Abstract
Intravenous immunoglobulin (IVIg) therapy has been suggested as a potential treatment option for hospitalised COVID‐19 patients. The aim of this systematic review and meta‐analysis was to investigate the potential impact of IVIg on mortality and length of hospitalisation in adult COVID‐19 patients. PubMed, Scopus, Web of Science and medRxiv were searched in the week of 20.12.2021 for English language, prospective trials, and retrospective studies with control groups, reporting on the use of intravenous immunoglobulin therapy in adult hospitalised COVID‐19 patients. Exclusion criteria were: studies evaluating the use of IVIg in paediatric COVID‐19 cases, trials using convalescent anti‐SARS‐CoV‐2 plasma or immunoglobulins derived from convalescent anti‐SARS‐CoV‐2 plasma. A random effects meta‐analysis with subgroup analyses regarding study design and patient disease severity according to WHO criteria was also performed. A total of 13 studies were included, of which 6 were prospective, on a total of 2313 (IVIg = 1104, control = 1209) patient outcomes. Meta‐analysis results indicated that IVIg therapy had no statistically significant effect on mortality (RR 0.91 [0.59; 1.39], p = 0.65, I 2 = 69% [46%; 83%]) or length of hospital stay (MD 0.51 [−2.80; 3.81], p = 0.76, I 2 = 96% [94%; 98%]). Subgroup analyses indicated no statistically significant impact on either outcome, but prospective studies' results suggested that IVIg may increase the length of hospitalisation in the severe COVID‐19 patient group (MD 2.66 [1.43; 3.90], p < 0.01, I 2 = 0% [0%; >90%]). The results of this meta‐analysis do not support use of IVIg in hospitalised adult COVID‐19 patients.
Keywords: COVID‐19, intravenous immunoglobulin, ivig
Abbreviations
- ARDS
acute respiratory distress syndrome
- COVID‐19
corona virus disease 2019
- INF
interferon
- IQR
interquartile range
- IV
inverse variance method
- IVIg
intravenous immunoglobulins
- MD
mean difference
- MH
Mantel‐Haenszel method
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta‐Analyses
- PRS
prospective studies
- RCS
retrospective cohort studies
- REML
restricted maximum likelihood
- RoB‐2
Risk of Bias 2 tool
- ROBINS‐I
Risk Of Bias In Non‐randomised Studies ‐ of Interventions tool
- RR
risk ratio
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- WHO
World Health Organization
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has caused a worldwide pandemic with over 323 million confirmed cases and over 5.5 million deaths reported by the World Health Organisation (WHO) as of 16 January 2022. 1 The pathogenesis of COVID‐19 seems to be driven not only by the direct viral damage, but also by an overly aggressive host inflammatory responses. 2 As such, anti‐inflammatory treatments ‐ dexamethasone, 3 IL‐6 receptor antagonists 4 and Janus kinase inhibitors 5 ‐have shown efficacy in reducing mortality in hospitalised COVID‐19 patients. Additionally, meta‐analyses results have found that Janus kinase inhibitors have been associated with an increased recovery rate, shorter time till recovery and a reduction of clinical deterioration risk in COVID‐19 patients, 6 while tocilizumab has been shown to have a beneficial effect on multiple biomarkers of COVID‐19 disease such as C‐reactive protein, d‐dimer, ferritin, procalcitonin and lymphocyte levels. 7
Intravenous immunoglobulin (IVIg) has been suggested and used as a treatment option in COVID‐19 patients, with the potential to target both the viral, as well as the inflammatory segment of the disease's pathogenesis. 8 IVIg has previously been successfully used in several inflammatory and autoimmune conditions with multiple proposed immunomodulatory mechanisms of action: neutralisation of autoantibodies, modulation of the synthesis and release of cytokines/chemokines, expansion of regulatory T‐cells, regulation of dendritic cell activity and other, 9 which were all suspected of having a potential beneficial effect in COVID‐19 patients as well. Additionally, as IVIg is a pooled plasma product from a large number of individuals, it includes neutralisation activity against various pathogens including SARS‐CoV‐2. Anti‐SARS‐CoV‐2 antibodies have been detected in IVIg donor plasma pools in Europe and the USA as early as May 2020, with reports of increasing antibody levels up to September 2021 with neutralisation activity directed against pseudoviruses representing the wild‐type virus as well as alpha, beta, gamma, and delta variants, 10 although the clinical relevance of such antibodies remains unknow.
A relatively large number of published retrospective studies suggested that IVIg has been extensively used in COVID‐19 patients, especially in China, while the clinical benefits of such practice remained unknown. A meta‐analysis on the topic published in April 2021 by Xiang H. et al. Concluded that IVIg could reduce mortality in hospitalised critically ill COVID‐19 patients, although further well designed clinical trials were required to confirm the results. 11 A newer meta‐analysis published in January 2022 by Focosi D. et al. Failed to find a beneficial effect of IVIg on patient mortality, but it demonstrated that IVIg significantly reduced the length of hospital stay when given to moderately ill COVID‐19 patients. 12 In the meantime, further studies on this topic were conducted, most notably a double‐blind, placebo‐controlled, phase 3 trial in severely ill COVID‐19 patients that was published in the Lancet Respiratory Medicine. 13 In contrast to the previously reported meta‐analysis’ results, 11 the study by Mazeraud et al. 13 found no benefit of IVIg treatment in critically ill COVID‐19 patients. Considering the availability of results of additional studies which were not included in the meta‐analyses conducted earlier, there was a need to further synthetise and analyse the published data regarding this topic. Thus, in this systematic review and meta‐analysis we aimed to assess whether intravenous immunoglobulin treatment (IVIg) has any impact on mortality or hospitalisation duration in hospitalised adult COVID‐19 patients.
2. METHODS
This systematic review and meta‐analysis was written in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) checklist. 14
2.1. Search strategy
Two investigators (RM and VMD) independently searched PubMed, Scopus, Web of Science and medRxiv with the search phrase (‘intravenous immunoglobulin’ OR ‘IVIG’ OR ‘IVIg’) AND (‘COVID‐19’ OR ‘SARS‐CoV‐2’ OR ‘SARS‐COV‐2’ OR ‘Coronavirus Disease 2019’) in the week of 20.12.2021.
2.2. Eligibility criteria
We included studies exploring whether the use of IVIg compared to the standard of care alone or plus placebo in adult hospitalised COVID‐19 patients had any impact on patient mortality or length of hospital stay. Regarding study design, we included prospective, randomised controlled trials and retrospective studies with control groups. Only English language articles were included. Exclusion criteria were: studies evaluating the use of IVIg in paediatric COVID‐19 cases, trials using convalescent anti‐SARS‐CoV‐2 plasma or immunoglobulins derived from convalescent anti‐SARS‐CoV‐2 plasma.
2.3. Data extraction and quality assessment
Outcomes of interest were mortality and duration of hospitalisation. Data extraction was done independently by 2 authors (RM and VMD). With regards to mortality, the total numbers of patients in the experimental (IVIg) and control groups as well as the number of deceased patients were extracted. If multiple timepoint mortality data was reported in a particular study, the 28‐day mortality was used. Regarding the duration of hospital stay, data reporting on the mean and standard deviation (SD) of days of hospitalisation for both groups were retrieved. If a study reported median and interquartile range (IQR) values, the mean and SD values were estimated by an online calculator (https://tinyurl.com/2p9db7mk) using methods described by Luo et al. 15 and Wan et al. 16 One author (IR) conducted the risk of bias assessment using the RoB‐2 17 tool for randomised studies and the ROBINS‐I 18 tool for nonrandomised ones.
2.4. Statistical analysis
Meta‐analysis was conducted in the R (v. 4.0.5) programing language using the ‘meta’ package. 19 A random effects model was used to conduct all meta‐analyses due to heterogeneity of included studies. Effect measures used were risk ratio (RR) for mortality and mean difference (MD) for length of hospitalisation, 95% confidence intervals were used to assess certainty of results. The mortality outcome meta‐analysis was conducted using the Mantel‐Haenszel method and length of hospitalisation outcome was calculated using the inverse variance method. Tau was estimated using the Paule‐Mandel estimator and a continuity correction of 0.5 was used in studies with zero cell frequencies. Heterogeneity was assessed by I2, its 95% confidence interval and the Chi test p value, with values of I2 less than 30% being considered as low heterogeneity, 30%–60% as moderate heterogeneity and greater than 60% as high heterogeneity. Simple meta‐regression was conducted using a mixed‐effects model (tau estimator: restricted maximum likelihood (REML)) to evaluate the potential impact of total estimated IVIg dose and the time of study conduction on outcomes of interest. In all meta‐regressions only the predictor and outcome variables were used. Subgroup analyses were conducted with regards to study types (retrospective and prospective) and patient disease severity. The patients' disease severity was assessed according to study inclusion criteria as well as to the World Health Organization definition of severity; Critical group: defined by the criteria for acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life‐sustaining therapies such as mechanical ventilation (invasive or non‐invasive) or vasopressor therapy. Severe group; defined by any of the following: oxygen saturation <90% on room air, signs of severe respiratory distress (accessory muscle use, inability to complete full sentences, respiratory rate >30 breaths per minute). Non‐severe group; defined as absence of any criteria for severe or critical COVID‐19. 20 Possible publication bias was evaluated using funnel plots and Egger's test.
3. RESULTS
3.1. Study selection and overview
Our online database search identified 949 studies, out of which 13 studies were identified after screening and included in this meta‐analysis. PRISMA flow diagram can be seen on Figure 1. A total of 6 studies were excluded for not reporting on an adequate control group, 21 , 22 , 23 , 24 , 25 , 26 while 3 studies reported on convalescent plasma or hyperimmune anti‐SARS‐CoV‐2 serum 27 , 28 , 29 and some assessed studies were reviews, commentaries or publications that did not report any new data.
FIGURE 1.
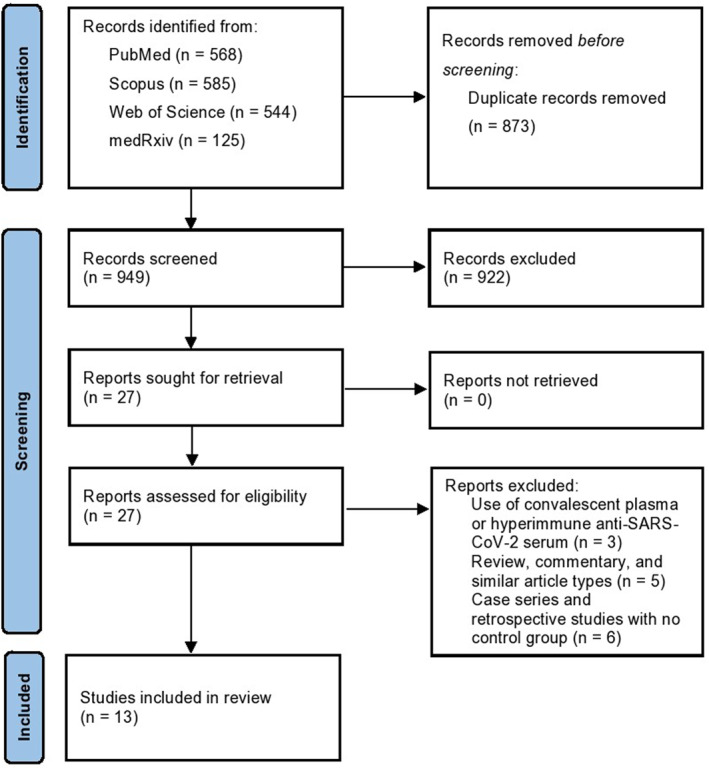
PRISMA flow diagram
Studies' characteristics can be seen in Table 1. From the 13 included studies, 7 were retrospective cohort studies 30 , 31 , 32 , 33 , 34 , 35 , 36 and 6 were prospective studies. 13 , 37 , 38 , 39 , 40 , 41 From the 6 prospective studies, 2 were randomised placebo controlled double‐blind trials, 2 randomised open‐label trials, 1 was a randomised placebo controlled open label trial and 1 was a non‐randomised open‐label prospective study. A relatively large number of studies were conducted in China (n = 5) and IVIg doses and therapy duration varied across studies, with the smallest approximated total dose being 59g and the largest 210g.
TABLE 1.
Overview of included studies
| No. | Title | First author | Publication year | Country | Study‐type | Patient severity according to WHO classification | Intervention (IVIg dose) | Results/Key findings | Study conduction/patient hospitalisation time | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Clinical outcomes of intravenous immunoglobulin therapy in COVID‐19 related acute respiratory distress syndrome: a Retrospective cohort study | Ali | 2021 | Qatar | Retrospective cohort study | Critical | Minimum one dose of 0.4 g/kg, further doses were given on consecutive days, to a maximum of 5 doses | Mortality was higher among IVIG‐treated patients (36.4% vs. 15.3%; sHR 3.5; 95% CI 1.98–6.19; p < 0.001). Ventilator‐free days and ICU‐free days at day‐28 were lower (p < 0.001 for both) | 7 March 2020–9 September 2020. | |
| 2. | High‐dose intravenous immunoglobulin in severe Coronavirus disease 2019: A multicenter retrospective study in China | Cao | 2021 | China | Retrospective cohort study | Severe | 2 g/kg, divided over 2–5 days. | The adjusted HR of 28‐day mortality in high‐dose IVIg group was 0.24 (95% CI 0.06–0.99, p < 0.001) | 7 February2020–30 March 2020 | |
| 3. | Effects of adjunct treatment with intravenous immunoglobulins on the course of severe COVID‐19: Results from a retrospective cohort study | Esen | 2021 | Turkey | Retrospective cohort study | Critical | 30 g/day for 5 consecutive days | Overall survival was 61% in the SIC + IVIG and 38% in the SIC only group (odds ratio: 2.2, 95% confidence interval: 0.9–5.4, p = 0.091 after controlling for baseline imbalances) | 19 March 2020–26 May 2020 | |
| 4. | Infliximab and intravenous Gammaglobulin in hospitalised severe COVID‐19 patients in intensive care unit | Farrokhpour | 2021 | Iran | Non‐randomised open‐label prospective study | Critical | 0.4 g/kg/d for 3–5 days | 26.1% in IVIg and 62.8% in the control group expired (p < 0.05). IVIg HR was 0.31 (95% CI: 0.12–0.76, p = 0.01) | March 2020 | |
| 5. | The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a Randomized placebo‐controlled double‐blind clinical trial | Gharebaghi | 2020 | Iran | Randomised placebo‐controlled double‐blind trial | Severe | 4 vials of 5 gm5 IVIg daily for three consecutive days | Significantly lower in‐hospital mortality rate in the IVIg group compared to control (6 [20.0%] vs. 14 [48.3%], respectively; p = 0.025). Multivariate regression analysis demonstrated that IVIg had a significant impact on mortality rate (aOR = 0.003 [95% CI: 0.001–0.815]; p = 0.042) | 9 May 2020–9 June 2020. | |
| 6. | Intravenous immunoglobulin‐based adjuvant therapy for severe COVID‐19: a single‐centre retrospective cohort study | Hou | 2021 | China | Retrospective cohort study | Severe | Not stated | After adjusting for confounding factors, differences in primary outcomes (death and initiation use of mechanical ventilation) between the two groups were not statistically significant (p = 0.167), however, patients in the IVIG group had longer hospital stay periods (p = 0.041) | 28 January2020–25 February 2020 | |
| 7. | Efficacy evaluation of intravenous immunoglobulin in non‐severe patients with COVID‐19: A retrospective cohort study based on propensity score matching | Houang | 2021 | China | Retrospective cohort study | Non‐severe | (1) 10 g/day for 3 days,8 patients; (2) 10 g/day for 5 days, 13 patients; (3) 20 g/day for 3 days, 16 patients; (4) 20 g/day for 5 days, 8 patients. | No statistically significant difference was found between the IVIg and control group in the duration of fever (p = 0.667), virus clearance time (p = 0.288), length of hospital stay (p = 0.469), or use of antibiotics (p = 0.901), progression to severe disease (p = 0.376) or mortality (p = 0.156). | Jan 202020–10 June 2020 | |
| 8. | Intravenous immunoglobulin treatment for patients with severe COVID‐19: a Retrospective multicentre study | Liu | 2021 | China | Retrospective cohort study | Severe | The median duration of IVIG treatment was 9.5 days, median doses were 9.85 g/day for survivors and 10.42 g/day for non‐survivors. | No significant difference in 28‐day mortality was observed after IPW analysis (average treatment effect (ATE) Ľ 0.008, 95% CI e0.081–0.097, p 0.863). | Not stated | |
| 9. | Intravenous immunoglobulins in patients with COVID‐19‐ associated moderate‐to severe acute respiratory distress syndrome (ICAR): Multicentre, double blind, placebo controlled, phase 3 trial | Mazeraud | 2021 | France | Randomised placebo‐controlled double‐blind trial | Critical | 2 g/kg divided into four perfusions of 0.5 g/kg over 4 days. | In patients with COVID‐19 who received invasive mechanical ventilation for moderate‐to‐severe ARDS, IVIG did not improve clinical outcomes at day 28 | 3 April 2020–20 October 2020 | |
| 10. | A phase II safety and efficacy study on prognosis of moderate pneumonia in Coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy | Raman | 2021 | India | Randomised open‐label trial | Non‐severe | 0.4 g/kg daily for 5 days | Duration of hospital stay was significantly shorter in the IVIg group compared with that of SOC alone (7.7 vs. 17.5 days). | Jul 2020–Sep 2020. | |
| 11. | Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID‐19: a Multicenter retrospective cohort study | Shao | 2020 | China | Retrospective cohort study | Both severe and critical group | The doses used differed among the different centres and physicians, ranging from 0.1 to 0.5 g/kg per day for infusion. The treatment period ranged from 5 to 15 days. | The 28‐day mortality was improved with IVIg after adjusting confounding in overall cohort (p = 0.0014), and the in‐hospital and the total duration of disease were longer in the IVIG group (p < 0.001). | Dec 2019–March 2020 | |
| 12. | Intravenous immunoglobulin (IVIG) significantly reduces respiratory morbidity in COVID‐19 pneumonia: A prospective randomized trial | Sakoulas | 2020 (medRxiv preprint) | California | Randomised placebo‐controlled open label trial | Severe | 0.5 g/kg daily for 3 days | The IVIG group showed i) a lower rate of progression to requiring mechanical ventilation (p = 0.038), ii) shorter median hospital length of stay (p = 0.01), iii) shorter median ICU stay (p = 0.006) than SOC. | 1 May 2020–16 June 2020 | |
| 13. | Evaluating the effects of intravenous immunoglobulin (IVIg) on the management of severe COVID‐19 cases: A randomized controlled trial | Tabarsi | 2021 | Iran | Randomised open‐label trial | Severe | 0.4 g/kg daily for 3 days | There was no significant difference between the two groups in terms of mortality rate (p = 0.8) and the need for mechanical ventilation (p = 0.39). The length of hospital stay was significantly lower for the control group than that of the intervention group (p = 0.003). | Not stated | |
3.2. Mortality
Results from a total of 13 studies reporting on 2313 (IVIg = 1104, control = 1209) patient outcomes have been included in this meta‐analysis. No statistically significant impact of IVIg treatment on mortality was observed (RR 0.91 [0.59; 1.39], p = 0.65), with high interstudy heterogeneity (I 2 = 69% [46%; 83%]), Figure 2.
FIGURE 2.
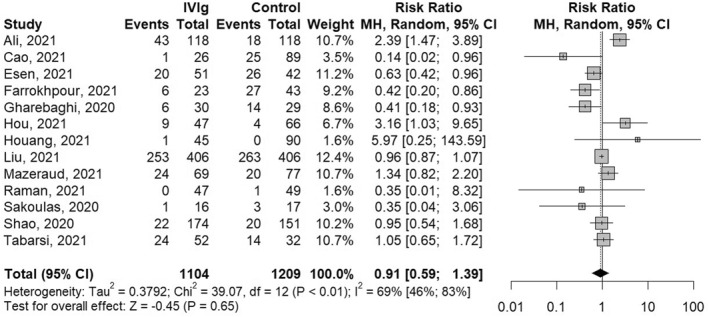
Meta‐analysis results and forest plot of the effect of intravenous immunoglobulin (IVIg) treatment on hospitalised COVID‐19 patient mortality. MH, Mantel‐Haenszel method
No significant difference (p = 0.31) was observed in the subgroup analysis between retrospective cohort studies (RR 1.13 [0.56; 2.30], p = 0.73, I 2 = 77% [53%; 89%]) and prospective studies' results (RR 0.73 [0.45; 1.18],p = 0.20, I 2 = 57% [0%; 83%]), Figure 3a. Subgroup analysis regarding patient severity according to the WHO disease severity criteria, Figure 3b, indicated there was no statistically significant effect of IVIg on mortality neither in non‐severe (RR 1.44 [0.09; 23.33], p = 0.80, I 2 = 35%), nor in severe (RR 0.82 [0.43; 1.57], p = 0.55, I 2 = 57% [0%; 81%]) nor in critically ill patients (RR 0.86 [0.45; 1.57], p = 0.56, I 2 = 87% [71%; 94%]), with no significant subgroup differences (p = 0.93). No statistically significant effect was found in the additional analysis regarding both the study type and patient disease severity, Figures 3c,d respectively. Meta‐regression found no statistically significant impact of approximated total IVIg dose or time of study conduction on the mortality outcome, Supplement Figures 3 and 5, respectively.
FIGURE 3.
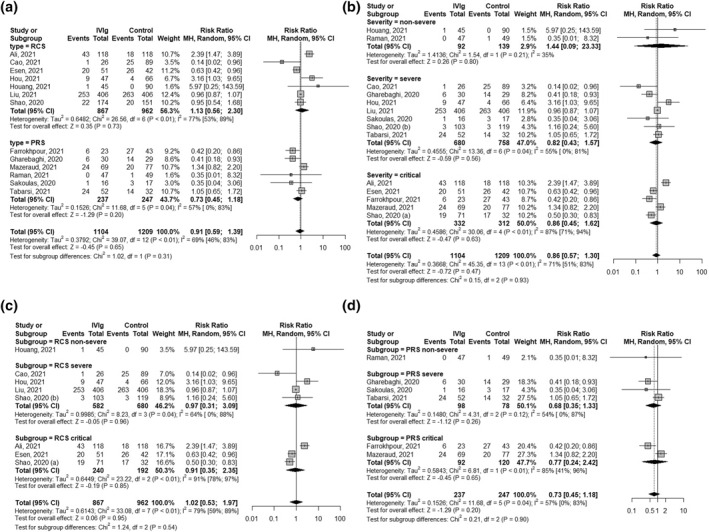
Subgroup analysis forest plots of the effect of intravenous immunoglobulin (IVIg) treatment on hospitalised COVID‐19 patient mortality. Figure 3(a) subgroup analysis according to study type, Figure 3(b) subgroup analysis according to patient severity, Figure 3(c) only retrospective cohort studies (RCS) subgroup analysis according to patient severity, Figure 3(d) only prospective studies (PRS) subgroup analysed according to patient severity. MH, Mantel‐Haenszel method; RCS, retrospective cohort studies; PRS, prospective studies
3.3. Length of hospitalisation
Only 10 out of 13 studies reported on the length of hospitalisation. Corresponding authors of the 3 studies have been contacted via email, however no reply was received. Consequently, a total of 1044 (IVIg = 492, control = 552) patient outcomes were included in the meta‐analysis regarding the length of hospital stay. No statistically significant impact of IVIg treatment on length of hospitalisation was observed (MD 0.51 [−2.80; 3.81], p = 0.76), with high interstudy heterogeneity (I 2 = 96% [94%; 98%]), Figure 4.
FIGURE 4.
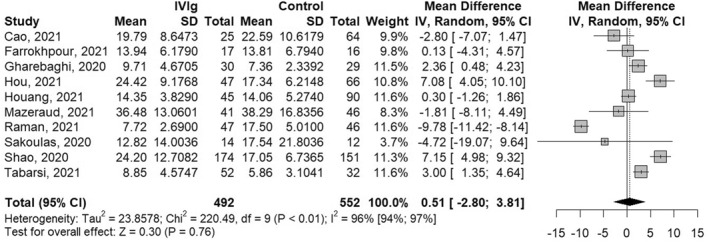
Meta‐analysis results and forest plot of the effect of intravenous immunoglobulin (IVIg) treatment on length of hospitalisation of COVID‐19 patients. IV, Inverse variance method
In the subgroup analysis, no significant difference (p = 0.17) was observed between retrospective cohort studies (MD 3.06 [−1.76; 7.87], p = 0.21, I 2 = 92% [84%; >96%]) and prospective studies' results (MD−1.45 [−5.62; 2.72],p = 0.50, I 2 = 97% [94%; 89%]), Figure 5a. Subgroup analysis regarding patient severity according to the WHO disease severity criteria, Figure 5b, showed no statistically significant effect of IVIg on the length of hospitalisation neither in the non‐severe (MD −4.74 [−14.61; 5.14], p = 0.35, I 2 = 99% [97%; 99%]), nor severe (MD 3.11 [−0.12; 6.35], p = 0.06, I 2 = 80% [57%; 91%]) nor in critically ill patients (MD 2.56 [−3.82; 8.95], p = 0.43, I 2 = 80% [38%; >94%]), with no significant subgroup differences (p = 0.33). Moreover, no statistically significant effect was found in further analysis regarding both the study type or patient disease severity regarding the retrospective cohort studies, Figure 5c, however the results from the prospective trials suggested that IVIg treatment in severe patients may increase the length of hospitalisation (MD 2.66 [1.43; 3.90], p < 0.01, I 2 = 0% [0%; >90%]), Figure 5d. Meta‐regression found no statistically significant impact of approximated total IVIg dosing or time of study conduct on the length of hospitalisation outcome, Supplement Figures 4 and 6, respectively.
FIGURE 5.
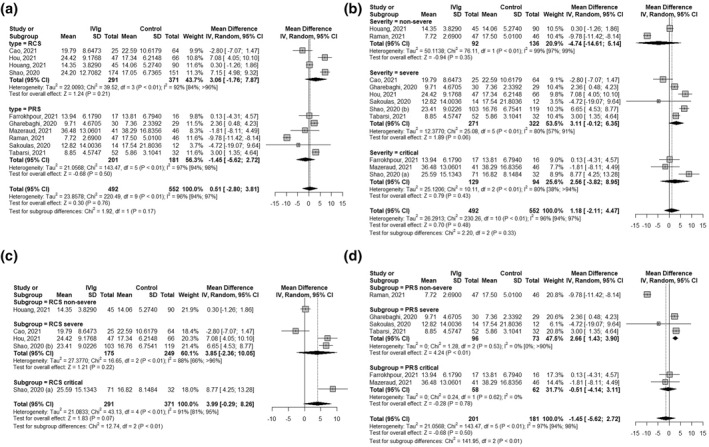
Subgroup analysis forest plots of the effect of intravenous immunoglobulin (IVIg) treatment on length of hospitalisation of COVID‐19 patients. Figure 5(a) subgroup analysis according to study type, Figure 5(b) subgroup analysis according to patient severity, Figure 5(c) only retrospective cohort studies (RCS) subgroup analysis according to patient severity, Figure 5(d) only prospective studies (PRS) subgroup analysed according to patient severity. IV, Inverse variance method; RCS, retrospective cohort studies; PRS, prospective studies
3.4. Bias assessment and certainty of evidence
Funnel plots and Eggers's test indicated no publication bias for mortality (p = 0.78) or length of hospitalisation (p = 0.8) outcomes, Figure 6. Overall risk of bias assessment of the included studies can be seen on Figure 7. randomised studies had a better risk assessment, with 2 studies having a low risk and 3 studies having some concerns. Non‐randomised studies had mostly a moderate risk of bias, although 3 studies had a serious risk of bias due to confounding. Looking at the conducted subgroup analysis according to study design, retrospective studies had an overall higher risk of bias, with no study having low risk, 5 studies having a moderate risk and 2 studies having a high risk of bias. On the other hand, the prospective studies subgroup had a more favourable risk of bias, with 2 studies having a low overall risk of bias, 3 having some concerns and 1 study (Farrokhpour) having serious risk of bias. Despite the marked difference in the risk of bias assessment, no statistically significant difference was observed in results between the 2 subgroups (p = 0.31). Furthermore, sensitivity analysis of the prospective study results also showed no difference in the meta‐analysis results due to the risk of bias of the included studies, Supplement Figure 7. The certainty of evidence is assessed to be low due to potential risk of bias and inconsistency due to high statistically significant heterogeneity of the included studies.
FIGURE 6.
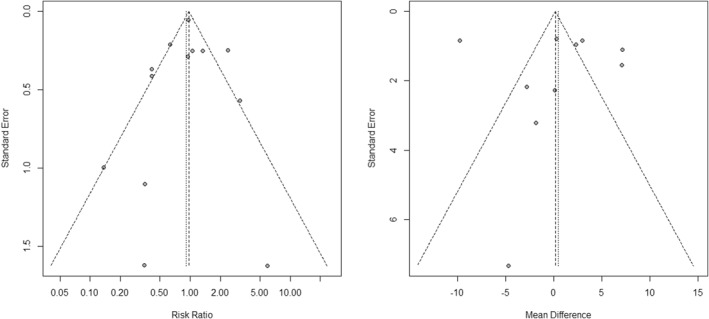
Funnel plots for mortality and length of hospitalisation
FIGURE 7.
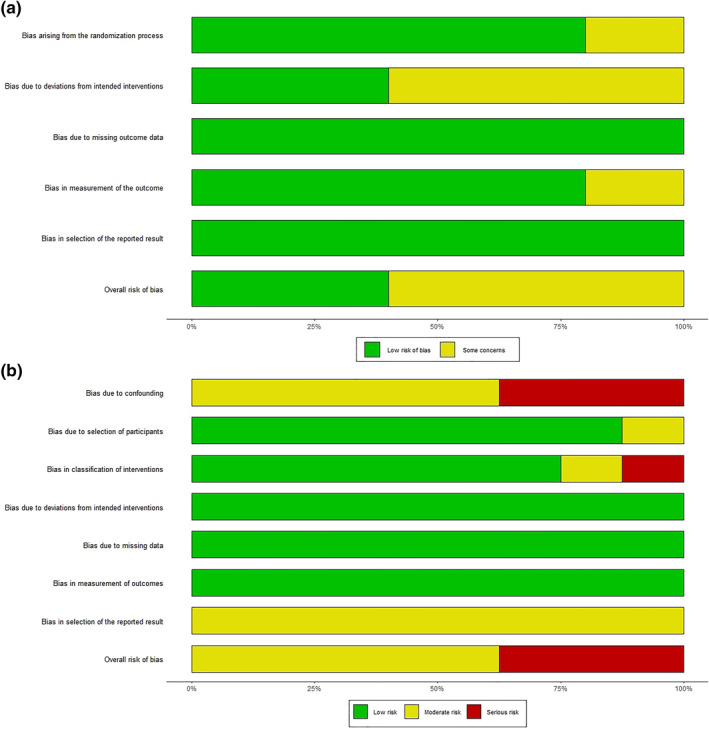
Risk of bias assessment of included studies. Figure 7(a) Bias assessment of randomised studies using the RoB 2 tool. Figure 7(b) Bias assessment of non‐randomised studies using the ROBINS‐I tool
4. DISCUSSION
Results from a total of 13 studies, reporting on the use of IVIg therapy in hospitalised adult COVID‐19 patients were pooled and analysed in this meta‐analysis. Overall, our results suggest that IVIg therapy provides no significant benefit regarding patient mortality or length of hospitalisation, with results from prospective studies indicating that IVIg therapy may actually increase the length of hospital stay in severe COVID‐19 patients.
The results of this meta‐analysis are partially in accord with a previously conducted meta‐analysis on the topic, but certain potential differences should be highlighted. The meta‐analysis conducted by Xiang et al. 11 analysed 7 studies (all of which were included in our analysis as well) using a fixed effect model and found no statistically significant benefit for the severe and non‐severe patient groups regarding mortality but found a significant effect (RR 0.57 [0.42; 0.79]) in the critically ill COVID‐19 patient group which favoured IVIg treatment. Our literature search identified 6 additional studies, 3 of which reported on critically ill COVID‐19, patients and found no significant effects (RR 0.86 [0.45; 1.57]). A newer meta‐analysis on the topic by Focosi D. et al. 12 analysed 10 studies (all of which are again included also in our analysis) and while they failed to find efficacy of IVIg regarding the patient mortality outcome, they did detect a beneficial statistically significant effect (MD −2.24 [−3.20; −1.27]) regarding the length of hospital stay in the moderate severity patient subgroup. Interestingly, the results of our meta‐analysis on the other hand suggest, that IVIg may increase the length of hospitalisation for severe COVID‐19 patients. As no significant effect was observed regarding mortality, it seems unlikely that this increase in the duration of hospital stay is a result of lower mortality in this patient subgroup. Another possible explanation are potential serious adverse events associated with IVIg treatment: renal impairment, thromboses, arrhythmias, aseptic meningitis, haemolytic anaemia, and transfusion‐related acute lung injury. 42 For instance, Mazeraud A. et al. 13 reported an increased frequency of serious adverse events in the IVIg treated patient group compared to the placebo group, although the difference was not statistically significant (p = 0.089). The rationale behind the idea of repurposing IVIG for the treatment of hospitalised COVID‐19 patients was sound, but unfortunately, according to our meta‐analysis’ results, it seems that IVIG lacks clinical efficacy in COVID‐19. Some of the potential reasons for the lack of efficacy could be that the new SARS‐CoV‐2 variants evade neutralising antibodies in IVIG and that a lack of standardised anti‐SARS‐CoV‐2 titres in the product leads to potential underdosing. Although anti‐SARS‐CoV‐2 antibodies with neutralising activity against pseudoviruses have been found in IVIG products, 10 they stem from vaccination aimed at the original Wuhan strain or from prior infection. The production process behind IVIG takes some time during which new immune evasive variants are continuously emerging, such as the Omicron BA.2.12.1, BA.4 and BA.5 subvariants which successfully evade neutralising antibodies arising after vaccination. 43 Furthermore, the amount of anti‐SARS‐CoV‐2 antibodies in different IVIG products is not standardised and no dose‐response studies have been performed thus the appropriate dosage in relation to the anti‐SARS‐CoV‐2 titters in the product is unknown. Moreover, the efficacy of antiviral therapeutics is better the earlier in the disease's course the therapy is administered. Therefore, in already hospitalised COVID‐19 patients the beneficial effect of anti‐SARS‐CoV‐2 antibodies seems limited and the immunomodulatory effects of IVIG may be too weak in comparison to other therapeutics which have so far demonstrated efficacy such as dexamethasone. 3
Highly statistically significant heterogeneity was observed among the included studies' results, with heterogeneity remaining high despite subgroup analysis according to the patients' severity and/or study design. Multiple other potential factors could have had an impact on individual study results and consequently interstudy heterogeneity, such as differences in IVIg treatment dose/duration and protocol, time of the study conduct, duration of COVID‐19 illness prior to IVIg administration, confounding effects of other used therapeutic treatments (especially in retrospective studies) and use of different non‐standardised IVIg formulations with unknown anti‐SARS‐CoV‐2 antibody titres/neutralisation activity. The dose and duration of IVIg treatment varied across the included studies, however the performed meta‐regression found no statistically significant effects of approximated total dose on either outcome. As reported earlier, the levels of SARS‐CoV‐2 antibodies found in IVIg donor plasma pools have been increasing parallel to the spread of the virus in the population, raising the possibility that IVIg preparations made later during the pandemic could have higher anti‐SARS‐CoV‐2 efficacy and clinical utility, although we found no significant effect in the meta‐regression with regards to the number of months between the start of the pandemic (taken as December 2019) and the study conduction date. Nevertheless, according to current trends regarding the increasing anti‐SARS‐CoV‐2 antibody titres, it is possible that the future IVIg products may potentially equate hyperimmune immunoglobulin preparations in terms of titres and efficacy, although at lower costs, with easier production and higher availability. 44 Therefore, additional research should be conducted in the future to again evaluate the potential clinical utility of IVIg prepared after or in the late stages of the COVID‐19 pandemic.
Further studies on the topic should concentrate on identifying potential patient subgroups which may nonetheless have a benefit from IVIg treatment. Patient subgroups benefiting from IVIg may be those with either primary or secondary hypogammaglobulinemia which are associated with an increased risk of bacterial superinfection, septic shock and death. 45 Another potentially important patient subgroup deserving further studies are those positive for neutralising autoantibodies against type I interferons (IFN). A recent study published in the Science found that at least 10.2% (n = 101/987) of patients with life‐threatening COVID‐19 pneumonia had neutralizing IgG auto‐antibodies against type 1 IFNs, while such auto‐antibodies were present in only 0.33% (n = 4/1227) healthy individuals. 46 Such a discrepancy could indicate that patients with anti‐IFN autoantibodies may be predisposed to more severe clinical outcomes. Studies evaluating the clinical utility of anti‐IFN autoantibodies as predictive biomarkers of COVID‐19 disease severity are urgently needed and such patients may also benefit from the immunomodulatory effects of IVIg treatment.
This meta‐analysis has several limitations. First, the systematic review included only English language studies, while it appears that IVIg therapy has been extensively used in China, so it is likely that additional Chinese language studies were not included. Second, the dosage and length of IVIg treatment varied between included studies, although it seems that, according to the conducted meta‐regression results, those differences had no significant impact on outcomes. Third, a relatively large number of included studies were retrospective (7) while only 2 studies were randomised placebo controlled double‐blind trials. Finally, a moderate to serious risk of bias was observed in the analysed non‐randomised studies and the protocol for this meta‐analysis has not been prospectively registered, so the bias on the part of investigators cannot be ruled out.
5. CONCLUSION
The results of this meta‐analysis do not support use of IVIg in hospitalised adult COVID‐19 patients. Further high‐quality, double‐blind, randomised, placebo‐controlled trials should concentrate on identifying specific patient subgroups which may nonetheless benefit from IVIg therapy.
AUTHOR CONTRIBUTIONS
Robert Marcec conceived the idea, conducted the analysis, and wrote the first manuscript draft. Robert Marcec and Vinko Michael Dodig conducted the systematic review and data extraction. Igor Radanovic conducted the risk of bias assessment. Igor Radanovic and Robert Likic provided critical feedback and suggestions. All authors participated in writing the final manuscript version.
CONFLICT OF INTEREST
No conflicts of interest to declare.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENT
No funding was received for this study.
Marcec R, Dodig VM, Radanovic I, Likic R. Intravenous immunoglobulin (IVIg) therapy in hospitalised adult COVID‐19 patients: a systematic review and meta‐analysis. Rev Med Virol. 2022;e2397. 10.1002/rmv.2397
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
REFERENCES
- 1. WHO. COVID‐19 weekly epidemiological update. World Heal Organ. 2021;58:1‐23. https://www.who.int/publications/m/item/covid‐19‐weekly‐epidemiological‐update [Google Scholar]
- 2. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The REMAP‐CAP Investigators . Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. N Engl J Med. 2021;384(16):1491‐1502. 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wijaya I, Andhika R, Huang I, et al. The use of Janus Kinase inhibitors in hospitalized patients with COVID‐19: systematic review and meta‐analysis. Clin Epidemiol Glob Heal. 11, 2021;11(May):100755. 10.1016/j.cegh.2021.100755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Limen RY, Sedono R, Sugiarto A, Hariyanto TI. Janus kinase (JAK)‐inhibitors and coronavirus disease 2019 (Covid‐19) outcomes: a systematic review and meta‐analysis. Expert Rev Anti Infect Ther. 2022;20(3):425‐434. 10.1080/14787210.2021.1982695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ivan Hariyanto T, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93(3):1832‐1836. 10.1002/jmv.26698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perricone C, Triggianese P, Bursi R, et al. Intravenous immunoglobulins at the Crossroad of autoimmunity and viral infections. Microorganisms. 2021;9(1):121. 10.3390/microorganisms9010121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaigne B, Mouthon L. Mechanisms of action of intravenous immunoglobulin. Transfus Apher Sci. 2017;56(1):45‐49. 10.1016/j.transci.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 10. Romero C, Díez J‐M, Gajardo R. Anti‐SARS‐CoV‐2 antibodies in healthy donor plasma pools and IVIG products—an update. Lancet Infect Dis. 2022;22(1):19. 10.1016/S1473-3099(21)00755-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiang H, Cheng X, Li Y, Luo W, Zhang Q, Peng W. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID‐19): a meta‐analysis. Int Immunopharm. 96, 2021;96(January):107732. 10.1016/j.intimp.2021.107732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Focosi D, Franchini M, Tuccori M, Cruciani M. Efficacy of high‐dose polyclonal intravenous immunoglobulin in COVID‐19: a systematic review. Vaccines. 2022;10(1):94. 10.3390/vaccines10010094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazeraud A, Jamme M, Mancusi RL, et al. Intravenous immunoglobulins in patients with COVID‐19‐associated moderate‐to‐severe acute respiratory distress syndrome (ICAR): multicentre, double‐blind, placebo‐controlled, phase 3 trial. Lancet Respir Med. November 10(2), 2021. 10.1016/S2213-2600(21)00440-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2018;27(6):1785‐1805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 16. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1‐13. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 18. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta‐analysis with R: a practical tutorial. Evid Base Ment Health. 2019;22(4):153‐160. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guideline WHO . Clinical Management of COVID‐19 Patients: Living Guideline, 18 November 2021. 2021. https://apps.who.int/iris/handle/10665/349321
- 21. Herth FJF, Sakoulas G, Haddad F. Use of intravenous immunoglobulin (prevagen or octagam) for the treatment of COVID‐19: retrospective case series. Respiration. 2020;99(12):1145‐1153. 10.1159/000511376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khodashahi R, Naderi HR, Sedaghat A, et al. Intravenous immunoglobulin for treatment of patients with COVID‐19: a case‐control study. Arch Clin Infect Dis. 2021;16(1). 10.5812/archcid.108068 [DOI] [Google Scholar]
- 23. Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID‐19. J Infect. 2020;81(2):318‐356. 10.1016/j.jinf.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omma A, Erden A, Armağan B, et al. A single center experience of intravenous immunoglobulin treatment in Covid‐19. Int Immunopharm. 2021;98:107891. 10.1016/j.intimp.2021.107891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reynaga E, Carrillo J, Santos JR, et al. Outcome of hospitalized patients with COVID‐19 pneumonia treated with high‐dose immunoglobulin therapy in a prospective case series. Clin Microbiol Infect. 2021;27(4):651‐652. 10.1016/j.cmi.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zantah M, Castillo ED, Gangemi AJ, et al. Anakinra and intravenous IgG versus tocilizumab in the treatment of COVID‐19 pneumonia. medRxiv. 2020. 10.1101/2020.09.11.20192401 [DOI] [Google Scholar]
- 27. Gonzalez JLB, González Gámez M, Mendoza Enciso EA, et al. Efficacy and safety of convalescent plasma and intravenous immunoglobulin in critically ill COVID‐19 patients. A controlled clinical trial. medRxiv. January 2021:2021.03.28.21254507. 10.1101/2021.03.28.21254507 [DOI] [Google Scholar]
- 28. Parikh D, Chaturvedi A, Shah N, Patel P, Patel R, Ray S. Safety and efficacy of COVID‐19 hyperimmune globulin (HIG) solution in the treatment of active COVID‐19 Infection‐ Findings from a Prospective, Randomized, Controlled, Multi‐Centric Trial. medRxiv. January 2021. 10.1101/2021.07.26.21261119 [DOI] [Google Scholar]
- 29. Ali S, Uddin SM, Shalim E, et al. Hyperimmune anti‐COVID‐19 IVIG (C‐IVIG) treatment in severe and critical COVID‐19 patients: a phase I/II randomized control trial. EClinicalMedicine. 2021;36:100926. 10.1016/j.eclinm.2021.100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ali HS, Elshafei MS, Saad MO, et al. Clinical outcomes of intravenous immunoglobulin therapy in COVID‐19 related acute respiratory distress syndrome: a retrospective cohort study. BMC Pulm Med. 2021;21(1):1‐7. 10.1186/s12890-021-01717-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C, Fei L, Li W, et al. Efficacy evaluation of intravenous immunoglobulin in non‐severe patients with COVID‐19: a retrospective cohort study based on propensity score matching. Int J Infect Dis. 2021;105(January):525‐531. 10.1016/j.ijid.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Esen F, Özcan PE, Orhun G, et al. Effects of adjunct treatment with intravenous immunoglobulins on the course of severe COVID‐19: results from a retrospective cohort study. Curr Med Res Opin. 2021;37(4):543‐548. 10.1080/03007995.2020.1856058 [DOI] [PubMed] [Google Scholar]
- 33. Hou X, Tian L, Zhou L, et al. Intravenous immunoglobulin‐based adjuvant therapy for severe COVID‐19: a single‐center retrospective cohort study. Virol J. 2021;18(1):101. 10.1186/s12985-021-01575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu J, Chen Y, Li R, et al. Intravenous immunoglobulin treatment for patients with severe COVID‐19: a retrospective multicentre study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(10):1488‐1493. 10.1016/j.cmi.2021.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID‐19: a multicenter retrospective cohort study. Clin Transl Immunol. 2020;9(10):2020.04.11.20061739. 10.1002/cti2.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao W, Liu X, Hong K, et al. High‐dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12:671443. 10.3389/fimmu.2021.627844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin (IVIG) significantly reduces respiratory morbidity in COVID‐19 pneumonia: a prospective randomized trial. medRxiv. 2021(165):1‐13. 10.1101/2020.07.20.20157891 [DOI] [Google Scholar]
- 38. Raman RS, Bhagwan Barge V, Anil Kumar D, et al. A phase II safety and efficacy study on prognosis of moderate pneumonia in coronavirus disease 2019 patients with regular intravenous immunoglobulin therapy. J Infect Dis. 2021;223(9):1538‐1543. 10.1093/infdis/jiab098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabarsi P, Barati S, Jamaati H, et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID‐19 cases: a randomized controlled trial. Int Immunopharm. 2021;90:107205. 10.1016/j.intimp.2020.107205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat‐Ebrahimi S.‐R, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo‐controlled double‐blind clinical trial. BMC Infect Dis. 2020;20(1):786. 10.1186/s12879-020-05507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farrokhpour M, Rezaie N, Moradi N, et al. Infliximab and intravenous gammaglobulin in hospitalized severe COVID‐19 patients in intensive care unit. Arch Iran Med. 2021;24(2):139‐143. 10.34172/aim.2021.22 [DOI] [PubMed] [Google Scholar]
- 42. Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;9(JUN):1‐13. 10.3389/fimmu.2018.01299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang Q, Guo Y, Iketani S, et al. Antibody evasion by SARS‐CoV‐2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603‐608. 10.1038/s41586-022-05053-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Focosi D, Franchini M. Passive immunotherapies for COVID‐19: the subtle line between standard and hyperimmune immunoglobulins is getting invisible. Rev Med Virol. 2022;32(4). 10.1002/rmv.2341 [DOI] [PubMed] [Google Scholar]
- 45. Wilfong EM, Matthay MA. Intravenous immunoglobulin therapy for COVID‐19 ARDS. Lancet Respir Med. 2021;10(2):123‐125. 10.1016/S2213-2600(21)00450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science. 2020;370(6515). 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).


