Abstract
Background
The COVID‐19 vaccine is effective in preventing severe cases of COVID‐19. For women, gynecological adverse events, such as menstrual irregularities and irregular bleeding, could be a concern after COVID‐19 vaccination. In this study, we investigated gynecological adverse events in the vaccinated Japanese female population.
Methods
We conducted a survey‐based study with health‐care workers, including medical doctors and nurses, medical coworkers, and medical university faculty, staff, and students, at a single medical school and affiliated hospital in Japan. We used McNemar's test and network analysis.
Results
Overall, we obtained 819 responses, and 424 were from females. After the exclusion of contradictory answers, 309 surveys were finally considered appropriate for the analysis. The frequencies of abnormal bleeding were 0.6%, 1.0%, and 3.0% for the first, second, and third doses, respectively. An irregular menstrual cycle was more common than abnormal bleeding: 1.9%, 4.9%, and 6.6% for the first, second, and third doses, respectively. Network analysis revealed that abnormal bleeding and an irregular menstrual cycle were not associated with other adverse reactions.
Conclusion
The present study showed that the effects of COVID‐19 vaccination on menstruation seem limited.
Keywords: adverse effect, COVID‐19 vaccination, menstrual abnormality
Introduction
The COVID‐19 vaccine is effective in preventing severe COVID‐19. However, adverse reactions to vaccination can often cause vaccine hesitancy. For females, gynecological adverse reactions, such as menstrual irregularities and irregular bleeding, could be serious concerns after COVID‐19 vaccination. There have been several reports on the association between COVID‐19 vaccination and menstruation. 1 For example, a total of 50 566 reports of suspected reactions related to menstrual disorders following COVID‐19 vaccination have been reported to the yellow card surveillance scheme by the UK Medicine and Healthcare Products Regulatory Agency (MHRA) following approximately 72.7 million COVID‐19 vaccine doses administered to women up to 2 March 2022. 2 According to the US Cohort study, the first dose of vaccine did not affect the subsequent menstruation period, while the second dose was associated with a small change in cycle length. 3 A study from the Norwegian Institute of Public Health showed that 37.8% of participants reported at least one change in menstrual changes, including unexpected breakthrough bleeding. 4 In Japan, there have been many voluntary reports of menstrual abnormalities or irregular bleeding experienced after COVID‐19 vaccination on social networking services (SNS) and other media, but no clinical or epidemiological reports have investigated this concern thus far. In addition, the relationship between systemic adverse reactions, such as fever and fatigue, and gynecological adverse reactions remains unclear. In this study, we investigated gynecological adverse events in the vaccinated Japanese female population. We conducted a survey‐based study on health‐care workers, including medical doctors and nurses; medical coworkers; and medical university faculty, staff, and students in a single medical school and affiliated hospital in Japan.
Materials and Methods
Study design and participants
We conducted a cross‐sectional survey using Google Forms between December 27, 2021, and March 5, 2022. The survey subjects were medical students, faculty members, and staff at Nihon University School of Medicine; nursing students and teachers at Nihon University Nursing School; and hospital staff of Nihon University School Hospital. The subjects included medical experts (medical doctors, dentists, nurses, other medical staff, medical researchers), administrative support staff, students, and office workers who received the BNT162b2 COVID‐19 vaccination. Informed consent to participate in this study was obtained from all of the potential participants before answering the questionnaire. This study was approved by the Ethics Committee of Nihon University School of Medicine (approval number: P21‐06‐1). All procedures were performed under the guidelines of our institutional ethics committee and adhered to the tenets of the Declaration of Helsinki.
Survey questions
The online survey questionnaires included the characteristics of the participants, such as age and gender. The COVID‐19 vaccine questions that were asked included the number of vaccine doses the participants had, COVID‐19 incidence, and adverse reactions. For premenopausal women, we asked whether they had irregular bleeding and abnormal menstrual cycles after each dose of the BNT162b2 vaccine. The contents of the questionnaire were shown in Table S1.
Statistical analyses
The change in the frequency of abnormal bleeding and irregular menstrual cycle between each dose was calculated with pairwise McNemar's test 5 with p‐value adjustment described by Benjamini and Hochberg 6 using the companion package of R 4.1.3. 7 p‐Values less than 0.05 were considered statistically significant.
Networks were used to visualize the connections of each adverse event. Networks were built with the IsingFit method in the IsingFit package, igraph, and qgraph of R 8 , 9 to assess the connection of abnormal bleeding and menstrual cycles to other adverse events. The model employed in the IsingFit package is a binary equivalent of the Gaussian approximation methods, which is applicable only to two‐state data, interactions are considered pairwise, and the data need to be cross‐sectional. This package builds a figure of a network. In this figure, each symptom is represented with a circle, or node, and nodes are connected with lines (edges). A green edge between two nodes represents a positive connection between the two symptoms, and a red edge shows a negative connection. The thickness of the edge indicates the strength of the connection between two symptoms. 10 , 11 Clusters of nodes were identified with the Walktrap algorithm. 12
Results
Characteristics of the responders
Overall, we obtained 819 responses, and 813 of these responses were deemed appropriate for analysis. Among the participants, 424 were females. We excluded postmenopausal females, pregnant, and breastfeeding women to determine the impact of vaccination on the menstrual cycle. In this study, none of the postmenopausal women reported experiencing irregular bleeding after vaccination. After the exclusion of contradictory answers, 309 surveys were considered appropriate for the final analysis (Figure 1). Among these, only one had never received the vaccine and was not analyzed for adverse events; the remaining 308 individuals received at least two doses of vaccine, and 305 received the third booster shot (Figure 1). The average age of the participants was 31.9 ± 10.9 years. Three individuals had been diagnosed with SARS‐CoV‐2 infection by this survey. The brief descriptive responder data are summarized in Table 1.
FIGURE 1.
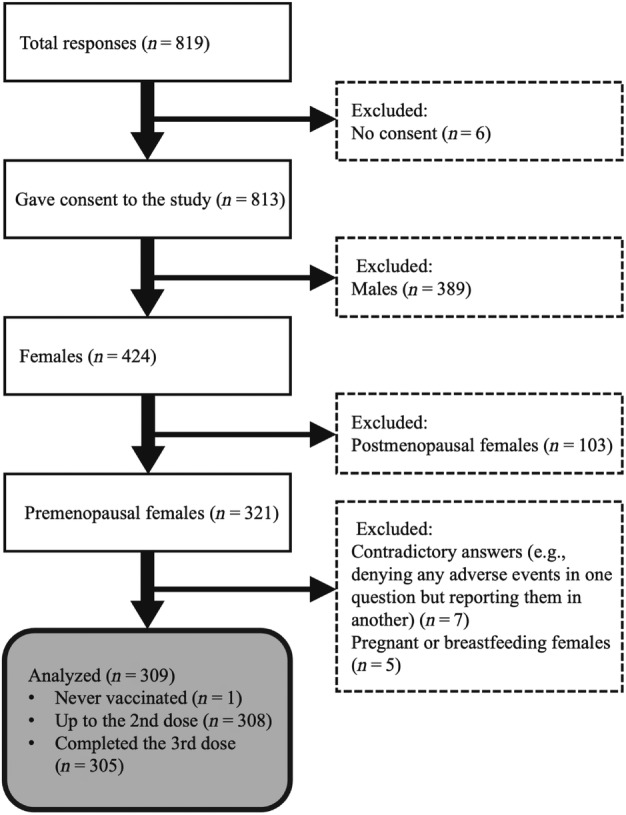
Flow diagram of the present study
TABLE 1.
A study subject summary data table
| Age (years) (n = 309) | 31.9 ± 10.9 | |||
|---|---|---|---|---|
| History of confirmed infection with SARS‐CoV‐2 or the diagnosis of COVID‐19 (% in n = 309) | 3 (0.0) | |||
| Observed event counts (%) | ||||
| First dose (n = 308) | Second dose (n = 308) | Third dose (n = 305) | ||
| Abnormal bleeding | 2 (0.6) | 3 (1.0) | 9 (3.0) | |
| Irregular menstrual cycle | 6 (1.9) | 15 (4.9) | 20 (6.6) | |
| Injection site local reactions | Pain | 258 (83.8) | 268 (87.0) | 263 (86.2) |
| Swelling | 117 (38.0) | 133 (43.2) | 120 (39.3) | |
| Redness | 65 (21.1) | 85 (27.6) | 62 (20.3) | |
| Itch | 41 (13.3) | 55 (17.9) | 54 (17.7) | |
| Systemic extra injection site reactions | Fatigue | 105 (34.1) | 207 (67.2) | 221 (72.4) |
| Headache | 53 (17.2) | 133 (43.2) | 150 (49.2) | |
| Myalgia | 41 (13.3) | 75 (24.4) | 101 (33.1) | |
| Chill | 36 (11.7) | 113 (36.7) | 141 (46.2) | |
| Fever | 56 (18.2) | 167 (54.2) | 186 (61.0) | |
| Pharyngeal irritation/pain | 5 (1.6) | 14 (4.5) | 32 (10.5) | |
| Arthralgia | 36 (11.7) | 96 (31.1) | 116 (38.0) | |
| Nausea/vomiting | 5 (1.6) | 24 (7.8) | 29 (9.5) | |
| Diarrhea | 7 (2.3) | 14 (4.5) | 16 (5.6) | |
| Abdominal pain | 5 (1.6) | 11 (3.6) | 11 (3.6) | |
| Skin rashes | 1 (0.3) | 2 (0.6) | 4 (1.3) | |
| Perioral abnormal sensation/numbness | 0 (0.0) | 1 (0.3) | 1 (0.3) | |
| Lymphadenopathy | 9 (2.9) | 21 (6.8) | 81 (26.6) | |
| Facial flush | 14 (4.5) | 28 (9.1) | 41 (13.4) | |
| Others | 1 (0.3) | 2 (0.6) | 13 (4.3) | |
Prevalence of gynecological adverse events after COVID‐19 vaccination
The frequencies of abnormal bleeding were 0.6% (2/308), 1.0% (3/308), and 3.0% (9/305) for the first, the second, and the third doses, respectively. Irregular menstrual cycles were more common than abnormal bleeding: 2.0% (6/308), 4.9% (15/308), and 6.6% (20/305) for the first, second, and third doses, respectively (Table 1).
Pairwise McNemar's tests showed that abnormal bleeding occurred more commonly after the third dose than after the first dose (p = 0.047) (Table 2). An irregular menstrual cycle was significantly more common after the second and third doses than after the first dose (p = 0.06 for each).
TABLE 2.
Comparison of the incidence of abnormal bleeding and irregular menstrual cycles after each dose of the BNT162b2 vaccine
| Comparison | p‐Value | Odds ratio | 95% confidence interval |
|---|---|---|---|
| Abnormal bleeding | |||
| 1st vs. 2nd | 1.000 | 153.5 | 38.2–616.5 |
| 1st vs. 3rd | 0.047* | 148.0 | 36.8–594.6 |
| 2nd vs. 3rd | 0.105 | 98.7 | 31.6–307.7 |
| Irregular menstrual cycle | |||
| 1st vs. 2nd | 0.006* | 48.3 | 21.5–108.5 |
| 1st vs. 3rd | 0.006* | 47.5 | 21.2–106.6 |
| 2nd vs. 3rd | 0.424 | 19.0 | 11.3–31.9 |
Statistically significant.
Network analysis
Next, we investigated the association between local and/or systemic adverse events and gynecological symptoms. To examine the relationships, we performed a network analysis. This analysis method can find factors that are related to each other. The network for the second dose revealed that there were four communities. The events and/or the symptoms within the community can be considered strongly correlated with each other. Although the irregular menstrual cycle belongs to the community consisting of systemic events, its connection with other adverse events is very weak (Figure 2). Abnormal bleeding was excluded from the network of the second dose because of the small number of events (n = 3, 1.0%). The network for the third dose revealed five clusters of adverse events, in which abnormal bleeding and an irregular menstrual cycle had a strong connection and constituted an isolated community (Figure 3). Both abnormal bleeding and an irregular menstrual cycle were observed in a very small number of participants. Therefore, we could not build the network after the first dose.
FIGURE 2.
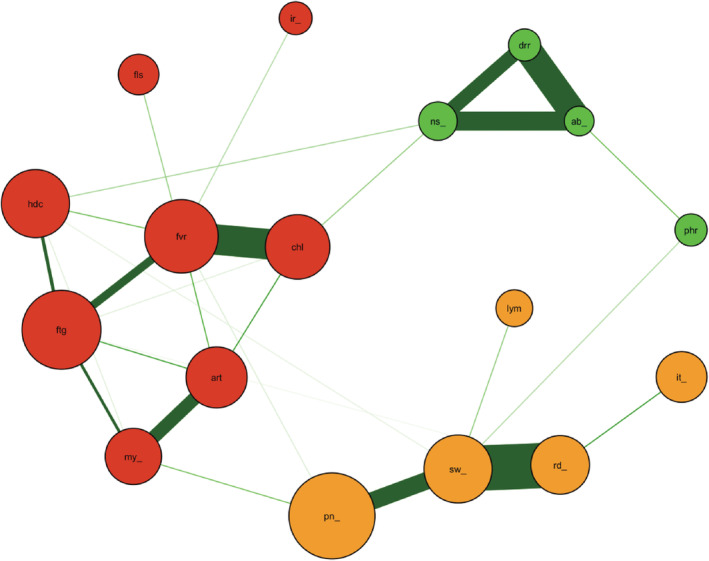
The network for the second dose of BNT162b2 vaccination. There were four identified clusters of symptoms (nodes) in the network after the second dose of vaccine. An irregular menstrual cycle belonged to a cluster that included systemic adverse events but the connections between irregular menstrual cycle and other systemic events in this cluster were very weak and connected with a very thin edge. Note that abnormal bleeding and some other symptoms are not included in this network because of the lack of observation. (abd_, abdominal pain; abn_, abnormal bleeding; art, arthralgia; chl, chill; drr, diarrhea; fls, facial flush; ftg, fatigue; fvr, fever; hdc, headache; ir_, irregular menstrual cycle; it_, itch in the injection site; lym, lymphadenopathy; my_, extrainjecyion site myalgia; ns_, nausea/vomiting; oth, other symptoms; phr, pharyngeal irritation/pain; pn_, pain in the injection site; rd_, redness in the injection site; sw_, swelling in the injection site).
FIGURE 3.
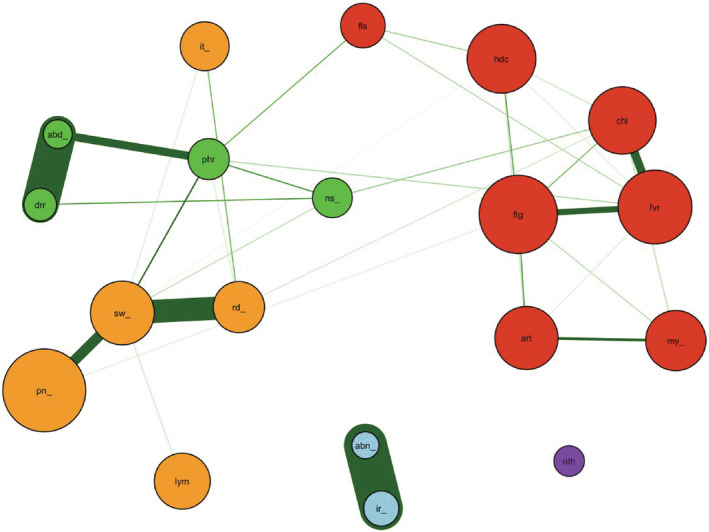
The network for the third dose of BNT162b2 vaccination. There were five identified clusters of symptoms in the network after the third dose of vaccine. The colors of the clusters with similar compositions after the second dose are shown in the same colors. Abnormal bleeding and irregular menstrual cycles belong to the same cluster (blue) with little connections to other symptoms. (abd_, abdominal pain; abn_, abnormal bleeding; art, arthralgia; chl, chill; drr, diarrhea; fls, facial flush; ftg, fatigue; fvr, fever; hdc, headache; ir_, irregular menstrual cycle; it_, itch in the injection site; lym, lymphadenopathy; my_, extrainjecyion site myalgia; ns_, nausea/vomiting; oth, other symptoms; phr, pharyngeal irritation/pain; pn_, pain in the injection site; rd_, redness in the injection site; sw_, swelling in the injection site).
Discussion
In the present study, we found that the effects of COVID‐19 vaccination on abnormal bleeding and menstrual cycle disruption are limited. However, there was an increasing trend of these adverse events following each booster shot. The frequency of abnormal bleeding following the first dose was 0.6% and increased following the second (1.0%) and third (3.0%) booster shots. Similarly, the frequency of irregular menstrual cycles also increased from 1.9% following the first dose to 4.9% and 6.6% following the second and the third doses, respectively. The incremental effect of booster shots on adverse events related to menstruation is consistent with previous reports. 3 , 13 According to a report from the United States, the COVID‐19 vaccine was associated with a less than 1‐day change in cycle length for first‐ and second‐dose cycles compared with prevaccine cycles. 3 A report from Saudi Arabia showed that menstrual abnormalities were observed in 0.98% of BNT162b2‐vaccinated women. 13 However, some papers have reported a much higher association of COVID‐19 vaccination with menstruation. 4 , 14 In a Norwegian survey, the relative risk of heavier than usual bleeding following the first dose of vaccination was 1.90 (95% CI: 1.69–2.13) and that following the second dose was 1.84 (1.66–2.03). 4 In a study in Italy, approximately 50%–60% and 60%–70% of women of reproductive age complained of menstrual cycle irregularities after the first and second vaccinations, respectively. 14 The possible reason for the different outcomes observed in these studies is the difference in survey methods. One of the most important concerns involving an online self‐report survey is reporting bias. 15 In the case of our study, women could be more attentive to their health after vaccination and more likely to perceive changes in their menstruation.
The menstrual cycle is regulated by the hypothalamic–pituitary–ovarian (HPO) axis through sex steroid hormones and disruption to this delicate system can easily result in a disturbance of the menstrual cycle. Thus, it is conceivable that severe inflammation, with its multiple effects on various organs, could also affect the menstrual cycle. Therefore, we investigated the associations of abnormalities in menstruation with other adverse events using network analysis. The result, however, showed the HPO axis and other systems are affected differently. As shown in Figures 2 and 3, abnormal bleeding and an irregular menstrual cycle were related but were independent of other adverse reactions in the network, such as fever, headache, and lymphadenopathy, which are usually considered indications of a robust systemic inflammatory reaction. In COVID‐19 patients, menstrual abnormalities, including a prolonged menstrual cycle and decreased menstrual volume, have been reported. 16 Although the median concentrations of sex hormones were reported to be marginally higher in both mildly and severely ill patients than in the control group, no significant difference was found between COVID‐19 patients and controls or between mild and severe patients. 16 Further research might be needed to clarify the association of COVID‐19 or its vaccines with gynecological abnormalities and the effects of the vaccines on the HPO axis.
There are some limitations to our research. First, this study was conducted on a limited number of people in a single institution. All participants were vaccinated with Pfizer/BioNTech's BNT162b2 mRNA vaccine, and none received any other vaccine, such as Moderna's mRNA‐1273 vaccine. Therefore, the present results can only examine the effect of the Pfizer vaccine. In addition, the target population for this survey included participants with medical backgrounds, such as medical doctors, medical students, and nurses. Their perceptions of adverse events following a vaccine could be different from those of the general population, potentially biasing the survey results. However, the frequency of adverse events observed in our study was not significantly divergent from those in previous reports; therefore, the bias was unlikely to be significant enough to invalidate our results. Second, the study employed a web‐based online survey system. Participation in the survey was voluntary. Therefore, there might have been some level of self‐selection bias. Third, the survey was a cross‐sectional study. We collected responses from the participants after the third vaccination. Thus, the responses only reflect the information available at that time. In addition, we did not examine the menstrual cycle of each participant. Thus, the relationship between the timing of vaccination during the menstrual period and menstrual abnormalities could not be investigated. Despite these limitations, we consider this study worthwhile because it is the first study to examine the association between COVID‐19 vaccination and menstrual abnormalities in Japan. In addition, previously reported studies have investigated adverse events up to the end of the second vaccination. This is the first report to investigate adverse events after the third vaccination, especially menstrual abnormalities and irregular bleeding in association with other adverse events.
COVID‐19 vaccines were the first approved mRNA vaccines and their rapid approval caused public concern for unknown adverse reactions worldwide. Possible abnormal menstruation after vaccination is a concern that is unique to women and is an important hindrance in vaccination programs. In this study, the effects of COVID‐19 vaccination on menstruation were limited. However, because irregularity in the menstrual cycle and abnormal bleeding decrease the quality of life of women, gynecologists should be well aware of these gynecological adverse events and are advised to provide enough information to patients based on the latest scientific data prior to vaccination. Since normal menstruation is important for women's health and reproductive health, further research is needed on the potential influence of COVID‐19 and the COVID‐19 vaccine on menstruation.
Author contributions
Conceptualization: Takahiro Namiki, Shihoko Komine‐Aizawa, Kazuhide Takada, Chika Takano, Quang Duy Trinh, and Satoshi Hayakawa; Data curation: Takahiro Namiki, Shihoko Komine‐Aizawa, and Kazuhide Takada; Formal analysis: Takahiro Namiki; Investigation: Takahiro Namiki, Shihoko Komine‐Aizawa, and Kazuhide Takada; Methodology: Takahiro Namiki, Shihoko Komine‐Aizawa; Project administration: Shihoko Komine‐Aizawa; Supervision: Satoshi Hayakawa; Visualization: Takahiro Namiki, Shihoko Komine‐Aizawa, and Kazuhide Takada, Writing—original draft: Takahiro Namiki, Shihoko Komine‐Aizawa; Writing—review & editing: Kazuhide Takada, Chika Takano, Quang Duy Trinh, and Satoshi Hayakawa.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Table S1. The contents of the questionnaire
Acknowledgments
We thank Professor Gotoda T. (Dean of Nihon University School of Medicine), Professor Ishihara H. (School Principal of Nihon University Nursing School), Professor Kaneita Y., Professor Gon Y., Professor Kawana K., Professor Nakayama T., Professor Hao H., Professor Takayama T., and Professor Takahashi S. (Nihon University School of Medicine) for their support during this study. We thank American Journal Experts for editing the English language in this manuscript.
Takahiro Namiki and Shihoko Komine‐Aizawa contributed equally to this study.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Male V. Menstruation and covid‐19 vaccination. BMJ. 2022;376:o142. [DOI] [PubMed] [Google Scholar]
- 2. summary MaHPRACvw, https://www.gov.uk/government/publications/coronaviruscovid- oYCrJ, 19‐vaccine‐adverse‐reactions/coronavirus‐vaccine‐summary‐of‐yellow‐card‐reporting#annex‐, 1‐vaccine‐analysis‐print Published online: E‐Pub.
- 3. Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID‐19) vaccination: a U.S. cohort. Obstet Gynecol. 2022;139:481–9. 10.1097/AOG.0000000000004695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lill Trogstad IL, Laake I, Anna HR, Mjaaland S, Caspersen IH, Juvet LK, et al. Increased occurrence of menstrual disturbances in 18‐ to 30‐year‐old women after COVID‐19 vaccination. Obstet Gynecol. 2022;139(5):940–1.35576361 [Google Scholar]
- 5. McNemar Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12:153–7. [DOI] [PubMed] [Google Scholar]
- 6. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995;57(1):289–300. [Google Scholar]
- 7. R Core Team . R: a language and environment for statistical computing. 2022. Accessed March 14 2022. Available from: https://www.r-project.org.
- 8. Csárdi G, Nepusz T. The igraph software package for complex network research. Int J Complex Syst. 2006;1695:1–9. [Google Scholar]
- 9. Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, Borsboom D. Qgraph: network visualizations of relationships in psychometric data. J Stat Softw. 2012;48(4):1–18. [Google Scholar]
- 10. van Borkulo CD, Borsboom D, Epskamp S, Blanken TF, Boschloo L, Schoevers RA, et al. A new method for constructing networks from binary data. Sci Rep. 2014;4:5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalege J, Borsboom D, van Harreveld F, van der Maas HLJ. Network analysis on attitudes: a brief tutorial. Soc Psychol Personal Sci. 2017;8(5):528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pons P, Latapy M. Computing communities in large networks using random walks. Berlin, Heidelberg: Springer; 2005. [Google Scholar]
- 13. Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel‐Moneim AS. BNT162b2 and ChAdOx1 SARS‐CoV‐2 post‐vaccination side‐effects among Saudi Vaccinees. Front Med (Lausanne). 2021;8:760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laganà AS, Veronesi G, Ghezzi F, Ferrario MM, Cromi A, Bizzarri M, et al. Evaluation of menstrual irregularities after COVID‐19 vaccination: results of the MECOVAC survey. Open Med (Wars). 2022;17(1):475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharp GC, Fraser A, Sawyer G, Kountourides G, Easey KE, Ford G, et al. The COVID‐19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2022;51:691–700. 10.1093/ije/dyab239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, et al. Analysis of sex hormones and menstruation in COVID‐19 women of child‐bearing age. Reprod Biomed Online. 2021;42(1):260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The contents of the questionnaire
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


