Abstract
Background
Occupational skin diseases (OSDs) are common in healthcare workers (HCWs).
Objectives
To investigate and compare the incidence and clinical features of OSDs among HCWs before and during the COVID‐19 pandemic.
Materials and Methods
Incident cases of OSDs were investigated in the cohort of HCWs at Trieste Hospitals from 1 July 2018 (3340 workers) to 31 October 2021 (137 532 person‐months).
Results
The monthly incidence was ranging from 0 to 11.90 cases per 10 000 person‐months in pre‐COVID‐19 period (cumulative incidence 4.22; 95% confidence interval [CI]: 2.9–6.0) and from 0 to 13.61 cases per 10 000 person‐months in COVID‐19 period (cumulative incidence 5.06; 95% CI: 3.6–6.9). The incidence rate ratio (IRR) between COVID‐19 and pre‐COVID‐19 period was 1.22 (95% CI: 0.73–1.98). The incidence of OSDs in the COVID‐19 period was 6.1 (4.2–8.6) and 2.7 (95% CI: 1.1–5.6) cases × 10 000 person‐months for women and men, respectively, with an IRR of 2.25 (95% CI: 0.98–5.9). Incidence in nurses in the COVID‐19 period was 6.7 (95% CI: 4.2–10.2) cases × 10 000 person‐months.
Conclusions
Incidence of OSDs was a little bit higher during the COVID‐19 pandemic compared to the previous period but fluctuation of numbers were mainly related to calendar period, with higher incidence in winter and spring. Incidence data were higher than that observed in 2004–2013 in the same cohort. Face dermatitis cases doubled after the start of COVID‐19 pandemic. Overall data demonstrated a non‐significant increase of OSDs in HCWs during the pandemic, probably due to the preventive strategies set up in our cohort over the years.
Keywords: contact dermatitis, COVID‐19, healthcare workers, incidence
Overall data demonstrated a non‐significant increase of occupational skin diseases in healthcare workers (HCWs) during the pandemic, probably due to the preventive strategies set up in our cohort over the years. When the COVID‐19 pandemic started, all HCWs had to use alcohol‐based gel reducing the use of soaps.
1. INTRODUCTION
Healthcare workers (HCWs) are at the frontline of the COVID‐19 outbreak response and are therefore at high risk for acquiring and transmitting SARS‐CoV‐2 infection. 1 , 2 Enhanced hand hygiene and adequate wearing of personal protective equipment (PPE), such as gloves, masks, goggles and face shields, are crucial for HCWs in order to prevent transmission of COVID‐19. 3
Due to frequent hand washing and prolonged glove wearing (wet work), occupational skin diseases (OSDs), mostly irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD), are common in HCWs. 4 Hence, the increased protective measures adopted by HCWs fighting against COVID‐19 might cause an increase in the occurrence and severity of their skin disorders.
According to studies from China, Saudi Arabia, Turkey, Ireland and Germany, the prevalence of skin reactions to PPE use and increased hygiene behaviour in frontline HCWs ranges from 46.4% to 97%, 5 , 6 , 7 , 8 , 9 , 10 , 11 which is higher than prevalence estimated before the pandemic. 12 , 13 In a cross‐sectional survey of Italian clinicians, the observed prevalence of occupational hand dermatitis (OHD) is 18%, lower than in above‐mentioned countries, while a high OHD incidence (80%) is found. 14 To date, no other data on incidence of OSDs during the COVID‐19 pandemic is available.
This study aims to investigate and compare the incidence and clinical features of OSDs among HCWs at Giuliano‐Isontina University Health Authority (ASUGI), northeastern Italy, before and during the COVID‐19 pandemic, in the cohort of health personnel periodically screened at Unit of Occupational Diseases of University of Trieste.
2. MATERIALS AND METHODS
Incident cases of OSDs in the cohort of ASUGI HCWs were investigated 20 months before and after the start of COVID‐19 pandemic (from July 2018 to November 2021). The study population consisted of HCWs occupied in the period considered. A total of 3340 workers represented the cohort on 1 July 2018, and by 3648 workers on 31 October 2021 for an overall 137 532 person‐months Calculation of incidence cases for each month was performed considering new cases of contact dermatitis in the considered month on total HCWs exposed that month × 10 000. Workers with previous contact dermatitis were excluded from the calculation of incidence.
At the time of hiring, all HCWs underwent clinical evaluation, were prick tested for common allergens and latex and filled in a questionnaire to investigate skin‐related diseases and atopic status. Every 2 years the participants underwent a new medical examination. In case of skin symptoms, HCWs could go to the Occupational Medicine Unit where they were evaluated by an occupational physician and tested when indicated. 15 New symptomatic cases were prick tested with the same latex extract and patch tested with standard and occupational haptens. Inclusion criteria was to work as HCW at ASUGI and to have required a medical examination at Units of Occupational Medicine for a possible work related hand contact dermatitis or face dermatitis 20 months before and after the start of COVID‐19 pandemic in Trieste (1 March 2020). Exclusion criteria was to present dermatitis in other body sites or without correlation with work activity.
Our study describes incident cases in the two period considered.
Patients were patch tested using Finn chambers on Scanpor tape (SmartPractice) and haptens produced by Chemotechnique Diagnostics, FIRMA and SmartPractice with the European baseline series and the Triveneto extended series. 16 , 17 Thirty‐six patients were tested with ‘healthcare series’ (amoxicillin trihydrate 10.0% pet.; lidocaine 15% pet.; bisphenol A 1.0% pet.; dichlorofene 0.5% pet.; hexachlorofene 1.0% pet.; glutaraldheyde 0.5% pet.; povidone iodine 10%% in water; chlorhexidine digluconate 0.5% pet.; benzalkonium chlroride 0.1% in water; p‐chlor‐m‐xylenol 1.0% pet.). Thirty‐nine patients were tested with ‘rubber series’ (N‐cyclohexyl‐2‐benzothiazolesulfenamide 1.0% pet., 2‐mercaptobenzothiazole 2.0% pet.; 1,3‐diphenylguanidine 1.0% pet.; N,N′‐diphenylthiourea 1.0% pet.; N‐phenyl‐2‐naphtylamine 1.0% pet., zinc diethyldithiocarbamate 1.0% pet.; zinc dibutyldithiocarbamate 1.0% pet., zinc dimethyldithiocarbamate 1.0% pet.; N,N′‐diethylthiourea 1.0% pet., N,N′‐dibutylthiourea 1.0% pet.; thiourea 0.1% pet).
All patches were applied on the upper back and removed after 48 h. The sites were examined on removal (day 2 [D2]) and after 72/96 h (D3/D4), according to the International Contact Dermatitis Research Group guidelines. 18 Patch tests with patients' own material were performed applying a piece of used gloves and masks, as reported by Santarossa et al. 19 Reactions of grades +, ++ and +++ were considered positive. Doubtful reactions (?+) were considered negative. Written informed consent was obtained from all the participants, and the study protocol was approved by the local ethics committee.
Skin prick tests (SPTs) included common inhalant allergens, perennial (Dermatophagoides farinae, Dermatophagoides pteronyssinus and dog and cat dander), and pollens (Gramineae, Parietariaspecies, Betulaceae and Oleaceae). Extracts of common allergens and latex were supplied by Lofarma Allergeni. The protein concentration of this latex extract was 12.5 mg/ml. The positive control was 1% histamine hydrogen chloride solution and the negative control was 1% glycerinate solution. SPTs were performed by trained registered nurses. Skin test sites were clearly marked, a drop of extract was placed on the skin, and this spot of skin was pricked with commercially available skin test lancets (Hollister Stier Laboratory). All tests were read and recorded after 15 min, and a wheal of at least 3 mm was considered a positive result. A single positive response to an inhalant allergen was considered the determining criterion for atopy (by SPT). 15
OSDs were defined when clinical data suggested an association with job tasks, and dermatitis improved out of work. ACD was defined when patch tests were positive and relevant.
Data analysis was performed using STATA 17.0. Continuous variables were summarized as median and interquartile range (IQR 25°–75° percentiles) due to non‐normal distribution. The difference between continuous variables was tested by Mann–Whitney test, whereas the difference between categorical data cross‐tabulated into contingency tables was tested by the χ 2 test. Incidence was calculated considering exposed subjects in person‐months and new symptomatic cases registered in each month of the follow‐up. We performed weighted regression analyses using duration of follow‐up as the weight to account for the increased risk of developing the outcomes associated with a longer follow‐up. For all statistical analyses, a 0.05 level of significance was used, and all p values were two‐sided.
3. RESULTS
3.1. Characteristics of the study population
From July 2018 to October 2021, 64 HCWs with hand or face dermatitis were studied for suspected OSDs (Figure 1 and Table 1). Twenty‐eight and thirty‐six HCWs were evaluated in the 20 months before (from July 2018 to February 2020) and after the COVID‐19 outbreak (from March 2020 to October 2021), respectively. Figure 2 reports the temporal trend and incidence calculated as cases on 10 000 person‐months showing a fluctuation of incidence in relationship with calendar months, with higher values in winter and spring months in 2019, a decrease in summer months and another increase from January to May 2021. The monthly incidence was ranging from 0 to 11.90 cases per 10 000 person‐months in pre‐COVID‐19 period (cumulative incidence was 4.22; 95% confidence interval [CI]: 2.9–6.0 cases per 10 000 person‐months) and from 0 to 13.61 cases per 10 000 person‐months in COVID‐19 period (cumulative incidence was 5.06; 95% CI: 3.6–6.9 cases per 10 000 person‐months). The incidence rate ratio (IRR) between COVID‐19 and pre‐COVID‐19 period was 1.22 (95% CI: 0.73–1.98).
FIGURE 1.
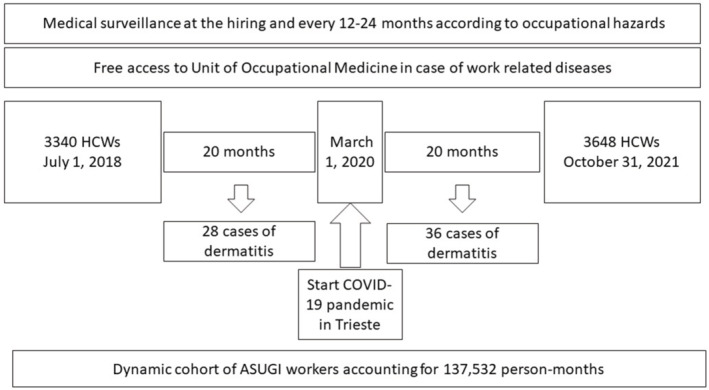
Layout of the study
TABLE 1.
Characteristics of subjects that developed an occupational skin disease before and during COVID‐19 pandemic
| Characteristics of the population | Before COVID‐19 N = 28 | During COVID‐19 N = 36 | Total N = 64 | p Value |
|---|---|---|---|---|
| Sex | ||||
| Females n (%) | 24 (85.7) | 30 (83.3) | 54 (84.4) | 0.795 |
| Males n (%) | 4 (14.3) | 6 (16.7) | 10 (15.6) | |
| Age, years median (25°–75° percentile) | 34 (28–49) | 36 (27.5–50.5) | 35.5 (28–49.5) | 0.455 |
| History of atopic dermatitis n (%) | 4 (14.3) | 5 (13.9) | 9 (14.1) | 0.990 |
| Occupation | ||||
| Physicians n (%) | 3 (10.7) | 4 (11.1) | 7 (10.9) | 0.622 |
| Nurses n (%) | 18 (64.3) | 19 (52.8) | 37 (57.8) | |
| Social‐health operators n (%) | 4 (14.3) | 10 (27.8) | 14 (21.9) | |
| Others n (%) | 3 (10.7) | 3 (8.3) | 6 (9.4) | |
| Hospital ward | ||||
| Medical area n (%) | 5 (17.9) | 25 (69.4) | 30 (46.9) | 0.001 |
| Surgical area n (%) | 8 (28.6) | 2 (5.6) | 10 (15.6) | |
| Laboratories n (%) | 2 (7.1) | 1 (2.8) | 3 (4.7) | |
| Others n (%) | 13 (46.4) | 8 (22.2) | 21 (32.8) | |
| Provinces (total n = 56) | ||||
| Trieste n (%) | 22 (88) | 30 (96.8) | 52 (92.9) | 0.218 |
| Gorizia n (%) | 3 (12) | 1 (3.2) | 4 (7.1) |
FIGURE 2.
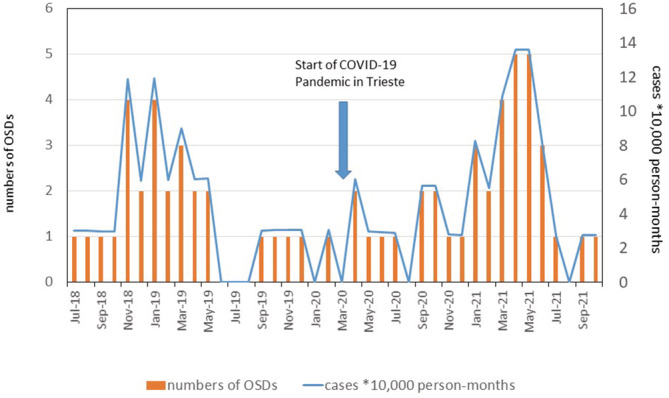
Temporal trend and incidence of cases of contact dermatitis in healthcare workers diagnosed in the period between July 2018 and October 2021: graphical representation of new monthly cases (left axes) and cases per 10 000 person‐months (right axes)
Hand dermatitis incidence was 3.92 and 4.21 cases per 10 000 person‐months before and during COVID‐19 period. IRR was 1.1 (95% CI: 0.64–1.82).
Face dermatitis accounted for 0.61 (95% CI: 0.19–1.45) and 1.12 (95% CI: 0.5–2.13) cases per 10 000 person‐months in pre and during COVID‐19 period, respectively. IRR was 1.40 (95% CI: 0.57–3.42).
Female subjects constituted the majority of the affected HCWs and the incidence of OSDs in the COVID‐19 period was 6.1 (4.2–8.6) and 2.7 (95% CI: 1.1–5.6) cases × 10 000 person‐months for women and men, respectively, with an IRR of 2.25 (95% CI: 0.98–5.9). Median age was 34 years (IQR 28–49) among the pre‐COVID‐19 group and 36 years (27.5–50.5) among the COVID‐19 one.
Nurses were the majority of HCWs with OSDs, being 64.3% (n = 18) and 52.8% (n = 19) of the subjects evaluated for OSDs before and after the beginning of the pandemic, respectively. Considering the cohort, the incidence in nurses was 6.8 (95% CI: 4.2–10.5) and 6.7 (95% CI: 4.2–10.2) cases × 10 000 person‐month, pre and during COVID‐19 period, respectively.
3.2. Dermatological conditions
With respect to OSDs localization among the pre‐COVID‐19 group, 20 (71.4%) affected the back of the hand, 6 (21.4%) the palm, 2 (7.4%) the interdigital spaces, 6 (21.4%) the fingers and 4 (14.3%) the face. Among the COVID‐19 group, 25 (69.4%) OSDs were on the back of the hand, 5 (13.9%) on the palm, 6 (16.7%) on interdigital spaces, 5 (13.9%) on fingers and 8 (22.2%) on the face. No statistically significant differences were found in localization, clinical presentation, duration of symptoms, used gloves and diagnosis between the two groups (Table 2).
TABLE 2.
Characteristics of occupational skin diseases observed in the study
| Characteristics | Before COVID‐19 N = 28 | During COVID‐19 N = 36 | Total N = 64 | p Value |
|---|---|---|---|---|
| Location | ||||
| Hand n (%) | 26 (92.9) | 30 (83.3) | 56 (87.5) | 0.285 |
| Back of the hand n (%) | 20 (71.4) | 25 (69.4) | 45 (70.3) | 0.863 |
| Palm of the hand n (%) | 6 (21.4) | 5 (13.9) | 11 (17.2) | 0.428 |
| Interdigital spaces n (%) | 2 (7.1) | 6 (16.7) | 8 (12.5) | 0.253 |
| Fingers n (%) | 6 (21.4) | 5 (13.9) | 11 (17.2) | 0.428 |
| Face n (%) | 4 (14.3) | 8 (22.2) | 12 (18.8) | 0.500 |
| Others n (%) | 4 (14.3) | 3 (8.3) | 7 (10.9) | 0.449 |
| Clinical presentation15 | ||||
| Erythema n (%) | 15 (53.6) | 18 (50.0) | 33 (51.6) | 0.890 |
| Dryness, desquamation, fissures n (%) | 21(75.0) | 28 (77.8) | 49 (76.6) | 0.795 |
| Papules and/or vesicles n (%) | 5 (17.9) | 7 (19.4) | 12 (18.8) | 0.872 |
| Itching n (%) | 13 (46.4) | 17 (47.2) | 30 (46.9) | 0.949 |
| Symptoms duration (in months) median (25°–75° percentile) | 3 (2–6) | 3 (2–6.5) | 3 (2–6) | 0.978 |
| Personal protective equipment | ||||
| Latex gloves n (%) | 14 (50) | 12 (33.3) | 26 (40.6) | 0.178 |
| Nitrile gloves n (%) | 8 (28.6) | 13 (36.1) | 21 (32.8) | 0.524 |
| Vinyl gloves n (%) | 8 (28.6) | 9 (25) | 17 (26.6) | 0.748 |
| Clinical diagnosis | ||||
| Irritant contact dermatitis n (%) | 15 (53.6) | 23 (63.9) | 38 (59.4) | 0.404 |
| Allergic contact dermatitis n (%) | 13 (46.4) | 13 (31.1) | 26 (40.6) |
3.3. Epicutaneous skin tests
Standard patch tests resulted positive in 15 (62.5%) HCWs before the pandemic and in 15 (57.7%) during the pandemic (p = 0.729). Specific series for disinfectants and drugs resulted positive only for two cases, one before and one during the pandemic (Table 3). Patch test with additional rubber series resulted positive in 5 (20.8%) and 2 (13.3%) workers before and during the pandemic, respectively. Patch tests with their own gloves were tested in 30 HCWs, and only one resulted positive to polyisoprene gloves (and negative to rubber additives). Twelve workers were tested with their own masks with negative results for all of them. All the allergens, which gave positive results, are reported in Table 4.
TABLE 3.
Results of patch tests
| Patch test | Before COVID‐19 Pos/tested (%) | During COVID‐19 Pos/tested (%) | Total | p Value |
|---|---|---|---|---|
| Standard | 15/24 (62.5) | 15/26 (57.7) | 30/50 (60) | 0.729 |
| Health care series | 1/20 (5) | 1/16 (6.3) | 2/36 (5.6) | 0.871 |
| Rubber series | 5/24 (20.8) | 2/15 (13.3) | 7/39 (17.9) | 0.131 |
| Own gloves | 1/11 (9.1) | 0/18 | 1/29 (3.5) | – |
| Own masks | 0/4 | 0/8 | 0/12 | – |
TABLE 4.
List of patch test giving a positive result between the two analysed groups
| Allergens | Before COVID‐19 | During COVID‐19 | Total |
|---|---|---|---|
| Positive/tested | Positive/tested | Positive/tested | |
| Nickel sulphate | 7/24 | 11/26 | 18/50 |
| Cobalt chloride | 6/24 | 3/26 | 9/50 |
| Carba‐mix | 5/24 | 3/26 | 8/50 |
| N‐cyclohexyl‐N‐phenyl‐p‐phenylenediamine | 4/24 | 1/15 | 5/39 |
| Thimerosal | 2/24 | 3/26 | 5/50 |
| Thiuram mix | 2/24 | 1/26 | 3/50 |
| P‐phenylenediamine | 1/24 | 2/26 | 3/50 |
| 1,3‐Diphenylguanidine | 1/24 | 2/15 | 3/39 |
| Fragrance mix‐I | 2/24 | 1/26 | 3/50 |
| Sodium bisulfite | 1/24 | 2/26 | 3/50 |
| Myroxlyon pereirae | 2/24 | 1/26 | 3/50 |
| Potassium dichromate | 3/24 | 1/26 | 4/50 |
| Rosin | 1/24 | 2/26 | 3/50 |
| Methylchloroisothiazolinone/methylisothiazolinone | 2/24 | 1/26 | 3/50 |
| Palladium chloride | 0/24 | 2/26 | 2/50 |
| Disperse blue | 0/24 | 2/26 | 2/50 |
| Neomycin sulphate | 1/24 | 0/26 | 1/50 |
| Diaminophenylmethane | 1/24 | 0/26 | 1/50 |
| Limonene | 1/24 | 0/26 | 1/50 |
| Lanolin alcohols | 0/24 | 1/26 | 1/50 |
| Povidone iodine | 1/24 | 0/26 | 1/50 |
| p‐Chloro‐m‐xylenol | 0/24 | 1/26 | 1/50 |
| Benzocaine | 0/24 | 1/26 | 1/50 |
A diagnosis of ACD was done for 13 and 13 workers pre and during COVID‐19 pandemic, respectively, and culprit occupational allergens were carbamates (n = 8), thiurams (n = 2), diphenilguanidine (n = 3), povidone iodine (n = 1), p‐chloro‐m‐xylenol (n = 1) and polyisoprene gloves (n = 1). Five workers were sensitized to N‐cyclohexyl‐N‐phenyl‐p‐phenylenediamine, a black rubber additive, two of them were co‐sensitized to carbamates and thiurams. Two were co‐sensitized to nickel and one to chromium. In 18 subjects prick test with latex extract was performed and none resulted positive.
3.4. Mask adverse cutaneous reactions
Due to the importance of masks during the pandemic, data about facial dermatitis are separately discussed and displayed in Table 5. In the considered period, 12 cases of facial adverse skin reactions were found (the 18.8% of the total involved sites of OSD), 4 of 28 in the pre‐COVID‐19 group and 8 of 36 in the COVID‐19 group (p = 0.420). In both cases, female subjects constituted the majority of the group, with a median age higher in the COVID‐19 group versus pre‐COVID‐19. Among the pre‐COVID‐19 group, two patients presented coexisting back of the hand dermatitis and one had a positive anamnesis for seborrheic dermatitis. Among the COVID‐19 group, three patients presented multiple dermatitis sites, two had back of the hand localization and another presented neck dermatitis; a patient had a positive anamnesis for psoriasis and rosacea, while another one for acne.
TABLE 5.
Characteristics of cases with facial dermatitis related to mask usage before and during COVID‐19 pandemic
| Before COVID‐19 | During COVID‐19 | Total | p Value | |
|---|---|---|---|---|
| N (%) | 4 (14.3) | 8 (22.2) | 12(18.8) | |
| Sex | ||||
| Females n (%) | 3 (75) | 8 (100) | 11(91.7) | 0.140 |
| Males n (%) | 1 (25) | 0 | 1 (8.3) | |
| Age, years median (25°–75° percentile) | 27 (25–30) | 42.5 (30.5–54.5) | 33 (25.5–60.5) | 0.109 |
| Occupation | ||||
| Physician n (%) | 1 (25) | 1 (12.5) | 2 (16.7) | 0.392 |
| Nurse n (%) | 3 (75) | 3 (37.5) | 6 (50) | |
| Social‐health operators n (%) | 0 | 3 (37.5) | 3 (25) | |
| Others n (%) | 0 | 1 (12.5) | 1 (8.3) | |
| Duration (in months) median (25°–75° percentile) | 1.5 (0.5–3.5) | 2 (0.12–2.5) | 2 (0.12–2.5) | 0.999 |
| Other associated sites n (%) |
2 (50) hands |
3 (37.5) 2 hands/1 neck |
5 (41.7) | 0.999 |
| Related pathologies n (%) |
1 (25) Seborroic dermatitis |
2 (25) Rosacea/acne |
3 (25) | 0.999 |
| Diagnosis | ||||
| Irritant contact dermatitis n (%) | 4 (100) | 7 (87.5) | 11(91.7) | 0.999 |
| Allergic contact dermatitis n (%) | 0 | 1 (12.5) | 1 (8.33) | |
| Patch test | ||||
|
Standard Positives n/tested |
0/2 |
2/5 Ni, Co, Fragrance mix |
2/7 | |
|
Health care series Positive n/tested |
0/1 | 0/1 | 0/2 | |
|
Rubber series Positives n/tested |
0/2 | 0/0 | 0/2 | |
|
Own mask Positives n/tested |
0/4 | 0/8 | 0/12 |
4. DISCUSSION
Our study investigated the incidence cases of hand and face OSDs 20 months before and 20 months after the beginning of COVID‐19 pandemic in HCWs at Trieste hospitals. The cumulative incidence was 4.22 (95% CI: 2.9–6.0) and 5.06 (95% CI: 3.6–6.9) cases per 10 000 person‐months, before and during COVID‐19 pandemic, respectively (p > 0.05). Considering only hand dermatitis the cumulative incidence was 3.92 and 4.21 cases per 10 000 person‐years. Observed values were a little bit higher than that reported in our previous study done in in years 2004–2013 where we found an incidence of 45 cases of hand dermatitis in 10 000 person year, that means 3.75 cases per 10 000 person‐months. 20
Face dermatitis cases doubled after the start of COVID‐19 pandemic (four cases before and eight cases after). In fact, it is well known that occlusion and increase of facial temperature can cause facial dermatitis such as rosacea or acne as well as ACD. 21 , 22 However, the observed increase did not reach the statistical significance and reported numbers were low.
Prolonged usage of PPE and increased skin disinfection practices for the prevention of SARS‐CoV‐2 spread were reported as risk factors for the development of cutaneous reactions. 4 Washing with soapy water is an excellent and traditionally operated hand decontamination solution, but at the same time, it may damage the integrity of the skin barrier, 23 and therefore favour the occurrence of ICD and ACD much more than alcohol‐based hand sanitizers. 24
Compared with OSDs cases recorded before the COVID‐19 pandemic among HCWs at ASUGI, our study pointed out a faint and not significant increase in cases during the pandemic in the period from January to May 2021. Moreover, peaks in the onset of the disease were recorded in March, April and May 2021, testifying how much the cold months contribute to the onset of skin diseases, with symptoms at the end of winter. However, the incidence rates did not present an increasing trend, but rather a biphasic distribution: the almost total absence of cases in the summer months and peaks during the late winter or spring months in both periods accounted. The seasonal variation was demonstrated also for facial dermatitis alone with the majority of cases (n = 8) in autumn/winter and 4 in spring.
These results are in contrast with the most recent papers that reported, in a cross‐sectional design, an increase of hand contact dermatitis in HCWs. Although the design of our study it is different, we did not find a significant increase of OSDs: to explain this, it is necessary to account the self‐selection bias and the usage of self‐administered questionnaires. 5 , 6 , 14 In fact, it is likely that HCWs suffering even of mild OSDs symptoms are more inclined to answer to the questionnaires but they would not search for medical or dermatological evaluation. It is also important to stress that in many cases a dermatological evaluation is missing.
In our study, HCWs could access during working hours to the Allergy Clinic and receive medical examination and patch tests when needed for free. However, it is possible that the surveillance system selected only the most severe cases because HCWs displaying only mild symptoms did not deem it necessary to seek further care and investigation.
Another reason for the low incidence of hand dermatitis in our workers might be related to the long experience in preventive actions for hand eczema that started more than 20 years ago after the outbreak of latex allergy in Trieste hospitals. 15 , 25 , 26 This has involved the use of less allergenic gloves, the use of detergents with less aggressive substances, the use of alcohol‐based gel as disinfectant during different work tasks, the availability of emollient creams to reduce damage induced by the repetitive use of strong detergents. When the COVID‐19 pandemic started, all HCWs had to use alcohol‐based gel reducing the use of soaps.
Therefore, it is possible that there has been an underestimation of the problem, however it is important to note that there has been a decreasing trend in OSDs diagnosis, as inferred from previous studies, 20 , 27 in the last years, probably due to improved personnel training and attention in hand protection like the widespread use of sanitizers and gloves with lesser allergenic and irritating action.
In our study, OSDs primarily affected the female sex, with an IRR of 2.25 (95% CI: 0.98–5.9). These data are consistent with many of the studies conducted during the pandemic. 5 , 9
However, it is necessary to consider the fact that in studies structured through the completion of a questionnaire, the respondents in most cases are represented by a predominantly female population; it is also worth remembering that in healthcare sector, the majority of HCWs are women; both of these factors could therefore lead to biased results. Rizzi et al. showed that age comprised between 30 and 49 years and prolonged use of gloves can be considered risk factors for the occurrence of OSDs, 14 and HCWs had to use gloves for many hours. Moreover, in our study, OSDs primarily affected nurses, with a similar trend in literature. 8 However, in the periods considered, no significant increase in OSDs was observed between the two groups.
It is also important to focus on the ward of belonging of HCWs with suspected OSDs. In fact, in our study this is the only aspect for which a significant difference was found: before the COVID‐19 outbreak, a greater involvement of the surgical and dental context was observed, while after the pandemic we observed an increase in cases in medical departments Surgeons and dentists have always been most at risk due to wet work conditions associating with the prolonged use of disposable gloves, which is also related to a greater probability of encountering an ACD, especially caused by sensitization to acrylates. 28 However, during the pandemic surgeons reduced their activity and many HCWs were occupied in COVID‐19 wards to supply shortage of personnel. For that reason, we showed a significant increase in OSDs cases among HCWs operating in medical departments after the pandemic outbreak, in accord with data in literature. 9 , 11
The majority of the population of our study (50 out of 64 evaluated HCWs) were subjected to patch tests despite the prevalent clinical diagnosis of ICD: as previously stated, in the occupational setting recognition of the trigger and possible correlation with work activity is fundamental. Thus, performing patch tests in operators at risk, despite a more evocative clinical diagnosis of ICD, is a correct management of the problem, since the objectivity often does not ensure an easy distinction of the two disorders. 29 In fact, clinical diagnosis in our study showed a higher proportion of ICDs compared to ACDs (72.2% vs. 27.8%) with more frequent localization on the back of the hand (69.4%) and with dehydration (dryness and or desquamation) (77.8%) and skin erythema (51.6%) as the most common manifestations, just as other works in literature. 8 , 10
It is interesting to note that none of the HCWs that underwent the patch tests with their own gloves and masks, during the COVID‐19 pandemic, presented an allergic reaction, justifying the fact that the lesions found were largely attributable to skin irritation caused by the occlusive effect secondary to the likely prolonged use of the device. 5
Our study did not show a significant difference in the occurrence of new cases of OSDs in HCWs discording with data already present in literature, despite similarities like the greater incidence among female gender, nurses and prevalent diagnosis of ICD. However, it may be that this has happened due to a great commitment to staff training and hospital management with the goal of minimizing the risk of OSDs, an underestimation of milder cases who did not go to Allergy Clinic at the Unit of Occupational Medicine in Trieste or a combination of the above.
AUTHOR CONTRIBUTIONS
Linda Piapan: Writing – original draft; writing – review and editing; investigation. Davide Bramuzzo: Writing – original draft; investigation. Francesca Rui: Data curation; conceptualization; resources. Francesca Larese Filon: Conceptualization; writing – review and editing; supervision; data curation; formal analysis; investigation; resources.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Piapan L, Bramuzzo D, Rui F, Filon FL. Incidence of skin diseases in healthcare workers before and during the COVID‐19 pandemic at Trieste hospitals (northeastern Italy). Contact Dermatitis. 2022;1‐8. doi: 10.1111/cod.14209
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID‐19 in health care workers—a systematic review and meta‐analysis. Am J Emerg Med. 2020;38(9):1727‐1731. doi: 10.1016/j.ajem.2020.05.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piapan L, De Michieli P, Ronchese F, et al. COVID‐19 outbreak in healthcare workers in hospitals in Trieste, north‐East Italy. J Hosp Infect. 2020;106(3):626‐628. doi: 10.1016/j.jhin.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Interim infection prevention and control recommendations for healthcare personnel during the coronavirus disease 2019 (COVID‐19) pandemic. 2022. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/infection‐control‐recommendations.html. Accesed June 6, 2022.
- 4. Larese Filon F, Pesce M, Paulo MS, et al. Incidence of occupational contact dermatitis in healthcare workers: a systematic review. J Eur Acad Dermatol Venereol. 2021;35(6):1285‐1289. doi: 10.1111/jdv.17096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin P, Zhu S, Huang Y, et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: a survey in Wuhan and its surrounding regions. Br J Dermatol. 2020;183(1):190‐192. doi: 10.1111/bjd.19089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease‐2019. J Am Acad Dermatol. 2020;82(5):1215‐1216. doi: 10.1016/j.jaad.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuan X, Xi H, Le Y, et al. Online survey on healthcare skin reactions for wearing medical‐grade protective equipment against COVID‐19 in Hubei Province, China. PLoS One. 2021;16(4):e0250869. doi: 10.1371/journal.pone.0250869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alluhayyan OB, Alshahri BK, Farhat AM, et al. Occupational‐related contact dermatitis: prevalence and risk factors among healthcare Workers in the Al'Qassim region, Saudi Arabia during the COVID‐19 pandemic. Cureus. 2020;12(10):e10975. doi: 10.7759/cureus.10975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erdem Y, Altunay IK, Aksu Çerman A, et al. The risk of hand eczema in healthcare workers during the COVID‐19 pandemic: do we need specific attention or prevention strategies? Contact Dermatitis. 2020;83(5):422‐423. doi: 10.1111/cod.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiely LF, Moloney E, O'Sullivan G, Eustace JA, Gallagher J, Bourke JF. Irritant contact dermatitis in healthcare workers as a result of the COVID‐19 pandemic: a cross‐sectional study. Clin Exp Dermatol. 2021;46(1):142‐144. doi: 10.1111/ced.14397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guertler A, Moellhoff N, Schenck TL, et al. Onset of occupational hand eczema among healthcare workers during the SARS‐CoV‐2 pandemic: comparing a single surgical site with a COVID‐19 intensive care unit. Contact Dermatitis. 2020;83(2):108‐114. doi: 10.1111/cod.13618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flyvholm MA, Bach B, Rose M, Jepsen KF. Self‐reported hand eczema in a hospital population. Contact Dermatitis. 2007;57(2):110‐115. doi: 10.1111/j.1600-0536.2007.01134.x [DOI] [PubMed] [Google Scholar]
- 13. Hamnerius N, Svedman C, Bergendorff O, Björk J, Bruze M, Pontén A. Wet work exposure and hand eczema among healthcare workers: a cross‐sectional study. Br J Dermatol. 2018;178(2):452‐461. doi: 10.1111/bjd.15813 [DOI] [PubMed] [Google Scholar]
- 14. Rizzi A, Inchingolo R, Viola M, et al. Occupational hand dermatitis web survey in a university hospital during COVID‐19 pandemic: the SHIELD study. Med Lav. 2021;112(4):320‐326. doi: 10.23749/mdl.v112i4.11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larese Filon F, Bochdanovits L, Capuzzo C, Cerchi R, Rui F. Ten years incidence of natural rubber latex sensitization and symptoms in a prospective cohort of health care workers using non‐powdered latex gloves 2000‐2009. Int Arch Occup Environ Health. 2014;87(5):463‐469. doi: 10.1007/s00420-013-0885-6 [DOI] [PubMed] [Google Scholar]
- 16. Rui F, Bovenzi M, Prodi A, et al. Nickel, cobalt and chromate sensitization and occupation. Contact Dermatitis. 2010;62(4):225‐231. doi: 10.1111/j.1600-0536.2009.01650.x [DOI] [PubMed] [Google Scholar]
- 17. Prodi A, Rui F, Fortina AB, Corradin MT, Filon FL. Healthcare workers and skin sensitization: north‐eastern Italian database. Occup Med. 2016;66(1):72‐74. doi: 10.1093/occmed/kqv139 [DOI] [PubMed] [Google Scholar]
- 18. Wilkinson DS, Fregert S, Magnusson B, et al. Terminology of contact dermatitis. Acta Derm Venereol. 1970;50(4):287‐292. [PubMed] [Google Scholar]
- 19. Santarossa M, Larese FF. Improving the diagnosis of allergic contact dermatitis using patch test with gloves. Dermatitis. 2018;29(1):49‐51. doi: 10.1097/DER.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 20. Larese Filon F, Plazzotta S, Rui F, Mauro M, De Michieli P, Negro C. Ten‐year incidence of contact dermatitis in a prospective cohort of healthcare workers in Trieste hospitals (north east of Italy) 2004‐2013. Br J Dermatol. 2017;177(2):560‐561. doi: 10.1111/bjd.15118 [DOI] [PubMed] [Google Scholar]
- 21. Niesert AC, Oppel EM, Nellessen T, et al. “Face mask dermatitis” due to compulsory facial masks during the SARS‐CoV‐2 pandemic: data from 550 health care and non‐health care workers in Germany. Eur J Dermatol. 2021;31(2):199‐204. doi: 10.1684/ejd.2021.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhatia R, Sindhuja T, Bhatia S, et al. Iatrogenic dermatitis in times of COVID‐19: a pandemic within a pandemic. J Eur Acad Dermatol Venereol. 2020;34(10):e563‐e566. doi: 10.1111/jdv.16710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magnano GC, Rui F, Larese Filon F. Skin decontamination procedures against potential hazards substances exposure. Chem Biol Interact. 2021;344:109481. doi: 10.1016/j.cbi.2021.109481 [DOI] [PubMed] [Google Scholar]
- 24. Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID‐19: recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83(6):1730‐1737. doi: 10.1016/j.jaad.2020.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larese Filon F, Bosco A, Fiorito A, Negro C, Barbina P. Latex symptoms and sensitisation in health care workers. Int Arch Occup Environ Health. 2001;74(3):219‐223. doi: 10.1007/s004200000208 [DOI] [PubMed] [Google Scholar]
- 26. Filon FL, Radman G. Latex allergy: a follow up study of 1040 healthcare workers. Occup Environ Med. 2006;63(2):121‐125. doi: 10.1136/oem.2003.011460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. França D, Sacadura‐Leite E, Fernandes‐Almeida C, Filipe P. Occupational dermatoses among healthcare workers in a hospital center in Portugal. Rev Bras Med Trab. 2020;17(3):285‐291. doi: 10.5327/Z1679443520190393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mikov I, Turkalj I, Jovanović M. Occupational contact allergic dermatitis in dentistry. Vojnosanit Pregl. 2011;68(6):523‐525. doi: 10.2298/vsp1106523m [DOI] [PubMed] [Google Scholar]
- 29. Rubins A, Romanova A, Septe M, Maddukuri S, Schwartz RA, Rubins S. Contact dermatitis: etiologies of the allergic and irritant type. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29(4):181‐184. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


