Abstract
Background and purpose
Clinical outcomes of multiple sclerosis (MS) patients affected by coronavirus disease 2019 (COVID‐19) have been thoroughly investigated, but a further analysis on main signs and symptoms and their risk factors still needs attention. The objective of this study was to group together and describe based on similarity the most common signs and symptoms of COVID‐19 in MS patients and identify all factors associated with their manifestation.
Method
Logistic and linear regression models were run to recognize factors associated with each pooled group of symptoms and their total number.
Results
From March 2020 to November 2021, data were collected from 1354 MS patients with confirmed infection of COVID‐19. Ageusia and anosmia was less frequent in older people (odds ratio [OR] 0.98; p = 0.005) and more in smoker patients (OR 1.39; p = 0.049). Smoke was also associated with an incremental number of symptoms (OR 1.24; p = 0.031), substance abuse (drugs or alcohol), conjunctivitis and rash (OR 5.20; p = 0.042) and the presence of at least one comorbidity with shortness of breath, tachycardia or chest pain (OR 1.24; p = 0.008). Some disease‐modifying therapies were associated with greater frequencies of certain COVID‐19 symptoms (association between anti‐CD20 therapies and increment in the number of concomitant symptoms: OR 1.29; p = 0.05). Differences in frequencies between the three waves were found for flu‐like symptoms (G1, p = 0.024), joint or muscle pain (G2, p = 0.013) and ageusia and anosmia (G5, p < 0.001). All cases should be referred to variants up to Delta.
Conclusion
Several factors along with the choice of specific therapeutic approaches might have a different impact on the occurrence of some COVID‐19 symptoms.
Keywords: COVID‐19, demyelinating diseases, disease‐modifying treatment, multiple sclerosis, neurological disorders
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), responsible for the coronavirus disease 2019 (COVID‐19) pandemic, has an extremely variable course, with cases ranging from asymptomatic to mild, moderate, severe as well as critical or fatal [1].
Symptoms usually appear within the first 2 weeks after exposure to the virus and some are more likely to be associated with COVID‐19 than others. The clinical manifestations of COVID‐19 are mainly fever or chills, cough, anosmia and ageusia, and difficulty breathing, but a variety of other symptoms are also involved: sore throat, loss of appetite, extreme fatigue, tiredness, headache, body aches, nausea, vomiting and diarrhoea [2]. However, new and unusual signs and symptoms have been found in newly infected patients from the beginning of the pandemic to date, following continuing viral mutations [3].
The association between symptoms and disease severity has already been explored in the general population. A recent study on 531 patients analysed the difference in symptoms of the coronavirus disease amongst hospitalized and non‐hospitalized patients: shortness of breath is more common in hospitalized patients than in those with mild forms of the disease, whereas fatigue, headache and myalgia are mostly reported by not hospitalized subjects [4].
For containing pandemic transmission, an early diagnosis is essential, by identifying sentinel symptoms so that infected persons can self‐isolate themselves just before they get contagious and start spreading the virus [5]. However, due to their high non‐specificity, neither the presence nor the absence of a single symptom can be considered indicative of COVID‐19 disease [6]. Some particular COVID‐19 symptoms are often related and tend to appear together, and therefore combined into specific groups may predict COVID‐19 very accurately [7].
In Italy there are more than 120,000 people with multiple sclerosis (MS) [8], most of them treated with disease‐modifying therapies which alter the components of the immune system and are associated with a greater infectious risk, especially viral [9]. In addition, the association between dysimmune phenomena underlying MS and the defective control of some pathogens has driven researchers around the world to focus on clinical outcomes in MS patients affected by COVID‐19 [10, 11, 12, 13, 14]. However, a more in‐depth analysis focusing on the major signs and symptoms and related risk factors for their occurrence still requires further examination. This is even more so since a wide comprehension of the main symptoms present during infection is essential for providing advice and for monitoring the severity of long‐term symptoms [15].
The aim of this study was to identify and group for similarity the most common signs and symptoms of COVID‐19 and evaluate all factors associated with their manifestation.
METHODS
All demographic and clinical information was extracted from a dedicated web‐based platform set up for the Italian MuSC‐19 project that collects clinically reported data regarding the impact of COVID‐19 infection on people affected by MS. Missing data on baseline characteristics were replaced with a procedure of multiple imputation by the chained equations algorithm, as detailed elsewhere [10].
Patients with a detailed history of MS and a confirmed (by reverse transcription polymerase chain reaction or serological test) symptomatic COVID‐19 infection were included in the sample. Descriptive data were presented as mean with standard deviation (SD), median with range or interquartile range (IQR) for continuous variables, and number with percentage for categorical variables, as appropriate.
Symptoms collected in the MuSC‐19 registry were based on patient reports along with physical examination performed during the routine visit. All of them were referred as symptoms associated with COVID infection, so presenting during or soon after COVID, and therefore they are supposed not to be present before COVID. Symptoms were pooled as per similarity into categories as proposed in a previous work [16] and the degree of agreement between different groups of symptoms was measured by Cohen's kappa. A patient was positive for a symptom group if he/she had at least one of the symptoms listed in that group.
Univariate and subsequent logistic regression models were run to recognize all factors associated with each group of symptoms by controlling for baseline characteristics. In addition, univariate and the following multivariate linear regression models were applied to identify all predicting factors for an increase in the total number of symptom groups. Only variables which have previously resulted in a significant association with at least one group of symptoms were included in the model as independent factors.
Finally, an analysis of the distribution of symptom groups between three pandemic waves was performed for discussing any differences in their frequencies of comparison (chi‐squared test).
The study was approved by the Regional Ethics Committee of Liguria (University of Genoa; no. 130/2020—DB id10433) and on a national level by Agenzia Italiana del Farmaco (AIFA).
RESULTS
From March 2020 to November 2021, data were collected from 2112 MS patients in care at 121 Italian sites. Of them 1354 had a confirmed infection of COVID‐19, presented at least one sign or symptom of disease, and showed a completed follow‐up until recovery (98.6%) or death (1.4%).
The mean age was 43.8 years (IQR 34.0–53.0), 67.3% were females and, of them, pregnancy was reported in 2%.
On average, patients lived with two cohabitants, without children (IQR 0.0–1.0), or with another COVID‐19‐positive cohabitant besides them (IQR 0.0–2.0). About lifestyle‐related factors, 198 (14.6%) were current smokers, 260 (19.2%) were former smokers and 896 (66.2%) had never smoked. Alcohol was consumed by 56.0% of patients, with regular consumption in 2.0%. Abuse of substance, defined by the National Cancer Institute as ‘the use of illegal drugs or the use of prescription or over‐the‐counter drugs or alcohol for purposes other than those for which they are meant to be used, or in excessive amounts’ was reported by 25 patients (1.8%).
Patients had a median Expanded Disability Status Scale (EDSS) of 2 (IQR 1–3.5), and the proportion of progressive patients was 13.8%. In all, 1152 (85.1%) patients were treated with a disease‐modifying therapy; recent use of methylprednisolone was declared for 84 patients (6.2%). About two‐thirds of patients (72.1%) had no comorbidities.
COVID‐19 severity was defined by three levels: 1152 (85.1%) patients with mild disease not requiring hospitalization, 173 (12.8%) cases of pneumonia and/or hospitalization, and 29 (2.1%) cases of admission in an intensive care unit and/or death (Table 1).
TABLE 1.
Baseline characteristics
| Sex, females | 911 (67.3%) |
| Age, mean ± SD | 43.8 ± 12.29 |
| Pregnancy (N = 911 females) | 18 (2.0%) |
| Number of cohabitants, median (IQR) | 2.0 (1.0–3.0) |
| Number of cohabitant children, median (IQR) | 0.0 (0.0–1.0) |
| Number of COVID‐positive cohabitants, median (IQR) | 1.0 (0.0–2.0) |
| Smoking habits | |
| Never smoked | 896 (66.2%) |
| Former smoker | 260 (19.2%) |
| Current smoker | 198 (14.6%) |
| Alcohol consumers | |
| No consumption | 596 (44.0%) |
| Occasional consumption | 731 (54.0%) |
| Regular consumption | 27 (2.0%) |
| Substance abusers | 25 (1.8%) |
| Presence of at least one comorbidity | 378 (27.9%) |
| Progressive MS | 187 (13.8%) |
| MS disease duration, mean ± SD | 10.4 ± 8.28 |
| Last EDSS, median (IQR) | 2.0 (1.0–3.5) |
| Last DMT | |
| Dimethyl fumarate | 256 (18.9%) |
| No therapy | 202 (14.9%) |
| Anti‐CD20 | 184 (13.6%) |
| Natalizumab | 176 (13.0%) |
| Fingolimod | 161 (11.9%) |
| Interferon | 127 (9.4%) |
| Glatiramer acetate | 97 (7.2%) |
| Teriflunomide | 91 (6.7%) |
| Cladribine | 24 (1.8%) |
| Other | 36 (2.7%) |
| Recent use of methylprednisolone | 84 (6.2%) |
| Previous vaccination against COVID‐19 | 24 (1.8%) |
| COVID‐19 severity | |
| Mild disease with no pneumonia nor requiring hospitalization | 1152 (85.1%) |
| Pneumonia and/or hospitalization | 173 (12.8%) |
| ICU and/or death | 29 (2.1%) |
Note: Results are expressed as mean ± SD, median (IQR), count (%).
Abbreviations: COVID‐19, coronavirus disease 2019; DMT, Disease modifying treatment; EDSS, expanded disability status scale; ICU, intensive care unit; IQR, interquartile range; MS, multiple sclerosis.
Reported symptoms were grouped as follows.
Group 1 (G1): Fever, chills, dyspnoea, cough, sputum production, lymph nodes enlarged, tonsil swelling, throat congestion, nasal congestion, sore throat, cold
Group 2 (G2): Arthralgia, myalgia, bone and joint pain, back pain
Group 3 (G3): Shortness of breath, tachycardia, chest pain
Group 4 (G4): Vomiting, nausea, diarrhoea, abdominal pain, loss of appetite, loss of weight, other gastrointestinal symptoms
Group 5 (G5): Ageusia, anosmia
Group 6 (G6): Symptoms of anxiety and depression, insomnia, headache, drowsiness, difficulty sleeping, loss of concentration, weakness, fatigue, asthenia
Group 7 (G7): Conjunctivitis, rash
The frequency of occurrence of single and grouped symptoms is reported in Figure 1, whereas Table 2 details their grade of agreement.
FIGURE 1.
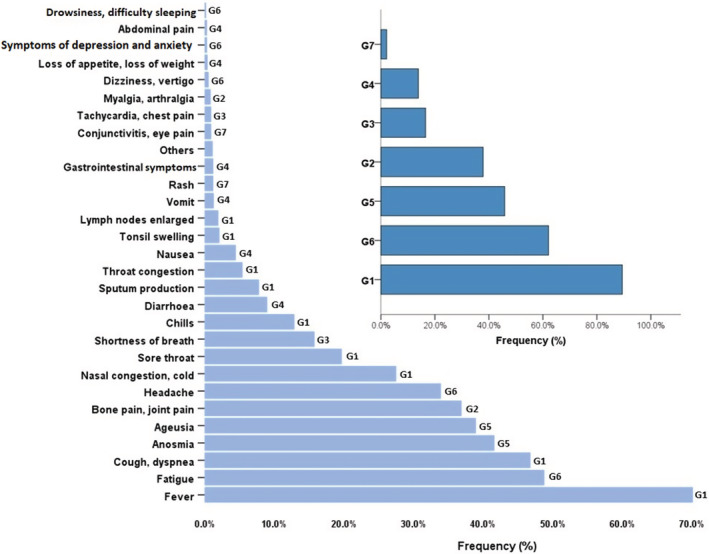
Frequency of single symptoms and grouped symptoms
TABLE 2.
Group of symptoms and their agreement degree
| N (%) | Cohen's kappa (p value) | ||||||
|---|---|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | G6 | G7 | ||
| 1211 (89.4%) | G1 | 0.05 (<0.001) | 0.03 (<0.001) | 0.01 (0.32) | −0.09 (<0.001) | 0.05 (0.007) | 0.00 (0.21) |
| 513 (37.9%) | G2 | – | 0.17 (<0.001) | 0.13 (<0.001) | 0.11 (<0.001) | 0.27 (<0.001) | 0.02 (0.12) |
| 224 (16.5%) | G3 | – | – | 0.08 (0.003) | 0.03 (0.13) | 0.09 (<0.001) | 0.03 (0.11) |
| 188 (13.9%) | G4 | – | – | – | 0.04 (0.044) | 0.06 (<0.001) | 0.07 (<0.001) |
| 621 (45.9%) | G5 | – | – | – | – | 0.06 (0.030) | 0.00 (0.79) |
| 841 (62.1%) | G6 | – | – | – | – | – | 0.01 (0.12) |
| 29 (2.1%) | G7 | – | – | – | – | – | – |
Note: G1: Fever, chills, dyspnoea, cough, sputum production, lymph nodes enlarged, tonsil swelling, throat congestion, nasal congestion, sore throat, cold. G2: Arthralgia, myalgia, bone and joint pain, back pain. G3: Shortness of breath, tachycardia, chest pain. G4: Vomiting, nausea, diarrhoea, abdominal pain, loss of appetite, loss of weight, other gastrointestinal symptoms. G5: Ageusia, anosmia. G6: Symptoms of anxiety and depression, insomnia, headache, drowsiness, difficulty sleeping, loss of concentration, weakness, fatigue, asthenia. G7: Conjunctivitis, rash.
Patients declared nearly three groups of symptoms (mean 2.7, range 1–7). The most common was G1 in 1211 patients (89.4%), followed by G6 in 841 (62.1%) of the patients. None or slight agreement (Cohen's kappa < 0.20) for each pairwise comparison was found (except a fair agreement between G2 and G6), confirming their independence from each other.
Figure 2 reports results from multivariate models regarding factors associated with each group of symptoms and factors associated with an increased number of symptoms. Males showed a lower risk for developing joint or muscle pain (G2) and gastrointestinal symptoms (G4) and for having an increment in the total number of symptom groups (OR 0.86, 95% CI 0.74–0.99; p = 0.037).
FIGURE 2.
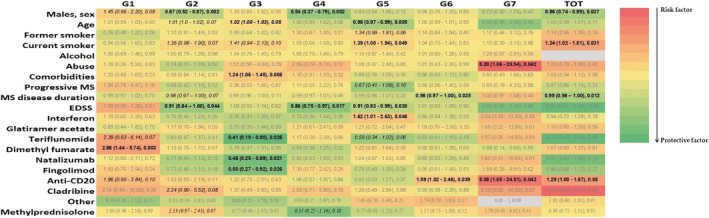
Heat map of correlation, as measured by odds ratio estimates, between factors and each symptom group. Values above 1 (shade of red) indicate risk factors, below 1 (shade of green) protective factors
Ageusia and anosmia (G5) were less frequently reported in older people (OR 0.98, 95% CI 0.97–0.99; p = 0.005) and more common in smoker patients (OR 1.39, 95% CI 1.00–1.94; p = 0.049). In addition, the smoking habit was demonstrated to be a risk factor for developing an increment in the total number of symptom groups (OR 1.24, 95% CI 1.02–1.51; p = 0.031). Substance abuse was associated with the occurrence of greater frequency of conjunctivitis and rash (G7; OR 5.20, 95% CI 1.06–25.54; p = 0.042), whereas the presence of at least one comorbidity was associated with shortness of breath, tachycardia or chest pain (G3; OR 1.24; 95% CI 1.06–1.45; p = 0.008).
An increasing MS disease duration and EDSS were significantly associated with lower occurrences of several symptoms (G2, G4, G5, G6). The use of some classes of MS therapies seems to be associated with the presence of some groups of symptoms; in particular the infusion of anti‐CD20 therapies was found to be associated with an increment in the number of concomitant symptoms (OR 1.29, 95% CI 1.00–1.67; p = 0.05). Twenty‐four patients (1.8%) were found to be previously immunized against COVID‐19. There were no differences in the frequency of occurrence of any of the symptom groups studied between vaccinated and unvaccinated patients before infection (always p > 0.10, data not shown).
Figure 3 illustrates the distribution of the group of symptoms during the three pandemic waves. In all the waves the most frequent groups of signs and symptoms were fever and flu‐like symptoms, although less common in the second wave (87.9%) compared to the first (93.4%) and third (92.6%; p = 0.024). Then, for all three waves, ‘mental’ symptoms (G6) were very common (>50% of patients), followed by ageusia and anosmia in the first wave (36.8%) and joint/muscle pain in the second (39.8%) and third waves (44.3%). In addition, differences in frequencies amongst the three waves were found for joint or muscle pain (G2, p = 0.013) and ageusia and anosmia (G5, p < 0.001; Table 3).
FIGURE 3.
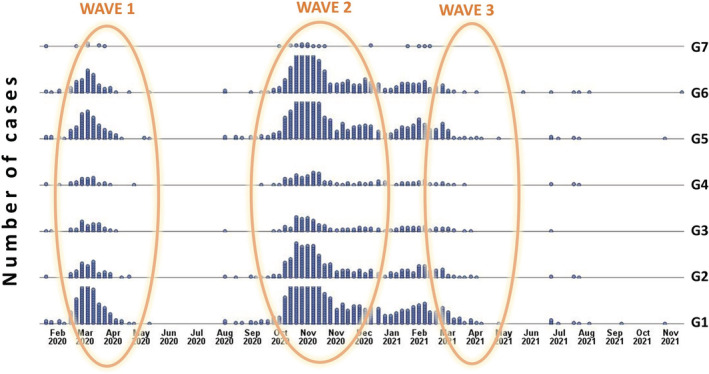
Distribution of symptom groups over time, with identification of the three pandemic waves
TABLE 3.
Distribution of group of symptoms during the three pandemic waves
| Group of symptoms | First wave (N = 238) | Second wave (N = 782) | Third wave (N = 122) | p |
|---|---|---|---|---|
| G1 | 226 (93.4%) | 687 (87.9%) | 113 (92.6%) | 0.024 |
| G2 | 74 (30.6%) | 311 (39.8%) | 54 (44.3%) | 0.013 |
| G3 | 47 (19.4%) | 115 (14.7%) | 24 (19.7%) | 0.12 |
| G4 | 33 (13.6%) | 102 (13.0%) | 16 (13.1%) | 0.97 |
| G5 | 89 (36.8%) | 392 (50.1%) | 42 (34.4%) | <0.001 |
| G6 | 137 (56.6%) | 483 (61.8%) | 81 (66.4%) | 0.16 |
| G7 | 7 (2.9%) | 16 (2.0%) | 2 (1.6%) | 0.67 |
Note: As reported by the Ministry of Health (Andamento della mortalità giornaliera [SiSMG] nelle città italiane in relazione all'epidemia di COVID‐19—1 Settembre 2020–16 Novembre 2021), first wave, 1 March–10 May 2020; second wave, 1 September 2020–9 January 2021; third wave, March 2021–May 2021. Bold values represent statistical significant results.
Finally, all cases should be referred to variants up to Delta. Indeed, Omicron was detected for the first time in Italy on 11 November 2021 and data were collected just from March 2020 to November 2021.
DISCUSSION
A recent review states that the most common symptoms were fever (68.8%), followed by cough (63.9%), fatigue/asthenia (51.2%) [17]. The present study reports in the same order fever and cough (89.4%) and fatigue and asthenia together with other ‘neurological’ symptoms (62.1%). However, other than fatigue (48.8%) and headache (34.0%), there are few central nervous system symptoms, even in MS.
Several associations between comparison of some group of symptoms and other characteristics have been detected. Males are less likely to develop some symptoms (i.e., bone and joint pain and gastrointestinal symptoms) and generally have a smaller set of symptoms than females. In a self‐reported survey, females declared a significantly higher frequency of gastrointestinal symptoms during COVID‐19 compared to men [18]. Even age influences the frequency of manifestation of some symptoms. In particular, the risk of developing anosmia decreased with increasing age [19]. However, this result could be explained because unawareness of olfactory dysfunction may increase with older age [20]. In contrast, as age increases, the probability of shortness of breath, tachycardia and chest pain increases. This symptomology is also much more common in subjects with pre‐existing conditions [21].
The effect of substance abuse on eyes and skin, already observed in other studies not COVID‐19 related, is noticeable [22, 23]. Smoking plays an important role in amplifying the cases of ageusia and anosmia, with an increasing trend from former to current smokers [24]. Also interesting is the effect (although not fully significant) on bone and joint pain and respiratory distress.
The impact of current smoking on COVID‐19 progression is debated. Two systematic reviews published at the beginning of the pandemic described the relationship between smoking and COVID‐19 suggesting the absence of association between active smoking and enhanced risk of progression of COVID‐19 [25, 26]. Subsequent papers have illustrated the most methodological problems with these studies (i.e., lack of detailed assessments of dose and duration for smoking, missing data), and promoted a worse COVID‐19 progression and outcomes in smokers compared to other persons [27, 28].
In the present study, smoking is shown to be a risk factor in increasing the number of multiple symptoms that occur during COVID‐19. The association between lower EDSS and the presence of some symptoms is probably due to an already underlying presence of some common symptoms in most critical MS patients, which consequently for them were not reported.
Teriflunomide, natalizumab and fingolimod appear to have some sort of protective role in the development of shortness of breath, tachycardia and chest pain, whereas the role of anti‐CD20 in increasing the likelihood of neurological disorders (G6) and the occurrence of conjunctivitis and rash (G7) as well as in determining increases in the total number of concomitant symptom groups is interesting. Dimethyl fumarate seems to increase flu or flu‐like symptoms, a common effect already reported in other studies [29].
It was demonstrated that interferon‐alpha interferes with the normal regenerative processes of the olfactory mucosa, causes depletion of oligominerals (such as zinc) involved in both taste and smell perception and exerts a neurotoxic action [30]. More serious cases of anosmia and ageusia in the present study have been found in patients treated with interferon. Similar cases of association between loss of the sense of smell and loss of the sense of taste associated with the use of interferon have been reported in the medical literature [31, 32, 33].
To date few studies have investigated and described clinical characteristics of COVID‐19 patients by comparing the three waves [34, 35]. The period between the second and third wave does not correspond to a new increase in cases but rather to a long stagnation [36]. A further analysis of symptom distribution amongst the three pandemic waves showed some differences. The cause for these variations is not yet identified, although different COVID‐19 variants may explain it [37], even if Omicron seems to be detected for the first time after the follow‐up period of this study. In addition, unfortunately, this study has not collected the molecular characterization of the COVID infection, and therefore it is not possible to know the strains of each infection. In addition, also the new treatments for patients introduced starting from the second wave may have played a role in symptom reshuffling [38].
Knowing possible risk factors, modifying some lifestyle behaviours and more accurately analysing the choice of certain therapeutic approaches might minimize the occurrence of COVID‐19 symptoms.
CONFLICT OF INTEREST
Schiavetti I received consulting fees from Hippocrates Research, NovaNeuro, Sakura Italia, ADL Farmaceutici, Associazione Commissione Difesa Vista Onlus. Carmisciano L has nothing to disclose. Ponzano M has nothing to disclose. Inglese M received research grants from NIH, DOD, NMSS, FISM, and Teva Neuroscience; received fees for participating in advisory boards from Roche, Biogen, Merck and Genzyme. Radaelli M received speaker honoraria from Biogen Idec, Sanofi‐Genzyme, Novartis and Merck Serono and funding for travel to scientific meetings from Biogen Idec, Sanofi‐Genzyme, Novartis, Merck Serono, Teva and Roche. De Rossi N received speaker honoraria from Biogen Idec, Genzyme, Novartis, Sanofi‐Aventis; received funding for participation in advisory board to Novartis, Biogen and Genzyme‐Sanofi and for travel to scientific meetings from Biogen Idec, Teva, Sanofi‐Genzyme, Roche, Almirall and Novartis. Immovilli P reports personal fees from Roche, personal fees from Biogen, personal fees from Merck, outside the submitted work. Capobianco M reports personal fees and non‐financial support from Biogen, personal fees and non‐financial support from Merck Serono, personal fees and non‐financial support from Roche, personal fees and non‐financial support from Novartis, personal fees and non‐financial support from Sanofi, personal fees from Almirall, outside the submitted work. Confalonieri Phas received honoraria for speaking or consultation fees from Novartis and Biogen, has received funding for travel to attend scientific events or speaker honoraria from Merck Serono, Biogen Idec, Teva and Roche. He has also received institutional research support from Merk‐Serono, Novartis and Roche. He is also principal investigator in clinical trials for Biogen, Merck Serono, Roche. Perini P has received honoraria for lectures and support for attending meetings and travel from Roche, Biogen, Merck, Novartis, Sanofi, Teva. Trojano M reports grants and personal fees from Biogen, grants and personal fees from Novartis, grants and personal fees from Roche, grants and personal fees from Merck, personal fees from Sanofi, personal fees from TEVA, from null, outside the submitted work. Comi G reports personal fees from Novartis, Teva Pharmaceutical Industries Ltd, Teva Italia Srl, Sanofi Genzyme, Genzyme Corporation, Genzyme Europe, Merck KGgA, Merck Serono SpA, Celgene Group, Biogen Idec, Biogen Italia Srl, F. Hoffman‐La Roche, Roche SpA, Almirall SpA, Forward Pharma, Medday, Excemed, outside the submitted work. Patti F reports grants from Biogen, grants from Merck, grants from FISM, grants from Onlus association, grants from University of Catania, personal fees from Almirall, personal fees from Bayer, personal fees from Biogen, personal fees from Merck, personal fees from Roche, personal fees from Sanofi, personal fees from TEVA, outside the submitted work. Salvetti M reports grants and personal fees from Biogen, grants and personal fees from Merck, grants and personal fees from Novartis, grants and personal fees from Roche, grants and personal fees from Sanofi, grants and personal fees from Teva, grants from Italian Multiple Sclerosis Foundation, grants from Sapienza University of Rome, outside the submitted work. Sormani MP reports grants from Roche, during the conduct of the study; personal fees from Biogen, Merck, Roche, Sanofi, Novartis, Medday, Geneuro, Celgene, Mylan outside the submitted work.
ETHICS STATEMENT
The study was approved by the Regional Ethics Committee of Liguria (University of Genoa) (no. 130/2020—DB id10433) and at a national level by Agenzia Italiana del Farmaco (AIFA).
Supporting information
Appendix S1
Schiavetti I, Carmisciano L, Ponzano M, et al. Signs and symptoms of COVID‐19 in patients with multiple sclerosis. Eur J Neurol. 2022;00:1‐9. doi: 10.1111/ene.15554
MuSC‐19 Study Group members are presented in Appendix S1.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vena A, Giacobbe DR, Di Biagio A, et al. Clinical characteristics, management and in‐hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect. 2020;26(11):1537‐1544. doi: 10.1016/j.cmi.2020.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Symptoms of COVID‐19. Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 3. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID‐19). StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 4. Pettrone K, Burnett E, Link‐Gelles R, et al. Characteristics and risk factors of hospitalized and nonhospitalized COVID‐19 patients, Atlanta, Georgia, USA, March–April 2020. Emerg Infect Dis. 2021;27(4):1164‐1168. doi: 10.3201/eid2704.204709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Najafloo R, Majidi J, Asghari A, et al. Mechanism of anosmia caused by symptoms of COVID‐19 and emerging treatments. ACS Chem Nerosci. 2021;12(20):3795‐3805. doi: 10.1021/acschemneuro.1c00477 [DOI] [PubMed] [Google Scholar]
- 6. Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 disease. Cochrane Database Syst Rev. 2020;7(7):CD013665. doi: 10.1002/14651858.CD013665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elliott J, Whitaker M, Bodinier B, et al. Predictive symptoms for COVID‐19 in the community: REACT‐1 study of over 1 million people. PLoS Med. 2021;18(9):e1003777. doi: 10.1371/journal.pmed.1003777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barometro della Sclerosi Multipla 2021. © AISM. ISBN 978‐88‐7148‐152‐4
- 9. Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK. Disease modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12:217‐233. [DOI] [PubMed] [Google Scholar]
- 10. Sormani MP, De Rossi N, Schiavetti I, et al. Disease‐modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780‐789. doi: 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079‐1088. doi: 10.1001/jamaneurol.2020.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fragoso YD, Schiavetti I, Carmisciano L, et al. Coronavirus disease 2019 in Latin American patients with multiple sclerosis. Mult Scler Relat Disord. 2021;55:103173. doi: 10.1016/j.msard.2021.103173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landtblom AM, Berntsson SG, Boström I, Iacobaeus E. Multiple sclerosis and COVID‐19: the Swedish experience. Acta Neurol Scand. 2021;144(3):229‐235. doi: 10.1111/ane.13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sen S, Karabudak R, Schiavetti I, et al. The outcome of a national MS‐COVID‐19 study: what the Turkish MS cohort reveals? Mult Scler Relat Disord. 2021;52:102968. doi: 10.1016/j.msard.2021.102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. doi: 10.1038/s41591-021-01283-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kratzer B, Trapin D, Ettel P, et al. Immunological imprint of COVID‐19 on human peripheral blood leukocyte populations. Allergy. 2021;76:751‐765. doi: 10.1111/all.14647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID‐19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1001. doi: 10.1212/NXI.0000000000001001 [published correction appears in Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1050]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sierpiński R, Pinkas J, Jankowski M, et al. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders in 1942 nonhospitalized patients with coronavirus disease 2019 (COVID‐19). Pol Arch Intern Med. 2020;130:501‐505. doi: 10.20452/pamw.15414 [DOI] [PubMed] [Google Scholar]
- 19. Sehanobish E, Barbi M, Fong V, et al. COVID‐19‐induced anosmia and ageusia are associated with younger age and lower blood eosinophil counts. Am J Rhinol Allergy. 2021;35(6):830‐839. doi: 10.1177/19458924211004800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol. 2011;26(3):260‐269. doi: 10.1093/arclin/acr019 [DOI] [PubMed] [Google Scholar]
- 21. Study ZOE COVID . Is shortness of breath a symptom of COVID‐19?. Accessed April 1, 2021. https://covid.joinzoe.com/post/covid‐symptoms‐shortness‐breath
- 22. Jain NP, Shao K, Stewart C, Grant‐Kels JM. The effects of alcohol and illicit drug use on the skin. Clin Dermatol. 2021;39(5):772‐783. doi: 10.1016/j.clindermatol.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 23. Gohil H, Miskovic M, Buxton JA, Holland SP, Strike C. Smoke gets in the eye: a systematic review of case reports of ocular complications of crack cocaine use. Drug Alcohol Rev. 2021;41(2):347‐355. doi: 10.1111/dar.13366 [DOI] [PubMed] [Google Scholar]
- 24. Katotomichelakis M, Balatsouras D, Tripsianis G, et al. The effect of smoking on the olfactory function. Rhinology. 2007;45(4):273‐280. [PubMed] [Google Scholar]
- 25. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med. 2020;75:107‐108. doi: 10.1016/j.ejim.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID‐19 patients: a systematic review and meta‐analysis. Ther Adv Chronic Dis. 2020;11:204062232093576. doi: 10.1177/2040622320935765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Westen‐Lagerweij NA, Meijer E, Meeuwsen EG, et al. Are smokers protected against SARS‐CoV‐2 infection (COVID‐19)? The origins of the myth. NPJ Prim Care Respir Med. 2021;31:10. doi: 10.1038/s41533-021-00223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID‐19: a systemic review and meta‐analysis. J Med Virol. 2020;92:1915‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernández Ó, Giovannoni G, Fox RJ, et al. Efficacy and safety of delayed‐release dimethyl fumarate for relapsing–remitting multiple sclerosis in prior interferon users: an integrated analysis of DEFINE and CONFIRM. Clin Ther. 2017;39(8):1671‐1679. doi: 10.1016/j.clinthera.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 30. Equils O, Lekaj K, Wu A, Fattani S, Liu G, Rink L. Intra‐nasal zinc level relationship to COVID‐19 anosmia and type 1 interferon response: a proposal. Laryngoscope Investig Otolaryngol. 2020;6(1):21‐24. doi: 10.1002/lio2.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mayet AY. Loss of smell (anosmia) and taste (ageusia) in a patient treated with pegylated interferon alfa and ribavirin. Curr Ther Res Clin Exp. 2007;68(4):271‐277. doi: 10.1016/j.curtheres.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kraus I, Vitezic D. Anosmia induced with alpha interferon in a patient with chronic hepatitis C. Int J Clin Pharmacol Ther. 2000;38(7):360‐361. doi: 10.5414/cpp38360 [DOI] [PubMed] [Google Scholar]
- 33. Maruyama S, Hirayama C, Kadowaki Y, Sagayama A, Omura H, Nakamoto M. Interferon‐induced anosmia in a patient with chronic hepatitis C. Am J Gastroenterol. 1998;93:122‐123. [DOI] [PubMed] [Google Scholar]
- 34. Taboada M, González M, Alvarez A, et al. First, second and third wave of COVID‐19. What have we changed in the ICU management of these patients? J Infect. 2021;82(6):e14‐e15. doi: 10.1016/j.jinf.2021.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borghesi A, Golemi S, Carapella N, Zigliani A, Farina D, Maroldi R. Lombardy, northern Italy: COVID‐19 second wave less severe and deadly than the first? A preliminary investigation. Infect Dis (Lond). 2021;53(5):370‐375. doi: 10.1080/23744235.2021.1884745 [DOI] [PubMed] [Google Scholar]
- 36. Jung C, Excoffier JB, Raphaël‐Rousseau M, Salaün‐Penquer N, Ortala M, Chouaid C. Evolution of hospitalized patient characteristics through the first three COVID‐19 waves in Paris area using machine learning analysis. PLoS One. 2022;17(2):e0263266. doi: 10.1371/journal.pone.0263266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hodcroft EB, Zuber M, Nadeau S, et al. Spread of a SARS‐CoV‐2 variant through Europe in the summer of 2020. Nature. 2021;595(7869):707‐712. doi: 10.1038/s41586-021-03677-y [DOI] [PubMed] [Google Scholar]
- 38. Lam S, Lombardi A, Ouanounou A. COVID‐19: a review of the proposed pharmacological treatments. Eur J Pharmacol. 2020;886:173451. doi: 10.1016/j.ejphar.2020.173451 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


