Summary
Lung damage caused by SARS‐Cov‐2 virus results in marked arterial hypoxia, accompanied in many cases by hypocapnia. The literature is inconclusive as to whether these conditions induce alteration of the affinity of haemoglobin for oxygen. We studied the oxyhaemoglobin dissociation curves (ODCs) of 517 patients hospitalized with coronavirus disease 2019 (COVID‐19) for whom arterial blood gas analysis (BGA) was performed upon hospitalization (i.e., before treatment). With respect to a conventional normal p50 (pO2 at 50% saturation of haemoglobin) of 27 mmHg, 76% had a lower standardized p50 (p50s) and 85% a lower in vivo p50 (p50i). In a 33‐patient subgroup with follow‐up BGAs after 3, 6, 9, 12, 15 and 18 days' treatment, p50s and p50i exhibited statistically significant differences between baseline values and values recorded at all these time points. The 30‐day Kaplan–Meier survival curves of COVID‐19 patients stratified by p50i level show a higher probability of survival among patients who at admission had p50 values below 27 mmHg (p = 0.012). Whether the observed alteration of the affinity of haemoglobin for oxygen in COVID‐19 patients is a direct or indirect effect of the virus on haemoglobin is unknown.
Keywords: clinical outcome, COVID‐19; dissociation curve; haemoglobin‐oxygen affinity; p50
Abbreviations
- (BGA)
blood gas analysis
- (eGFR)
estimated glomerular filtration rate
- (ICU)
intensive care unit
- (Hb)
haemoglobin
- (Hb‐O2)
oxyhaemoglobin
- (OCD)
oxyhaemoglobin dissociation curve
- (p50i)
in vivo p50
- (p50s)
standardized p50
- (RT‐PCR)
real‐time polymerase chain reaction
- (SARS‐Cov‐2)
severe acute respiratory syndrome coronavirus 2
INTRODUCTION
It is well known that in patients with coronavirus disease 2019 (COVID‐19) respiratory function is impaired, which reduces oxygen uptake in the lungs, leading to hypoxaemia. 1 , 2 It has been suggested 3 that compensatory effects on oxygen transport in the blood should be expected, primarily increased affinity of haemoglobin for oxygen. This would show up as a left shift in the oxyhaemoglobin dissociation curve (ODC), in particular a reduction in p50, the value of paO2 at 50% saturation of haemoglobin with O2. 4 However, of the few, small studies of the affinity of oxygen for Hb in patients with COVID‐19, 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 only two found increased affinity of Hb for oxygen. 6 , 12 Suspecting that the discrepancies might be due not only to small study size but also to the inclusion of patients receiving treatment (oxygen therapy, bronchodilators, fluid therapy, corticoids, intensive care, etc.), we studied p50 in a large sample of patients at admission to hospital, before the start of physical and pharmacological treatments.
PATIENTS AND METHODS
Participants
The University Hospital Complex, Santiago de Compostela, Spain, is a tertiary centre serving an almost exclusively white population of approximately 450,000 people. This study primarily concerned patients severely infected with acute respiratory syndrome coronavirus 2 (SARS‐Cov‐2, confirmed by polymerase chain reaction testing of nasopharyngeal specimens) who were admitted to the hospital between March 2020 and November 2021 and who met the following additional criteria: (1) age >18 years; (2) blood gas analysis (BGA) was performed at admission, before any treatment or transfer to the ICU; and (3) the endpoint (discharge or death) was reached before the end of the study.
Changes in the affinity of haemoglobin for O2 after the initiation of treatment were assessed in a subgroup of patients with post‐admission BGA determinations 3, 6, 9, 12, 15 and 18 days after the initial at‐admission BGA. For comparison, we also studied a group of patients who were admitted for respiratory problems (pneumonia, asthma, acute respiratory distress syndrome secondary to other aetiologies, etc.) but tested negative for SARS‐Cov‐2; these were selected randomly and synchronously with the COVID‐19 group, and were likewise older than 18 years and had a BGA at admission prior to any treatment.
Anonymised information was entered in a database hosted on a secure server. Individual consent was not required for anonymous data. The study was reviewed and approved by the Clinical Research Ethics Committee of the Consellería de Sanidade, Xunta de Galicia, Spain, and was carried out in accordance with the current Declaration of Helsinki.
Data collection and methods
We retrospectively collected medical record data concerning demographic characteristics, comorbidities, complications and clinical outcome (discharge or death while hospitalized). Comorbidities included hypertension, coronary artery disease, past or active cancer, chronic kidney disease associated with stage 3–5 kidney failure (estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation), obesity (body mass index [BMI] ≥30 kg/m2) and diabetes status.
The presence of SARS‐CoV‐2 in respiratory specimens was detected by “real‐time” RT‐PCR methods. Blood gas analyses (BGAs) were performed using a RAPIDPoint 500 analyser (Siemens Healthcare Diagnostics, Barcelona, Spain). In this system, the partial pressure of carbon dioxide (pCO2), partial pressure of oxygen (pO2) and pH were determined with electrochemical measurements, and the oxygen saturation of haemoglobin, carboxyhaemoglobin, methaemoglobin and total haemoglobin with a multiwavelength spectrophotometer; deoxyhaemoglobin, oxygen content and base excess were calculated parameters.
The value of pO2 for 50% O2 saturation of haemoglobin (p50) was calculated as both standardized p50 p50s, a value calculated for a blood temperature of 37°C, blood pH 7.40, pCO2 40 mmHg and carboxyhaemoglobin <2%, 13 , 14 as in vivo p50 (p50i; p50 for the blood pH, temperature, pCO2 and carboxyhaemoglobin levels of the patient); both equations were used as presented by Vogel et al. 6 Following the RapidPoint 500 manufacturer's recommendations for p50 calculation, BGAs with saturations ≥97% were not used for this study. The normal value of p50 for human blood is about 27 mmHg. 15
Statistical analysis
All variables were checked for normality. The statistical significance of differences between groups was estimated by Student's t‐test for normally distributed variables, and nonparametrically otherwise (Mann–Whitney U‐test). Descriptive statistics were calculated for all variables, with mean and SD or median and interquartile range reported for continuous variables, and number and percentage of total for categorical and integer variables. Comparisons of blood gas components between at‐admission and post‐admission values and between patients with and without COVID‐19, and comparisons of clinical characteristics and outcomes between patients with p50 levels <27 or ≥ 27 mmHg, were performed using the Student t‐test for continuous variables (a two‐tailed p value ≤0.05 was deemed statistically significant) and the Pearson χ 2 test for categorical variables. Spearman's correlation coefficient (r) was employed to examine the relation between p50 and haemoglobin. Kaplan–Meier analysis of survival for 30 days after admission was performed for COVID‐19 subgroups defined by at‐admission p50 (<27 or ≥ 27 mmHg); a Log‐rank test was used to compare survival distributions. To assess the associations of preadmission attributes with in‐hospital mortality, we performed logistic regression analyses with death as the outcome variable. Variables with a probability value <0.1 at univariate analysis and several potential confounding factors were then entered into a multivariate analysis to identify the independent predictors. Multivariate logistic regression analysis with backward elimination was conducted (OR, elimination threshold p > 0.1) to determine the predictors of death, including age, gender, hypertension, dyslipidaemia, current or former smoking, obesity, chronic kidney disease, coronary artery disease, cancer and p50 (p50i for one analysis, p50s for another). The odds ratios are reported together with their 95% CIs. All calculations were performed using SPSS version 28 statistical software.
RESULTS
During the study period, 667 patients testing positive for SARS‐CoV‐2 required hospitalization. After exclusion of 150 patients for the reasons indicated in Figure S1, 517 were included in the study; Table 1 summarizes their characteristics. Some 85.3% had an at‐admission p50i value less than a conventional normal 27 mmHg. Compared with these low‐p50 patients, the others had more comorbidities (3 vs. 2, p < 0.001), with prevalences greater by factors of 2.9 for chronic kidney disease, 1.8 for coronary artery disease, 1.7 for diabetes, 1.4 for hypertension, and 1.3 for obesity (p < 0.05 in all cases). Those with p50i <27 mmHg had a higher average arterial oxygen content than those with p50i ≥27 mmHg, 17.2 (15.7–18.7) vs. 15.2 (13.0–16.6) ml/dl (p < 0.001). Anaemia significantly increased both p50i and p50s (p < 0.001 in both cases; Table S1), in keeping with which haemoglobin was inversely correlated with both p50i (r = 0.309, p < 0.001) and p50s (r = 0.217, p < 0.001).
TABLE 1.
Demographic and clinical characteristics, and outcomes, of patients hospitalized with COVID‐19
| Characteristic | Total (n = 517) | p50i at admission (mmHg) | ||
|---|---|---|---|---|
| < 27 (n = 441) | ≥27 (n = 76) | p value* | ||
| Demographic | ||||
| Age (years) | 70 ± 14 | 70 ± 14 | 72 ± 15 | 0.22 |
| >60 years | 387 (74.9) | 327 (74.4) | 60 (78.9) | 0.37 |
| Female | 253 (48.9) | 215 (48.8) | 38 (50.0) | 0.84 |
| Oxygen content (ml/dl) | 16.6 ± 2.7 | 17.0 ± 2.5 | 14.7 ± 3.0 | <0.001 |
| Comorbidities | 2 (1–3) | 2 (1–3) | 3 (2–4) | <0.001 |
| Hypertension | 287 (55.5) | 232 (52.6) | 55 (72.4) | 0.001 |
| Dyslipidaemia | 257 (49.7) | 220 (49.9) | 37 (48.7) | 0.85 |
| Chronic kidney disease | 66 (12.8) | 44 (10.0) | 22 (28.9) | <0.001 |
| Coronary artery disease | 54 (10.4) | 41 (9.3) | 13 (17.1) | 0.04 |
| Cancer | 54 (10.4) | 46 (10.4) | 8 (10.5) | 0.98 |
| Current or former smoking | 132 (25.5) | 114 (25.9) | 18 (23.7) | 0.69 |
| BMI (kg/m2) | 30.2 ± 5.4 | 29.9 ± 5.0 | 32.0 ± 6.9 | 0.003 |
| Obesity (BMI ≥30 kg/m2) | 246 (47.6) | 200 (45.4) | 45 (59.7) | 0.025 |
| Diabetes | 128 (24.8) | 99 (22.4) | 29 (38.2) | 0.003 |
| Outcomes | ||||
| Length of stay (days) | 10 (7–17) | 10 (7–17) | 11 (8–21 | 0.059 |
| Mortality | 75 (14.5) | 57 (12.9) | 18 (23.7) | 0.014 |
Note: Data are mean ± SD, median (IQR) or n(%).
p value for comparison between groups with p50i <27 or ≥ 27 mmHg.
In a subgroup comprising 112 COVID‐19 patients with a mean 42 BGAs per patient (3250 post‐admission BGAs – 29 per patient ‐ after discarding 1339 with Hb saturation > 97%), 83.9% of the at‐admission samples had p50i values <27 mmHg, as against 35.4% of the 3250 post‐admission samples.
The evolution of p50 was followed in greater detail in a group of 33 patients who had BGA data obtained every 3 days between day 0 (admission) and day 18 (Figure 1). At admission, 78.8% of these patients had p50i <27 mmHg, compared to 21.2% 6 days later or 45.5% 18 days post‐admission. At all times post‐admission, mean p50i was significantly greater, and the percentage of patients with p50i <27 mmHg significant less, than at admission (p < 0.001 for all comparisons with the at‐admission data). Figure S2 shows the difference between day 0 and day 6 for each of the 33 patients. Similar trends emerge in regard to different follow‐up times with more or fewer patients: the corresponding changes in p50s were less pronounced, but the trends were the same.
FIGURE 1.
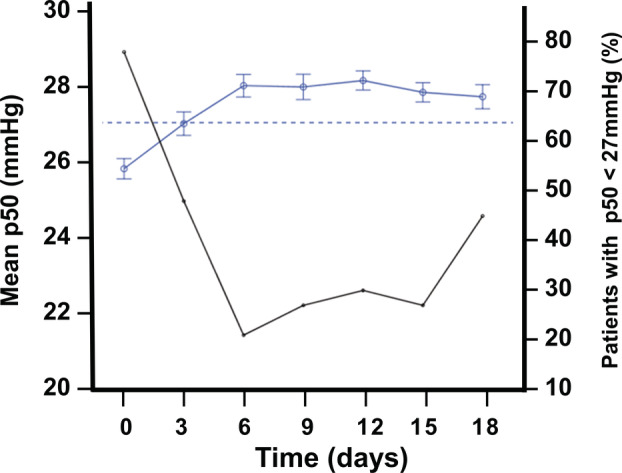
Time‐course of mean p50i in 33 Covid‐19 patients from admission to day 18. Data of p50i are presented as means and standard error (blue line and circles) and percentage of patients with p50i <27 mmHg (black line). The dotted line represents the conventional normal p50 value (27 mmHg).
To examine whether the pre‐treatment at‐admission BGA characteristics of the COVID‐19 patients differed from those of other patients with lung pathology, we compared them with those of a group of patients with other respiratory problems (Table S2). The COVID‐19 group had greater prevalences of hypoxaemia (73.3% vs 57.3%, p < 0.001), hypocapnia (59.4% vs 35.7%, p < 0.001), combined hypoxaemia and hypocapnia (59.4% vs 27.8%, p < 0.001) and alkalosis (53.4% vs 30.9%) (Table 2). There were no differences in total oxygen content between the two groups, but the COVID‐19 group had significantly lower median p50i and p50s than the non‐COVID group, and the percentage of patients with p50 < 27 mmHg was significantly greater in the COVID‐19 group (85.3% vs. 57.3% for p50i, 76.0% vs, 59.9% for p50s; p < 0.001 in both cases). Figure S3 shows the greater shift to the left in the distribution of p50i values in patients with COVID‐19.
TABLE 2.
Blood gas characteristics of COVID‐19 and non‐COVID respiratory patients at admission
| COVID‐19 (n = 517) | Non‐COVID‐19 (n = 314) | p value* | |
|---|---|---|---|
| Age (years) | 70 ± 14 | 72 ± 18 | 0.06 |
| Female | 253 (48.9) | 145 (46.2) | 0.44 |
| Blood gas variables | |||
| pO2 (mmHg) | 62.8 ± 11.9 | 67.0 ± 14.5 | <0.001 |
| pCO2 (mmHg) | 34.6 ± 6.4 | 38.9 ± 8.7 | <0.001 |
| pH | 7.45 ± 0.05 | 7.42 ± 0.06 | <0.001 |
| Haemoglobin (g/dl) | 13.0 ± 1.8 | 13.0 ± 2.3 | 0.80 |
| Oxygen saturation (%) | 90.9 ± 6.4 | 90.8 ± 7.2 | 0.78 |
| Oxyhaemoglobin (%) | 90.0 ± 6.4 | 89.3 ± 8.5 | 0.16 |
| Oxygen content (ml/dl) | 16.6 ± 2.7 | 16.7 ± 3.2 | 0.97 |
| Carboxyhaemoglobin (%) | 0.5 (0.3–1.0) | 1.0 (0.6–1.6) | <0.001 |
| Methaemoglobin (%) | 0.3 (0.2–0.4) | 0.2 (0.1–0.3) | <0.001 |
| Deoxyhaemoglobin (%) | 7.1 (5.5–10.0) | 6.9 (4.7–10.7) | 0.12 |
| Base excess (mmol/L) | 0.0 (−1.4 to 1.7) | 0.0 (−2.0 to 1.9) | 0.91 |
| p50 (mmHg) | |||
| In vivo | 25.7 ± 1.4 | 27.1 ± 2.8 | <0.001 |
| <27 | 441 (85.3) | 180 (57.3) | <0.001 |
| Standardized | 26.3 ± 1.2 | 26.8 ± 2.9 | <0.001 |
| <27 | 393 (76.0) | 188 (59.9) | <0.001 |
Note: Data are mean ± SD, median (IQR) or n(%).
p value for comparison between groups with and without COVID‐19.
Table 3 lists the results of univariate and multivariable analyses for factors associated with COVID19 mortality. Age, chronic kidney disease, cancer and p50s were significant independent predictors of mortality. With p50i the significance is slightly lower than with p50s (OR 1.23, 95% CI 0.98–1.44, p = 0.063 for p50i; OR 1.28, 95% CI 1.01–1.63, p = 0.042 for p50s). The Kaplan–Meier 30‐day survival curves of COVID‐19 patients stratified by p50i level show a significantly greater probability of survival among patients with admission p50i <27 mmHg (Figure 2; log‐rank test p = 0.012), and the death rate in this group, 12.9%, was lower than among the patients with admission p50i ≥27 mmHg (23.7%, p = 0.014). Similar results are observed in regard to p50s (Figure S4; log‐rank test p = 0.045).
TABLE 3.
Univariate and multivariate analysis of factors associated with COVID‐19 death
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age (years) | 1.09 (1.06–1.12) | <0.001 | 1.08 (1.05–1.12) | <0.001 |
| Sex (male vs. female) | 1.70 (0.91–2.45) | 0.063 | ||
| Hypertension | 4.02 (2.09–7.73) | <0.001 | ||
| Dyslipidaemia | 1.46 (0.81–2.82) | 0.54 | ||
| Coronary artery disease | 2.56 (1.29–5.10) | 0.007 | ||
| Chronic kidney disease | 4.43 (2.42–8.12) | <0.001 | 2.83 (1.47–5.44) | 0.002 |
| Current or former smoking | 1.27 (0.71–2.26) | 0.42 | ||
| Cancer | 2.89 (1.47–5.68) | 0.002 | 2.39 (1.14–5.01) | 0.020 |
| Diabetes | 1.66 (0.87–3.14) | 0.17 | ||
| Obesity a | 1.30 (0.76–2.24) | 0.34 | ||
| p50s (mmHg) | 1.27 (1.03–1.58) | 0.028 | 1.29 (1.02–1.63) | 0.035 |
Obesity was defined as having a body mass index greater than 30.
FIGURE 2.
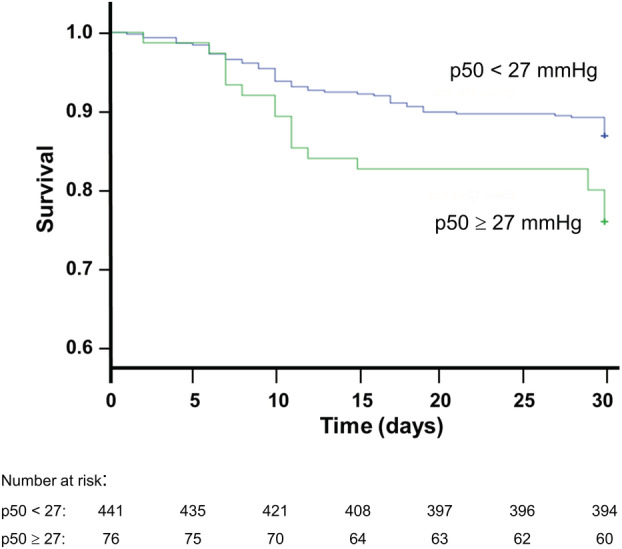
Kaplan–Meier analysis showing survival during hospitalization in 517 COVID‐19 patients, stratified by at‐admission p50 levels (log‐rank test p = 0.012).
DISCUSSION
In this observational study of a large sample of patients hospitalized for COVID‐19, Hb‐O2 affinity was in general markedly greater than normal at the time of admission to hospital, but clearly less than normal after a few days of treatment. Patients presenting with this increased Hb‐O2 affinity tended to have fewer comorbidities, and had a greater probability of survival, than those who did not. Specifically, higher p50s was an independent predictor of death.
Observation of Hb‐O2 affinity at admission of COVID‐19 patients to hospital has the advantage, over later determinations, that any alteration due to the virus will have suffered no hospital treatment and possibly less reduction of viral viability, which as far as is known is no longer than 20 days from symptom onset and typically 15 days in critically ill and immunocompromised patients. 16
Previous studies of Hb‐O2 affinity in COVID‐19 patients have used BGAs at various times post‐admission but for the most part not before treatment. 5 , 6 , 7 , 8 , 9 , 10 , 11 In our study, we found that, after admission, most BGA components (pO2, pCO2, pH, sO2, p50i, p50s) changed significantly with respect to the values measured at admission; in particular, the group of 33 patients evaluated regularly for 18 days showed an increase of more than 2 mmHg in mean p50i. Other authors who have examined the difference between first and later BGAs 6 , 8 may have obtained biased results due to the progressive reduction of the study group by death or discharge.
The BGAs of our non‐COVID respiratory patients, like those of the COVID‐19 group, were performed using samples of arterial blood obtained on admission, discarding those with SO2 >97%. Under these conditions, the COVID‐19 patients had higher pH and lower pCO2, in keeping with which their p50 values were lower, whereas Vogel et al. 6 reported lower p50 values in association with lower pH and higher pCO2.
Since p50 frequently increases during anaemia due to stimulation of 2,3‐bisphosphoglycerate synthesis, surprise has been expressed 3 that this has not occurred in COVID‐19 groups that were anaemic compared to their control groups. 5 , 6 In this study there was no difference in Hb concentration between the COVID‐19 and non‐COVID groups, but COVID‐19 patients with anaemia had significantly higher p50i and p50s than COVID‐19 patients without anaemia, in keeping with which there was significant inverse correlation between p50 and haemoglobin in the total group of COVID‐19 patients. Gille et al. 8 found no such correlation.
In COVID‐19 we are faced with hindered oxygen uptake, and one would expect that higher Hb‐O2 affinity could be helpful. 3 , 17 Our COVID‐19 patients with left‐shifted ODCs at admission did indeed have a lower death rate than the others. Our results might therefore be seen as supporting the use of treatments that increase Hb‐O2 affinity, such as 5‐hydroxymethyl‐2‐furfural, which does this by binding to valine 1α of the haemoglobin molecule. 18
On the other hand, the lower p50 values observed in most of our COVID‐19 patients at admission are likely attributable to respiratory alkalosis (a drop in pCO2) consequent upon tachypnoea and hyperpnoea caused by hypoxaemia. It is therefore also possible that what they reflect is maintenance of the ability to hyperventilate, and that the cause of this preserved ability (perhaps the presence of relatively few comorbidities, or relatively mild alteration of the lung) is what gives rise to better prognosis.
Increased Hb‐O2 affinity explains the relatively well‐preserved oxygen saturations at very low pO2. 19 Thus in our COVID‐19 patients, the oxygen content of blood was similar to that observed in the group without COVID‐19. However, although the absence of ACE2 receptors on the surface of red blood cells should in principle prevent SARS‐Cov‐2 from entering these cells and contacting Hb, a number of experimental studies have found indications of red blood cell damage due to interaction between the virus and haemoglobin. 20 , 21 , 22 , 23 , 24 Whether the observed alteration in Hb‐O2 affinity in COVID‐19 patients is a direct or indirect effect of the virus on Hb thus remains an open question.
A limitation of this study is that we had no truly normal group with which to compare p50 values, as the ODCs of our non‐COVID group may also have deviated from normal, albeit less than those of the COVID‐19 group. Another limitation is that during the study a number of SARS‐Cov‐2 variants appeared, so our patients may not all have been responding to exactly the same virus. The main strength of this study is the systematic use of BGA samples taken at the time of admission, when uninfluenced by hospital care.
CONCLUSION
In COVID‐19 patients, Hb‐O2 affinity varies markedly with the time of sampling: in this large study of hospitalized patients, more than 80% had a left‐shifted ODC at the time of admission, as against only 35% of post‐admission samples. The change is clearly noticeable from the third day after admission onwards. The finding that patients with a right shift at admission (14.7% in this study) have a lower survival rate makes their identification of great clinical importance.
AUTHOR CONTRIBUTIONS
Andrea Valle and Santiago Rodriguez‐Segade conceived the study, developed the statistical analysis and drafted the manuscript. Both are the guarantors of this work, and as such, had full access to the data in the study and take responsibility for the integrity and the accuracy of the data. All authors contributed to research data and critically reviewed the manuscript for important intellectual content. Miguel Rodriguez‐Segade and Santiago Rodriguez‐Segade take responsability for specific software and tools used in data analyses. Felix Camiña, Andrea Valle and Santiago Rodriguez‐Segade edited the final version of the manuscript and made the artwork. The final version of the manuscript was approved by all authors.
CONFLICT OF INTEREST
The authors have no competing interests.
DISCLOSURE
None.
Supporting information
Figure S1
Figure S2
Figure S3
Table S1
Table S2
ACKNOWLEDGEMENTS
This research received no specific grant from funding agencies in the public, commerial or not‐for‐profit sectors.
Valle A, Rodriguez J, Camiña F, Rodriguez‐Segade M, Ortola JB, Rodriguez‐Segade S. The oxyhaemoglobin dissociation curve is generally left‐shifted in COVID‐19 patients at admission to hospital, and this is associated with lower mortality. Br J Haematol. 2022;00:1–7. 10.1111/bjh.18431
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Böning D, Kuebler WM, Bloch W. The oxygen dissociation curve of blood in COVID‐19. Am J Physiol Lung Cell Mol Physiol. 2021;321:L349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:599–602. [DOI] [PubMed] [Google Scholar]
- 5. Daniel Y, Hunt BJ, Retter A, Henderson K, Wilson S, Sharpe CC, et al. Haemoglobin oxygen affinity in patients with severe COVID‐19 infection. Br J Haematol. 2020;190:e126–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vogel DJ, Formenti F, Retter AJ, Vasques F, Camporota L. A left shift in the oxyhaemoglobin dissociation curve in patients with severe coronavirus disease 2019 (COVID‐19). Br J Haematol. 2020;191:390–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeMartino AW, Rose JJ, Amdahl MB, Dent MR, Shah FA, Bain W, et al. No evidence of hemoglobin damage by SARS‐CoV‐2 infection. Haematologica. 2020;105:2769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gille T, Sese L, Aubourg E, Fabre EE, Cymbalista F, Ratnam KC, et al. The affinity of hemoglobin for oxygen is not altered during COVID‐19. Front Physiol. 2021;12:578708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pascual‐Guardia S, Ferrer A, Diaz O, Caguana AO, Tejedor E, Bellido‐Calduch S, et al. Absence of relevant clinical effects of SARS‐CoV‐2 on the affinity of hemoglobin for O2 in patients with COVID‐19. Arch Bronconeumol (Engl Ed). 2021;57:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Renoux C, Fort R, Nader E, Boisson C, Joly P, Stauffer E, et al. Impact of COVID‐19 on red blood cell rheology. Br J Haematol. 2021;192:e108–11. [DOI] [PubMed] [Google Scholar]
- 11. Laredo M, Curis E, Masson‐Fron E, Voicu S, Mégarbane B. Does COVID‐19 alter the oxyhemoglobin dissociation curve? – an observational cohort study using a mixed‐effect modelling. Clin Chem Lab Med. 2021;59:e416–9. [DOI] [PubMed] [Google Scholar]
- 12. Ceruti S, Minotti B, Glotta A, Biggiogero M, Bona G, Marzano M, et al. Temporal changes in the oxyhemoglobin dissociation curve of critically ill COVID‐19 patients. J Clin Med. 2022;788(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas LJ Jr. Algorithms for selected blood acid‐base and blood gas calculations. J Appl Physiol. 1972;33:154–8. [DOI] [PubMed] [Google Scholar]
- 14. Kelman GR, Nunn JF. Nomograms for correction of blood Po2, Pco2, pH, and base excess for time and temperature. J Appl Physiol. 1966;21:1484–90. [DOI] [PubMed] [Google Scholar]
- 15. West JB, Luks AM. West's Respiratory Physiology. 10th ed. Philadelphia: Wolters Kluwer; 2016. [Google Scholar]
- 16. Rhee C, Kanjilal S, Baker M, Klompas M. Duration of servere acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis. 2021;8:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulow VA, Fähling M. How to increase cellular oxygen availability in COVID‐19? Acta Physiol. 2021;5:e13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woyke S, Rauch S, Strohle M, Gatterer H. Modulation of Hb‐O2 affinity to improve hypoxemia in COVID‐19 patients. Clin Nutr. 2020;40:38–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harutyunyan G, Harutyunyan G, Mkhoyan G, Harutyunyan V, Soghomonyan S. Haemoglobin oxygen affinity in patients with severe COVID‐19 infection: still unclear. Br J Haematol. 2020;190:725–6. [DOI] [PubMed] [Google Scholar]
- 20. Venter C, Bezuidenhout JA, Laubscher GJ, Lourens PJ, Steenkamp J, Kell DB, et al. Erythrocyte, platelet, serum ferritin, and p‐selectin pathophysiology implicated in severe hypercoa‐gulation and vascular complications in COVID‐19. Int J Mol Sci. 2020;21:8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerard D, Ben Brahim S, Lesesve JF, Perrin J. Are mushroom shaped erythrocytes an indicator of COVID‐19? Br J Haematol. 2021;192:230. [DOI] [PubMed] [Google Scholar]
- 22. Thomas T, Stefanoni D, Dzieciatkowska M, Issaian A, Nemkov T, Hill RC, et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID‐19 patients. J Proteome Res. 2020;19:4455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Messner CB, Demichev V, Wendisch D, Michalick L, White M, Freiwald A, et al. Ultra‐high‐throughput clinical proteomics reveals classifiers of COVID‐19 infection. Cell Syst. 2020;11:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lechuga GC, Souza‐Silva F, Sacramento CQ, Trugilho MRO, Valente RH, Napoleão‐Pêgo P, et al. SARS‐CoV‐2 proteins bind to hemoglobin and its metabolites. Int J Mol Sci. 2021;22:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


