Abstract
Favipiravir is one of the most used antiviral agents for the treatment of coronavirus disease 2019 infection in many countries, including Thailand. This study aimed to investigate the effect of favipiravir‐warfarin interaction in terms of changes in international normalized ratio (INR) of patients. Medication charts of all inpatients in a hospital in Thailand between April 2021 and March 2022 were reviewed. Patients who received either warfarin with standard care or warfarin with favipiravir were included. The INR levels of patients were monitored at baseline and the earliest date following treatment, as well as other laboratory parameters. There were 43 and 53 patients in the warfarin‐favipiravir and the warfarin‐only groups, respectively. Baseline characteristics, such as sex, age, body mass index, and warfarin dose, were not significantly different between the 2 groups. The results showed that the mean INR of patients using favipiravir and warfarin was increased from 2.14 to 3.88 (P < .001), while the patients using warfarin alone had no increase in the mean INR (1.93 vs 1.91; P = .906). Other parameters were not significantly changed, including white blood cell count, red blood cell count, hemoglobin, hematocrit, and liver function. However, an increase in platelet count was observed in the favipiravir‐warfarin group, but not in the control group. This real‐world study highlighted a significant increase in the INR levels of patients who used favipiravir together with warfarin, compared to patients who used only warfarin. However, the interaction did not affect other laboratory parameters, except an increase in platelet count.
Keywords: COVID‐19, drug interaction, favipiravir, warfarin
Coronavirus disease (COVID‐19) is a pandemic infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 Although there are currently many vaccines developed to prevent infection, antiviral agents are still important in the treatment of the disease. The recently approved antiviral treatment of COVID‐19 includes favipiravir, remdesivir, molnupiravir, and paxlovid. 2 , 3 , 4 , 5 Favipiravir is an antiviral agent that was approved for medical use in Japan in 2014 for the treatment of pandemic influenza virus infections. 6 This drug is widely used for the treatment of COVID‐19 infection in many countries and currently is the first‐line treatment in Thailand.
Favipiravir is a prodrug of purine base analog. It is converted by intracellular phosphoribosylation to be favipiravir ribofuranosyl‐5B‐triphosphate, which is the active form. The mechanism of action of this drug is a selective and potent inhibitor of RNA‐dependent RNA polymerase of RNA viruses, resulting in the inhibition of RNA synthesis of SARS‐CoV‐2 in the infected cells, and therefore the infected cells cannot duplicate. 7 As favipiravir was developed and approved for other diseases, the information of this drug is not fully studied, especially its pharmacokinetic profile. According to the data from the manufacturer, favipiravir is mainly metabolized in the liver, and mainly by the aldehyde oxidase enzyme. 8 In addition, this drug is shown to inhibit cytochrome P450 (CYP) 2C8, so coadministration of favipiravir with any drugs that are metabolized by CYP2C8 should be closely monitored. 8 , 9
There are several drugs that are known to be metabolized via CYP2C8, such as pioglitazone, rosiglitazone, loperamide, and warfarin. 10 Warfarin is an anticoagulant drug that has many indications, for example, atrial fibrillation, venous thromboembolism, and pulmonary embolism. 11 Warfarin that is available in the market consists of a racemic mixture of 2 optical isomers (S‐warfarin and R‐warfarin, 1:1). R‐isomer is mainly metabolized by CYP1A2 and CYP3A4, while S‐isomer is mainly metabolized by CYP2C9. 12 Moreover, both R‐ and S‐isomers are metabolized by CYP2C8. 13 Consequently, warfarin is one of the drugs that have numerous reports on drug interaction.
Since favipiravir can inhibit CYP2C8 and warfarin is metabolized by the same enzyme, theoretically, these 2 drugs can have drug interaction. However, there have never been reports that described favipiravir‐warfarin interaction, except for a case report in Japan. 14 Therefore, this study aimed to investigate the interaction between favipiravir and warfarin in patients with indications of both drugs.
Methods
Ethical Approval
This study was approved by the Human Research Ethics Committee of Songkhla Hospital (Registration number: SKH IRB 2022‐Pharm‐IN3‐1016). The requirement of patient informed consent was waived because it was a retrospective study that did not directly involve any patient.
Study Design
This was a retrospective observational study. All patients who were admitted at a hospital in Thailand between April 2021 and March 2022 were screened. The inclusion criteria of this study were patients who were aged ≥18 years and were prescribed warfarin for the treatment of underlying diseases at admission. Patients who were prescribed favipiravir for the treatment of COVID‐19 were collected as an intervention group.
Due to the regulation of the Ministry of Public Health, Thailand, all patients with cardiovascular diseases who were infected with SARS‐CoV‐2 virus had to receive favipiravir regardless of their symptoms 15 ; thus, it was impossible to find patients with COVID‐19 who did not use favipiravir to be a control group. The control group in this study, therefore, was the patients who were admitted to the hospital due to any causes and received warfarin but not favipiravir. These patients might receive any standard‐of‐care treatment for COVID‐19 infection. Patients were excluded if they had incomplete data listed for analysis.
Electronic medication records of all recruited patients were reviewed. The relevant variables were collected, including age, sex, weight, height, comorbid diseases, concurrent medication, and laboratory results, that is, international normalized ratio (INR), white blood cell (WBC), red blood cell (RBC), platelet, hemoglobin, and hematocrit levels. Serum creatinine, aspartate transaminase (AST), alanine transferase (ALT), and serum albumin were also collected.
The characteristics of patients were recorded on the first date of admission. Other parameters including concurrent medication and laboratory results were collected twice throughout the admission: the first date of prescription of warfarin and/or favipiravir and the first date that had laboratory data after the treatment. Incomplete data were defined as no INR level at pretreatment of warfarin and favipiravir, no INR level following treatment, or no other relevant parameters. The primary outcome of this study was the mean change in INR after treatment with favipiravir compared with no favipiravir. The secondary outcomes were the mean changes in hematologic parameters, including hemoglobin, hematocrit, platelet, RBC, and WBC levels.
Statistical Analysis
Baseline characteristics of all patients were analyzed using descriptive statistics and reported as numbers, means, and percentages. The Wilcoxon signed‐rank test was used to compare INR levels before and after treatment within the groups, as well as other continuous variables. Chi‐square was used for the analysis of all categorical variables. The Mann‐Whitney U test was used to compare the differences in INR levels between patients who were treated and were not treated with favipiravir. All statistical analyses were performed using SPSS Statistics software version 28.0.0.0 (IBM, Armonk, New York), and statistical significance was set at P < .05.
Results
Baseline Characteristics
Between April 2021 and March 2022, there were 108 patients who were screened in the study setting. Of them, only 96 patients had the complete clinical data and were recruited for the analysis (Figure 1). A total of 43 patients were assigned to the intervention group (warfarin and favipiravir), and 53 patients were assigned to the control group (warfarin and standard care).
Figure 1.
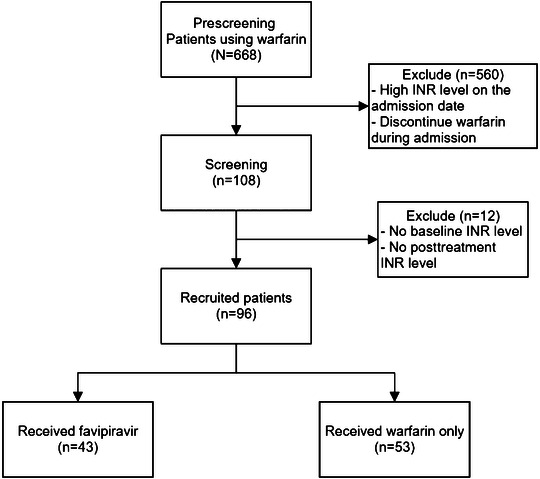
Flow diagram of the patient recruitment process. INR, international normalized ratio.
The baseline characteristics of both groups are described in Table 1. There was no statistically significant difference between the intervention and control groups in almost all baseline variables, except WBC count. The patients in both groups contained a similar number of men and women with an average age of ≥60 years. The mean body mass index of both groups was <23.00 kg/m2. The mean warfarin dose was 19.84 mg/week in the intervention group and 16.54 mg/week in the control group. Additionally, no statistically significant difference in RBC, hemoglobin, hematocrit, platelet, serum creatinine, AST, ALT, and serum albumin level was observed in this study. The mean WBC count of the intervention group was 6.85 × 103 cell/mm3 (95%CI, 5.90‐7.79) which was different from 7.58 × 103 cell/mm3 (95%CI, 6.04‐9.13) of the control group (P = .036).
Table 1.
Baseline Characteristics of Patients Who Were Admitted to the Clinical Setting and Received Warfarin Prior to Receiving Either Favipiravir or Standard Care (N = 96)
| Variable | Normal Range | Warfarin and Favipiravir (n = 43) | Warfarin and Standard Care (n = 53) | P Value |
|---|---|---|---|---|
| Sex, male, n (%) | – | 20 (46.5) | 27 (50.9) | .666 |
| Age, y, median (range) | – | 69 (38‐87) | 73 (45‐87) | .979 |
| Body mass index, kg/m2, mean (95%CI) | – | 22.77 (21.44‐24.12) | 22.37 (19.95‐24.79) | .614 |
| Warfarin dose, mg/week, mean (95%CI) | – | 19.84 (16.40‐23.29) | 16.54 (13.64‐19.45) | .293 |
| White blood cell count, ×103 cells/mm3, mean (95%CI) | 4.5‐10.0 | 6.85 (5.90‐7.79) | 7.58 (6.04‐9.13) | .036 |
| Red blood cell count, ×106 cells/mm3, mean (95%CI) | 4.2‐5.5 | 4.34 (4.06‐4.61) | 4.13 (3.89‐4.37) | .396 |
| Hemoglobin, g/dL, mean (95%CI) | 12‐16 | 11.74 (10.92‐12.57) | 11.35 (10.38‐12.33) | .692 |
| Hematocrit, %, mean (95%CI) | 36‐48 | 36.13 (33.51‐38.74) | 35.04 (32.33‐37.76) | .685 |
| Platelet count, ×103 cells/mm3, mean (95%CI) | 140‐400 | 214.41 (182.18‐246.63) | 193.13 (160.93‐225.33) | .713 |
| Serum creatinine, mg/dL, mean (95%CI) | 0.55‐1.02 | 1.60 (0.95‐2.45) | 1.30 (0.90‐1.70) | .613 |
| Aspartate transaminase, IU, mean (95%CI) | <35 | 42.78 (30.37‐55.19) | 34.52 (23.37‐45.67) | .103 |
| Alanine transferase, IU, mean (95%CI) | <35 | 21.28 (13.71‐28.85) | 20.52 (13.28‐27.76) | .729 |
| Serum albumin, mg/dL, mean (95%CI) | 3.5‐5.2 | 3.60 (3.43‐3.77) | 3.55 (3.34‐3.75) | .990 |
The difference in sex was calculated using chi‐square, while other differences were calculated using the Mann‐Whitney U test.
Regarding the concurrent medication, Table 2 shows the medication that each patient received in the hospital admission as standard care. Markedly, the patients in both groups were similarly prescribed medicine (ie, enoxaparin, piperacillin/tazobactam, clopidogrel, and sertraline). In addition, the patients in both groups had other medications, such as simvastatin, amiodarone, allopurinol, levofloxacin, and ceftazidime. There was no statistically significant difference in medication between the intervention and control groups. However, only the intervention group received azithromycin, cotrimoxazole, and rosuvastatin. Metronidazole, dicloxacillin, and apixaban were especially received in the control group.
Table 2.
Medication of Standard Treatment That Patients in Both Groups Received While Admitted to the Hospital
| Medication | Warfarin and Favipiravir, n (%) (n = 43) | Warfarin and Standard Care, n (%) (n = 53) | P Value |
|---|---|---|---|
| Simvastatin | 24 (55.8) | 29 (54.7) | .692 |
| Enoxaparin | 5 (11.6) | 5 (9.4) | .951 |
| Aspirin | 6 (13.9) | 12 (22.6) | .176 |
| Amiodarone | 4 (9.3) | 6 (11.3) | .468 |
| Allopurinol | 2 (4.7) | 3 (5.7) | .617 |
| Azithromycin | 1 (2.3) | 0 | .324 |
| Levofloxacin | 2 (4.7) | 1 (1.9) | .580 |
| Ceftazidime | 1 (2.3) | 2 (3.8) | .536 |
| Cotrimoxazole | 1 (2.3) | 0 | .324 |
| Rosuvastatin | 1 (2.3) | 0 | .324 |
| Piperacillin/Tazobactam | 4 (9.3) | 4 (7.6) | .957 |
| Clopidogrel | 7 (16.3) | 7 (13.2) | .941 |
| Sertraline | 1 (2.3) | 1 (1.9) | .979 |
| Metronidazole | 0 | 1 (1.9) | .306 |
| Dicloxacillin | 0 | 1 (1.9) | .306 |
| Apixaban | 0 | 1 (1.9) | .306 |
Each patient could receive >1 medicine.
Effects on Patient INR
Figure 2 describes the changes in INR levels of all 96 patients—43 patients in the intervention group (Figure 2a) and 53 patients in the control group (Figure 2b). At baseline, the average INR levels of patients in the intervention and the control groups were 2.14 (95%CI, 1.84‐2.45) and 1.93 (95%CI, 1.72‐2.14), respectively (Figure 3). The pretreatment INR levels of both groups were not significantly different (P = .446). Following treatment, the average INR of patients in the intervention group significantly increased to 3.88 (95%CI, 3.22‐4.55) (P < .001). However, the mean posttreatment INR of the control group did not significantly differ from the baseline (mean, 1.91; 95%CI, 1.73‐2.09) (P = .906). Furthermore, the increases in posttreatment INR of both groups were significantly different, at P < .001.
Figure 2.
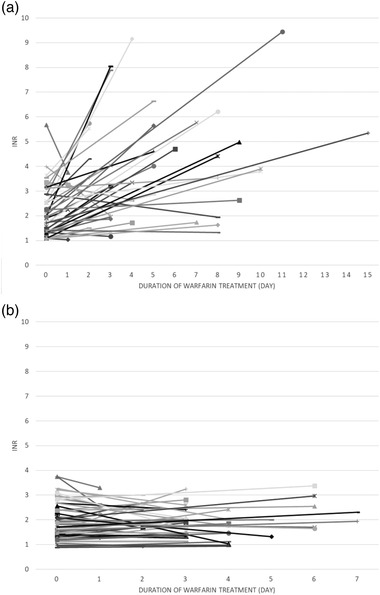
The changes in international normalized ratio of individual patients who were treated with warfarin and favipiravir (a) (n = 43) and warfarin without favipiravir (b) (n = 53).
Figure 3.
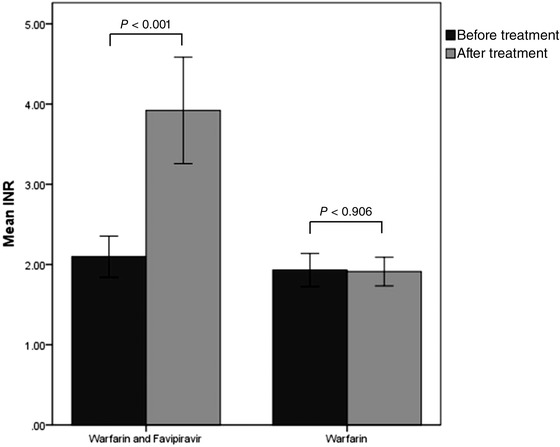
Comparison of the average INR before and after treatment of patients who received warfarin with and without favipiravir (n = 53). INR, international normalized ratio.
Effects on Other Parameters
The comparison of all laboratory parameters showed no statistically significant difference before and after treatment in both the intervention and control groups, except platelet count in patients who received favipiravir (Table 3). In the intervention group, the mean changes in WBC, RBC, hemoglobin, hematocrit, and serum creatinine levels before and after treatment were 7.30 × 103 cells/mm3, −0.22 × 106 cells/mm3, −0.40 g/dL, −1.38%, and 0.05 mg/dL, respectively. AST, ALT, and serum albumin levels were not assessed in this group because the sample size was too small. For platelet count, the results showed a significant increase in mean platelet count from 214.41 to 256.91 × 103 cells/mm3 (P = .018).
Table 3.
Differences in Laboratory Parameters Before and After Treatment With Warfarin With Favipiravir and Warfarin With Standard Care (N = 96)
| Mean Difference (P Value) | ||
|---|---|---|
| Variable a |
Warfarin and Favipiravir (n = 43) |
Warfarin and Standard care (n = 53) |
| White blood cell count, ×103 cells/mm3 (4.5‐10.0 × 103 cells/mm3) | 7.30 (0.050) | −.04 (0.310) |
| Red blood cell count, ×106 cells/mm3 (4.2‐5.5 × 106 cell/mm3) | −.22 (0.484) | .05 (0.612) |
| Hemoglobin, g/dL (12.0‐16.0 g/dL) | −.40 (0.483) | .19 (0.600) |
| Hematocrit, % (36%‐48%) | −1.38 (0.397) | .71 (0.599) |
| Platelet count, ×103 cells/mm3 (140‐400 × 103 cell/mm3) | 42.50 (0.018) | −12.00 (0.237) |
| Serum creatinine, mg/dL (0.55‐1.02 mg/dL) | .05 (0.465) | −.02 (0.711) |
| Aspartate transaminase (IU) (<35 IU) | N/A | 7.50 (0.180) |
| Alanine transferase, IU (<35 IU) | N/A | 9.50 (0.180) |
| Serum albumin, mg/dL (3.5‐5.2 mg/dL) | N/A | −.35 (0.317) |
N/A, not applicable because some variables could not calculate the differences due to very low sample sizes.
aNormal ranges are shown in parentheses.
Likewise, in the control group, the mean differences of all laboratory parameters were not significant, including WBC, RBC, hemoglobin, hematocrit, platelet, serum creatinine, AST, ALT, and serum albumin levels. For instance, WBC count was changed from 7.58 to 7.54 × 103 cells/mm3 (P = .310). Hemoglobin increased from 11.35 to 11.54 g/dL (P = .600), while platelet count decreased from 193.13 to 181.13 × 103 cells/mm3 (P = .237).
Discussion
The results of this study indicated significant INR elevation in patients who were prescribed favipiravir together with warfarin compared to patients with warfarin only. This interaction did not affect other parameters including WBCs, RBCs, hemoglobin, hematocrit, serum creatinine, AST, ALT, and serum albumin. However, platelets seemed to be increased in patients who received favipiravir and warfarin, but this did not affect patient INR levels.
Favipiravir is a novel antiviral agent that is currently used for the treatment of COVID‐19 infection in many countries, including Thailand. 2 , 16 Therefore, this drug must be used in various populations of patients. In patients who are receiving warfarin and are COVID‐19 positive, concurrent use of favipiravir and warfarin is feasible, resulting in a potential drug interaction.
According to the previous studies, favipiravir was shown to inhibit CYP2C8, 8 , 9 which was the enzyme that partly involved in warfarin metabolism. 13 The inhibition of this enzyme could increase the level of warfarin and therefore potentially increase the INR level in patients. The data sheet suggested that favipiravir could inhibit CYP2C8 with a half maximal inhibitory concentration value of 74.9 µg/mL. 8 The pharmacokinetic profile indicated that administration of 1800 mg of favipiravir—the starting dose of favipiravir in Thai patients—provided maximal concentration of 74.7‐85.5 µg/mL, 17 which is likely to inhibit CYP2C8 and result in an increase in warfarin level. Nonetheless, the exact mechanism of this interaction is still unknown and needs more studies, as well as the actual effects of favipiravir on patient INR.
Although this study was a retrospective study with several possible confounding factors, many factors were compared between the intervention and control groups and showed no significant difference. The results from a previous study demonstrated that hematocrit was an important determinant of the viscosity of blood and might affect the INR level, 18 but this parameter was similar in both groups. The baseline platelet count that affects a blood clot 19 was not significantly different between the 2 groups. In addition, the liver function tests showed normal results in mean AST and ALT levels at baseline and following treatment of all groups. Hepatic impairment is known to be a cause of the altered response to warfarin due to the impaired synthesis of clotting factors and the decrease in metabolism of warfarin. The unchanged AST and ALT levels after treatment therefore suggested that the increased INR levels might not result from the impaired liver function.
Concurrent drugs were the other most critical factors that could affect patient INR. However, the patients in both groups were not able to receive the same medication as standard of care due to the retrospective methods of this study together with different underlying diseases of the patients. For instance, some patients were administered aspirin or clopidogrel, which can augment the effect of warfarin, resulting in an increase in INR. 11 , 20 , 21 On the other hand, some drugs such as dicloxacillin and spironolactone could decrease the warfarin level and INR. 11 , 22 , 23 Thus, the statistical analysis was performed to ensure that the patients in both groups received the same drugs, particularly the drugs that were known to have a robust interaction with warfarin. Nevertheless, it should be noted that some patients with favipiravir treatment had different concurrent medication from the others and might result in the decreases in INR (Figure 2a), while most patients had the increased INR.
Apart from the INR levels, the results in this study showed no other effect of favipiravir‐warfarin interaction. However, interestingly, this study found that the platelet count was significantly increased in patients treated with favipiravir. This phenomenon may guide in the follow‐up of favipiravir treatment and in determining the prognosis. It is known that a low number of platelets is related to longer duration of blood clots, and vice versa. 24 , 25 Therefore, patients in this study who had a higher mean platelet count should theoretically have lower INR, but the mean INR of such patients was significantly higher after using favipiravir. In spite of that, the INR of patients was significantly increased.
Several limitations should be noted for the results of this study. First, there are numerous factors that are known to affect warfarin and/or INR levels, so it was impossible for the retrospective methods to control all potential confounding factors. Although this study best tried to compare as many factors as possible, it was truly believed that there might be some other underlying factors that might affect the INR, such as food. However, as all patients were admitted to the same hospital, almost all food that patients received was from the hospital kitchen and should have been very similar. Moreover, some patients were prescribed medicines that can have clinical interaction with warfarin, such as amiodarone, allopurinol, metronidazole, and statins. These concomitant medications could alter the INR levels. Second, the INR levels of all patients were not collected at the same period of time; meaning that the effects of warfarin in this study were not from the same levels of warfarin. As there is no official guideline to monitor the INR in this setting, blood samples of the patients were collected only following doctors’ orders. Thus, some patients had the first posttreatment INR at the 10th day of admission. Third, there were no other blood clotting parameters collected in this study, such as prothrombin time, partial thromboplastin time, blood clotting duration, and coagulation factors. Furthermore, this study did not observe any pharmacokinetic parameters of the patients, such as peak and trough concentrations, time to peak concentration, and area under the concentration‐time curve.
Future research should emphasize the mechanism of interaction of favipiravir and warfarin, as well as other drugs that are metabolized via CYP2C8. The expression of the relevant proteins should be measured together with the concentration of favipiravir in the body to confirm the interaction. However, according to the previous case report of interaction 14 and the results of this study, coadministration of favipiravir and drugs that are metabolized by the CYP2C8 system should be done with care until the mechanism is better understood. 26
Conclusion
This study suggested that patients who received favipiravir with warfarin had significantly higher INR, compared with those who received only warfarin. Other parameters were not affected by this interaction, including WBC, RBC, hemoglobin, hematocrit, serum creatinine, AST, ALT, and albumin levels. The mechanism of interaction is currently unknown, but there is a high possibility that it involves CYP2C8 inhibitory effect of favipiravir. Further studies are needed to indicate the actual mechanism; in the meantime, patients who received favipiravir for the treatment of COVID‐19 together with warfarin should have their INR closely monitored as well as signs of bleeding.
Author Contributions
S.U. designed the research, analyzed data, and wrote the manuscript. K.W. and N.L. collected and analyzed data. P.K. wrote the manuscript.
Conflicts of Interest
All authors report no conflicts of interest.
Data Sharing Statement
All patient data that were used in this study can be requested from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all staff at Songkhla Hospital for their help in collecting data.
References
- 1. World Health Organization . Coronavirus disease (COVID‐19). https://www.who.int/health‐topics/coronavirus. Accessed March 22, 2022.
- 2. Radhakrishnan A, Arunachalam R, Elango A. Critical review and analysis of approval of favipiravir for restricted emergency use in mild‐to‐moderate COVID‐19. J Pharmacol Pharmacother. 2020;11(1):1‐7. [Google Scholar]
- 3. Food and Drug Administration . Remdesivir (Veklury). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s000lbl.pdf. Published 2020. Accessed March 26, 2022.
- 4. Food and Drug Administration . Fact sheet for healthcare providers: Emergency Use Authorization (EUA) for molnupiravir. https://www.fda.gov/media/155054/download. Published 2022. Accessed March 26, 2022.
- 5. Food and Drug Administration . Fact sheet for healthcare providers: Emergency Use Authorization (EUA) for paxlovid. https://www.fda.gov/media/155050/download. Published 2021. Accessed March 26, 2022.
- 6. Shiraki K, Daikoku T. Favipiravir, an anti‐influenza drug against life‐threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baranovich T, Wong SS, Armstrong J, et al. T‐705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. 2013;87(7):3741‐3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toyama Chemicals . Pharmaceuticals and medical devices agency: avigan (favipiravir) review report. https://www.pmda.go.jp/files/000210319.pdf. Published 2014. Accessed March 29, 2022.
- 9. Madelain V, Nguyen TH, Olivo A, et al. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet. 2016;55(8):907‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Backman JT, Filppula AM, Niemi M, Neuvonen PJ. Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016;68(1):168‐241. [DOI] [PubMed] [Google Scholar]
- 11. Bristol‐Myers Squibb Pharma Company . Product monograph: Coumadin. https://pdf.hres.ca/dpd_pm/00047189.pdf. Published 2018. Accessed March 22, 2022.
- 12. Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Ther. 1997;73(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 13. Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sekimoto M, Imai T, Hidaka S, et al. Elevated INR in a COVID‐19 patient after concomitant administration of favipiravir and warfarin: A case report. J Clin Pharm Ther. 2022;47(3):407‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Department of Medical Services . Practice guideline for the diagnosis, treatment, and prevention of COVID‐19 infection for doctors and health professionals in hospitals: Revised version. https://covid19.dms.go.th/Content/Select_Landding_page?contentId=164. Published 2021. Accessed March 22, 2022. [Google Scholar]
- 16. Agrawal U, Raju R, Udwadia ZF. Favipiravir: a new and emerging antiviral option in COVID‐19. Med J Armed Forces India. 2020;76(4):370‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siripongboonsitti T, Ungtrakul T, Watanapokasin N, et al. Pharmacokinetic comparison of favipiravir oral solution and tablet formulations in healthy thai volunteers. Clin Pharmacol Drug Dev. 2022; 10.1002/cpdd.1149. [DOI] [PubMed] [Google Scholar]
- 18. Martín‐Pérez M, Gaist D, de Abajo FJ, García Rodríguez LA. Predictors of over‐anticoagulation in warfarin users in the UK general population: a nested case‐control study in a primary health care database. Thromb Haemost. 2019;119(1):66‐76. [DOI] [PubMed] [Google Scholar]
- 19. Ho CH. White blood cell and platelet counts could affect whole blood viscosity. J Chin Med Assoc. 2004;67(8):394‐397. [PubMed] [Google Scholar]
- 20. Hurlen M, Erikssen J, Smith P, Arnesen H, Rollag A. Comparison of bleeding complications of warfarin and warfarin plus acetylsalicylic acid: a study in 3166 outpatients. J Intern Med. 1994;236(3):299‐304. [DOI] [PubMed] [Google Scholar]
- 21. Johnson SG, Rogers K, Delate T, Witt DM. Outcomes associated with combined antiplatelet and anticoagulant therapy. Chest. 2008;133(4):948‐954. [DOI] [PubMed] [Google Scholar]
- 22. Lacey CS. Interaction of dicloxacillin with warfarin. Ann Pharmacother. 2004;38(5):898. [DOI] [PubMed] [Google Scholar]
- 23. O'Reilly RA. Spironolactone and warfarin interaction. Clin Pharmacol Ther. 1980;27(2):198‐201. [DOI] [PubMed] [Google Scholar]
- 24. Yaylaci S, Dheir H, Şenocak D, et al. The effects of favipiravir on hematological parameters of covıd‐19 patients. Rev Assoc Med Bras (1992). 2020;66(2):65‐70. [DOI] [PubMed] [Google Scholar]
- 25. Ozbas HM, Kayan T, Yakarisik M, et al. Role of Favipiravir on the hematologic parameters in patients with COVID‐19 infection. Med Sci Discov. 2021;8(10):577‐80. [Google Scholar]
- 26. Corritori S, Savchuk N, Pauza CD. Risk/benefit profiles of currently approved oral antivirals for treatment of COVID‐19: similarities and differences. COVID. 2022; 2(8):1057‐1076. [Google Scholar]


