceptualization, Methodology, Writing – Review & Editing. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was financially supported by: LA/P/0045/2020 (ALiCE) and UIDB/00511/2020–UIDP/00511/2020 (LEPABE) funded by national funds through FCT/MCTES (PIDDAC); Project PTDC/EAM‐AMB/32391/2017, funded by FEDER funds through COMPETE2020 – Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES. Priscilla G. Silva thanks the Portuguese Foundation for Science and Technology – FCT for the financial support of her PhD work (2020.07806.BD, CRM: 0026504) contract through the DOCTORATES 4 COVID‐19 program. Sofia I.V. Sousa thanks the Portuguese Foundation for Science and Technology (FCT) for the financial support of her work contract through the Scientific Employment Stimulus—Individual Call—CEECIND/02477/2017.
Silva PG, Branco PTBS, Soares RRG, Mesquita JR, Sousa SIV. SARS‐CoV‐2 air sampling: A systematic review on the methodologies for detection and infectivity. Indoor Air. 2022;32:e13083. doi: 10.1111/ina.13083
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. WHO . Transmission of SARS‐CoV‐2: implications for infection prevention precautions; 2020. WHO Glob. Accessed September 28, 2020. https://www.who.int/news‐room/commentaries/detail/transmission‐of‐sars‐cov‐2‐implications‐for‐infection‐prevention‐precautions
- 2. Chang T, Wu J, Chang L. Particle sizes of infectious aerosols: implications for infection control. J Formos Med Assoc. 2020;8:19‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ISDA . Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel: Update 2010. The National Academies Press; 2011. doi: 10.17226/13027 [DOI] [PubMed] [Google Scholar]
- 4. Singanayagam A, Zhou J, Elderfield RA, et al. Characterising viable virus from air exhaled by H1N1 influenza‐infected ferrets reveals the importance of haemagglutinin stability for airborne infectivity. PLoS Pathog. 2020;16:1‐21. doi: 10.1371/journal.ppat.1008362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verreault D, Moineau S, Duchaine C. Methods for sampling of airborne viruses. Microbiol Mol Biol Rev. 2008;72:413‐444. doi: 10.1128/mmbr.00002-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan M, Lednicky JA, Wu CY. Collection, particle sizing and detection of airborne viruses. J Appl Microbiol. 2019;127:1596‐1611. doi: 10.1111/jam.14278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barker J, Jones MV. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J Appl Microbiol. 2005;99:339‐347. doi: 10.1111/j.1365-2672.2005.02610.x [DOI] [PubMed] [Google Scholar]
- 8. McDermott CV, Alicic RZ, Harden N, Cox EJ, Scanlan JM. Put a lid on it: are faecal bio‐aerosols a route of transmission for SARS‐CoV‐2? J Hosp Infect. 2020;105:397‐398. doi: 10.1016/j.jhin.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng X, Huang X, Zhou P, Li C, Wu A. Alert for SARS‐CoV‐2 infection caused by fecal aerosols in rural areas in China. Infect Control Hosp Epidemiol. 2020;41:987. doi: 10.1017/ice.2020.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallis C, Melnick JL, Rao VC, Sox TE. Method for detecting viruses in aerosols. Appl Environ Microbiol. 1985;50:1181‐1186. doi: 10.1128/AEM.50.5.1181-1186.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brinkman NE, Fout GS, Keely SP. Retrospective surveillance of wastewater to examine seasonal dynamics of Enterovirus infections. mSphere. 2017;2:e00099‐17. doi: 10.1128/mSphere.00099-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carducci A, Arrighi S, Ruschi A. Detection of coliphages and enteroviruses in sewage and aerosol from an activated sludge wastewater treatment plant. Lett Appl Microbiol. 1995;21:207‐209. doi: 10.1111/j.1472-765X.1995.tb01042.x [DOI] [PubMed] [Google Scholar]
- 13. Drossinos Y, Stilianakis NI. What aerosol physics tells us about airborne pathogen transmission. Aerosol Sci Tech. 2020;54:639‐643. doi: 10.1080/02786826.2020.1751055 [DOI] [Google Scholar]
- 14. Fannin KF, Spendlove JC, Cochran KW, Gannon JJ. Airborne coliphages from wastewater treatment facilities. Appl Environ Microbiol. 1976;31:705‐710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adams AP, Spendlove JC. Coliform aerosols emitted by sewage treatment plants. Science. 1970;169:1218‐1220. doi: 10.1126/science.169.3951.1218 [DOI] [PubMed] [Google Scholar]
- 16. Burge WD, Marsh PB. Infectious disease hazards of Landspreading sewage wastes. J Environ Qual. 1978;7:1‐9. doi: 10.2134/jeq1978.00472425000700010001x [DOI] [Google Scholar]
- 17. Moore BE, Sagik BP, Sorber CA. Procedure for the recovery of airborne human enteric viruses during spray irrigation of treated wastewater. Appl Environ Microbiol. 1979;38(4):688‐693. doi: 10.1128/aem.38.4.688-693.1979 PMID: 231937; PMCID: PMC243561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teltsch B, Katzenelson E. Airborne enteric bacteria and viruses from spray irrigation with wastewater. Appl Environ Microbiol. 1978;35(2):290‐296. doi: 10.1128/aem.35.2.290-296.1978. PMID: 345967; PMCID: PMC242828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Labadie T, Batéjat C, Leclercq I, Manuguerra J‐C. Historical discoveries on viruses in the environment and their impact on public health. Intervirology. 2020;63:17‐32. doi: 10.1159/000511575 [DOI] [PubMed] [Google Scholar]
- 20. Firquet S, Beaujard S, Lobert PE, et al. Survival of enveloped and non‐enveloped viruses on inanimate surfaces. Microbes Environ. 2015;30:140‐144. doi: 10.1264/jsme2.ME14145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shigematsu S, Dublineau A, Sawoo O, et al. Influenza a virus survival in water is influenced by the origin species of the host cell. Influenza Other Respir Viruses. 2014;8:123‐130. doi: 10.1111/irv.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cosset F‐L, Lavillette D. Cell entry of enveloped viruses. Adv Genet. 2011;73:121‐183. doi: 10.1016/B978-0-12-380860-8.00004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasickova P, Pavlik I, Verani M, Carducci A. Issues concerning survival of viruses on surfaces. Food Environ Virol. 2010;2:24‐34. doi: 10.1007/s12560-010-9025-6 [DOI] [Google Scholar]
- 24. Kormuth KA, Lin K, Prussin AJ, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J Infect Dis. 2018;218:739‐747. doi: 10.1093/infdis/jiy221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. La Rosa G, Fratini M, Della Libera S, Iaconelli M, Muscillo M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann Ist Super Sanità. 2013;49:124‐132. doi: 10.4415/ANN [DOI] [PubMed] [Google Scholar]
- 26. Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor air. 2006;16:335‐347. doi: 10.1111/j.1600-0668.2006.00432.x [DOI] [PubMed] [Google Scholar]
- 27. Alzyood M, Jackson D, Aveyard H, Brooke J. COVID‐19 reinforces the importance of handwashing. J Clin Nurs. 2020;29:2760‐2761. doi: 10.1111/jocn.15313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asadi S, Cappa CD, Barreda S, Wexler AS, Bouvier NM, Ristenpart WD. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 2020;10:1‐13. doi: 10.1038/s41598-020-72798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma QX, Shan H, Zhang HL, Li GM, Yang RM, Chen JM. Potential utilities of mask‐wearing and instant hand hygiene for fighting SARS‐CoV‐2. J Med Virol. 2020;92:1567‐1571. doi: 10.1002/jmv.25805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moosa IA. The effectiveness of social distancing in containing Covid‐19. Appl Econ. 2020;52:1‐14. doi: 10.1080/00036846.2020.1789061 [DOI] [Google Scholar]
- 31. Morawska L, Cao J. Airborne transmission of SARS‐CoV‐2: the world should face the reality. Environ Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Somsen GA, van Rijn C, Kooij S, Bem RA, Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS‐CoV‐2 transmission. Lancet Respir Med. 2020;8:658‐659. doi: 10.1016/S2213-2600(20)30245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. CDC . SARS‐CoV‐2 transmission; 2021.
- 34. WHO . Coronavirus disease (COVID‐19): How is it transmitted? 2021. Accessed August 28, 2021 https://www.who.int/news‐room/q‐a‐detail/coronavirus‐disease‐covid‐19‐how‐is‐it‐transmitted
- 35. de Man P, Paltansing S, Ong DSY, Vaessen N, van Nielen G, Koeleman JGM. Outbreak of Coronavirus Disease 2019 (COVID‐19) in a nursing home associated with aerosol transmission as a result of inadequate ventilation. Clin Infect Dis. 2021;73(1):170‐171. doi: 10.1093/cid/ciaa1270 PMID: 32857130; PMCID: PMC7499506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamner L, Dubbel P, Capron I, et al. High SARS‐CoV‐2 attack rate following exposure at a choir practice. Morb Mortal Wkly Rep High. 2020;69:606‐610. doi: 10.15585/mmwr.mm6919e6 [DOI] [PubMed] [Google Scholar]
- 37. Lu J, Gu J, Li K, et al. COVID‐19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628‐1631. doi: 10.3201/eid2607.200764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller SL, Nazaroff WW, Jimenez JL, et al. Transmission of SARS‐CoV‐2 by inhalation of respiratory aerosol in the Skagit Valley chorale superspreading event. Indoor Air. 2021;31:314‐323. doi: 10.1111/ina.12751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park SY, Kim Y‐M, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26:1666‐1670. doi: 10.3201/eid2608.201274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen Y, Li C, Dong H, et al. Community outbreak investigation of SARS‐CoV‐2 transmission among bus riders in eastern China. JAMA Intern Med. 2020;180:1665‐1671. doi: 10.1001/jamainternmed.2020.5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang JW, Bahnfleth WP, Bluyssen PM, et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Hosp Infect. 2021;110:89‐96. doi: 10.1016/j.jhin.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lewis D. The superspreading problem. Nature. 2021;590:544‐546. [DOI] [PubMed] [Google Scholar]
- 43. Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS‐CoV‐2. Lancet. 2021;397:1603‐1605. doi: 10.1016/S0140-6736(21)00869-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morawska L, Milton DK. It is time to address airborne transmission of COVID‐19. Clin Infect Dis. 2020;71(9):2311‐2313. doi: 10.1093/cid/ciaa939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lednicky J, Lauzard M, Fan ZH, et al. Viable SARS‐CoV‐2 in the air of a hospital room with COVID‐19 patients. Int J Infect Dis. 2020;100:476‐482. doi: 10.1016/j.ijid.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lednicky JA, Lauzardo M, Alam MM, et al. Isolation of SARS‐CoV‐2 from the air in a car driven by a COVID patient with mild illness. Int J Infect Dis. 2021;108:212‐216. doi: 10.1016/j.ijid.2021.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lednicky JA, Shankar SN, Elbadry MA, et al. Collection of SARS‐CoV‐2 virus from the air of a clinic within a university student health care center and analyses of the viral genomic sequence. Aerosol Air Qual Res. 2020;20:1167‐1171. doi: 10.4209/aaqr.2020.05.0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Santarpia JL, Rivera DN, Herrera VL, et al. Aerosol and surface contamination of SARS‐CoV‐2 observed in quarantine and isolation care. Sci Rep. 2020;10:1‐8. doi: 10.1038/s41598-020-69286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chirizzi D, Conte M, Feltracco M, et al. SARS‐CoV‐2 concentrations and virus‐laden aerosol size distributions in outdoor air in north and south of Italy. Environ Int. 2021;146:106255. doi: 10.1016/j.envint.2020.106255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stern RA, Koutrakis P, Martins MAG, et al. Characterization of hospital airborne SARS‐CoV‐2. Respir Res. 2021;22:1‐8. doi: 10.1186/s12931-021-01637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Birgand G, Peiffer‐Smadja N, Fournier S, Kerneis S, Lescure F‐X, Lucet J‐C. Assessment of air contamination by SARS‐CoV‐2 in hospital settings. JAMA Netw Open. 2020;3:e2033232. doi: 10.1001/jamanetworkopen.2020.33232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rahmani AR, Leili M, Azarian G, Poormohammadi A. Sampling and detection of corona viruses in air: a mini review. Sci Total Environ. 2020;740:140207. doi: 10.1016/j.scitotenv.2020.140207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Robotto A, Quaglino P, Lembo D, et al. SARS‐CoV‐2 and indoor/outdoor air samples: a methodological approach to have consistent and comparable results. Environ Res. 2021;195:110847. doi: 10.1016/j.envres.2021.110847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aghalari Z, Dahms H‐U, Sosa‐Hernandez JE, Oyervides‐Muñoz MA, Parra‐Saldívar R. Evaluation of SARS‐COV‐2 transmission through indoor air in hospitals and prevention methods: a systematic review. Environ Res. 2021;195:110841. doi: 10.1016/j.envres.2021.110841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dinoi A, Feltracco M, Chirizzi D, et al. A review on measurements of SARS‐CoV‐2 genetic material in air in outdoor and indoor environments: Implication for airborne transmission. Sci Total Environ. 2022;809:151137. doi: 10.1016/j.scitotenv.2021.151137 Epub 2021 Oct 23. PMID: 34699823; PMCID: PMC8539199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. WHO . Coronavirus disease (COVID‐19) pandemic; 2020. Coronavirus Dis Outbreak Situat. Accessed June 1, 2021. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019
- 57. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Binder RA, Alarja NA, Robie ER, et al. Environmental and aerosolized severe acute respiratory syndrome coronavirus 2 among hospitalized COVID‐19 patients. J Infect Dis. 2020;222:1798‐1806. doi: 10.1093/infdis/jiaa575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS‐CoV‐2 in hospital rooms of infected patients. Nat Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dunker S, Hornick T, Szczepankiewicz G, et al. No SARS‐CoV‐2 detected in air samples (pollen and particulate matter) in Leipzig during the first spread. Sci Total Environ. 2021;755:142881. doi: 10.1016/j.scitotenv.2020.142881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng B, Xu K, Gu S, et al. Multi‐route transmission potential of SARS‐CoV‐2 in healthcare facilities. J Hazard Mater. 2021;402:123771. doi: 10.1016/j.jhazmat.2020.123771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ge XY, Pu Y, Liao CH, et al. Evaluation of the exposure risk of SARS‐CoV‐2 in different hospital environment. Sustain Cities Soc. 2020;61:102413. doi: 10.1016/j.scs.2020.102413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ghaffari HR, Farshidi H, Alipour V, et al. Detection of SARS‐CoV‐2 in the indoor air of intensive care unit (ICU) for severe COVID‐19 patients and its surroundings: considering the role of environmental conditions. Environ Sci Pollut Res Int. 2021;1‐7. doi: 10.1007/s11356-021-16010-x Epub ahead of print. PMID: 34482469; PMCID: PMC8418690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lane MA, Brownsword EA, Babiker A, et al. Bioaerosol sampling for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in a referral center with critically ill coronavirus disease 2019 (COVID‐19) patients march–may 2020. Clin Infect Dis. 2021;73(7):e1790‐e1794. doi: 10.1093/cid/ciaa1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lane MA, Brownsword EA, Morgan JS, et al. Bioaerosol sampling of a ventilated patient with COVID‐19. Am J Infect Control. 2020;48:1540‐1542. doi: 10.1016/j.ajic.2020.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lei H, Ye F, Liu X, et al. SARS‐CoV‐2 environmental contamination associated with persistently infected COVID‐19 patients. Influenza Other Respir Viruses. 2020;14:688‐699. doi: 10.1111/irv.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. López JH, Romo ÁS, Molina DC, et al. Detection of Sars‐Cov‐2 in the air of two hospitals in Hermosillo, Sonora, México, utilizing a low‐cost environmental monitoring system. Int J Infect Dis. 2021;102:478‐482. doi: 10.1016/j.ijid.2020.10.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ong SWX, Tan YK, Coleman KK, et al. Lack of viable severe acute respiratory coronavirus virus 2 (SARS‐CoV‐2) among PCR‐positive air samples from hospital rooms and community isolation facilities. Infect Control Hosp Epidemiol. 2021;42(11):1327‐1332. doi: 10.1017/ice.2021.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pivato A, Amoruso I, Formenton G, et al. Evaluating the presence of SARS‐CoV‐2 RNA in the particulate matters during the peak of COVID‐19 in Padua, northern Italy. Sci Total Environ. 2021;784:147129. doi: 10.1016/j.scitotenv.2021.147129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Robie ER, Abdelgadir A, Binder RA, Gray GC. Live SARS‐CoV‐2 is difficult to detect in patient aerosols. Influenza Other Respir Viruses. 2021;15:554‐557. doi: 10.1111/irv.12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Setti L, Passarini F, De Gennaro G, et al. SARS‐Cov‐2 RNA found on particulate matter of Bergamo in northern Italy: first evidence. Environ Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stern RA, Al‐Hemoud A, Alahmad B, Koutrakis P. Levels and particle size distribution of airborne SARS‐CoV‐2 at a healthcare facility in Kuwait. Sci Total Environ. 2021;782:146799. doi: 10.1016/j.scitotenv.2021.146799 [DOI] [Google Scholar]
- 73. Habibi N, Uddin S, Al‐Salameen F, et al. SARS‐CoV‐2, other respiratory viruses and bacteria in aerosols: report from Kuwait's hospitals. Indoor Air. 2021;31:1815‐1825. doi: 10.1111/ina.12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu W, Li D, Yang C, et al. Environmental contamination with SARS‐CoV‐2 in COVID‐19 hospitals in Wuhan, China, 2020. Environ Microbiol. 2021. doi: 10.1111/1462-2920.15695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Conte M, Feltracco M, Chirizzi D, et al. Airborne concentrations of SARS‐CoV‐2 in indoor community environments in Italy. Environ Sci Pollut Res Int. 2022;29(10):13905‐13916. doi: 10.1007/s11356-021-16737-7 Epub 2021 Oct 1. PMID: 34599449; PMCID: PMC8486635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Santarpia JL, Herrera VL, Rivera DN, et al. The size and culturability of patient‐generated SARS‐CoV‐2 aerosol. J Expo Sci Environ Epidemiol. 2021. doi: 10.1038/s41370-021-00376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS‐CoV‐2 in two Wuhan hospitals. Nature. 2020;582:557‐560. doi: 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 78. Passos RG, Silveira MB, Abrahão JS. Exploratory assessment of the occurrence of SARS‐CoV‐2 in aerosols in hospital facilities and public spaces of a metropolitan center in Brazil. Environ Res. 2021;195:110808. doi: 10.1016/j.envres.2021.110808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. da Silva PG, Nascimento MSJ, Soares RRG, Sousa SIV, Mesquita JR. Airborne spread of infectious SARS‐CoV‐2: moving forward using lessons from SARS‐CoV and MERS‐CoV. Sci Total Environ. 2020;764:142802. doi: 10.1016/j.scitotenv.2020.142802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Aerosol Devices Inc . BioSpot‐VIVAS™ – an enhanced bioaerosol sampler: Model BSS310; 2021. Accessed July 5, 2021. https://aerosoldevices.com/biospot‐vivas‐sampler/
- 81. Pan M, Eiguren‐Fernandez A, Hsieh H, et al. Efficient collection of viable virus aerosol through laminar‐flow, water‐based condensational particle growth. J Appl Microbiol. 2016;120:805‐815. doi: 10.1111/jam.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lednicky J, Pan M, Loeb J, et al. Highly efficient collection of infectious pandemic influenza H1N1 virus (2009) through laminar‐flow water based condensation. Aerosol Sci Tech. 2016;50:i‐iv. doi: 10.1080/02786826.2016.1179254 [DOI] [Google Scholar]
- 83. Pan M, Bonny TS, Loeb J, et al. Collection of viable aerosolized influenza virus and other respiratory viruses in a student health care center through water‐based condensation growth. Appl Environ Sci. 2017;2:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Walls HJ, Ensor DS, Harvey LA, et al. Generation and sampling of nanoscale infectious viral aerosols. Aerosol Sci Tech. 2016;50:802‐811. doi: 10.1080/02786826.2016.1191617 [DOI] [Google Scholar]
- 85. CDC . Background for environmental sampling; 2015.
- 86. Hogan CJJ, Kettleson EM, Lee M‐H, Ramaswami B, Angenent LT, Biswas P. Sampling methodologies and dosage assessment techniques for submicrometre and ultrafine virus aerosol particles. J Appl Microbiol. 2005;99:1422‐1434. doi: 10.1111/j.1365-2672.2005.02720.x [DOI] [PubMed] [Google Scholar]
- 87. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. doi: 10.1056/nejmoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Newton S, Sellström U, Harrad S, Yu G, de Wit CA. Comparisons of indoor active and passive air sampling methods for emerging and legacy halogenated flame retardants in Beijing, China offices. Emerg Contam. 2016;2:80‐88. doi: 10.1016/j.emcon.2016.02.001 [DOI] [Google Scholar]
- 89. Roux JM, Sarda‐Estève R, Delapierre G, Nadal MH, Bossuet C, Olmedo L. Development of a new portable air sampler based on electrostatic precipitation. Environ Sci Pollut Res. 2016;23:8175‐8183. doi: 10.1007/s11356-015-5522-3 [DOI] [PubMed] [Google Scholar]
- 90. Orr C Jr, Gordon MT, Kordecki MC. Thermal precipitation for sampling air‐borne microorganisms. Appl Microbiol. 1956;4:116‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lindsley WG, Green BJ, Blachere FM, et al. Sampling and characterization of bioaerosols. NIOSH Manual of Analytical Methods. Centers for Disease Control and Prevention (CDC); 2017. [Google Scholar]
- 92. Dolskiy AA, Grishchenko IV, Yudkin DV. Cell cultures for virology: usability, advantages, and prospects. Int J Mol Sci. 2020;21(21):7978. doi: 10.3390/ijms21217978 PMID: 33121109; PMCID: PMC7662242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Morley PS, Pusterla N. Viral respiratory disease in athletic horses. In: Hinchcliff KW, Kaneps AJ, Geor RJ, eds. Equine Sports Medicine and Surgery: Basic and Clinical Sciences for the Athletic Horse. 2nd ed. nd ed W.B. Saunders; 2014:649‐664. doi: 10.1016/B978-0-7020-4771-8.00030-2 [DOI] [Google Scholar]
- 94. Bindari YR, Walkden‐Brown SW, Gerber PF. Methods to prevent PCR amplification of DNA from non‐viable virus were not successful for infectious laryngotracheitis virus. PLoS One. 2020;15:e0232571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Randazzo W, Vasquez‐García A, Aznar R, Sánchez G. Viability RT‐qPCR to distinguish between HEV and HAV with intact and altered capsids. Front Microbiol. 2018;9:1‐8. doi: 10.3389/fmicb.2018.01973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Smee DF, Hurst BL, Evans WJ, et al. Evaluation of cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J Virol Methods. 2017;246:51‐57. doi: 10.1016/j.jviromet.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang X, Jia R, Zhou J, Wang M, Yin Z, Cheng A. Capsid‐targeted viral inactivation: a novel tactic for inhibiting replication in viral infections. Viruses. 2016;8:258. doi: 10.3390/v8090258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Raynor PC, Adesina A, Aboubakr HA, Yang M, Torremorell M, Goyal SM. Comparison of samplers collecting airborne influenza viruses: 1 Primarily Impingers and Cyclones. PLoS One. 2021;16:e0244977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lednicky JA, Hamilton SB, Tuttle RS, Sosna WA, Daniels DE, Swayne DE. Ferrets develop fatal influenza after inhaling small particle aerosols of highly pathogenic avian influenza virus a/Vietnam/1203/2004 (H5N1). Virol J. 2010;7:231. doi: 10.1186/1743-422X-7-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Alonso C, Raynor PC, Davies PR, Torremorell M. Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS One. 2015;10:1‐12. doi: 10.1371/journal.pone.0135675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Di Carlo P, Falasca K, Ucciferri C, et al. Normal breathing releases SARS‐CoV‐2 into the air. J Med Microbiol. 2021;70:1‐5. doi: 10.1099/jmm.0.001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Edwards DA, Ausiello D, Salzman J, et al. Exhaled aerosol increases with COVID‐19 infection, age, and obesity. Proc Natl Acad Sci USA. 2021;118:e2021830118. doi: 10.1073/pnas.2021830118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ma J, Qi X, Chen H, et al. Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clin Infect Dis. 2021;72:e652‐e654. doi: 10.1093/cid/ciaa1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Malik M, Kunze A‐C, Bahmer T, Herget‐Rosenthal S, Kunze T. SARS‐CoV‐2: viral loads of exhaled breath and Oronasopharyngeal specimens in hospitalized patients with COVID‐19. Int J Infect Dis. 2021;110:105‐110. doi: 10.1016/j.jhin.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhou L, Yao M, Zhang X, et al. Breath‐, air‐ and surface‐borne SARS‐CoV‐2 in hospitals. J Aerosol Sci. 2021;152:105693. doi: 10.1016/j.jaerosci.2020.105693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Faridi S, Niazi S, Sadeghi K, et al. A field indoor air measurement of SARS‐CoV‐2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baldovin T, Amoruso I, Fonzo M, et al. SARS‐CoV‐2 RNA detection and persistence in wastewater samples: an experimental network for COVID‐19 environmental surveillance in Padua, Veneto region (NE Italy). Sci Total Environ. 2020;760:143329. doi: 10.1016/j.scitotenv.2020.143329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bar‐Or I, Weil M, Indenbaum V, et al. Detection of SARS‐CoV‐2 variants by genomic analysis of wastewater samples in Israel. Sci Total Environ. 2021;789:148002. doi: 10.1016/j.scitotenv.2021.148002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bivins A, Greaves J, Fischer R, et al. Persistence of SARS‐CoV‐2 in water and wastewater. Environ Sci Technol Lett. 2020;7:937‐942. doi: 10.1021/acs.estlett.0c00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nemudryi A, Nemudraia A, Wiegand T, et al. Temporal detection and phylogenetic assessment of SARS‐CoV‐2 in municipal wastewater. Cell Rep Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tomasino MP, Semedo M, Vieira e Moreira P, et al. SARS‐CoV‐2 RNA detected in urban wastewater from Porto, Portugal: method optimization and continuous 25‐week monitoring. Sci Total Environ. 2021;792:148467. doi: 10.1016/j.scitotenv.2021.148467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wu F, Zhang J, Xiao A, et al. SARS‐CoV‐2 titers in wastewater are higher than expected. mSystems. 2020;5:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Usman M, Farooq M, Farooq M. Exposure to SARS‐CoV‐2 in aerosolized wastewater: toilet Flushing, wastewater treatment, and sprinkler irrigation. Water. 2021;13:436. doi: 10.3390/w13040436 [DOI] [Google Scholar]
- 114. Cheng VC‐C, Wong S‐C, Chen JH‐K, et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID‐19) due to SARS‐CoV‐2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41:493‐498. doi: 10.1017/ice.2020.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim UJ, Lee SY, Lee JY, et al. Air and environmental contamination caused by COVID‐19 patients: a multi‐center study. J Korean Med Sci. 2020;35:e332. doi: 10.3346/jkms.2020.35.e332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Li YH, Fan YZ, Jiang L, Wang HB. Aerosol and environmental surface monitoring for SARS‐CoV‐2 RNA in a designated hospital for severe COVID‐19 patients. Epidemiol Infect. 2020;148. doi: 10.1017/S0950268820001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. JAMA. 2020;3‐5. doi: 10.1001/jama.2020.3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wei L, Huang W, Lu X, et al. Contamination of SARS‐CoV‐2 in patient surroundings and on personal protective equipment in a non‐ICU isolation ward for COVID‐19 patients with prolonged PCR positive status. Antimicrob Resist Infect Control. 2020;9:1‐5. doi: 10.1186/s13756-020-00839-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Stern RA, Koutrakis P, Martins MAG, et al. Characterization of hospital airborne SARS‐CoV‐2. Respir Res. 2021;22:1‐8. doi: 10.1186/s12931-021-01637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stern RA, Al‐Hemoud A, Alahmad B, Koutrakis P. Levels and particle size distribution of airborne SARS‐CoV‐2 at a healthcare facility in Kuwait. Sci Total Environ. 2021;782:146799. doi: 10.1016/j.scitotenv.2021.146799 [DOI] [Google Scholar]
- 121. Song ZG, Chen YM, Wu F, et al. Identifying the risk of SARS‐CoV‐2 infection and environmental monitoring in airborne infectious isolation rooms (AIIRs). Virol Sin. 2020;35:785‐792. doi: 10.1007/s12250-020-00301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Morioka S, Nakamura K, Iida S, et al. Possibility of transmission of severe acute respiratory syndrome coronavirus 2 in a tertiary care hospital setting: a case study. Infect Prev Pract. 2020;2:100079. doi: 10.1016/j.infpip.2020.100079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Masoumbeigi H, Ghanizadeh G, Yousefi Arfaei R, et al. Investigation of hospital indoor air quality for the presence of SARS‐Cov‐2. J Environ Health Sci Eng. 2020;18:1259‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Declementi M, Godono A, Mansour I, et al. Assessment of air and surfaces contamination in a COVID‐19 non‐intensive care unit. Med Lav. 2020;111:372‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dumont‐Leblond N, Veillette M, Bhérer L, et al. Positive no‐touch surfaces and undetectable SARS‐CoV‐2 aerosols in long‐term care facilities: an attempt to understand the contributing factors and the importance of timing in air sampling campaigns. Am J Infect Control. 2021;49:701‐706. doi: 10.1016/j.ajic.2021.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vosoughi M, Karami C, Dargahi A, et al. Investigation of SARS‐CoV‐2 in hospital indoor air of COVID‐19 patients’ ward with impinger method. Environ Sci Pollut Res. 2021;28:58812‐58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Chui J, Wong C, Hapuarachchi HC, et al. Environmental contamination of SARS‐CoV‐2 in a Non‐healthcare setting. Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Razzini K, Castrica M, Menchetti L, et al. SARS‐CoV‐2 RNA detection in the air and on surfaces in the COVID‐19 ward of a hospital in Milan, Italy. Sci Total Environ. 2020;742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Kenarkoohi A, Noorimotlagh Z, Falahi S, et al. Hospital indoor air quality monitoring for the detection of SARS‐CoV‐2 (COVID‐19) virus. Sci Total Environ. 2020;748:141324. doi: 10.1016/j.scitotenv.2020.141324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mouchtouri VA, Koureas M, Kyritsi M, et al. Environmental contamination of SARS‐CoV‐2 on surfaces, air‐conditioner and ventilation systems. Int J Hyg Environ Health. 2020;230:113599. doi: 10.1016/j.ijheh.2020.113599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Guo Z‐D, Wang Z‐Y, Zhang S‐F, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jin T, Li J, Yang J, et al. SARS‐CoV‐2 presented in the air of an intensive care unit (ICU). Sustain Cities Soc. 2021;65. http://www.sciencedirect.com/science/article/pii/S2210670720306661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hadei M, Mohebbi SR, Hopke PK, et al. Presence of SARS‐CoV‐2 in the air of public places and transportation. Atmos Pollut Res. 2021;12:302‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ding Z, Qian H, Ph D, Xu B, Huang Y, Miao T. Toilets dominate environmental detection of SARS‐CoV‐2 virus in a hospital. Sci Total Environ. 2021;753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Amato‐Lourenço LF, de Souza Xavier Costa N, Dantas KC, et al. Quantification of airborne SARS‐CoV‐2 genomic particles in different hospital settings. Sci Rep. 2021;11:21284. doi: 10.1038/s41598-021-00761-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hemati S, Mobini GR, Heidari M, et al. Simultaneous monitoring of SARS‐CoV‐2, bacteria, and fungi in indoor air of hospital: a study on Hajar Hospital in Shahrekord, Iran. Environ Sci Pollut Res. 2021;28:43792‐43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Baboli Z, Neisi N, Babaei AA, et al. On the airborne transmission of SARS‐CoV‐2 and relationship with indoor conditions at a hospital. Atmos Environ. 2021;261:118563. doi: 10.1016/j.atmosenv.2021.118563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Moreno T, Pintó RM, Bosch A, et al. Tracing surface and airborne SARS‐CoV‐2 RNA inside public buses and subway trains. Environ Int. 2021;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Semelka CT, Ornelles DA, O’Connell NS, et al. Detection of environmental spread of SARS‐CoV‐2 and associated patient characteristics. Open Forum Infect Dis. 2021;8:2‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Capeyron O, Squinazi F, Dumoulin P, et al. A simple method for SARS‐CoV‐2 RNA detection in the air of an enclosed space. J Hosp Infect. September 2021:S0195‐6701(21)00316‐9. September 20, 2021. https://pubmed.ncbi.nlm.nih.gov/34547322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ang AXY, Luhung I, Ahidjo BA, et al. Airborne SARS‐CoV‐2 surveillance in hospital environment using high‐flow rate air samplers and its comparison to surface sampling. Indoor Air. 2021;1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bazzazpour S, Rahmatinia M, Mohebbi SR, et al. The detection of SARS‐CoV‐2 RNA in indoor air of dental clinics during the COVID‐19 pandemic. Environ Sci Pollut Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sousan S, Fan M, Outlaw K, Williams S, Roper RL. SARS‐CoV‐2 detection in air samples from inside heating, ventilation, and air conditioning (HVAC) systems‐ COVID surveillance in student dorms. Am J Infect Control. October 2021:S0196‐6553(21)00674‐X. October 21, 2021. https://pubmed.ncbi.nlm.nih.gov/34688726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Akin H, Karabay O, Toptan H, et al. Investigation of the presence of SARS‐CoV‐2 in aerosol after dental treatment. Int Dent J. 2021;2021:1‐5. doi: 10.1016/j.identj.2021.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Nannu Shankar S, Witanachchi CT, Morea AF, et al. SARS‐CoV‐2 in residential rooms of two self‐isolating persons with COVID‐19. J Aerosol Sci. 2022;159:105870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Silva PG, Gonçalves J, Lopes AI, et al. Evidence of air and surface contamination with SARS‐CoV‐2 in a major hospital in Portugal. Int J Environ Res Public Health. 2022;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Moore G, Rickard H, Stevenson D, et al. Detection of SARS‐CoV‐2 within the healthcare environment: a multi‐centre study conducted during the first wave of the COVID‐19 outbreak in England. J Hosp Infect. 2021;108:189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Dumont‐Leblond N, Veillette M, Mubareka S, et al. Low incidence of airborne SARS‐CoV‐2 in acute care hospital rooms with optimized ventilation. Emerg Microbes Infect. 2020;9:2597‐2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yamagishi T, Ohnishi M, Matsunaga N, et al. Environmental sampling for severe acute respiratory syndrome coronavirus 2 during a COVID‐19 outbreak on the diamond princess cruise ship. J Infect Dis. 2020;222:1098‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ben‐Shmuel A, Brosh‐Nissimov T, Glinert I, et al. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) environmental contamination in isolation units and quarantine facilities. Clin Microbiol Infect. 2020;26:1658‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhou J, Otter JA, Price JR, et al. Investigating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) surface and air contamination in an acute healthcare setting during the peak of the Coronavirus Disease 2019 (COVID‐19) pandemic in London. Clin Infect Dis. 2021;73:e1870‐e1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Barbieri P, Zupin L, Licen S, et al. Molecular detection of SARS‐CoV‐2 from indoor air samples in environmental monitoring needs adequate temporal coverage and infectivity assessment. Environ Res. 2021;198:111200. doi: 10.1016/j.envres.2021.111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mallach G, Kasloff SB, Kovesi T, et al. Aerosol SARS‐CoV‐2 in hospitals and long‐term care homes during the COVID‐19 pandemic. PLoS One. 2021;16:e0258151. doi: 10.1371/journal.pone.0258151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kim HR, An S, Hwang J, Park JH, Byeon JH. In situ lysis droplet supply to efficiently extract ATP from dust particles for near‐real‐time bioaerosol monitoring. J Hazard Mater. 2019;369:684‐690. doi: 10.1016/j.jhazmat.2019.02.088 [DOI] [PubMed] [Google Scholar]
- 155. Demokritou P, Gupta T, Ferguson S, Koutrakis P. Development and laboratory performance evaluation of a personal cascade impactor. J Air Waste Manag Assoc. 2002;52:1230‐1237. [DOI] [PubMed] [Google Scholar]
- 156. Cheng V, Wong S‐C, Chiu K, Yip C, Wong S, Yuen K‐Y. Detection of norovirus in air samples in a non‐vomiting patient: implications of testing saliva for norovirus in an immunocompromised host. J Hosp Infect. 2019;103:357‐358. [DOI] [PubMed] [Google Scholar]
- 157. Cheng VCC, Wong S‐C, Wong SCY, et al. Measles outbreak from Hong Kong International Airport to the hospital due to secondary vaccine failure in healthcare workers. Infect Control Hosp Epidemiol. 2019;40:1407‐1415. [DOI] [PubMed] [Google Scholar]
- 158. Wei L, Lin J, Duan X, et al. Asymptomatic COVID‐19 patients can contaminate their surroundings: an environment sampling study. Am Soc Microbiol. 2020b;5:3‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


