Abstract
Co‐infection of SARS‐CoV‐2 and influenza viruses has been reported worldwide in humans. Wild birds are natural reservoir hosts for coronaviruses (CoVs) and avian influenza viruses (AIVs). It is unknown whether co‐infection with these two types of viruses occurs in wild birds. In this study, the prevalence of co‐infection with CoV and AIV in wild birds in Shanghai, China during 2020–2021 was investigated by detecting these viruses in cloacal, tracheal, and faecal samples. Results showed that the overall rate of samples positive for both CoV and AIV was 3.3% (82/2510; 95% confidence interval [CI]: 2.6%–4.0%), and that was mainly from Anseriformes. In CoV‐positive samples, 38.9% (82/211; 95% CI: 32.5%–45.6%) of them had both CoVs and AIVs, whereas only 26.9% (82/305; 95% CI: 22.2%–32.1%) of AIV‐positive samples had both CoVs and AIVs. These results suggest that CoV infection in wild birds renders them more susceptible to AIV infection. Phylogenetic analysis based on partial RNA‐dependent RNA polymerase (RdRp) gene sequences of CoVs revealed that gamma‐CoVs mainly cluster with duck CoVs and that delta‐CoVs are more diversified and cluster with those of various wild birds. Continual surveillance is necessity to monitor the transmission and evolution of co‐infection of these two types of viruses in their natural hosts.
Keywords: avian influenza virus, co‐infection, coronavirus, wild birds
1. INTRODUCTION
The ongoing pandemic of coronavirus disease 2019 (COVID‐19) has posed a great challenge to human health globally with more than 571 million confirmed cases and 6.38 million deaths as of 28 July 2022 (WHO Coronavirus [COVID‐19] Dashboard [https://covid19.who.int/]). As coronaviruses (CoVs) are important pathogens of birds and mammals, interspecies spill‐over from wild to domestic animals occurs frequently. It is speculated that the porcine delta‐CoV is originated in birds (Wille & Holmes, 2020).
Wild birds are effective long‐distance vectors for the spread of various zoonotic diseases. The H5N8 influenza virus is a prime example of intercontinental spread of avian influenza A virus (AIV) by migratory birds (Lee et al., 2015). In addition to AIVs, wild birds are also natural reservoir hosts for of gamma‐ and delta‐CoVs (Wille & Holmes, 2020). The infectious bronchitis virus (IBV) is a gamma‐CoV and is frequently found to co‐infect with AIVs in chickens, causing significant financial losses in poultry industry (Hassan et al., 2016). Humans co‐infected with SARS‐CoV‐2 and influenza A virus have recently been reported (Rizzo et al., 2021; Wu et al., 2020). Such co‐infection usually causes more intense inflammatory responses with increased morbidity and mortality rates (Cuadrado‐Payán et al., 2020; Kong et al., 2021). Little is known about the prevalence of CoV and AIV co‐infections in wild birds and the impact of such co‐infections on wild bird populations. Shanghai is located at the Yangtze River Estuary and is an important stopover site for migratory birds along the East Asian–Australasian Flyway. To investigate the role of wild birds in zoonoses, we determined the prevalence of conducted CoV and AIV infections in wild birds in Shanghai during 2020–2021.
2. MATERIALS AND METHODS
From March 2020 to December 2021, a total of 2510 samples, including 1646 cloacal and tracheal swabs and 904 faeces, were collected from Chongming Dongtan (31°91ʹ N, 121°96ʹ E) and Nanhui Dongtan (30°90ʹ N, 121°98ʹ E) wetlands and Jiuduansha Natural Reservation Zone (31°36ʹ N, 121°74ʹ E), Shanghai, China. Wild birds were captured with mist nets and the bird species were identified morphologically by expert ornithologists. Swabs samples were collected with the help and permission of the Shanghai Forestry Station and Management Office (2018[125]), and birds were released immediately after sampling.
Viral RNA was extracted from the samples with a Magmax‐96 Express instrument (Applied Biosystems) using the MagMAX™ Pathogen RNA/DNA Kit (Applied Biosystems, USA). The extracted RNA was stored in a −80°C freezer until used. For CoVs, viral RNA was reverse transcribed to cDNA using random hexamers. The cDNA was then subjected to a pancoronavirus nested PCR (nPCR) to amplify a portion of the RNA‐dependent RNA polymerase (RdRp) gene of CoVs as described previously (Chu et al., 2011). Primers used for the first round nPCR were forward primer 5′‐GGK TGG GAY TAY CCK AAR TG‐3′ and reverse primer 5′‐TGY TGT SWR CAR AAY TCR TG‐3′, and those for the second round nPCR were forward primer 5′‐GGT TGG GAC TAT CCT AAG TGT GA‐3′ and reverse primer 5′‐CCA TCA TCA GAT AGA ATC ATC AT‐3′. For AIVs, viral RNA was detected using quantitative real‐time reverse transcription PCR with primers specific for the matrix gene on a 7500 Real‐Time PCR instrument (Applied Biosystems, USA) according to the method of the World Health Organization (WHO, 2002). AIV subtypes were determined by PCR using primers specific for haemagglutinin (HA) and neuraminidase (NA) genes (Huang et al., 2013; Tsukamoto et al., 2008). All final PCR products of AIVs and CoVs were sequenced and subject to BLASTN homology analysis using sequence data in the GenBank (https://www.ncbi.nlm.nih.gov/). The maximum‐likelihood phylogenetic tree of CoVs was constructed using the Kimura 2‐parameter model in the software package Mega version 6.0(http://www.megasoftwarenet/) with 1000 bootstrap replicates. All experiments were conducted under biosafety level 2 (BSL‐2) conditions.
The prevalence of CoVs and AIVs infections in wild birds was determined by calculating the ratio of the number of samples tested positive for CoV, AIV, or both to total sample number and the binomial 95% confidence interval (CI). Correspondence analysis for the determination of the relationships among CoVs and AIVs and their hosts was performed with VassarStats, which is available from http://www.vassarstats.net/. All other statistical analyses were carried out with the SPSS software (version 23.0, SPSS Inc., Chicago, IL, USA). For all analyses, p < .05 was considered significant. Graphs were produced using the software Origin (Pro), version 2022 (OriginLab Corporation, Northampton, MA, USA).
3. RESULTS AND DISCUSSION
Of the wild bird samples, 8.4% (211/2510; 95% CI: 7.4%–9.6%) were positive for CoVs, including 175 (82.9%) gamma‐CoVs and 36 (17.1%) delta‐CoVs (Table 1). The gamma‐CoVs were more frequently identified than delta‐CoVs in these sampled wild birds; the difference might be attributed to the bias for the CoVs infection in different species of wild birds (Wille & Holmes, 2020). The overall prevalence of AIVs in these wild bird samples was 12.2% (305/2510; 95% CI: 10.9%–13.5%). Nine HA subtypes (H1, H3–H6, H9–H12) and four NA subtypes (N1, N2, N5, and N8) were identified with 13 different combinations (Table 1). 3.3% (82/2510; 95% CI: 2.6%–4.0%) of total samples were co‐infected with CoVs and AIVs, and the rates of co‐infection in swab and feacal samples are significantly different (p < .001), 4.7% (76/1606; 95% CI: 3.8%–5.9%) for swab and 0.7% (6/904; 95% CI: 0.3%–1.4%) for faecal samples. In CoV‐positive samples, 38.9% (82/211; 95% CI: 32.5%–45.6%) of all samples, 42.0% (76/181; 95% CI: 35.0%–49.3%) of swab samples, and 20% (6/30; 95% CI: 9.5%–37.3%) of faecal samples had AIVs. In AIV‐positive samples, only 26.9% (82/305; 95% CI: 22.2%–32.1%) of all samples, 25.9% (76/293; 95% CI: 21.3%–31.3%) of swab samples, and 50% (6/12; 95% CI: 25.4%–74.6%) of faecal samples had CoVs. These results suggest that CoV infections may predispose wild birds to AIV infections.
TABLE 1.
CoV and AIV hosts and number of samples positive for CoV, AIV, or both from wild birds in Shanghai in 2020–2021
| Order | Common name | Scientific name | No. of samples | AIV prevalence % (95% CI; No. of AIV+ samples/total sample no) | CoV prevalence % (95% CI; No. of CoV+ samples/total sample no) | Prevalence % of CoV and AIV co‐infection (95% CI; No. of CoV+ and AIV+ samples/total sample no) | AIV subtypes (No.) | CoV genus (No.) |
|---|---|---|---|---|---|---|---|---|
| Anseriformes | Common Teal | Anas crecca | 390 | 25.6 (21.6–30.2; 100/390) | 18.7 (15.2–22.9; 73/390) | 7.4 (5.2–10.5; 29/390) | H5N1(21) H5N8(7) N1(7) H5(4) H4(3) H4N2(3) H6N2(3) H9N2(3) N2(3) H5N2(2) H10(1) Unknown b (43) |
γ(62) δ(11) |
| Mallard | Anas platyrhynchos | 169 | 35.5 (28.7–43.0; 60/169) | 14.2 (9.7–20.3; 24/169) | 8.9 (5.5–14.1; 15/169) | H5N8(18) H5N1(4) H5(2) N8(2) N2(2) N1(2) H5N2(1) H3N2(1) H6N2(1) H6N8(1) Unknown (26) |
γ(23) δ(1) |
|
| Spot‐billed Duck | Anas poecilorhyncha | 122 | 48.4 (40.0–57.1; 59/122) | 22.1 (15.7–30.3; 27/122) | 15.6 (10.2–23.0; 19/122) | H5N8(23) H5(7) H1N1(3) H5N1(3) N2(3) N1(2) H12N5(1) H9N2(1) H6N5(1) H10(1) Unknown (14) |
γ(27) δ(0) |
|
| Eurasian Wigeon | Anas penelope | 73 | 27.4 (18.5–38.6; 20/73) | 16.4 (9.7–26.6; 12/73) | 6.8 (3.0–15.1; 5/73) | H9N2(2) H5N1(2) H5N8(1) Unknown (15) |
γ(10) δ(2) |
|
| Northern Shoveler | Anas clypeata | 54 | 22.2 (13.2–34.9; 12/54) | 9.3 (4.0–19.9; 5/54) | 3.7 (1.0–12.5; 2/54) | H5N1(6) H5N8(1) N8(1) H10N2(1) Unknown (3) |
γ(2) δ(3) |
|
| Gadwall | Anas strepera | 37 | 24.3 (13.4–40.1; 9/37) | 18.9 (9.5–34.2; 7/37) | 5.4 (1.5–17.7; 2/37) | H5N8(3) H9N2(1) N2(1) H5N1(1) Unknown (3) |
γ(5) δ(2) |
|
| Northern Pintail | Anas acuta | 24 | 29.2 (14.9–49.2; 7/24) | 25.0 (12.0–44.9; 6/24) | 8.3 (2.3–25.8; 2/24) | H5N8(4) H3N2(1) H9N2(1) Unknown (1) |
γ(6) δ(0) |
|
| Common Pochard | Aythya ferina | 13 | 30.8 (12.7–57.6; 4/13) | 30.8 (12.7–57.6; 4/13) |
0 |
H5N8(2) Unknown (2) |
γ(3) δ(1) |
|
| Falcated Teal | Mareca falcata | 13 | 69.2 (42.4–87.3; 9/13) | 15.4 (4.3–42.2; 2/13) | 15.4 (4.3–42.2; 2/13) | H5N1(2) H9N2(1) H5N8(1) Unknown (5) |
γ(2) δ(0) |
|
| Mandarin | Aix galericulata | 12 | 41.7 (19.3–68.1; 5/12) | 16.7 (4.7–44.8; 2/12) | 0 | H6N2(1) H10(1) Unknown (3) |
γ(2) δ(0) |
|
| Charadriiformes | Great Knot | Calidris tenuirostris | 212 | 2.8 (1.3–6.0; 6/212) | 1.4 (0.5–4.1; 3/212) | 0 | Unknown (6) |
γ(0) δ(3) |
| Dunlin | Calidris alpina | 90 | 0 | 3.3 (1.1–9.3; 3/90) | 0 | / |
γ(2) δ(1) |
|
| Bar‐tailed Godwit | Limosa lapponica | 53 | 1.9 (0.3–10.0; 1/53) | 5.7 (1.9–15.4; 3/53) | 0 | Unknown (1) |
γ(3) δ(0) |
|
| Common Redshank | Tringa totanus | 35 | 0 | 2.9 (0.5–14.5; 1/35) | 0 | / |
γ(1) δ(0) |
|
| Broad‐billed Sandpiper | Limosa lapponica | 14 | 0 | 14.3 (4.0–40.0; 2/14) | 0 | / |
γ(0) δ(2) |
|
| Ruddy Turnstone | Arenaria interpres | 12 | 0 | 8.3 (1.5–35.4; 1/12) | 0 | / |
γ(0) δ(1) |
|
| Ciconiiformes | Little Egret | Egretta | 7 | 0 | 42.9 (15.8–75.0; 3/7) | 0 | / |
γ(0) δ(3) |
| Night Heron | Nycticorax nycticorax | 7 | 14.3 (2.6–51.3; 1/7) | 28.6 (8.2–64.1; 2/7) | 0 | H5N8(1) |
γ(0) δ(2) |
|
| Columbiformes | Red Turtle Dove | Streptopelia tranquebarica | 16 | 0 | 6.3 (1.1–28.3; 1/16) | 0 | / |
γ(0) δ(1) |
| / a | / | / | 904 | 1.3 (0.8–2.3; 12/904) | 3.3 (2.3–4.7; 30/904) |
0.7 (0.3–1.4; 6/904) |
H3N8(2) H3N2(1) H6N1(1) H11(1) Unknown (7) |
γ(27) δ(3) |
| Total | / | / | 2510 | 12.2 (10.9–13.5; 305/2510) | 8.4 (7.4–9.6; 211/2510) | 3.3 (2.6–4.0; 82/2510) | 305 | 211 |
Samples collected from faeces.
AIV positive, but the subtype is unknown.
The highest prevalence of CoVs was found in October 2020 (38.8%; 95% CI: 26.4%–52.8%) and November 2021 (18.6%; 95% CI: 15.6%–22.0%), and the highest prevalence of AIVs was subsequently found in November and December 2020 (Figure 1). The CoVs infection is positively correlated with the co‐infection (p = .002), while there was no correlation between AIVs and co‐infection. It has been shown that co‐infection with infectious bronchitis virus (IBV), a gamma‐CoV, may reduce the stability of HA of H9N2 AIV in diseased flocks (AboElkhair et al., 2021) or induce a severe inflammatory response in chickens (Kong et al., 2021). Whether CoV infections play a major role in AIV epidemic in wild birds remains to be investigated, and the molecular mechanisms of co‐infection with CoVs and AIVs should also deserve more attention.
FIGURE 1.
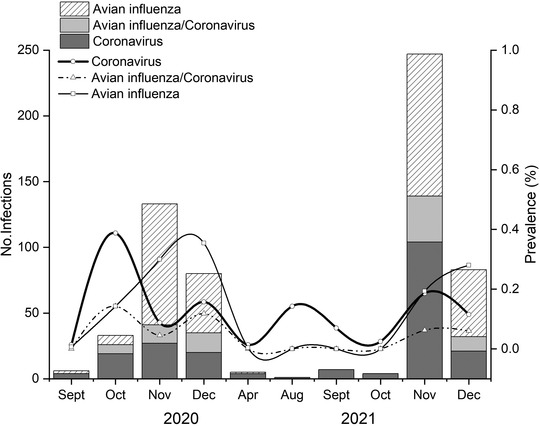
Number of samples positive for CoV, AIV, or both and their prevalence (%) in wild birds in Shanghai, China, in 2020–2021. Only the months in which samples positive for the viruses are shown.
Among the 211 CoVs in this study, 205 partial RdRp sequences were obtained, including those of 170 gamma‐CoVs and 35 delta‐CoVs. The sequences of five delta‐CoVs and three gamma‐CoVs were selected based on their isolation time, host, and location as representative sequences to construct a phylogenetic tree by comparing the sequences of RdRp with those in the GenBank database (Figure 2). Results showed that gamma‐CoVs were mainly clustered with duck CoVs and may have the same ancestor as other CoVs. The five delta‐CoVs were found to belong to four different subclades with one belonging to the sparrow and porcine CoVs clade. As the RdRp sequence determined in this study only represents only about 1% of the CoVs genome, the relationships shown in the phylogenetic tree should be confirmed using the whole genome, and the possibility of CoV transmission to mammals should be closely monitored.
FIGURE 2.
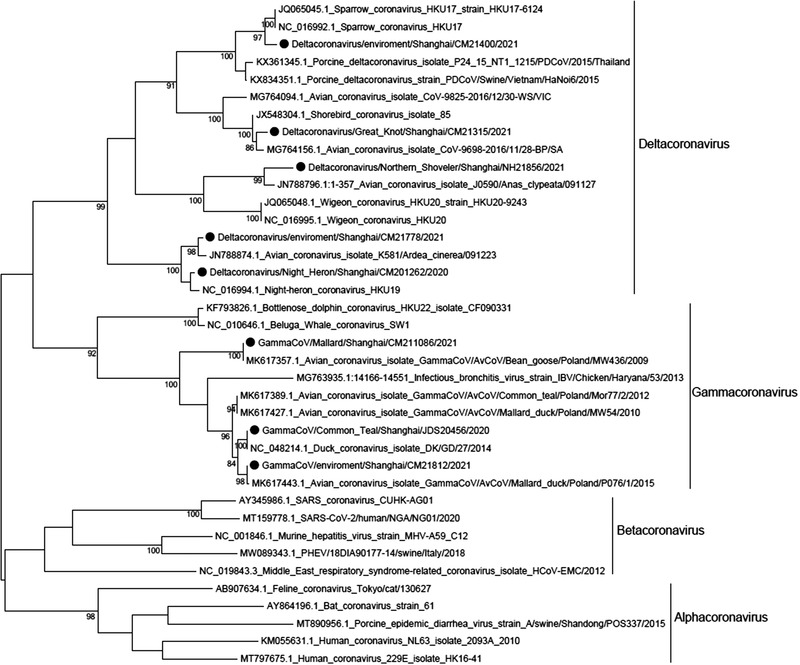
Phylogenetic tree based on partial RdRp (295 bp) gene sequences of coronaviruses from wild birds. The viral sequences obtained in this study are indicated with black dots. The maximum likelihood tree was constructed using the Kimura 2‐parameter model in MEGA software version 6 (http://www.megasoftwarenet/). Bootstrap values were calculated with 1000 replicates, and those bootstrap values less than 80% are not shown.
The swab samples were collected from 59 species of wild birds in nine avian genetic orders. Except for very low sample sizes (<7 individuals in a species), samples positive for both CoVs and AIVs were only found in those of Anseriformes, and the highest detection rate was from spot‐billed duck (Anas poecilorhyncha) (Table 1). As spot‐billed ducks are the most abundant migratory ducks in Shanghai, they should be closely monitored for causing CoV and or AIV epidemics in the future. Our previous surveillance has shown that Anseriformes are more readily infected with AIVs than members of other orders (Tang et al., 2020), which may account for the higher CoV and AIV co‐infection rates in Anseriformes determined in this study. As delta‐CoVs were mainly found in Ciconiiformes and Columbiformes, which are the dominant resident bird species in Shanghai, the potential risk of their spill‐over to domestic poultry and other mammals should be closely monitored.
In summary, the present study demonstrated that CoV and AIV co‐infection is highly prevalent in wild birds. As they are long‐distance vector for spreading various viruses, the risk of CoV and AIV spill‐over to domestic poultry and other mammals is high. Long‐term continual surveillance of such co‐infections in wild birds is required to better understand the ecology and epidemiology of these viruses.
AUTHOR CONTRIBUTIONS
Min Ma, Lei Ji, Le Ming, Yuting Xu, and Chenyao Zhao collected the samples and carried out the laboratory experiments. Min Ma, Lei Ji, and Guimei He analysed the data and wrote the manuscript. Tianhou Wang and Guimei He designed and supervised the experiments and edited the manuscript. The final version of the manuscript was read and approved by all authors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The authors confirm that this manuscript complies with the ethical policies of the journal. Wild birds were captured and released with the permission of the Shanghai Forestry Station.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (32172943), Shanghai Science and Technology Committee Project (grant no. 18DZ2293800), and the Shanghai Wildlife‐borne Infectious Disease Monitoring Program. We thank staffs of Shanghai Forestry Station for assistance in field sampling.
Ma, M. , Ji, L. , Ming, L. , Xu, Y. , Zhao, C. , Wang, T. , & He, G. (2022). Co‐circulation of coronavirus and avian influenza virus in wild birds in Shanghai (2020–2021). Transboundary and Emerging Diseases, 00, 1–7. 10.1111/tbed.14694
Min Ma, Lei Ji and Le Ming contributed equally to this work.
DATA AVAILABILITY STATEMENT
All data of this study are available from the corresponding author upon request.
REFERENCES
- Aboelkhair, M. A. , Hasan, M. E. , Mousa, A. , Moharam, I. , Sultan, H. , Malik, Y. , & Sakr, M. A. (2021). In‐silico evidence for enhancement of avian influenza virus H9N2 virulence by modulation of its hemagglutinin (HA) antigen function and stability during co‐infection with infectious bronchitis virus in chickens. Virus Disease, 32, 548–558. 10.1007/s13337-021-00688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D. K. W. , Leung, C. Y. H. , Gilbert, M. , Joyner, P. H. , Ng, E. M. , Tse, T. M. , Guan, Y. , Peiris, J. S. M. , & Poon, L. L. M. (2011). Avian coronavirus in wild aquatic birds. Journal of Virology, 85, 12815–12820. 10.1128/JVI.05838-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado‐Payán, E. , Montagud‐Marrahi, E. , Torres‐Elorza, M. , Bodro, M. , Blasco, M. , Poch, E. , Soriano, A. , & Piñeiro, G. J. (2020). SARS‐CoV‐2 and influenza virus co‐infection. Lancet, 395, e84. 10.1016/S0140-6736(20)31052-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, K. E. , Shany, S. A. S. , Ali, A. , Dahshan, A.‐H. M. , El‐Sawah, A. A. , & El‐Kady, M. F. (2016). Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poultry Science, 95, 1271–1280. 10.3382/ps/pew068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Khan, M. , & Mandoiu, I. I. (2013). Neuraminidase subtyping of avian influenza viruses with primerhunter‐designed primers and quadruplicate primer pools. PLoS ONE, 8, e81842. 10.1371/journal.pone.0081842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L. , You, R. , Zhang, D. , Yuan, Q. , Xiang, B. , Liang, J. , Lin, Q. , Ding, C. , Liao, M. , Chen, L. , & Ren, T. (2021). Infectious bronchitis virus infection increases pathogenicity of H9N2 avian influenza virus by inducing severe inflammatory response. Frontiers in Veterinary Science, 8, 824179. 10.3389/fvets.2021.824179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.‐H. , Torchetti, M. K. , Winker, K. , Ip, H. S. , Song, C.‐S. , & Swayne, D. E. (2015). Intercontinental spread of Asian‐origin H5N8 to North America through Beringia by migratory birds. Journal of Virology, 89, 6521–6524. 10.1128/JVI.00728-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, K. R. , Hoover, C. , Jain, S. , Sun, M. , Myers, J. F. , Bregman, B. , Dominguez, D. M. , Jacobsen, A. , Jenkins, G. J. , Hennessy‐Burt, T. , & Murray, E. L. (2021). Influenza and SARS‐CoV‐2 co‐infections in California, USA, September 2020–April 2021. Emerging Infectious Diseases, 27(11), 2923–2926. 10.3201/eid2711.211129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, L. , Tang, W. , Li, X. , Hu, C. , Wu, D. , Wang, T. , & He, G. (2020). Avian influenza virus prevalence and subtype diversity in wild birds in Shanghai, China. Viruses, 12, 1031. 10.3390/v1209103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto, K. , Ashizawa, H. , Nakanishi, K. , Kaji, N. , Suzuki, K. , Okamatsu, M. , Yamaguchi, S. , & Mase, M. (2008). Subtyping of avian influenza viruses H1 to H15 on the basis of hemagglutinin genes by PCR assay and molecular determination of pathogenic potential. Journal of Clinical Microbiology, 46, 3048–3055. 10.1128/JCM.02386-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2002). WHO manual on animal influenza diagnosis and surveillance . http://apps.who.int/iris/bitstream/handle/10665/68026/WHO_CDS_CSR_NCS_2002.5.pdf?sequence51&isAllowed5y
- Wille, M. , & Holmes, E. C. (2020). Wild birds as reservoirs for diverse and abundant gamma and deltacoronaviruses. EMS Microbiology Reviews, 44, 631–644. 10.1093/femsre/fuaa026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X. , Cai, Y. , Huang, X. , Yu, X. , Zhao, L. , Wang, F. , Li, Q. , Gu, S. , Xu, T. , Li, Y. , Lu, B. , & Zhan, Q. (2020). Co‐infection with SARSCoV‐2 and influenza a virus in patient with pneumonia, China. Emerging Infectious Diseases, 26, 1324–1326. 10.3201/eid2606.200299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data of this study are available from the corresponding author upon request.


