Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BAU
binding antibody units
- CNI
calcineurin inhibitor
- COVID‐19
coronavirus disease 2019
- HCC
hepatocellular carcinoma
- IgG
immunoglobulin G
- LT
liver transplantation
- MMF
mycophenolate mofetil
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplantation
PATIENTS AND METHODS
We conducted a prospective monocentric study at the Montpellier University Hospital Center. All patients with LT had the determination of antibody titers against SARS‐CoV‐2 30 days after the third dose of mRNA vaccine. Patients with antispike immunoglobulin G (IgG) titers below 260 binding antibody units (BAU)/ml were considered for prophylactic antibodies (tixagevimab/cilgavimab or casirivimab/imdevimab). Among these patients, those ineligible or refusing prophylactic antibody infusion were consecutively included in our study. We collected the following clinical and biological characteristics: age, sex, time from LT and indication, immunosuppressive regimen, previous SARS‐CoV‐2 vaccinations, liver function tests, and antispike IgG level after three doses of a vaccine (two‐dose series followed by a third dose booster). For each patient, MMF was stopped by the investigator at a mean time of 25 days before the fourth dose, and serological control was performed 2 to 4 weeks after the fourth dose of vaccine (Pfizer‐BioNTech BNT162b2). MMF was resumed 2 weeks after the fourth dose. Anti–SARS‐CoV‐2 spike protein antibody detection was mostly performed using Elecsys Anti–SARS‐CoV‐2 S (Roche). In the case of liver function test abnormalities, a liver biopsy was performed to rule out acute rejection. This study was approved by the Institutional Review Board of the Montpelier University Hospital (202201087). All patients gave their consent to participate in this study.
RESULTS
Between October 21, 2021, and January 12, 2022, 14 patients were eligible for the study. A total of 10 patients (6 men and 4 women) were included in the study with a mean age of 62 years (range, 53–71 years). The median length of time since LT was 38 months (range, 15–214 months). The main LT indication was alcohol‐associated liver cirrhosis, and the main immunosuppressive regimen was tacrolimus associated with MMF (mean tacrolimus trough level at inclusion, 5.35 μg/L; range, 2.2–9.9 μg/L; Table 1). Two patients had past histories of nonsevere graft rejection (4 and 10 years prior to MMF discontinuation). Nine patients had received three doses of a SARS‐CoV‐2 vaccine before MMF discontinuation; one received four doses but was still a nonresponder. Mean antispike IgG titers were 43.01 BAU/ml (range, 0–237 BAU/ml) before MMF discontinuation. Patient characteristics and the main results are presented in Table 1. No history of COVID‐19 was reported among the included patients, and SARS‐CoV‐2 nucleocapsid antibodies were negative for all patients. The mean duration between MMF discontinuation and the fourth dose of vaccine was 25 days (range, 7–34 days). The mean duration between the fourth dose and the biological sample for antispike IgG titers was 23 days (range, 14–35 days). No safety concerns were reported after the fourth dose of the vaccine. The antispike IgG titers increased in all patients, with a mean titer of 1437 BAU/ml (range, 166–3000 BAU/ml; Figure 1A,B). All patients became responders after the fourth dose of the vaccine when considering a threshold of 143 BAU/ml (Figure 1A,B). No patient developed COVID‐19 during the follow‐up period. No abnormalities were noted in the liver function tests after MMF discontinuation (Table 1).
TABLE 1.
Characteristics of the patients and SARS‐Cov‐2 vaccine data
| Patient | Sex, age (years) | LT | Prior SARS‐CoV‐2 vaccines | Antibody levels after complete vaccination (BAU/ml) | MMF discontinuation—fourth injection (days) | Liver blood test before MMF discontinuation (AST/ALT; UI/L) | Fourth injection—antibody determination (days) | Antibody levels (BAU/ml) | Liver blood test after vaccination (AST/ALT; UI/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delay (months) | Primary indication | Medical history of liver graft rejection | Tacrolimus concentration (μg/L) | |||||||||
| 1 | Female, 53 | 27 | HCC | No | 4,2 | BNT162b2 a ×3 | 0,4 | 16 | 18/8 | 33 | 451 | 18/9 |
| 2 | Male, 68 | 38 | HCC | No | 6,5 | BNT162b2 a ×3 | 7,1 | 34 | 12/10 | 25 | 669 | 17/15 |
| 3 | Female, 58 | 123 | HCC | No | 2,8 | BNT162b2 a ×3 | 4,4 | 7 | 14/10 | 31 | 3000 | 13/9 |
| 4 | Male, 53 | 52 | Alcohol‐associated cirrhosis | No | 9,9 | BNT162b2 a ×3 | 0 | 29 | 16/24 | 1120 | 16/24 | |
| 5 | Male, 68 | 141 | HCC | Yes (2012) | 4,9 | BNT162b2 a ×4 | 237 | 31 | 24/29 | 16 | 2080 | 38/45 |
| 6 | Male, 57 | 38 | Alcohol‐associated cirrhosis | Yes (2018) | 4,1 | mRNA b ×2 + ChAdOx1‐S ×1 | 29,5 | 19 | 13/27 | 35 | 2000 | 15/12 |
| 7 | Female, 67 | 27 | Alcohol‐associated cirrhosis | No | 9,1 | BNT162b2 a ×3 | 0,4 | 24 | 24/35 | 16 | 166 | 26/46 |
| 8 | Female, 57 | 214 | Transthyretin amyloidosis | No | 7,9 | BNT162b2 a ×3 | 16,9 | 29 | 23/29 | 14 | 2080 | 31/39 |
| 9 | Male, 71 | 19 | HCC | No | 2,2 | mRNA b ×2 + BNT162b2 ×1 | 132,8 | 32 | 8/8 | 15 | 2500 | 11/12 |
| 10 | Male, 68 | 15 | HCC | No | 6,5 | BNT162b2 a ×3 | 1,7 | 33 | 15/12 | 14 | 304 | 13/12 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BAU, binding antibody units; ChAdOx1‐S, Vaxzevria AstraZeneca; HCC, hepatocellular carcinoma; MMF, mycophenolate‐mofetil.
Pfizer‐BioNTech BNT162b2.
mRNA‐1273.
FIGURE 1.
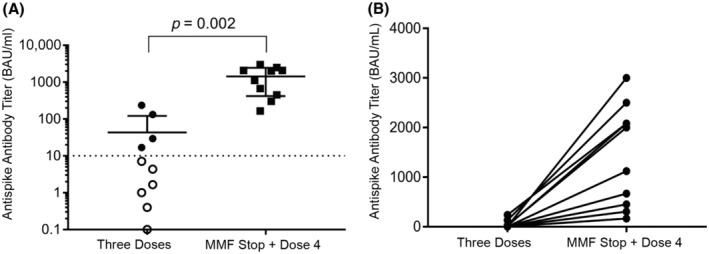
Immunogenicity. (A) Antispike IgG antibodies after three doses of the SARS‐CoV‐2 vaccination and after MMF discontinuation and Dose 4. (B) Antispike IgG antibody evolution in LT recipients after MMF discontinuation and Dose 4
DISCUSSION
To our knowledge, this is the first study to show that MMF discontinuation in LT recipients improves the SARS‐CoV‐2 vaccine response. MMF discontinuation allowed an increase in antispike IgG titers in all LT recipients 2 to 4 weeks after the fourth dose of the SARS‐CoV‐2 vaccine. All patients developed an antispike IgG level above 143 BAU/ml. Consistent with previous studies, our results confirm the key role of MMF in the lack of vaccine response in LT recipients.[ 4 ] MMF preferentially depletes guanosine nucleotides in T and B lymphocytes and inhibits their proliferation, thereby suppressing cell‐mediated immune responses and antibody formation. This mechanism of action probably explains the decreased humoral response and increased severity of COVID‐19 in MMF‐treated LT recipients.[ 1 ]
We preferentially selected patients who were nonresponders or poor responders to vaccination; we can therefore speculate that antispike IgG titers can be increased even in responders after MMF discontinuation and a fourth dose of the vaccine.
The temporary MMF discontinuation in our patients did not lead to any complications. Moreover, in LT, calcineurin inhibitors (CNIs) are the mainstay of maintenance immunosuppressive treatment and antiproliferative agents as MMF allows CNI dose reductions.[ 5 ] However, caution should be taken when MMF is resumed in other SOTs (e.g., kidney, lung, heart).
Our study is limited by the small number of patients and the lack of a control group. However, we felt that it was important to rapidly report these major results for patients who are immunocompromised. Several points still need to be clarified, such as the optimal length of MMF discontinuation before vaccination and timing before resuming. Unresolved issues remain, such as the relevance of MMF discontinuation at each booster injection and the place of this vaccine strategy as an alternative to prophylactic antibodies. These results are a proof of concept that could be transposed to other SOT recipients or patients treated with antimetabolites. Discontinuation of MMF should be decided on a case‐by‐case basis according to the time after transplantation and the assessment of rejection risk.
Our results are promising for reducing the number of SOT patients who do not respond to vaccination and for limiting the indications of prophylactic infusion of monoclonal anti–SARS‐CoV‐2 antibodies.
AUTHOR CONTRIBUTIONS
Lucy Meunier and Magdalena Meszaros contributed to writing the manuscript. Emilie Malezieux contributed to the data collection. José Ursic Bedoya contributed to the statistical analysis. Lucy Meunier, Emilie Malezieux, José Ursic Bedoya, Stéphanie Faure, Maxime Echenne, Antoine Debourdeau, Magdalena Meszaros, and Georges Philippe Pageaux contributed to the study concept design, data collection, and manuscript review.
FUNDING INFORMATION
Funding support came solely from institutional and/or departmental sources (Montpellier University Hospital, 34000, France).
CONFLICT OF INTEREST
Stéphanie Faure received grants from Gilead. José Ursic Bedoya received grants from Astellas.
To the editor,
Patients receiving solid organ transplantation (SOT) are at an increased risk of mortality and morbidity attributed to coronavirus disease 2019 (COVID‐19).[ 1 ] Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccination reduces the risk of severe disease and death.[ 2 ] Several studies have shown a lack of vaccine response in liver transplantation (LT) recipients after two or three doses of the vaccine.[ 3 ] Predictors for a negative response among LT recipients include older age, lower glomerular filtration rate, and treatment with mycophenolate mofetil (MMF).[ 4 ] The rate of protection in SOT recipients after three doses of the vaccine remains insufficient. For these patients, prophylactic infusion of monoclonal anti–SARS‐CoV‐2 antibodies can be offered, although we have less insight regarding the long‐term efficacy when compared with the vaccines. The immunosuppression regimen classically administered to LT recipients is a combination of tacrolimus and MMF.[ 5 ] However, in various situations, MMF can be stopped or discontinued without increasing the risk of acute graft rejection, especially in low‐risk patients. In this study, we aimed to evaluate the impact of a temporary discontinuation of MMF on vaccine response after a fourth dose of vaccine in nonresponding LT recipients.
REFERENCES
- 1. Colmenero J, Rodríguez‐Perálvarez M, Salcedo M, Arias‐Milla A, Muñoz‐Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID‐19 in liver transplant patients. J Hepatol. 2021;74:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid‐19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Efficacy of covid‐19 vaccines in immunocompromised patients: systematic review and meta‐analysis. BMJ. 2022;376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabinowich L, Grupper A, Baruch R, Ben‐Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlton M, Levitsky J, Aqel B, O'Grady J, Hemibach J, Rinella M, et al. International liver transplantation society consensus statement on immunosuppression in liver transplant recipients. Transplantation. 2018;102:727–43. [DOI] [PubMed] [Google Scholar]


